Abstract
Diabetic peripheral neuropathy is the most prominent microvascular complication of diabetes mellitus and the leading cause of ulceration, amputation, and extended hospitalization. Evidence regarding the magnitude and factors associated with diabetic peripheral neuropathy is not well documented in Ethiopia, particularly in the study area. A facility-based cross-sectional study was conducted among 293 adult type 2 diabetic patients who were on treatment and follow-up from May to June 31, 2023. To select participants in the study, a systematic random sampling method was utilized. Data were collected using semi-structured questionnaires and medical record reviews. The Michigan Neuropathy Screening Instrument (MNSI) was employed to assess diabetic peripheral neuropathy. To model the association between diabetic peripheral neuropathy and independent variables, binary logistic regression model was used. An adjusted odds ratio with a 95% confidence interval was used to estimate the association and statistical significance was proclaimed at a p-value < 0.05. The magnitude of diabetic peripheral neuropathy was 14.3% (95% CI 10.4–18.0). It was 13.4% (95% CI 8.4–19.1) among males and 15.4% (95% CI 10.1–22.2) among females. Age above 60 years (AOR = 5.06, 95% CI 1.60–15.96), being rural resident (AOR = 2.41; 95% CI 1.15–5.06), duration of diabetes above 5 years (AOR = 2.48, 95% CI 1.16–5.27) and having comorbid hypertension (AOR = 2.56, 95% CI 1.24–5.28) were independently associated with diabetic peripheral neuropathy. One in seven adult type 2 diabetes patients in the study area had diabetic peripheral neuropathy. Factors such as age, place of residence, duration of diabetes, and comorbid hypertension showed positive associations with diabetic peripheral neuropathy. Thus, it is imperative to give special consideration to diabetic patients who are elderly, living in rural areas, experiencing a prolonged duration of diabetes, or dealing with comorbid hypertension.
Keywords: Diabetic neuropathy, Diabetes mellitus, Adama, Ethiopia
Subject terms: Diabetes complications, Type 2 diabetes, Epidemiology
Introduction
Diabetes mellitus (DM) remains a serious public health problem, and type 2 DM is progressing rapidly, impacting individuals in all regions of the world. Every year, diabetes is directly responsible for 1.5 million deaths1. In 2021, it was estimated that 537 million adults globally had DM; this figure is expected to climb to 643 million by 2030 and 783 million by 2045, with low- and middle-income nations bearing the brunt of the predicted growth. Elevated mortality and morbidity rates, affecting both developing and developed nations, primarily stem from DM, with type 2 DM playing a leading role in this health burden. Ethiopia is one of the top five African countries pertaining to DM, with an estimated 1.9 million people suffering from it1–4.
Diabetes complications are expected to rise in tandem with the rise in the incidence of DM5. Blindness, renal failure, and peripheral neuropathy are all mainly triggered by DM. A quarter of individuals diagnosed with type 2 DM exhibit signs of DM complications right from the outset. Diabetic peripheral neuropathy (DPN) is the most prominent microvascular complication of DM and the most common cause of foot ulcers and amputations6,7. Nearly 30–50% of people with DM develop DPN throughout their lifetime and it accounts for 80% of foot ulcers, 50–75% of non-traumatic amputations, and frequent hospitalization8–11.
DPN is a global health issue that affects both developed and developing nations. Globally, the prevalence of DPN ranges from 22 to 47%12–14, while in Africa it is estimated to be 22%–66%15–17. In Ethiopia, the prevalence ranges from 1.9 to 53.6%, with variations contingent on geographical region and time7,18. Owing to late diagnosis, a paucity of screening and diagnostic resources, and a lack of proper DM care services, the prevalence of DPN is high in developing countries19,20.
Earlier studies have demonstrated that the development of DPN in type 2 DM patients is influenced by several factors, including age, the duration of diabetes, glycemic control, fasting blood sugar (FBS) level, body mass index (BMI), hypertension, diabetic retinopathy, and smoking7,21–26.
To reduce the detrimental consequences of DPN complications, it is crucial to screen type 2 DM patients for DPN at the earliest possible time. It is suggested that each adult with DM be checked for DPN at the time of diagnosis and then once a year after that. Preventing the complications and morbidity associated with DPN requires early recognition, detection, and management of risk factors27,28. Nevertheless, there is insufficient evidence, particularly within the study area, to demonstrate the magnitude and factors associated with DPN. Recognizing the escalating global burden of DM and its complications, our study aims to fill a critical gap in the current understanding of the magnitude and contributing factors of DPN specific to the study area. By shedding light on the nuanced dynamics of DPN in the local context, our findings are poised to play a pivotal role in informing targeted interventions, enhancing patient care, and ultimately contributing to the broader discourse on diabetes management. Therefore, this study aimed to determine the magnitude of DPN and associated factors among adult type 2 DM patients in Adama, central Ethiopia.
Methods and materials
Study design, setting, and period
A facility-based cross-sectional study was conducted in Adama city from May 01 to June 30, 2023. Adama city is 99 km to the southeast of Addis Ababa and is situated in one of the special zones of the Oromia region (East Shoa). The city covers 29.86 km2, with a population exceeding 500,000. It features one public hospital, five private hospitals, 160 private clinics, and eight health centers. They collectively provide services to catchment areas with over five million people and act as a referral hub for zones and regions nearby29.
Population and eligibility criteria
The source population was all adult type 2 DM patients on follow-up at Public health facilities in Adama city. The study population was all DM patients who were on follow-up at randomly selected Public health facilities in Adama city during the study period. Patients under the age of 18 years, who were critically and mentally ill, pregnant women, and patients diagnosed during the study period were excluded.
Sample size determination
The sample size for this study was determined using the single population proportion formula considering the assumptions of a 95% confidence level (the critical value Zα/2 = 1.96), a 5% margin of error (d = 0.05), and a 22% (p = 0.22) proportion of DPN18.
where: n = the required sample size, Z α/2 = the standardized normal distribution curve value for the 95% confidence interval, p = the proportion of DPN, and d = degree of precision.
By adding 10% for nonresponse, the final sample size of the study becomes 293.
Sampling procedure and technique
Public health facilities were stratified into hospitals and health centers. From eight health centers, two were selected by a simple random sampling method, and the hospital was selected purposively. Samples were proportionally allocated to the chosen health facilities based on their previous three-month average patient flow for DM follow-up. To recruit study participants from each facility, a systematic random sampling method was used. The sampling interval was set at each facility by dividing the average number of DM patients in the previous three months at the chosen facilities by the required sample size (Fig. 1).
Figure 1.
Schematic presentation of the sampling technique used to select the study participants from public health facilities of Adama, Ethiopia, 2023.
Operational definitions and study measurements
Diabetic peripheral neuropathy: the presence of DPN was assessed using the Michigan neuropathy screening instrument (MNSI). DPN was deemed to be present if the patient's history version of MNSI questionnaire score was ≥ 7 and/or if the examination version of MNSI scores was ≥ 2.57,30,31.
The tool consists of two complementary parts. The first part is the history version. It encompasses 15 items, of which only 13 were used to assess symptoms of DPN. The total score ranges from 0 to 13 points, and a score of ≥ 7 indicates the presence of DPN.
The second part contains a physical examination of the feet. It involves inspection of the feet appearance, vibration perception at the distal great toe using a 128-Hz C-tuning fork, examination of the ankle reflex using a standard triangular rubber-headed hammer, and a 5.07/10-g Semmes–Weinstein monofilament test.
On appearance, a foot with any abnormality received a score of one, and if not 0, a foot with an ulcer received a score of 1, and if not 0, absent vibration perception received a score of 1, reduced to 0.5, and if the vibration is present, 0. If ankle reflex was present, it was scored as 0, while if absent, the patient was told to do the Jendrassic maneuver, and if present, scored 0.5 and otherwise scored 1. On the monofilament test, nine sites on the plantar surface and one on the dorsum of the foot were examined. A score of 8 correct responses out of 10 applications received 0, 1–7 correct responses received 0.5, and no correct answers received 1. After each foot was evaluated independently, the results were combined. If the patient's score on the examination version of the MNSI was ≥ 2.5 on a scale of 10, they were deemed to have DPN.
Physical activity: Participants were deemed physically active if they engaged in physical exercise for at least 30 min per day, five days per week (≥ 150 min per week)7,32.
Smoking: participant who has never smoked, or who has smoked less than 100 cigarettes in his or her lifetime was considered non-smoker.
Alcohol intake: Male participants who drank more than 2 units of alcohol per day and female participants who drank more than 1 unit of alcohol per day were deemed alcohol consumers.
Data collection procedure and quality control
Data were collected using interviewer-administered semi-structured questionnaires and patient medical record reviews. The questionnaire was derived from validated scales and published studies and tailored to the study’s context7,8,19,21,22,24,26,30. A data extraction checklist was utilized to retrieve the necessary clinical related information from patients' records.
The questionnaire was prepared in English, then translated into local languages and back into English to ensure consistency. A pre-test was conducted on 5% of the total sample of patients (n = 15) at non-selected public health institutions to assess the quality and compatibility of the data abstraction format and questionnaire with the study's objective, and a correction was made accordingly. In addition, during data collection, Supervisors evaluated each questionnaire for completeness and consistency, and the principal investigator double-checked it daily and all collected data were cross-checked during data entry to clarify any missing data.
Data processing and analysis
Data were exported to the Statistical Package for Social Sciences (SPSS) Version 27 for cleaning and analysis after being coded and entered into Epi-Info Vision 7. To describe the study population in the context of pertinent characteristics, descriptive statistics were used. The association between the independent and outcome variables was modeled using binary logistic regression. The model's statistical assumptions were checked, and no major violations were seen. In the bivariable logistic regression model, a p-value of 0.25 was chosen as a cut-off value to select variables for multivariable logistic regression analysis to control the effects of confounders. The multicollinearity between the explanatory variables was explored using the variance inflation factor and tolerance, and it was determined to be within the range (1.013–1.028) showing no existence of multicollinearity. The adjusted odds ratio (AOR) with a 95% confidence interval (CI) was utilized to identify factors independently associated with DPN in the multivariable logistic regression. The model was fitted using the standard model-building method. Hosmer and Lemeshow's goodness-of-fit test was used to assess the model's fitness, and the result was significant with a p-value of 0.471 indicating a good fit. In the final model, variables with a p-value less than 0.05 were considered statistically significant.
Ethical approval and consent to participate
Ethical approval was obtained from the Institutional Ethical Review Board of Adama Hospital Medical College, and permission to access patient records and conduct the study was obtained from the medical directors of each selected health facility. Participants were informed of the study's purpose and benefits during the data collection period and informed written consent was obtained to ensure their decision to participate or refuse. To uphold respondents' rights and ensure confidentiality, anonymity, and privacy, safeguards were enacted, and all of the study's procedures followed the principles outlined in the Helsinki Declaration33.
Results
Socio-demographic characteristics
A total of 293 type 2 DM patients participated in this study giving a response rate of 100%. The mean age of the participants was 51.1 (SD: ± 12.2) years. Of the total participants, 157 (53.6%) were males, 274 (73.0%) urban residents, 223 (76.1%) married, and 119 (40.6%) were employed (Table 1).
Table 1.
Sociodemographic characteristics of type 2 diabetes mellitus patients on follow-up at public health facilities in Adama, Ethiopia, 2023 (n = 293).
| Variables | Category | Frequency | Percentage |
|---|---|---|---|
| Sex | Male | 157 | 53.6 |
| Female | 136 | 46.4 | |
| Age | ≤ 40 | 69 | 23.5 |
| 41–60 | 156 | 53.2 | |
| > 60 | 68 | 23.2 | |
| Mean 51.1 (SD: ± 12.2) | |||
| Place of residence | Urban | 274 | 73.0 |
| Rural | 79 | 27.0 | |
| Marital status | Currently married | 223 | 76.1 |
| Currently unmarried | 70 | 22.8 | |
| Religion | Orthodox | 148 | 50.5 |
| Muslim | 106 | 36.2 | |
| Protestant | 39 | 13.3 | |
| Educational status | No formal education | 27 | 9.2 |
| Elementary (1–8) | 62 | 21.2 | |
| Secondary (9–12) | 87 | 29.7 | |
| College and above | 117 | 39.9 | |
| Occupational status | Employed | 119 | 40.6 |
| Merchant | 64 | 21.8 | |
| Farmer | 39 | 13.3 | |
| Retired | 47 | 16.1 | |
| Othersa | 24 | 8.2 | |
aHousewife, student, daily laborer.
Behavioral and clinical characteristics
Among the participants included in this study, 151 (51.5%) were taking oral hypoglycemic agents (OHAs) and the majority of DM patients, 212 (72.4%), had good glycemic control. The median FBS level was 220 (IQR = 190–292) while the median duration of DM was 3.8 (IQR = 1.8–6.1), and of the participants, 216 (73.2%) were physically inactive and 116 (39.6%) had a history of missed follow-up (Table 2). In this study, hypertension 117 (39.9%) was the most common comorbidity, followed by chronic heart disease (CHD) 30 (10.2%) and chronic kidney disease 24 (8.2%). The commonest complication was diabetic foot ulcer 23 (7.8%) (Fig. 2).
Table 2.
Behavioral and clinical characteristics of type 2 diabetes mellitus patients on follow-up at public health facilities in Adama, Ethiopia, 2023 (n = 293).
| Variables | Category | Frequency | Percentage |
|---|---|---|---|
| Type of treatment | Oral hypoglycemic agents | 151 | 51.5 |
| Insulin | 37 | 12.6 | |
| Mixed | 105 | 35.8 | |
| Oral hypoglycemic agents | Metformin | 133 | 45.4 |
| Glibenclimide | 45 | 15.4 | |
| Both | 115 | 39.2 | |
| Fasting blood sugar level (mg/dl) | < 200 | 101 | 34.5 |
| ≥ 200 | 192 | 65.5 | |
| Median 220 (IQR = 190–292) | |||
| Glycemic control | Good | 212 | 72.4 |
| Poor | 81 | 27.6 | |
| Body mass index (kg/m2) | Underweight | 32 | 10.9 |
| Normal (18.5–24.99) | 116 | 39.6 | |
| Overweight (25–29.99) | 78 | 26.6 | |
| Obese (≥ 30) | 67 | 22.9 | |
| Family history of DM | Yes | 21 | 8.2 |
| No | 272 | 92.8 | |
| Duration of DM | < 5 years | 223 | 76.1 |
| ≥ 5 years | 70 | 23.9 | |
| Median 3.8 (IQR = 1.8–6.1) | |||
| Physical activity | Active | 77 | 26.3 |
| Inactive | 216 | 73.2 | |
| Alcohol intake | Yes | 36 | 12.3 |
| No | 257 | 87.7 | |
| History of Smoking | Yes | 9 | 3.1 |
| No | 284 | 96.9 | |
| Follow-up miss | Yes | 116 | 39.6 |
| No | 177 | 60.4 | |
Figure 2.
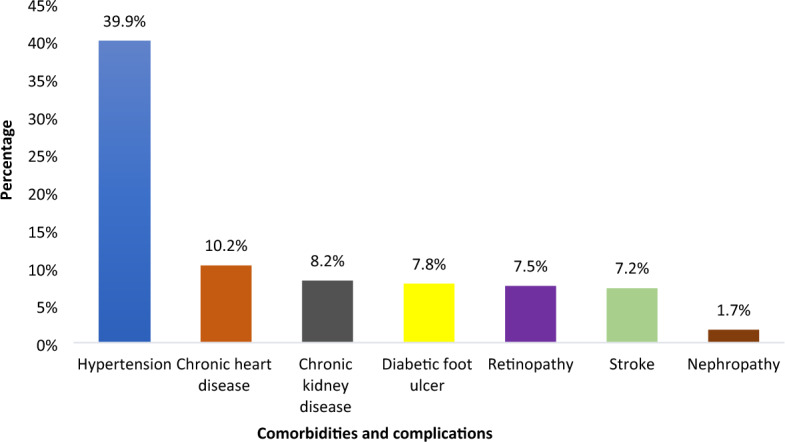
Comorbidity and complication-related characteristics of type 2 diabetes mellitus patients on follow-up at public health facilities in Adama, Ethiopia,2023 (n = 293).
The magnitude of diabetic peripheral neuropathy
The proportion of DPN among type 2 DM participants was 14.3% with (95% CI 10.4–18.0). It was found to be 13.4% (95% CI 8.4–19.1) among males and 15.4% (95% CI 10.1–22.2) among females respectively (Fig. 3).
Figure 3.
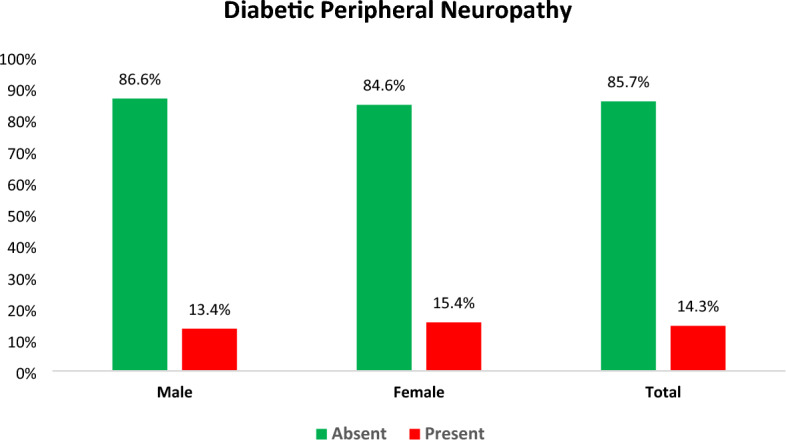
The proportion of diabetic peripheral neuropathy among type 2 diabetes mellitus patients on follow-up at public health facilities in Adama, Ethiopia, 2023 (n = 293).
Factors associated with diabetic peripheral neuropathy
In bivariable analysis, age, place of residence, type of treatment, glycemic control duration of DM, and comorbid HTN demonstrated a significant association. After adjusting for potential confounders using multivariable binary logistic regression analysis age, place of residence, duration of DM, and comorbid HTN persisted as statistically significant factors associated with DPN at a p-value < 0.05.
The odds of developing DPN were 5 times greater among DM patients above the age of 60 compared to patients 40 years of age or younger (AOR = 5.06, 95% CI 1.60–15.96). Patients who resided in rural areas had 2.4 times the odds of experiencing DPN than those living in urban areas (AOR = 2.41; 95% CI 1.15–5.06). Compared to patients with DM for 5 years or less, those with DM for more than 5 years had 2.4 times the odds of developing DPN (AOR = 2.48, 95% CI 1.16–5.27). Furthermore, the odds of developing DPN among DM patients with comorbid HTN were 2.5 times higher compared to their counterparts (AOR = 2.56, 95% CI 1.24–5.28) (Table 3).
Table 3.
Factors associated with diabetic peripheral neuropathy among type 2 diabetes mellitus patients on follow-up at public health facilities in Adama, Ethiopia, 2023 (n = 293).
| Variables | Category | DPN | COR (95% CI) | AOR (95% CI) | |
|---|---|---|---|---|---|
| Present (%) | Absent (%) | ||||
| Age (years) | ≤ 40 | 5 (7.2) | 64 (92.8) | 1 | 1 |
| 41–60 | 20 (12.8) | 136 (87.2) | 1.88 (0.68–5.24)* | 1.77 (0.60–5.23) | |
| > 60 | 17 (25.0) | 51 (75.0) | 4.27 (1.47–12.35)* | 5.06 (1.60–15.96)** | |
| Residence | Urban | 25 (11.7) | 189 (88.3) | 1 | 1 |
| Rural | 17 (21.5) | 62 (78.5) | 2.07 (1.05–4.09)* | 2.41 (1.15–5.06)** | |
| Type of treatment | OHA | 24 (15.9) | 127 (84.1) | 1 | 1 |
| Insulin | 3 (8.1) | 34 (91.9) | 0.47 (0.13–1.64)* | 0.53 (0.14–2.05) | |
| Mixed | 15 (14.3) | 90 (85.7) | 0.88 (0.44–1.78) | 0.81 (0.38–1.72) | |
| Glycemic control | Good | 25 (11.8) | 187 (88.2) | 0.50 (0.26–0.99)* | 0.46 (0.22–1.01) |
| Poor | 17 (21.0) | 64 (79.0) | 1 | 1 | |
| Duration of DM (years) | ≤ 5 | 26 (11.7) | 197(88.3) | 1 | 1 |
| > 5 | 16 (22.9) | 54(77.1) | 2.25(1.12–4.84)* | 2.48(1.16–5.27)** | |
| Comorbid HTN | Yes | 24 (20.8) | 93 (79.5) | 2.27 (1.17–4.39)* | 2.56 (1.24–5.28)** |
| No | 18 (10.2) | 158(89.8) | 1 | 1 | |
Discussion
This health facility-based cross-sectional study assessed the magnitude and factors associated with DPN among adult type 2 DM patients in Adama, Ethiopia.
This study revealed that the magnitude of DPN was 14.3% (95% CI 10.4–18.0) which is comparable with studies conducted in Spain (12.9%), Ghana (16.6%)34, and Northwest Ethiopia (16.63%)24. Conversely, the finding of the current study is lower than a global systematic review and metanalysis (35.78%)13, a systematic review and metanalysis done in Africa (46%)17, studies done in Singapore (28%)35, Vietnam (26.6%)36 Jimma University Medical Center, Ethiopia (53.6%)7, and Bahirdar, Ethiopia (52.2%)26. Moreover, the magnitude of DPN in this study is higher than in studies conducted in Nigeria (7.5%)37 and China (8.4%)38. These discrepancies could be attributed to variations in neuropathy diagnostic criteria and differences in the study population, with the present study encompassing individuals with type 2 DM. Additionally, divergences in socioeconomic status, study period, sample size, and study settings may offer another plausible explanation for the noted differences.
In line with studies conducted in China39, Singapore35, India40, Romania41, Egypt42, Uganda43, Tanzania44, Northwest Ethiopia24,25, Jimma University Medical Center, Ethiopia7, and Bahirdar Ethiopia26. The odds of developing DPN were fivefold greater among DM patients above the age of 60 compared to patients 40 years of age or younger. The justification lies in the age-related increase in oxidative stress, counter-regulatory signaling stimulation, and mitochondrial dysfunction. These factors heighten the risk of inflammation and peripheral nerve damage, consequently escalating the likelihood of DPN. Additionally, aging exposes the neural system to continual metabolic stress and declining physiological health, further elevating the risk of contracting DPN45–47.
Place of residence was another significant factor associated with DPN. In this study, Patients who resided in rural areas had 2.4 times the odds of experiencing DPN than those living in urban areas. This finding is consistent with previous studies conducted in Sri Lanka20 and India48. This may be due to a lack of better healthcare facilities to detect and treat diabetic problems in rural settings. On the contrary, studies done in Cameroon19 and Gamo and Gofa zones, Ethiopia22 showed higher odds of DPN among urban dwellers. This disparity may be attributable to the majority of urban people maintaining sedentary lifestyles, unhealthy eating habits, and less physical activity all of which can contribute to DPN49–51.
In this study, there were also statistically significant associations between the duration of DM and higher odds of DPN. So, compared to patients with DM for 5 years or less, those with DM for more than 5 years had 2.4 times the odds of developing DPN. This finding is similar to a systematic review and meta-analysis of 16 studies23,52; studies done in China38, Jordan53, India54 Egypt55, Iran56, Jimma University Medical Center, Ethiopia7; Bahirdar, Ethiopia26; Gamo and Gofa zones, Ethiopia22. This can be explained by the fact that the prolonged duration of DM is linked with chronic hyperglycemia, which activates several metabolic pathways, causing oxidative stress in diabetic neurons and leading to nerve damage and neuronal ischemia57,58. Moreover, living with diseases for an extended time may raise the likelihood of being exposed to unhealthy lifestyles and foods raising the risk of developing complications22. Conversely, a study conducted in Northwest Ethiopia showed a contrasting finding24.
The finding of this study indicated that the odds of DPN were greater among DM patients with comorbid HTN compared to their counterparts. This is supported by findings from a systematic review and meta-analysis of 16 studies52, a finding from a systematic review59, and studies conducted in China39,60, Taiwan61, Egypt42, and Northwest Ethiopia25. Increased oxidative stress, inflammation, activation of the sympathetic nervous system, stimulation of the renin–angiotensin–aldosterone system, endothelial cell dysfunction, vascular rigidity leading to reduced blood supply to the nerve cell, slowed conduction, and axonal atrophy—each of these factors may contribute to the increased susceptibility to developing DPN as a result of HTN62,63. The mechanism of HTN in DPN is still obscure, and further studies are required to define the nature of the physiologic interaction, as well as the relationship between HTN and DPN in diabetics.
Limitations of the study
Given the cross-sectional nature of the study, it is difficult to draw a causal relationship, and the gold standard diagnostic test for DPN was not used due to its high cost.
Conclusion
This study revealed that one in seven DM patients had DPN. Age, place of residence, duration of DM, and comorbid HTN were factors positively associated with DPN. Therefore, it is essential to prioritize diabetic patients, including the elderly, those in rural areas, individuals with a longer duration of diabetes, and those with comorbid hypertension, during routine follow-up visits. Tailored care and focused monitoring for these groups are crucial for effective healthcare management, thereby enhancing diabetes management effectiveness.
Acknowledgements
The authors like to acknowledge Adama town public health facility officials for providing all necessary assistance. We also are thankful for the data collectors, supervisors, and all the study’s participants.
Abbreviations
- AHMC
Adama Hospital Medical College
- AHR
Adjusted hazard ratio
- CI
Confidence interval
- DM
Diabetes mellitus
- DPN
Diabetic peripheral neuropathy
- FBS
Fasting blood sugar
- HTN
Hypertension
- MNSI
Michigan neuropathy screening instrument
- OHA
Oral hypoglycemic agent
Author contributions
Conceptualization, N.Y.M.; data curation, N.Y.M.; formal analysis, N.Y.M.; project administration, N.Y.M., and B.T.N.; supervision, N.Y.M. and B.T.N.; writing-original draft, N.Y.M. and B.T.N.; writing—review and editing, N.Y.M. All authors contributed to the article and approved the submitted version.
Data availability
All data and materials are available from the corresponding author without undue reservation.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ong KL, Stafford LK, McLaughlin SA, Boyko EJ, Vollset SE, Smith AE, et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402(10397):203–234. doi: 10.1016/S0140-6736(23)01301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 3.Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nanayakkara N, Curtis AJ, Heritier S, Gadowski AM, Pavkov ME, Kenealy T, et al. Impact of age at type 2 diabetes mellitus diagnosis on mortality and vascular complications: Systematic review and meta-analyses. Diabetologia. 2021;64(2):275–287. doi: 10.1007/s00125-020-05319-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 6.Lu Y, Xing P, Cai X, Luo D, Li R, Lloyd C, et al. Prevalence and risk factors for diabetic peripheral neuropathy in type 2 diabetic patients from 14 countries: Estimates of the INTERPRET-DD study. Front. Public Health. 2020;8:534372. doi: 10.3389/fpubh.2020.534372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdissa D, Hamba N, Kene K, Bedane DA, Etana G, Muleta D, et al. Prevalence and determinants of peripheral neuropathy among type 2 adult diabetes patients attending Jimma University Medical Center, Southwest Ethiopia, 2019, an institutional-based cross-sectional study. J. Diabetes Res. 2020;29(2020):1–8. doi: 10.1155/2020/9562920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ko SH, Cha BY. Diabetic peripheral neuropathy in type 2 diabetes mellitus in Korea. Diabetes Metab. J. 2012;36(1):6–12. doi: 10.4093/dmj.2012.36.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hicks CW, Selvin E. Epidemiology of peripheral neuropathy and lower extremity disease in diabetes. Curr. Diab. Rep. 2019;19(10):86. doi: 10.1007/s11892-019-1212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alleman CJM, Westerhout KY, Hensen M, Chambers C, Stoker M, Long S, et al. Humanistic and economic burden of painful diabetic peripheral neuropathy in Europe: A review of the literature. Diabetes Res. Clin. Pract. 2015;109(2):215–225. doi: 10.1016/j.diabres.2015.04.031. [DOI] [PubMed] [Google Scholar]
- 11.Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, et al. Diabetic neuropathy. Nat. Rev. Dis. Primers. 2019;5(1):41. doi: 10.1038/s41572-019-0092-1. [DOI] [PubMed] [Google Scholar]
- 12.Abbott CA, Malik RA, Van Ross ER, Kulkarni J, Boulton AJ. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the UK. Diabetes Care. 2011;34(10):2220–2224. doi: 10.2337/dc11-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Souza, L. R., Debiasi, D., Ceretta, L. B., SimÃμes, P. W. & Tuon, L. Meta-analysis and meta-regression of the prevalence of diabetic peripheral neuropathy among patients with type 2 diabetes mellitus. Int. Arch. Med.9, 1–13. 10.3823/1936 (2016).
- 14.Yovera-Aldana M, Velásquez-Rimachi V, Huerta-Rosario A, More-Yupanqui MD, Osores-Flores M, Espinoza R, et al. Prevalence and incidence of diabetic peripheral neuropathy in Latin America and the Caribbean: A systematic review and meta-analysis. PLoS ONE. 2021;16(5):e0251642. doi: 10.1371/journal.pone.0251642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garoushi S, Johnson MI, Tashani OA. Point prevalence of painful diabetic neuropathy in the Middle East and North Africa region: A systematic review with meta-analysis. Libyan J. Med. Sci. 2018;2(3):85–94. [Google Scholar]
- 16.Hall V, Thomsen RW, Henriksen O, Lohse N. Diabetes in Sub Saharan Africa 1999–2011: Epidemiology and public health implications. A systematic review. BMC Public Health. 2011;11(1):1–12. doi: 10.1186/1471-2458-11-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiferaw WS, Akalu TY, Work Y, Aynalem YA. Prevalence of diabetic peripheral neuropathy in Africa: A systematic review and meta-analysis. BMC Endocr. Disord. 2020;20:1–9. doi: 10.1186/s12902-020-0534-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tadesse DB, Gebrewahd GT, Hailay A, Aberhe W, Mebrahtom G, Zereabruk K, et al. Diabetic peripheral neuropathy in Ethiopia: A systematic review and meta-analysis. J. Diabetes Res. 2021;2021:1–13. doi: 10.1155/2021/5304124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuate-Tegueu C, Temfack E, Ngankou S, Doumbe J, Djientcheu VP, Kengne AP. Prevalence and determinants of diabetic polyneuropathy in a sub-Saharan African referral hospital. J. Neurol. Sci. 2015;355(1–2):108–112. doi: 10.1016/j.jns.2015.05.035. [DOI] [PubMed] [Google Scholar]
- 20.Katulanda P, Ranasinghe P, Jayawardena R, Constantine GR, Sheriff MHR, Matthews DR. The prevalence, patterns and predictors of diabetic peripheral neuropathy in a developing country. Diabetol. Metab. Syndr. 2012;4(1):21. doi: 10.1186/1758-5996-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdissa D, Sorsa R, Gerbi A, Hamba N, Banjaw Z. Magnitude and associated factors of peripheral neuropathy among diabetes patients attending Jimma University Medical Center, Southwest Ethiopia. Heliyon. 2021;7(11):e08460. doi: 10.1016/j.heliyon.2021.e08460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gebabo TF, Zewdie TH, Shagaro SS, Haile F. Determinants of peripheral neuropathy among diabetic patients under follow-up in chronic care clinics of public hospitals at Gamo and Gofa zones, southern Ethiopia. PLoS ONE. 2021;16(2):e0246722. doi: 10.1371/journal.pone.0246722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu X, Xu Y, An M, Zeng Q. The risk factors for diabetic peripheral neuropathy: A meta-analysis. PLoS ONE. 2019;14(2):e0212574. doi: 10.1371/journal.pone.0212574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kebede SA, Tusa BS, Weldesenbet AB, Tessema ZT, Ayele TA. Time to diabetic neuropathy and its predictors among newly diagnosed type 2 diabetes mellitus patients in Northwest Ethiopia. Egypt J. Neurol. Psychiatry Neurosurg. 2021;57(1):147. doi: 10.1186/s41983-021-00402-4. [DOI] [Google Scholar]
- 25.Tantigegn S, Ewunetie AA, Agazhe M, Aschale A, Gebrie M, Diress G, et al. Time to diabetic neuropathy and its predictors among adult type 2 diabetes mellitus patients in Amhara regional state Comprehensive Specialized Hospitals, Northwest Ethiopia, 2022: A retrospective follow up study. PLoS ONE. 2023;18(4):e0284568. doi: 10.1371/journal.pone.0284568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jember G, Melsew YA, Fisseha B, Sany K, Gelaw AY, Janakiraman B. Peripheral Sensory Neuropathy and associated factors among adult diabetes mellitus patients in Bahr Dar, Ethiopia. J. Diabetes Metab. Disord. 2017;16:16. doi: 10.1186/s40200-017-0295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Association AD 11. Microvascular complications and foot care: Standards of medical care in diabetes-2020. Diabetes Care. 2020;43(1):S135–S151. doi: 10.2337/dc20-S011. [DOI] [PubMed] [Google Scholar]
- 28.Staudt MD, Prabhala T, Sheldon BL, Quaranta N, Zakher M, Bhullar R, et al. Current strategies for the management of painful diabetic neuropathy. J. Diabetes Sci. Technol. 2022;16(2):341–352. doi: 10.1177/1932296820951829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Negussie YM, Getahun MS, Bekele NT. Factors associated with diabetes concordant comorbidities among adult diabetic patients in Central Ethiopia: A cross-sectional study. Front. Clin. Diabetes Healthc. 2023;13(4):1307463. doi: 10.3389/fcdhc.2023.1307463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herman WH, Pop-Busui R, Braffett BH, Martin CL, Cleary PA, Albers JW, et al. Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in Type 1 diabetes: Results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet. Med. 2012;29(7):937–944. doi: 10.1111/j.1464-5491.2012.03644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moghtaderi A, Bakhshipour A, Rashidi H. Validation of Michigan neuropathy screening instrument for diabetic peripheral neuropathy. Clin. Neurol. Neurosurg. 2006;108(5):477–481. doi: 10.1016/j.clineuro.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Tola A, Regassa LD, Ayele Y. Prevalence and associated factors of diabetic foot ulcers among type 2 diabetic patients attending chronic follow-up clinics at governmental hospitals of Harari Region, Eastern Ethiopia: A 5-year (2013–2017) retrospective study. SAGE Open Med. 2021;9:205031212098738. doi: 10.1177/2050312120987385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Medical Association World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull World Health Organ. 2001;79(4):373–374. [PMC free article] [PubMed] [Google Scholar]
- 34.Yeboah K, Agyekum JA, Owusu Mensah RNA, Affrim PK, Adu-Gyamfi L, Doughan RO, et al. Arterial stiffness is associated with peripheral sensory neuropathy in diabetes patients in Ghana. J. Diabetes Res. 2018;2018:2320737. doi: 10.1155/2018/2320737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu Hanquan A, Teo Li Wen MR. Prevalence of diabetic peripheral neuropathy in patients with type 2 diabetes mellitus at a Tertiary Referral Centre in Singapore. Proc. Sing. Healthc. 2021;30(4):265–270. doi: 10.1177/2010105820978993. [DOI] [Google Scholar]
- 36.Dinh Le T, Phi Thi Nguyen N, Tran TTH, Luong Cong T, Ho Thi Nguyen L, Do Nhu B, et al. Diabetic peripheral neuropathy associated with cardiovascular risk factors and glucagon-like peptide-1 concentrations among newly diagnosed patients with type 2 diabetes mellitus. DMSO. 2022;15:35–44. doi: 10.2147/DMSO.S344532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mba I. Diabetes mellitus in Abia State University Teaching Hospital Aba, a 30 months retrospective study. J. Med. Investig. Pract. 2001;2:48–51. [Google Scholar]
- 38.Lu B, Hu J, Wen J, Zhang Z, Zhou L, Li Y, et al. Determination of peripheral neuropathy prevalence and associated factors in Chinese subjects with diabetes and pre-diabetes—ShangHai Diabetic neuRopathy Epidemiology and Molecular Genetics Study (SH-DREAMS) PLoS ONE. 2013;8(4):e61053. doi: 10.1371/journal.pone.0061053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng Y, Cao W, Zhang J, Wang J, Liu X, Wu Q, et al. Determinants of diabetic peripheral neuropathy and their clinical significance: A retrospective cohort study. Front. Endocrinol. 2022;13:934020. doi: 10.3389/fendo.2022.934020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abu Bashar, M. D. & Verma, M. Prevalence and Determinants of Diabetic Peripheral Neuropathy/Foot Syndrome in the Rural Population of North India. (2020) https://zenodo.org/record/4393501 (Accessed 24 Oct 2023).
- 41.Baderca F, Timar B, Popescu S, Simu M, Diaconu L, Velea I, et al. Age as an independent factor for the development of neuropathy in diabetic patients. CIA. 2016 doi: 10.2147/CIA.S97295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahmoud Hewedi K, Farag El-Adawy A, Ahmed Rezk A, Gamal Ahmed Yassen A. Risk factors associated with peripheral neuropathy in type II diabetic patients. Al-Azhar Med. J. 2020;49(2):387–398. doi: 10.21608/amj.2020.67803. [DOI] [Google Scholar]
- 43.Kisozi T, Mutebi E, Kisekka M, Lhatoo S, Sajatovic M, Kaddumukasa M, et al. Prevalence, severity and factors associated with peripheral neuropathy among newly diagnosed diabetic patients attending Mulago hospital: A cross-sectional study. Afr. Health Sci. 2017;17(2):463–473. doi: 10.4314/ahs.v17i2.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amour AA, Chamba N, Kayandabila J, Lyaruu IA, Marieke D, Shao ER, et al. Prevalence, patterns, and factors associated with peripheral neuropathies among diabetic patients at tertiary Hospital in the Kilimanjaro Region: Descriptive cross-sectional study from north-eastern Tanzania. Int. J. Endocrinol. 2019;2019:5404781. doi: 10.1155/2019/5404781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vinik AI, Strotmeyer ES, Nakave AA, Patel CV. Diabetic neuropathy in older adults. Clin. Geriatr. Med. 2008;24(3):407–35,v. doi: 10.1016/j.cger.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Román-Pintos LM, Villegas-Rivera G, Rodríguez-Carrizalez AD, Miranda-Díaz AG, Cardona-Muñoz EG. Diabetic polyneuropathy in type 2 diabetes mellitus: Inflammation, oxidative stress, and mitochondrial function. J. Diabetes Res. 2016;2016:1–16. doi: 10.1155/2016/3425617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corriere M, Rooparinesingh N, Kalyani RR. Epidemiology of diabetes and diabetes complications in the elderly: An emerging public health burden. Curr. Diab. Rep. 2013;13(6):805–813. doi: 10.1007/s11892-013-0425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jasmine A, GV A, Durai V, Shriraam V, et al. Prevalence of peripheral neuropathy among type 2 diabetes mellitus patients in a rural health centre in South India. Int. J. Diabetes Dev. Ctries. 2021;41(2):293–300. doi: 10.1007/s13410-020-00885-6. [DOI] [Google Scholar]
- 49.Leitzmann, M. Physical activity, sedentary behaviour, and obesity. In Energy Balance and Obesity (eds Romieu, I. et al.). (International Agency for Research on Cancer, 2017) (Accessed 3 Oct 2023) (IARC Working Group Reports). http://www.ncbi.nlm.nih.gov/books/NBK565813/. [PubMed]
- 50.Bavuma CM, Musafiri S, Rutayisire PC, Ng’ang’a LM, McQuillan R, Wild SH. Socio-demographic and clinical characteristics of diabetes mellitus in rural Rwanda: Time to contextualize the interventions? A cross-sectional study. BMC Endocr. Disord. 2020;20(1):180. doi: 10.1186/s12902-020-00660-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Du GL, Su YX, Yao H, Zhu J, Ma Q, Tuerdi A, et al. Metabolic risk factors of type 2 diabetes mellitus and correlated glycemic control/complications: A cross-sectional study between rural and urban Uygur Residents in Xinjiang Uygur Autonomous Region. PLoS ONE. 2016;11(9):e0162611. doi: 10.1371/journal.pone.0162611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fakkel TM, Çakici N, Coert JH, Verhagen AP, Bramer WM, Van Neck JW. Risk factors for developing diabetic peripheral neuropathy: A meta-analysis. SN Compr. Clin. Med. 2020;2(10):1853–1864. doi: 10.1007/s42399-020-00480-0. [DOI] [Google Scholar]
- 53.Khawaja N, Abu-Shennar J, Saleh M, Dahbour SS, Khader YS, Ajlouni KM. The prevalence and risk factors of peripheral neuropathy among patients with type 2 diabetes mellitus; the case of Jordan. Diabetol. Metab. Syndr. 2018;10(1):8. doi: 10.1186/s13098-018-0309-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bansal D, Gudala K, Muthyala H, Esam HP, Nayakallu R, Bhansali A. Prevalence and risk factors of development of peripheral diabetic neuropathy in type 2 diabetes mellitus in a tertiary care setting. J. Diabetes Investig. 2014;5(6):714–721. doi: 10.1111/jdi.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kasim K, Amar M, Sadek AAE, Gawad SA. Peripheral neuropathy in type-II diabetic patients attending diabetic clinics in Al-Azhar University Hospitals, Egypt. Int. J. Diabetes Mellitus. 2010;2(1):20–23. doi: 10.1016/j.ijdm.2009.10.002. [DOI] [Google Scholar]
- 56.Kiani J, Moghimbeigi A, Azizkhani H, Kosarifard S. The prevalence and associated risk factors of peripheral diabetic neuropathy in Hamedan, Iran. Arch. Iran Med. 2013;16(1):17–19. [PubMed] [Google Scholar]
- 57.Edwards JL, Vincent AM, Cheng HT, Feldman EL. Diabetic neuropathy: Mechanisms to management. Pharmacol. Ther. 2008;120(1):1–34. doi: 10.1016/j.pharmthera.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nisar MU, Asad A, Waqas A, Ali N, Nisar A, Qayyum MA, et al. Association of diabetic neuropathy with duration of type 2 diabetes and glycemic control. Cureus. 2015;7(8):e302. doi: 10.7759/cureus.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sethi Y, Uniyal N, Vora V, Agarwal P, Murli H, Joshi A, et al. Hypertension the “missed modifiable risk factor” for diabetic neuropathy: A systematic review. Curr. Probl. Cardiol. 2023;48(4):101581. doi: 10.1016/j.cpcardiol.2022.101581. [DOI] [PubMed] [Google Scholar]
- 60.Li C, Wang W, Ji Q, Ran X, Kuang H, Yu X, et al. Prevalence of painful diabetic peripheral neuropathy in type 2 diabetes mellitus and diabetic peripheral neuropathy: A nationwide cross-sectional study in mainland China. Diabetes Res. Clin. Pract. 2023;198:110602. doi: 10.1016/j.diabres.2023.110602. [DOI] [PubMed] [Google Scholar]
- 61.Yang CP, Lin CC, Li CI, Liu CS, Lin WY, Hwang KL, et al. Cardiovascular risk factors increase the risks of diabetic peripheral neuropathy in patients with type 2 diabetes mellitus: The Taiwan Diabetes Study. Medicine. 2015;94(42):e1783. doi: 10.1097/MD.0000000000001783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Buren PN, Toto R. Hypertension in diabetic nephropathy: Epidemiology, mechanisms, and management. Adv. Chronic Kidney Dis. 2011;18(1):28–41. doi: 10.1053/j.ackd.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gregory JA, Jolivalt CG, Goor J, Mizisin AP, Calcutt NA. Hypertension-induced peripheral neuropathy and the combined effects of hypertension and diabetes on nerve structure and function in rats. Acta Neuropathol. 2012;124(4):561–573. doi: 10.1007/s00401-012-1012-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials are available from the corresponding author without undue reservation.



