ABSTRACT
Vulvovaginal candidiasis (VVC) is a common condition among women. Fluconazole remains the dominant treatment option for VVC. Oteseconazole is a highly selective inhibitor of fungal CYP51. This randomized, double-blinded, phase 3 trial was conducted to evaluate the efficacy and safety of oteseconazole compared with fluconazole in treating severe VVC. Female subjects presenting with vulvovaginal signs and symptoms score of ≥7 and positive Candida infection determined by potassium hydroxide test or Gram staining were randomly assigned to receive oteseconazole (600 mg on D1 and 450 mg on D2) or fluconazole (150 mg on D1 and D4) in a 1:1 ratio. The primary endpoint was the proportion of subjects achieving therapeutic cure [defined as achieving both clinical cure (absence of signs and symptoms of VVC) and mycological cure (negative culture of Candida species)] at D28. A total of 322 subjects were randomized and 321 subjects were treated. At D28, a statistically significantly higher proportion of subjects achieved therapeutic cure in the oteseconazole group than in the fluconazole group (66.88% vs 45.91%; P = 0.0002). Oteseconazole treatment resulted in an increased proportion of subjects achieving mycological cure (82.50% vs 59.12%; P < 0.0001) and clinical cure (71.25% vs 55.97%; P = 0.0046) compared with fluconazole. The incidence of treatment-emergent adverse events was similar between the two groups. No subjects discontinued study treatment or withdrew study due to adverse events. Oteseconazole showed statistically significant and clinically meaningful superiority over fluconazole for the treatment of severe VVC and was generally tolerated.
KEYWORDS: oteseconazole, Candida albicans, severe vulvovaginal candidiasis, fluconazole, Candida glabrata
INTRODUCTION
Vulvovaginal candidiasis (VVC) is a mucosal fungal infectious disease that causes vulvovaginal itching, irritation, burning, soreness, fissuring, redness, vaginal discharge, and dyspareunia (1). Approximately 80% of women experience at least one occurrence of VVC in their lifetime, with a much higher incidence in women of reproductive age than menopausal women, seriously impacting their quality of life (2, 3). Despite that VVC is predominantly caused by Candida albicans (C. albicans), non-C. albicans species, especially Candida glabrata (C. glabrata), have been increasingly recognized as pathogens associated with VVC (4). Oral fluconazole remains the dominant treatment option for VVC and has achieved great success (5, 6). However, concerns have been raised regarding the development of drug resistance and reduced activity against non-C. albicans species (7, 8). In a previous in vitro antifungal susceptibility study of Candida species, it was observed that the resistance to fluconazole was noticeably higher in C. glabrata compared to C. albicans, with resistance rates of 73.3% and 16.6%, respectively (9). In addition, despite the remarkable therapeutic efficacy observed during fluconazole treatment, approximately 50% of the women experience disease relapse within 6 months of fluconazole cessation (7, 8). Therefore, novel antifungal drugs are still in great need.
Oteseconazole (formerly designated as VT-1161) is a novel, oral, highly selective inhibitor of fungal CYP51. Oteseconazole demonstrated a more than 2,000-fold selectivity for fungal CYP51 over human CYPs, thus potentially reducing the safety concerns caused by off-target toxicities (10). In an antifungal activity test against a panel of clinical isolates of Candida species, oteseconazole showed superior activity, with an average potency of a more than 40-fold greater than fluconazole for most species (11). More importantly, for fluconazole-resistant C. glabrata, the minimal inhibitory concentration (MIC) of oteseconazole was 64-fold lower than fluconazole (11, 12).
Previous phase 2 and phase 3 global studies with oteseconazole have demonstrated clinically meaningful efficacy in treating recurrent vulvovaginal candidiasis (RVVC), which was defined as ≥3 episodes of VVC within ≤12 months (13 – 16). In this phase 3 study, we compared the efficacy and safety of oteseconazole versus fluconazole for the treatment of severe VVC in Chinese women.
MATERIALS AND METHODS
Subject population
Female subjects aged ≥18 and ≤75 years with vulvovaginal signs and symptoms (VSS) score ≥7 were enrolled. The VSS score is determined with a standardized and predefined scoring system in which each sign (congestion and edema, scratches, rhagades and erosions, secretion volume) and symptom (itching, pain) was given a numerical rating based on severity. The severity of each item was graded from 0 to 3 (absent = 0, mild = 1, moderate = 2, and severe = 3), except for the item “scratches, rhagades and erosions” (absent = 0 and present = 3) (17). In addition, positive Candida species determined by the microscopic examination of a wet mount of vaginal discharge with potassium hydroxide (KOH) or Gram staining were required. For women of child-bearing potential, a negative pregnancy test at screening was a prerequisite, as well as the usage of contraception throughout the study and 6 months after the last administration of treatments. Subjects with RVVC or a history of RVVC or with concomitant vulvovaginitis caused by other pathogens were excluded. Other major exclusion criteria included the use of antifungal treatments (systemic and/or topical), CYP3A4 substrates, CYP3A4 inducers or inhibitors, and vulvovaginal corticosteroids; estrogen replacement therapy within 7 days before randomization; use of systemic corticosteroid treatments within 30 days before randomization or systemic immunosuppressant treatments within 90 days before randomization; a history of cervical cancer; or moderate to severe hepatic and/or renal disorders.
This study was performed between April 2021 and October 2021 at 26 sites in China. The study was conducted in accordance with the Declaration of Helsinki and the Guidelines for Good Clinical Practice. Each study site obtained independent ethics committee approval before the study initiation, and each subject provided written informed consent before participating in the study. This study was registered in ClinicalTrials.gov (NCT04956419).
Study design and procedures
Subjects were randomly assigned in a 1:1 ratio to the oteseconazole group or the fluconazole group. Subjects, investigators, site personnel, and the sponsor were all blinded to the treatment. To maintain blinding, all randomized subjects received matching oteseconazole or fluconazole placebo based on the treatment regimen, and study treatments had the same appearance.
In the oteseconazole group, subjects were orally administered with oteseconazole 600 mg (150 mg per capsule) and fluconazole matching placebo 150 mg (50 mg per capsule) on D1, oteseconazole 450 mg on D2, and fluconazole matching placebo 150 mg on D4. In the fluconazole group, subjects were orally administered with fluconazole 150 mg and oteseconazole matching placebo 600 mg on D1, oteseconazole matching placebo 450 mg on D2, and fluconazole 150 mg on D4. If clinically meaningful relief was not achieved, symptoms worsened, or signs of intolerance appeared and microscopic examination confirmed positive Candida infection 1 week after the initial dose, rescue therapy with clotrimazole vaginal tablet (Canesten, Bayer), 500 mg on D1 and D4, could be provided at the discretion of the investigator. A subject diary was used to record adverse events (AEs), study drug exposure, and concomitant medications from screening till the last visit and was retrieved at each visit.
Assessments and outcome measures
At baseline, D14, and D28, assessments of efficacy were performed in terms of VVC VSS scores (17); vaginal secretion fungal culture, strain identification, and in vitro antifungal susceptibility testing conducted by qualified central laboratory per Clinical and Laboratory Standards Institute M59 and M60 guidelines (18, 19) and European Committee on Antimicrobial Susceptibility Testing methods; and microscopic examination of vaginal secretion pathogen by KOH or Gram staining. The baseline was defined as the last measurement before treatment administration.
Safety was assessed by monitoring AEs, physical examinations, laboratory tests (hematology, clinical chemistry, and urinalysis), vital signs, and electrocardiograms (ECGs) from the time of signed consent to the end of the study. For women with child-bearing potential, a blood pregnancy test was performed at screening and at the D28 visit.
Endpoints
The primary efficacy endpoint was the proportion of subjects achieving therapeutic cure at D28, defined as the achievement of both clinical cure (absence of signs and symptoms of VVC) and mycological cure (negative culture of vaginal swabs for growth of Candida species). The secondary efficacy endpoints included the proportion of subjects reaching therapeutic cure at D14, the proportion of subjects achieving clinical cure at D14 and D28, the proportion of subjects achieving mycological cure at D14 and D28, and the percentage of subjects taking rescue medication during study treatment.
Statistical analyses
Efficacy analyses were performed in the modified intention-to-treat (mITT) population, which included all randomized subjects with positive mycological culture of Candida species at screening. For efficacy analyses, categorical data were summarized as percentages, with 95% confidence intervals (CIs) calculated by the Clopper-Pearson method. Treatment differences between the two groups were detected using Fisher’s exact probability test, and the 95% CI of rate difference was calculated by the normal approximate method. For the endpoints of therapeutic cure, clinical cure, and mycological cure rates, analyses in the mITT subpopulation of subjects testing positive for C. albicans were also performed. Safety analysis set included all randomized subjects who received at least one dose of study treatment. AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 24.1 and summarized by preferred term (PT) with a breakdown of treatment groups. All analyses were conducted using SAS software version 9.4 (SAS Institute Inc, Cary, North Carolina).
A sample size of 320 subjects (1:1 randomization) was required to provide 85% power to detect a non-inferiority margin of 12% between the oteseconazole group and the fluconazole group, with a one-sided α of 0.025 of Fisher’s exact probability test, a 5% negative rate of vaginal swabs culture, a 10% dropout rate, and assuming therapeutic cure rates of 70% and 65% for the oteseconazole and fluconazole groups at D28 visit, respectively (13).
If the lower limit of the 95% CI for the between group difference was greater than −12% in the primary endpoint analysis, non-inferiority test was met. If non-inferiority was demonstrated, oteseconazole was to be tested for superiority compared with fluconazole for the primary endpoint. All statistical tests for the efficacy endpoints, except for the non-inferiority test in the primary analysis, were tested using a two-sided α of 0.05.
RESULTS
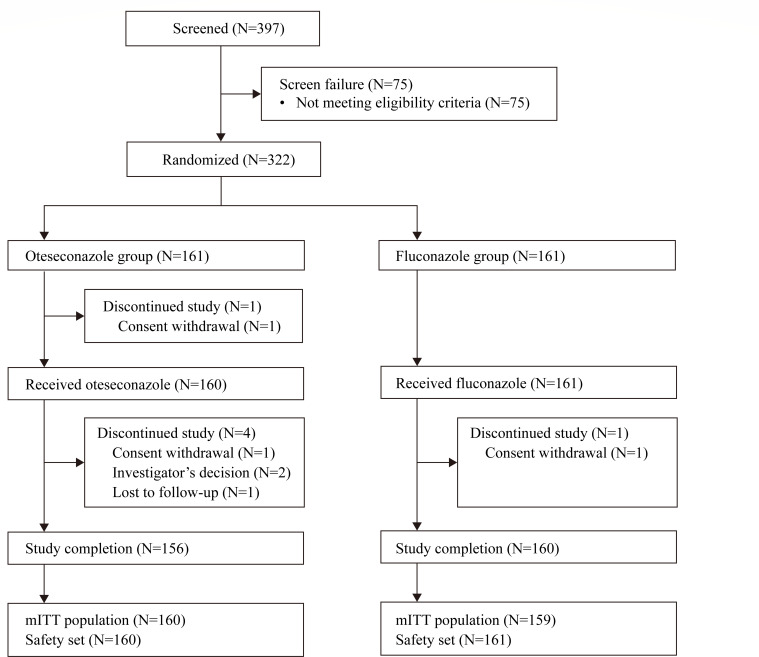
A total of 322 female subjects were randomized, with 161 subjects each in the oteseconazole and fluconazole groups, respectively. A total of 319 subjects had a confirmed positive culture for Candida species at screening and were included in the mITT population for efficacy evaluations. All subjects received treatments and were included in the safety analysis set, except for one subject in the oteseconazole group. A total of 316 subjects completed the study, with the reasons for premature study withdrawal being consent withdrawal (n = 3), investigator’s decisions (n = 2), and lost to follow-up (n = 1) (Fig. 1).
Fig 1.
Subject disposition. Abbreviations: N, number of subjects; mITT, modified intention-to-treat.
The demographics and baseline disease characteristics were generally balanced between the two groups. Baseline mean VSS score was comparable between the oteseconazole (8.7) and fluconazole groups (8.4), with a range of 7–14 in both groups. Most subjects tested positive culture for C. albicans (78.1%), followed by C. glabrata (15.4%). In vitro susceptibility testing demonstrated that 309 and 233 strains were sensitive to oteseconazole and fluconazole, 6 and 32 strains were resistant to oteseconazole and fluconazole, 0 and 53 strains were dose-dependently sensitive to oteseconazole and fluconazole, respectively (Table 1). Oteseconazole yielded a lower MIC value for 90% of the organisms (MIC90) than fluconazole for clinical isolates of C. albicans (0.25 vs 4 µg/mL) and C. glabrata (4 vs 16 µg/mL) (Table S1).
TABLE 1.
Demographics and baseline disease characteristics (mITT) c
| Oteseconazole (N = 160) | Fluconazole (N = 159) | |
|---|---|---|
| Age (year) | ||
| Mean (SD) | 29.9 (8.0) | 31.2 (7.5) |
| Median (min, max) | 29 (18, 70) | 31 (18, 50) |
| Ethnicity | ||
| Han | 152 (95.0) | 149 (93.7) |
| Other | 8 (5.0) | 10 (6.3) |
| Weight (kg) | ||
| Mean (SD) | 56.6 (9.2) | 55.6 (8.7) |
| Median (min, max) | 55.0 (42.0, 95.0) | 55.0 (40.0, 90.0) |
| BMI (kg/m2) | ||
| Mean (SD) | 21.4 (3.5) | 21.5 (3.2) |
| Median (min, max) | 20.8 (15.6, 37.1) | 20.8 (16.4, 32.9) |
| Composite VSS score | ||
| Mean (SD) | 8.7 (1.8) | 8.4 (1.8) |
| Median (min, max) | 8.0 (7, 14) | 8.0 (7, 14) |
| Candida species a | ||
| Candida albicans | 128 (80.0) | 121 (76.1) |
| Candida glabrata | 22 (13.8) | 27 (17.0) |
| Candida tropicalis | 5 (3.1) | 3 (1.9) |
| Candida krusei | 1 (0.6) | 2 (1.3) |
| Candida spherical | 2 (1.3) | 2 (1.3) |
| Candida parapsilosis | 1 (0.6) | 2 (1.3) |
| Kodamaea ohmeri | 0 | 1 (0.6) |
| Candida dubliniensis | 1 (0.6) | 0 |
| Saccharomyces cerevisiae | 0 | 1 (0.6) |
| Candida lusitaniae | 0 | 1 (0.6) |
| Susceptibility testing b | ||
| Sensitive | 309 (96.6) | 233 (72.8) |
| Resistant | 6 (1.9) | 32 (10.0) |
| Dose-dependently sensitive | 0 | 53 (16.6) |
| Wild strain | 2 (0.6) | 2 (0.6) |
| Unknown | 3 (0.9) | 0 |
Determined by mycological culture of vaginal secretion.
A total of 320 fungal strains were isolated from 319 subjects and 320 was set as the denominator. One subject in the fluconazole group presented with two strains (Candida parapsilosis and Kodamaea ohmeri).
Data are n (%) unless otherwise indicated. Abbreviations: BMI, body mass index; max, maximum; min, minimum; mITT, modified intention-to-treat; N, number of subjects in the mITT population; SD, standard deviation; VSS, vulvovaginal signs and symptoms.
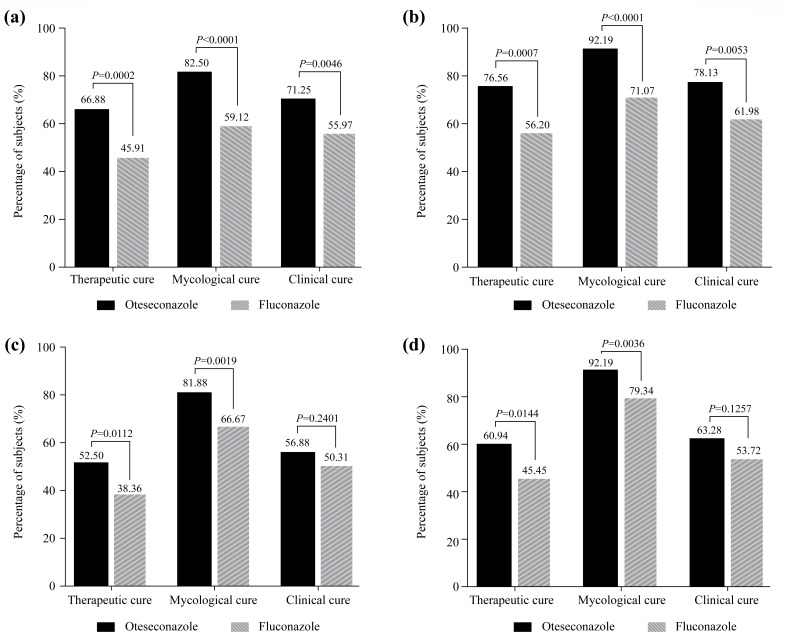
At D28, a statistically significantly higher proportion of subjects achieved therapeutic cure in the oteseconazole group than in the fluconazole group [66.88% vs 45.91%; difference (95% CI): 20.96% (10.32, 31.60); P = 0.0002] (Fig. 2a; Table S2). Consistent results were observed in subjects with a positive culture of C. albicans, where therapeutic cure was reported in 76.56% of subjects in the oteseconazole group versus 56.20% in the fluconazole group at D28 [difference (95% CI): 20.36% (8.87, 31.85); P = 0.0007] (Fig. 2b; Table S2).
Fig 2.
Efficacy results at D28 in the mITT population (a), at D28 in the mITT subpopulation with positive culture for Candida albicans (b), at D14 in the mITT population (c),and at D14 in the mITT subpopulation with positive culture for Candida albicans (d). Abbreviation: mITT, modified intention-to-treat.
Results of the secondary endpoints were supportive of the primary endpoint. At D28, the proportion of subjects achieving mycological cure was significantly higher in the oteseconazole group than in the fluconazole group [82.50% vs 59.12%; difference (95% CI): 23.38% (13.73, 33.03); P < 0.0001], which was also true in the subjects with a positive culture of C. albicans [92.19% vs 71.07%; difference (95% CI): 21.11% (11.79, 30.43); P < 0.0001]. A significantly higher clinical cure rate at D28 was shown with the treatment of oteseconazole versus fluconazole, in the overall mITT population [71.25% vs 55.97%; difference (95% CI): 15.28% (4.85, 25.70); P = 0.0046], as well as in the subpopulation of subjects with positive C. albicans [78.13% vs 61.98%; difference (95% CI): 16.14% (4.91, 27.37); P = 0.0053] (Fig. 2a and b; Table S2).
At D14, the proportions of subjects experiencing therapeutic cure and mycological cure were significantly higher in the oteseconazole group than in the fluconazole group [therapeutic cure rate: 52.50% vs 38.36%; difference (95% CI): 14.14% (3.32, 24.95); P = 0.0112 and mycological cure rate: 81.88% vs 66.67%; difference (95% CI): 15.21% (5.76, 24.66); P = 0.0019] (Fig. 2c; Table S3). Consistent results were observed in the subpopulation of subjects with positive C. albicans [therapeutic cure rate: 60.94% vs 45.45%; difference (95% CI): 15.48% (3.23, 27.74); P = 0.0144 and mycological cure rate: 92.19% vs 79.34%; difference (95% CI): 12.85% (4.27, 21.43); P = 0.0036] (Fig. 2d; Table S3). However, the clinical cure rate at D14 was similar between the two groups in the overall mITT population (56.88% in the oteseconazole group vs 50.31% in the fluconazole group, P = 0.2401) and in subjects with positive C. albicans (63.28% in the oteseconazole group vs 53.72% in the fluconazole group, P = 0.1257) (Table S3).
Antifungal rescue medication usage was reported in 3.75% of subjects receiving oteseconazole versus 14.47% of subjects receiving fluconazole, and such difference was statistically significant (P = 0.0009).
In the oteseconazole group, one subject missed one capsule of oteseconazole on D1 and another subject missed one capsule of fluconazole matching placebo on D4, and all the other subjects administered treatments per the protocol, indicating a high compliance. There were 51.3% and 42.9% of subjects in the oteseconazole group and the fluconazole group, respectively, reporting at least one treatment-emergent adverse event (TEAE), among whom 21.9% and 19.9% of subjects experienced at least one treatment-related adverse event (TRAE), respectively. All TEAEs were mild or moderate in severity. In the oteseconazole group, the most commonly reported TEAEs by PT were urinary tract infection (8.1%), bacterial vulvovaginitis (4.4%), dizziness (3.8%), and headache (3.8%); while in the fluconazole group, the most commonly reported TEAEs by PT were bacterial vulvovaginitis (7.5%), bacterial vaginosis (6.2%), urinary tract infection (4.3%), and nausea (3.1%) (Table 2). No subjects discontinued treatments or study due to TEAEs. No deaths occurred in this study. One subject in the fluconazole group reported a serious TEAE of threatened abortion, which was deemed as unrelated to the treatment by the investigator. During the study, there were no clinically significant changes in the vital signs, physical examination findings, ECGs, or laboratory parameters in either group.
TABLE 2.
Treatment-emergent adverse events with an incidence >1% in either group by preferred term (safety set) a
| Preferred term | Oteseconazole (N = 160) | Fluconazole (N = 161) |
|---|---|---|
| Urinary tract infection | 13 (8.1) | 7 (4.3) |
| Bacterial vulvovaginitis | 7 (4.4) | 12 (7.5) |
| Dizziness | 6 (3.8) | 3 (1.9) |
| Headache | 6 (3.8) | 1 (0.6) |
| Nausea | 5 (3.1) | 5 (3.1) |
| Upper respiratory tract infection | 5 (3.1) | 4 (2.5) |
| Abdominal discomfort | 4 (2.5) | 1 (0.6) |
| Abdominal pain | 3 (1.9) | 1 (0.6) |
| Blood creatine phosphokinase increased | 3 (1.9) | 2 (1.2) |
| Diarrhoea | 3 (1.9) | 2 (1.2) |
| Lethargy | 3 (1.9) | 2 (1.2) |
| Abnormal uterine bleeding | 2 (1.3) | 0 |
| Aspartate aminotransferase increased | 2 (1.3) | 1 (0.6) |
| Atrial escape rhythm | 2 (1.3) | 0 |
| Bacterial vaginosis | 2 (1.3) | 10 (6.2) |
| Bilirubin conjugated increased | 2 (1.3) | 2 (1.2) |
| Blood cholesterol increased | 2 (1.3) | 1 (0.6) |
| Blood triglycerides increased | 2 (1.3) | 1 (0.6) |
| Blood uric acid increased | 2 (1.3) | 3 (1.9) |
| Dry mouth | 2 (1.3) | 0 |
| Lipids increased | 2 (1.3) | 0 |
| Menstrual disorder | 2 (1.3) | 0 |
| Mycoplasma infection | 2 (1.3) | 1 (0.6) |
| Nasopharyngitis | 2 (1.3) | 0 |
| Proteinuria | 2 (1.3) | 0 |
| Rash | 2 (1.3) | 3 (1.9) |
| Urine ketone body present | 2 (1.3) | 0 |
| White blood cells urine positive | 2 (1.3) | 0 |
| Anaemia | 1 (0.6) | 4 (2.5) |
| Asthenia | 1 (0.6) | 3 (1.9) |
| Back pain | 1 (0.6) | 2 (1.2) |
| White blood cell count decreased | 1 (0.6) | 2 (1.2) |
| Abdominal pain upper | 0 | 2 (1.2) |
| Decreased appetite | 0 | 2 (1.2) |
| Neutrophil count decreased | 0 | 2 (1.2) |
| QRS axis abnormal | 0 | 2 (1.2) |
Data are n (%). Abbreviations: N, number of subjects in the safety set.
DISCUSSION
Primarily based on the global pivotal phase 3 studies of VIOLET (NCT03562156 and NCT03561701) and ultraVIOLET (NCT03840616), oteseconazole (Vivjoa) was approved by the Food and Drug Administration (FDA) to reduce the incidence of RVVC in April 2022. This randomized, double-blinded, positive-controlled, phase 3 study was conducted to evaluate the efficacy and safety of oteseconazole versus fluconazole in treating severe VVC in Chinese women.
After adequate discussion with and agreed upon by the investigators and health authority, we used a locally standardized VSS scoring system for screening and efficacy evaluations according to the current VVC diagnosis and treatment guidance in China (17). This scoring system is modified based on the global standard and is rendered to better suit the Chinese women suffering from VVC and reflect their conditions more accurately. The decision of choosing the time point of Day 28 to assess the efficacy primarily was in accordance with the FDA guidance on drug development for VVC treatments issued in 2019 (20), in which the primary endpoint for the efficacy evaluations of systemic drugs with long half-time was recommended to be assessed at D21 to D30.
The results of MICs for clinical isolates of Candida species and in vitro susceptibility test favored oteseconazole over fluconazole. At D28, oteseconazole demonstrated statistically significant and clinically meaningful superiority over fluconazole (66.88% vs 45.91%; P = 0.0002) in the primary endpoint of therapeutic cure rate, and consistent results were observed in the secondary endpoints of clinical cure and mycological cure rates at D28. In line with the reported epidemiology in VVC, C. albicans was the causative agent for the majority of subjects (78.1%) in this study (1). As expected, the results of therapeutic cure, clinical cure, and mycological cure rates at D28 in subjects tested positive for C. albicans were consistent with those in the overall mITT population. In addition to potent antifungal clinical activity in the treatment of VVC, oteseconazole treatment was potentially associated with reduced VVC recurrence and sustained treatment effect (13). In a phase 2 study in VVC subjects, subjects in the oteseconazole group showed no evidence of mycological recurrence whereas 28.6% and 46.1% of subjects in the fluconazole group had mycological recurrence at D84 and D168, respectively (13).
Oteseconazole was generally tolerated in subjects with severe VVC. The incidence of TEAEs was similar between the oteseconazole and the fluconazole groups. In the oteseconazole group, the most commonly reported TEAEs were urinary tract infection and bacterial vulvovaginitis, which were expected symptoms in subjects with severe VVC. Other commonly reported TEAEs in the oteseconazole group were mainly in the infection and infestations, gastrointestinal disorders, nervous system disorders, and system organ classes, which were also typical events of azole antifungal agents. In the fluconazole group, the most frequently observed TEAEs were bacterial vulvovaginitis, bacterial vaginosis, and urinary tract infection, which were similar to that reported in the previous study (15).
The potential pregnancy risk in the use of azole agents is an important concern for patients with VVC. The FDA has alerted the public that the use of chronic, high doses of fluconazole during the first trimester of pregnancy may be associated with birth defects (21). Based on the previous rat studies, oteseconazole is contraindicated in females of reproductive potential and in pregnant or lactating women (22). In the present study, nine subjects (two in the oteseconazole group and seven in the fluconazole group) reported pregnancy during the study, all chose elective termination. No treatment-related pregnancy risks were reported in this study or previous studies. At the time being, there are limited data on pregnant women who were exposed to oteseconazole during clinical trials, and these data are insufficient to exclude potential risks to human infants. Further studies are warranted to investigate the impact of oteseconazole on reproductivity and fertility and inform the optimal usage and relative precautions for women of reproductive potential.
This study had several limitations. Firstly, we did not perform long-term follow-up to evaluate the effect of oteseconazole on reducing VVC recurrence. However, in the three completed phase 3 studies (NCT03562156, NCT03561701, and NCT03840616) where subjects with RVVC were treated and followed up for more than 1 year, oteseconazole significantly reduced VVC recurrence rate. In addition, long-term follow-up did not result in any increase in toxicity, with no serious TRAEs or deaths reported. There were only few severe TEAEs leading to treatment discontinuations observed (15, 22, 23). Another limitation was that this study did not assess the antifungal effect of oteseconazole and fluconazole against non-C. albicans species due to the very small number of isolates. However, it is worth noting that preclinical studies of oteseconazole showed potent antifungal activity against non-C. albicans species both in vitro and in vivo (11, 12). Nonetheless, additional clinical data demonstrating such efficacy would be very insightful, especially considering that infections caused by non-C. albicans species are on the rise (24, 25). In the future, a global, multicenter trial to investigate the efficacy and safety of oteseconazole among a diverse population of patients presenting with various Candida species from diverse ethnic backgrounds is substantial.
In conclusion, this phase 3 study demonstrated statistically significant and clinically meaningful superiority of oteseconazole over fluconazole in the treatment of severe VVC. Additionally, oteseconazole was generally tolerated. The totality of evidence suggested that oteseconazole could serve as a treatment option in women suffering from severe VVC, with improved efficacy and a desirable safety profile.
ACKNOWLEDGMENTS
We sincerely thank all the subjects and their families, investigators, and investigational staff for participating in this study. We would like to acknowledge the contributions of Xiaopeng Wang (Jiangsu Hengrui Pharmaceuticals Co., Ltd) in the study design and Hao Yu (Department of Biostatistics, School of Public Health, Nanjing Medical University, China) in statistical analyses and outputs programming support. Medical writing support was provided by Haofeng Ding (Jiangsu Hengrui Pharmaceuticals Co., Ltd) per Good Publication Practice Guidelines.
This study was supported by Jiangsu Hengrui Pharmaceuticals Co., Ltd. (Shanghai, China), who contributed to the study conduction as the sponsor and provided assistance in writing the manuscript.
X.Q.W. was the coordinating investigator and was involved in the study execution, data analysis and interpretation, and manuscript writing. Q.P.L. was the principal investigator, and she was involved in the study conception, protocol development and review, data analysis, and manuscript review. Q.R.W. and F.W. were involved in the study design, study oversight, data analysis and interpretation, and manuscript writing and review. J.L.B. was involved in the statistical analyses plan development, outputs programming, data analysis, and manuscript review. All the other authors were the investigators, and they were involved in protocol review, study execution, data analysis, and manuscript review. All authors contributed substantially to the work. All authors had full access to the data and were responsible for the integrity and accuracy of the data. All authors approved the final version of the manuscript to be published.
Contributor Information
Qinping Liao, Email: qinping_liao@163.com.
Damian J. Krysan, University of Iowa, Iowa City, Iowa, USA
DATA AVAILABILITY
All the data underlying the findings of this manuscript are available from the corresponding author. Qualified scientific and medical researchers may request access to the data by sending an email to the corresponding author (qinping_liao@163.com); the corresponding author and sponsor will grant access to the data if the request is deemed appropriate.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/aac.00778-23.
Susceptibility analysis and summary of efficacy results.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Sobel JD. 2007. Vulvovaginal candidosis. Lancet 369:1961–1971. doi: 10.1016/S0140-6736(07)60917-9 [DOI] [PubMed] [Google Scholar]
- 2. Yano J, Sobel JD, Nyirjesy P, Sobel R, Williams VL, Yu Q, Noverr MC, Fidel PL. 2019. Current patient perspectives of vulvovaginal candidiasis: Incidence, symptoms, management and post-treatment outcomes. BMC Womens Health 19:48. doi: 10.1186/s12905-019-0748-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gonçalves B, Ferreira C, Alves CT, Henriques M, Azeredo J, Silva S. 2016. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol 42:905–927. doi: 10.3109/1040841X.2015.1091805 [DOI] [PubMed] [Google Scholar]
- 4. Denning DW, Kneale M, Sobel JD, Rautemaa-Richardson R. 2018. Global burden of recurrent vulvovaginal candidiasis: a systematic review. Lancet Infect Dis 18:e339–e347. doi: 10.1016/S1473-3099(18)30103-8 [DOI] [PubMed] [Google Scholar]
- 5. Sherrard J, Wilson J, Donders G, Mendling W, Jensen JS. 2018. European (IUSTI/WHO) International Union against Sexually Transmitted Infections (IUSTI) World Health Organisation (WHO) guideline on the management of vaginal discharge. Int J STD AIDS 29:1258–1272. doi: 10.1177/0956462418785451 [DOI] [PubMed] [Google Scholar]
- 6. Workowski KA, Bachmann LH, Chan PA, Johnston CM, Muzny CA, Park I, Reno H, Zenilman JM, Bolan GA. 2021. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep 70:1–187. doi: 10.15585/mmwr.rr7004a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sobel JD, Wiesenfeld HC, Martens M, Danna P, Hooton TM, Rompalo A, Sperling M, Livengood C III, Horowitz B, Von Thron J, Edwards L, Panzer H, Chu T-C. 2004. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med 351:876–883. doi: 10.1056/NEJMoa033114 [DOI] [PubMed] [Google Scholar]
- 8. Collins LM, Moore R, Sobel JD. 2020. Prognosis and long-term outcome of women with idiopathic recurrent vulvovaginal candidiasis caused by Candida albicans. J Low Genit Tract Dis 24:48–52. doi: 10.1097/LGT.0000000000000496 [DOI] [PubMed] [Google Scholar]
- 9. Wang F-J, Zhang D, Liu Z-H, Wu W-X, Bai H-H, Dong H-Y. 2016. Species distribution and in vitro antifungal susceptibility of vulvovaginal Candida isolates in China. Chin Med J (Engl) 129:1161–1165. doi: 10.4103/0366-6999.181964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Warrilow AGS, Hull CM, Parker JE, Garvey EP, Hoekstra WJ, Moore WR, Schotzinger RJ, Kelly DE, Kelly SL. 2014. The clinical candidate VT-1161 is a highly potent inhibitor of Candida albicans CYP51 but fails to bind the human enzyme. Antimicrob Agents Chemother 58:7121–7127. doi: 10.1128/AAC.03707-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sobel JD, Nyirjesy P. 2021. Oteseconazole: an advance in treatment of recurrent vulvovaginal candidiasis. Future Microbiol 16:1453–1461. doi: 10.2217/fmb-2021-0173 [DOI] [PubMed] [Google Scholar]
- 12. Schell WA, Jones AM, Garvey EP, Hoekstra WJ, Schotzinger RJ, Alexander BD. 2017. Fungal CYP51 inhibitors VT-1161 and VT-1129 exhibit strong in vitro activity against Candida glabrata and C. krusei isolates clinically resistant to azole and echinocandin antifungal compounds. Antimicrob Agents Chemother 61:e01817-16. doi: 10.1128/AAC.01817-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brand SR, Sobel JD, Nyirjesy P, Ghannoum MA, Schotzinger RJ, Degenhardt TP. 2021. A randomized phase 2 study of VT-1161 for the treatment of acute vulvovaginal candidiasis. Clin Infect Dis 73:e1518–e1524. doi: 10.1093/cid/ciaa1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brand SR, Degenhardt TP, Person K, Sobel JD, Nyirjesy P, Schotzinger RJ, Tavakkol A. 2018. A phase 2, randomized, double-blind, placebo-controlled, dose-ranging study to evaluate the efficacy and safety of orally administered VT-1161 in the treatment of recurrent vulvovaginal candidiasis. Am J Obstet Gynecol 218:624. doi: 10.1016/j.ajog.2018.03.001 [DOI] [PubMed] [Google Scholar]
- 15. Martens MG, Maximos B, Degenhardt T, Person K, Curelop S, Ghannoum M, Flynt A, Brand SR. 2022. Phase 3 study evaluating the safety and efficacy of oteseconazole in the treatment of recurrent vulvovaginal candidiasis and acute vulvovaginal candidiasis infections. Am J Obstet Gynecol 227:880. doi: 10.1016/j.ajog.2022.07.023 [DOI] [PubMed] [Google Scholar]
- 16. de Cássia Orlandi Sardi J, Silva DR, Anibal PC, de Campos Baldin JJCM, Ramalho SR, Rosalen PL, Macedo MLR, Hofling JF. 2021. Vulvovaginal candidiasis: epidemiology and risk factors, pathogenesis resistance, and new therapeutic options. Curr Fungal Infect Rep 15:32–40. doi: 10.1007/s12281-021-00415-9 [DOI] [Google Scholar]
- 17. Liu Z, Liao Q. 2012. Amendment of the standards for the diagnosis and treatment of vulvovaginal candidiasis (VVC). Chin Med J 28:401–402. [Google Scholar]
- 18. CLSI . 2018. CLSI supplement M59. In Epidemiological cutoff values for antifungal susceptibility testing, 2nd ed. Clinical and Laboratory Standards Institute, Wayne, PA. https://clsi.org/media/1934/m59ed2_sample-updated.pdf. [Google Scholar]
- 19. CLSI . 2017. CLSI supplement M60. In Performance standards for antifungal susceptibility testing of yeasts, 1st ed. Clinical and Laboratory Standards Institute, Wayne, PA. https://clsi.org/media/1895/m60ed1_sample.pdf. [Google Scholar]
- 20. US Food and Drug Administration . Guidance for industry. Vulvovaginal candidiasis: developing drugs for treatment. Available from: https://www.fda.gov/. Accessed 09 April 2023
- 21. US Food and Drug Administration . 2011. FDA Drug Safety Communication:Use of long-term, high-dose Diflucan (fluconazole) during pregnancy may be associated with birth defects in infants. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communicationuse-long-term-high-dose-diflucan-fluconazole-during-pregnancy-may-be. Accessed 07 Aug 2023.
- 22. US Food and Drug Administration . 2022. Vivjoa (oteseconazole capsules). Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215888s000lbl.pdf. Accessed 09 Apr 2023.
- 23. Sobel JD, Donders G, Degenhardt T, Person K, Curelop S, Ghannoum M, Brand SR. 2022. Efficacy and safety of oteseconazole in recurrent vulvovaginal candidiasis. NEJM Evidence 1. doi: 10.1056/EVIDoa2100055 [DOI] [PubMed] [Google Scholar]
- 24. Makanjuola O, Bongomin F, Fayemiwo SA. 2018. An update on the roles of non-albicans Candida species in vulvovaginitis. J Fungi (Basel) 4:121. doi: 10.3390/jof4040121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mintz JD, Martens MG. 2013. Prevalence of non-albicans Candida infections in women with recurrent vulvovaginal symptomatology. Adv Infect Dis 03:238–242. doi: 10.4236/aid.2013.34035 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Susceptibility analysis and summary of efficacy results.
Data Availability Statement
All the data underlying the findings of this manuscript are available from the corresponding author. Qualified scientific and medical researchers may request access to the data by sending an email to the corresponding author (qinping_liao@163.com); the corresponding author and sponsor will grant access to the data if the request is deemed appropriate.