Abstract
Methylphenidate (MPH) is highly efficacious in reducing symptoms of attention-deficit/hyperactivity disorder (ADHD) in children. Generally increased doses are found to result in better symptom control; however, it remains unclear whether this pattern can be observed at the individual level, given the large heterogeneity in individual dose–response relationships and observed placebo responses. A double-blind, randomized, placebo-controlled cross-over trial was used to compare weekly treatment with placebo and 5, 10, 15 and 20 mg of MPH twice daily on parent and teacher ratings of child ADHD symptoms and side effects. Participants were 5–13-year-old children with a DSM-5 diagnosis of ADHD (N = 45). MPH response was assessed at group and individual levels and predictors of individual dose–response curves were examined. Mixed model analysis showed positive linear dose–response curves at group level for parent and teacher rated ADHD symptoms and parent rated side effects, but not for teacher rated side effects. Teachers reported all dosages to improve ADHD symptoms compared to placebo, while parents only reported > 5 mg/dose as effective. At the individual level, most (73–88%) children, but not all, showed positive linear dose–response curves. Higher severity of hyperactive-impulsive symptoms and lower internalizing problems, lower weight, younger age and more positive opinions towards diagnosis and medication partly predicted steeper linear individual dose–response curves. Our study confirms that increased doses of MPH yield greater symptom control at a group level. However, large interindividual variation in the dose–response relationship was found and increased doses did not lead to greater symptom improvement for all children. This trial was registered in the Netherlands trial register (# NL8121).
Supplementary Information
The online version contains supplementary material available at 10.1007/s00787-023-02176-x.
Keywords: Attention-deficit, Hyperactivity disorder, Methylphenidate, Dose–response, Placebo-response
Introduction
Attention-deficit hyperactivity disorder (ADHD) is the most diagnosed childhood-onset psychiatric disorder [1]. Methylphenidate (MPH) is highly efficacious in reducing ADHD symptoms, with effect sizes close to 1.0 [2]. International guidelines [3, 4] recommend ‘stepwise titration’ to determine the therapeutic dose in which treatment starts with a low dose and is gradually increased until the most effective dose with acceptable side effects [3–6]. Absolute rather than weight-based dosing is recommended, because body mass fails to predict optimal dose [7].
Currently, the efficacy of stepwise MPH titration is challenged by two issues. First, stepwise titration assumes that higher individual doses lead to greater ADHD symptom reduction in a linear manner. Several studies [6, 8, 9] have validated this assumption, showing that children who do not respond to lower doses have a higher probability of symptomatic improvement with increasing dose. However, studies focusing on individual outcomes [10–13] found that optimal doses varied between 10 and 50 mg/day and between 5 and 20 mg per administration, translating into a fourfold difference between the lowest and highest optimal dose. These findings cannot be explained by the weight of the child, given that small children (low in weight) may require relatively high doses, while more heavier children may require relatively small doses [13, 14]. Also differences in absorption and metabolism do not provide an explanation for the heterogeneity in optimal doses, since children who respond to low doses (5 mg per administration) may show low serum concentrations of MPH (4–5 ng/ml at Tmax) and those who respond to high doses (20 mg per administration) may show high serum concentrations (12–15 ng/ml) [13, 14]. Moreover, studies [15, 16] investigating individual dose–response relations reported nonlinear dose–response curves for the effects of MPH, implying that increased doses may not always lead to better symptom control [15, 16]. Taken together at a group level, increased doses of stimulants are associated with improved efficacy however we may question, that high doses always lead to the greatest ADHD symptom reduction at an individual level.
Second, stepwise titration does not offer a comparison with placebo. A recent meta-analysis by Faraone and colleagues [17] showed a significant improvement of ADHD symptoms under placebo treatment in controlled pharmacological trials with effect sizes ranging between 0.36 and 0.75 SMD, depending on the rater. In the Multimodal Treatment of Attention Deficit Hyperactivity Disorder Study (MTA study) 10% of children were classified as placebo responders, with these children showing no additional beneficial effects of MPH to placebo [18]. If no comparison with placebo is made, placebo responders are less likely to be identified and are exposed to MPH side effects without profiting from MPH specific effects.
An alternative method to investigate dose–response is double-blind, placebo-controlled titration (PCT). Different doses of MPH and placebo are prescribed double-blind and in random order. The effects of each dose are assessed in terms of symptom reduction and side effects [19]. Subsequently, it can be determined whether the child is a non-responder, placebo-responder or responder. For responders, the optimal dose provides most optimal symptom control with minimal side effects.
Another point that complicates MPH titration is the lack of clinically useful predictors for individual response. Studies aiming to predict children’s response to MPH, showed weaker responses to MPH in older children, children with more internalizing symptoms and in those with less severe symptoms of ADHD [20, 21]. However, most of these studies did not include the impact of dose on efficaciousness. One exception is the MTA study [12] that showed steeper dose–response curves for younger and lighter children, but did not confirm the effect of internalizing symptoms. Thus far little attention has been given to placebo-related effects. However, variables associated with placebo effects are important when investigating predictors for the dose–response relationship for MPH. Placebo effects have a prominent role in the overall effect of MPH [22, 23] and are highly related to patients expectations [24]. These expectations even lead to increases of synaptic dopamine, without MPH administration, which has been shown in a PET-study by Volkow et al. [23] Thus, variables measuring expectations and attitudes towards treatment could be interesting in predicting dose–response relations of MPH. Taken together, little is known about possible predictors of the dose–response relation of MPH that may facilitate more personalized MPH titration.
The aim of the current study was to gain more insight into the dose–response curves of MPH in terms of ADHD symptom reduction and side effects using PCT. The blinded, randomized and placebo-controlled titration trials offered the unique opportunity to compare dose–response curves at group and individual level. First, dose–response curves were studied at group level by determining the function best describing the dose–response relationship and comparing the effects of doses of 5, 10, 15 and 20 mg and placebo. Likewise, individual dose–response curves were determined. Finally, variables that showed promise in clinically predicting response to MPH, were explored as possible predictors of the individual dose–response curves.
Methods
Study design
Children were recruited from mental health clinics in The Netherlands between May 2017 and December 2019. Inclusion criteria were as follows: (a) a clinical diagnosis of ADHD according to DSM-5, (b) 5–13 years of age, (c) IQ > 70, (d) indication for MPH treatment, as determined by the treating physician, and (e) no pharmacological treatment for ADHD 4 weeks prior to study entry. Comorbid diagnoses were not an exclusion criterion. Diagnostic status was confirmed by the first author (K.V.) using the (1) Kiddie–Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS), a semi-structured standardized, investigator-based parent interview [25] and (2) teacher rated Disruptive Behavior Disorder rating scale (DBDRS) assessing the presence and severity of symptoms of ADHD [26].
The PCT protocol was based on the titration protocol used in the MTA study [18], modified to improve clinical usability by weekly instead of daily dose changes [27]. All participants received the following treatment conditions: placebo and 5, 10, 15 and 20 mg of MPH twice daily (20 mg only for children > 25 kg [28] in a semi-randomized order. The randomization and blinding procedure is described in more detail in Supplement S1. We used dosing twice daily versus three times daily as this is the dominant Dutch clinical practice. The titration procedure started with a lead-in phase [29], consisting of 4 days in which all doses were administered in ascending order. If a dose was not tolerated it was excluded from the PCT. Duration of the PCT was 3 to 5 weeks, depending on the child’s weight and MPH doses tolerated. During PCT, treatment with a particular dose started on a Saturday and was administered for seven consecutive days, twice daily, at breakfast (around 8 a.m.) and at lunch time (around 12 a.m.).
At baseline and at the end of each week, parents and teachers completed the Strength and Weakness of ADHD symptoms and Normal Behavior Rating Scale (SWAN) [30] rating scale and an adapted version of the MTA Side Effect Rating Scale (see description below) [19]. The local ethics committee approved the study (METC VUMC, # 2016.594 & Netherlands trial register # NL8121).
Outcome variables
ADHD symptoms
ADHD symptom severity was measured with the SWAN [30], adapted to measures symptom severity in the past week. This questionnaire contains the following two scales: the Inattention scale and the Hyperactivity/Impulsivity scale, each comprising nine items based on the DSM-IV symptoms of ADHD. Items are scored on a 7-point Likert scale ranging from − 3 to 3, with lower ratings reflecting worse symptoms. Items reflect both ends (strong and weak) of the behavior captured in each symptom. Therefore, it has the potential to reveal clearer and additional dose–response effects due to assessment across the full range of positive and negative manifestations of the behavior underling the symptoms of ADHD [31].
Side effects
Side effects were reported using an adapted version of the MTA Side Effect Rating Scale [19], using a scoring system according to Wigal [32]. Commonly reported side effects were rated on a 4-point scale (0 = not at all, 1 = just a little, 2 = pretty much and 3 = very much) measuring side effect severity in the past week. The total score was used in the analyses.
Predictors of individual MPH dose–response curves
To explore clinically useful predictors, a range of variables that have been associated with dose–response effects [12, 20, 21, 24] and can be routinely identified in standard clinical practice, were assessed at baseline. Candidate predictors included clinical characteristics, demographic variables and attitudes towards ADHD diagnoses and treatment and are described in the Supplement S2 and S3.
Statistical analyses
Analyses were performed using STATA (version 16.0). To examine the effects of MPH dose on ADHD symptoms and side effects, mixed model analyses were conducted given the hierarchical data-structure. To investigate the dose–response curves at group level, two analyses were performed. First, to determine the function best describing the dose–response relationship linear dose–response curves were fitted as function of absolute dose (mg) for each of the following six outcome measures: parent- and teacher-rated inattention symptoms, hyperactivity-impulsivity symptoms and side effects. Next, it was evaluated if a second-, or third order polynomial better described the relationship by adding a quadric and S-shaped component, respectively. Additionally, the analysis were repeated with relative dose (mg/kg). Second, we treated dose as a categorical variable, comparing the effects of 5, 10, 15, and 20 mg (children > 25 kg only) on each of the six outcomes with a mixed model analysis.
As linear dose–response curves provided the best description of the dose–response curves at the group level, linear dose–response curves were also fitted to the individual dose–response curves. The regression coefficient of these individual dose–response curves was used to quantify the dose–response relationship for each outcome measure. Positive β-values indicate that increased doses of MPH were related to steeper linear dose–response curves, with increased doses resulting in larger reductions in ADHD symptoms or a smaller increase in side effects. Non-positive β-values indicate that higher MPH doses were related to more shallow linear dose–response curves, with increased doses resulting in smaller reductions in ADHD symptoms or a smaller increase in side effects.
Finally, the possibility to predict the variation in the individual linear dose–response curves, using variables assessed at study entry was explored. Mixed model analyses, with univariate preselection of significant predictors and backward selection procedure, were used to construct prediction models. Complying with the convention in this type of analysis, the threshold for significance was set at p-values < 0.10 [33]. The amount of variance explained (R.2) was calculated, with 1%, 9% and 25% used as the thresholds for small, medium and large effects, respectively [34].
Results
Sample
Forty-one clinicians from 13 youth mental health clinics across the Netherlands participated. Forty-five children were included in the analyses. Table 1 displays the participants’ demographic and clinical characteristics. One child did not receive the 15 and 20 mg due to a combination of severe side effects and dosing restriction (< 25 kg), eight children did not receive the 20 mg dose, six because of the dosing restrictions (< 25 kg) and two due to severe side effects. All clinicians fully adhered to the protocol with each tested MPH dose being prescribed for seven consecutive days, twice daily.
Table 1.
Demographic and clinical characteristics of participating children (n = 45)
| M | SD | Min | Max | |
|---|---|---|---|---|
| Age (years) | 9.53 | 1.70 | 5.91 | 12.77 |
| Sex (% male) | 67% | |||
| Weight (kg) | 32.7 | 8.41 | 18 | 64 |
| KSADS | ||||
| Inattention symptoms | 7.07 | 1.62 | 0 | 8 |
| Hyperactivity-impulsivity symptoms | 5.84 | 2.29 | 2 | 9 |
| ODD symptoms | 2.11 | 2.24 | 0 | 8 |
| SWAN inattention | ||||
| Parent | − 14.30 | 6.59 | − 23 | 4 |
| Teacher | − 13 | 6.02 | − 26 | 10 |
| SWAN hyperactivity-impulsivity | ||||
| Parent | − 13.42 | 7.58 | − 27 | 17 |
| Teacher | − 9.49 | 10.62 | − 26 | 13 |
| CBCL internalizing problems | 57.49 | 9.98 | 33 | 74 |
| Opinion on treatment (child) | 4.35 | 1.67 | 1 | 9 |
| Aversion towards medication (child) | 1.60 | 0.79 | 1 | 3 |
| Agreement with therapy | ||||
| Parent | 17.91 | 2.73 | 11 | 20 |
| Teacher | 18.14 | 2.46 | 11 | 20 |
| Treatment expectations | ||||
| Parent | 4.25 | 0.81 | 2 | 5 |
| Teacher | 4.08 | 0.73 | 3 | 5 |
Dose–response curves at group level
Results of the mixed model analyses on the dose–response curves (with absolute dose) at group level are depicted in Tables 2, 3 and 4. Regression coefficients of the second- and third order polynomial were non-significant (data available from the author). The relationship best describing the dose–response was a positive linear relationship for MPH dose and both parent and teacher ratings on the SWAN scales Inattention and Hyperactivity/Impulsivity, indicating that higher MPH doses resulted in a larger improvements in ADHD symptoms. For side effects measured with the parent-reported Side Effect Rating Scale, the relationship was best described by a positive linear effect, indicating that increased doses resulted in more side effects as observed by parents. No significant relationship was found between MPH dose and teacher-reported side effects. Additional analysis with relative dose (mg/kg) resulted in similar results (data available from the author).
Table 2.
Effects of MPH on inattention, hyperactivity/impulsivity and side effects
| Placebo | 5 mg | 10 mg | 15 mg | 20 mg | |
|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | |
| SWAN inattention | |||||
| Parent | − 8.93 (8.89) | − 9.53 (7.83) | − 6.16 (7.78) | − 4.86 (9.40) | − 4.54 (8.04) |
| Teacher | − 12.27 (7.17) | − 9 (7.18) | − 6.88 (9.08) | − 4.3 (9.41) | − 4.41 (7.25) |
| SWAN hyperactivity/impulsivity | |||||
| Parent | − 8.48 (8.58) | − 8.84 (8.46) | − 5.2 (6.70) | − 4.34 (8.66) | − 2.97 (7.86) |
| Teacher | − 11.46 (8.02) | − 7.19 (9.53) | − 4.90 (10.89) | − 2.88 (9.13) | − 3.34 (8.18) |
| Side effects | |||||
| Parent | 4.98 (4.03) | 6.07 (4.70) | 5.64 (3.95) | 6.86 (4.81) | 5.79 (4.06) |
| Teacher | 2.71 (3.03) | 3.35 (2.85) | 2.75 (3.16) | 3.35 (3.54) | 2.63 (2.63) |
SWAN scores range from − 27 to 27, Side effects scores range from 0 to 39
Table 3.
Dose–response curves fitted as function of dose at group level
| Outcome | Linear trend |
|---|---|
| Coef. (95% CI) | |
| SWAN inattention | |
| Parent | 0.29*** (0.15 to 0.42) |
| Teacher | 0.42*** (0.28 to 0.55) |
| SWAN hyperactivity/impulsivity | |
| Parent | 0.33*** (0.19 to 0.46) |
| Teacher | 0.43*** (0.29 to 0.56) |
| Side effects | |
| Parent | 0.08* (0.01 to 0.15) |
| Teacher | 0.01 (− 0.04 to 0.05) |
*p < 0.05; **p < 0.01; ***p < 0.001
Table 4.
Effects of different MPH doses versus placebo
| Outcome | 5 mg | 10 mg | 15 mg | 20 mg |
|---|---|---|---|---|
| Coef. (95% CI) | Coef. (95% CI) | Coef. (95% CI) | Coef. (95% CI) | |
| SWAN inattention | ||||
| Parent | − 0.62 (− 3.46 to 2.23) | 2.76 (− 0.08 to 5.61) | 3.93** (1.07 to 6.80) | 4.82** (1.75 to 7.88) |
| Teacher | 3.22* (0.39 to 6.05) | 5.06** (2.20 to 7.93) | 7.36*** (4.45 to 10.27) | 8.20*** (5.05 to 11.36) |
| SWAN hyperactivity/impulsivity | ||||
| Parent | − 0.31 (− 3.08 to 2.47) | 3.34* (0.56 to 6.11) | 4.18** (1.38 to 6.97) | 5.91*** (2.92 to 8.90) |
| Teacher | 3.91** (1.15 to 6.68) | 6.10*** (3.32 to 8.90) | 7.76*** (4.95 to 10.57) | 8.52*** (5.52 to 11.53) |
| Side effects | ||||
| Parent | 1.16 (− 0.27 to 2.61) | 1.03 (− 0.48 to 2.53) | 2.01** (0.52 to 3.55) | 1.40 (− 0.24 to 3.04) |
| Teacher | 0.48 (− 0.49 to 1.45) | 0.02 (− 0.93 to 0.98) | 0.87 (− 0.10 to 1.84) | − 0.08 (− 1.09 to 0.94) |
The regression coefficient represents the difference in the outcome between the MPH dose and placebo
*p < .0.05; **p < 0.01; ***p < 0.001
Next, we treated dose as a categorical variable comparing the effects of each of the MPH doses and placebo on the six outcomes. Parents reported significant improvement with MPH doses of 10, 15 and 20 mg compared to placebo on the SWAN hyperactivity/impulsivity scale and for 15 and 20 mg doses on the SWAN inattention scale. Additionally parents reported significantly higher scores on the Side Effect Rating Scale for 15 mg compared to placebo. Teachers reported significant improvement on both SWAN scales for all MPH doses compared to placebo. For side effects measured with the teacher-reported Side Effect Rating Scale, none of the MPH doses differed significantly from placebo. None of the other comparisons between the tested MPH doses was significant.
Dose–response curves at individual level
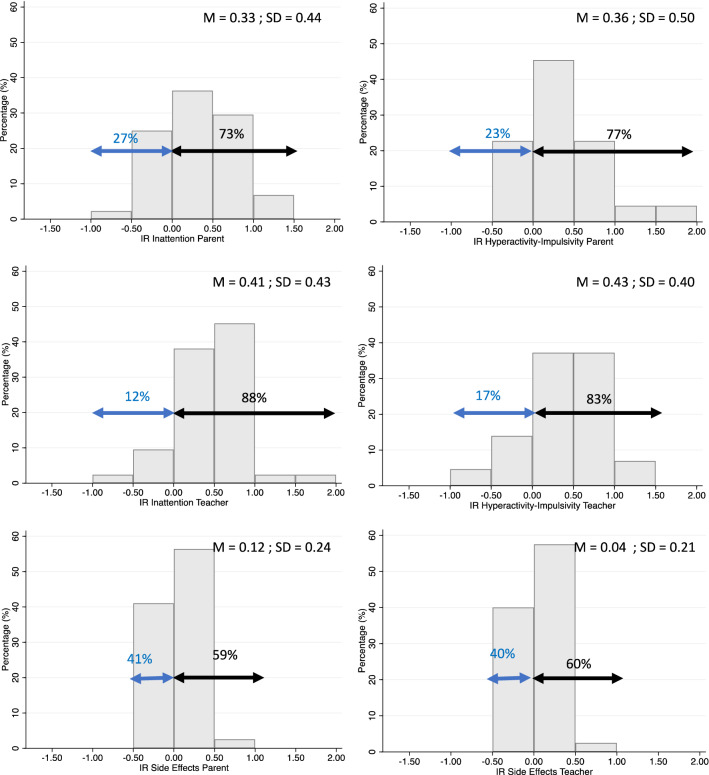
To investigate individual response to MPH, a linear trend was fitted to the individual dose–response curves. The regression coefficients of these linear curves were used to quantify the individual dose–response relationships. Figure 1 shows the distribution of the regression coefficients for each of the six outcome measures. In line with the findings at group level, for parent- and teacher-reported inattention and hyperactivity/impulsivity on the SWAN, the majority of subjects (73–88%), showed positive regression coefficients. However, 12 to 27% of the dose–response curves yielded non-positive regression coefficients, indicating that in these children, higher MPH doses did not result in lower levels of ADHD symptoms.
Fig. 1.
Histogram of regression coefficients obtained from the individual linear dose–response curves. The left arrows represent the percentage of children with negative dose–response curves, while the right arrows represent the percentage of children with positive dose–response curves
For both parent- and teacher-reported side effects, the majority of subjects (59 and 60%, respectively) showed positive regression coefficients, indicating greater severity of side effects with increased doses. This indicates that 40–41% of the children did not show an increase in side effects when increasing the dose. Note that for both parent- and teacher-reported side effects less than 10% of the children show beta coefficients > 0.5, suggesting that most children showed shallow dose-side effect curves.
Prediction models for regression coefficients of linear dose–response
Table 5 shows the prediction models. Higher child-reported aversion towards medication was significantly related, with a medium effect (R2 = 17%), to a shallower linear dose–response curves for parent-reported inattention on the SWAN. Higher parent-reported KSADS hyperactivity-impulsivity was significantly related, with a medium effect (R2 = 14%), to steeper linear dose–response curves for parent-reported hyperactivity-impulsivity on the SWAN. Higher Child Behavior Checklist (CBCL) Internalizing symptoms and lower parent- and teacher-reported agreement with diagnosis and therapy were significantly related to shallower linear dose–response curves for teacher-reported inattention and hyperactivity-impulsivity on the SWAN, with large effects (respectively R2 = 27% and 48%). Regarding side effects, older age was significantly related, with a medium effect (R2 = 9%), to shallower linear dose–response curves for parent-reported side-effects. Lower weight and lower CBCL internalizing symptoms were significantly related, with medium effects (R2 = 28%), to shallower linear dose–response curves for teacher-reported side-effects.
Table 5.
Results of the regression analysis testing the relationship between demographic and clinical characteristics and coefficients of the linear individual dose–response curves
| Outcome | Predictors | Multivariable results | Variance explained |
|---|---|---|---|
| SWAN inattention | |||
| Parent | Aversion towards taking medication (child) | β(SE) = − 0.22 (0.08), p = 0.004 | R2 = 17% |
| Teacher | Teacher-reported agreement with diagnosis and therapy | β(SE) = 0.05 (0.02), p = 0.03 | R2 = 27% |
| CBCL internalizing problems | β(SE) = − 0.01 (0.01), p = 0.06 | ||
| SWAN hyperactivity/impulsivity | |||
| Parent | KSADS hyperactivity-impulsivity | β(SE) = 0.08 (0.03), p = 0.009 | R2 = 14% |
| Teacher | CBCL internalizing symptoms | β(SE) = − 0.01 (0.01), p = 0.01 | R2 = 48% |
| Parent-reported agreement with diagnosis and therapy | β(SE) = 0.05 (0.02), p = 0.02 | ||
| Teacher-reported agreement with diagnosis and therapy | β(SE) = 0.05 (0.02), p = 0.02 | ||
| Side effects | |||
| Parent | Age | β(SE) = − 0.04 (0.02), p = 0.08 | R2 = 9% |
| Teacher | Weight | β(SE) = − 0.01 (0.00), p = 0.06 | R2 = 28% |
| CBCL internalizing symptoms | β(SE) = − 0.01 (0.00), p < 0.001 | ||
Negative β-values indicate that a higher score on the predictor was related to a more shallow linear dose–response curve, whereas positive β-values indicate that a higher score on the predictor was related to a steeper linear dose–response curve. R2 = amount of variance explained
For all analyses, sensitivity analyses were conducted, excluding children with clinical scores on the internalizing scale of the parent reported Child Behavior Checklist (CBCL) because internalizing symptoms might be perceived as ADHD-symptoms and therefore might have biased our results. This did not change our main findings (data available from the author).
Discussion
This study aimed to gain more insight into dose effects of MPH on ADHD symptoms and side effects using a double-blinded, randomized, placebo-controlled crossover design. Our results demonstrated that, at group level, the dose–response relationship for parent- and teacher-rated ADHD symptoms can best be described as a positive linear relationship, indicating that higher MPH doses resulted in a larger improvements in ADHD symptoms. Regarding side effects, parents reported increased doses to result in more side effects, although this trend was not observed by teachers. Comparisons of the effects of placebo and the tested MPH doses showed that doses of 10 mg and higher yielded significant reductions in parent-reported ADHD symptoms, whereas all tested doses yielded significant reductions in teacher-reported ADHD symptoms. Regarding side effects, parents reported more side effects with doses 15 mg compared to placebo, whereas teachers observed no differences between any of the MPH doses and placebo. Examining individual dose–response curves showed that a significant part (12–27%) of children did not show a positive linear dose–response curve. For side effects, only 59–60% of children showed an increase of side effects with increasing doses. Explorative analyses showed that higher severity of hyperactive-impulsive symptoms and lower internalizing problems, lower weight, younger age and more positive opinions towards diagnosis and medication contributed to predicting steeper linear individual dose–response curves.
The positive linear dose–response curves observed at group level and the finding that only high doses show parent-reported symptom reduction are consistent with the idea that increased doses of MPH lead to a larger reductions in ADHD symptoms. However, our results clearly demonstrate that a significant subgroup does not show larger symptom reduction with increased doses. Thus, the current study replicates that the effects of MPH on ADHD symptoms can be best described as a positive linear dose–response relationship, but also shows that divergent responses to dose increase are not incidental findings.
At group level, higher MPH doses were related to more parent-reported, but not to higher teacher-reported side effects. This is in line with the findings from the MTA-study that showed parents to report more dose-related adverse effects, making them the best reporters of side effects [19]. For the majority of subjects, increased doses of MPH are related to greater severity in side effects, although this inverse relationship between dose and side effects was not observed in a substantial part of the children (39–40%). For most children our data support the advice to explore the full range of MPH doses [7], with a low risk of clinical important side effects.
This study explored predictors for the individual dose–response to MPH. To the best of our knowledge, the current study is the first to explore the role of attitudes towards diagnosis and medication in the response to MPH. Interestingly, we found that higher parent and teacher agreement with the diagnosis and therapy were related to larger improvements in teacher-reported ADHD symptoms with increasing dose. Furthermore, larger aversion of the child towards taking medication, was related to smaller improvements in ADHD symptoms with increasing dose. There are two possible explanations for these findings. On the one hand this suggests that negative attitudes towards the diagnosis and medication may result in smaller placebo effects, which in turn may diminish the beneficial effects. On the other hand it’s also possible that negative attitudes towards diagnosis and medication may result in more accurate ratings of ADHD symptoms following pharmacological intervention, because these ratings are less biased by the expectation of symptom improvement.
Additionally, more severe hyperactivity–impulsivity symptoms and lower internalizing problems were associated with a larger reduction of ADHD symptoms with increasing MPH doses. This is in line with previous research [20, 21, 35] showing these measures to be associated with a larger response to MPH regardless of dose. In contrast with the results from the MTA-study [12], age was not significantly related to the dose–response to MPH. Side effects, higher age, weight and internalizing symptoms, were related with more shallow dose response curves, and thus a smaller increase of side effects with increasing dose.
Our findings should be viewed with some limitations in mind. First, our results only pertain to the short-term effects of MPH dose. Follow-up from the MTA-study has shown that initial titration does not prevent the need for subsequent maintenance adjustments; however, end-of-titration optimal dose and maintenance dose were strongly related [10]. Second, no instruments were used to report treatment fidelity. Children who had greater aversion to taking medication may not have taken medication as prescribed which, in turn, could affect symptom changes. Third, MPH was administered twice daily, in accordance with dominant Dutch clinical practice. The observed differences between dose–response effects for teacher and parent ratings might be affected by the time–response effects and the time of assessment by observations during the day (by teachers) and after school (by parents). Fourth, only the clinical diagnosis of ADHD and symptoms of ODD were assessed using standardized assessments conducted by the researchers. No standardized assessment of other comorbid disorders was conducted. Consequently, we were not able to test the possible predictive power of comorbidity for dose–response effects
Despite these limitations our study is one of few studies that attempted to analyze dose–response curves of MPH in ADHD. Last, we explored predictors for individual dose response as a first attempt to study what dosage works for whom. We explored multiple potential predictors in a relatively small sample, which generated important hypotheses regarding the response to MPH. However, due to limited power, weaker predictors may have remained undetected. Further research is needed to replicate these findings and draw stronger conclusions regarding subgroups that respond differently to MPH dosages, such as subgroups that differ in attitudes towards diagnoses and treatment for ADHD.
Conclusion
Although increased doses of MPH yield greater symptom control at a group level, there is large inter-individual variation in the dose–response relationship. As dose–response curves can only be partly explained based on patient characteristics, our findings emphasize the need to use an objective evaluation of the full range of the possible therapeutic doses for all individual patients as substantiated in the MTA-study [18] and in the recent meta-analyses by Farhat and colleagues [6]. Side effects should be monitored when increasing the dose [6], although exploring the clinical effect of the full dose range is often possible without increasing side effects. Physicians should be mindful about attitudes towards the diagnosis and medication as they might contribute to MPH treatment effects.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We like to thank all participating children and their families for their contribution, as well as all research interns for their valuable support. Furthermore, we would like to thank the participating centers of child and adolescent psychiatry and the Innovation fund of health insurances (Innovatiefonds Zorgverzekeraars).
Abbreviations
- ADHD
Attention-deficit/hyperactivity disorder
- MPH
Methylphenidate
- K-SADS
Kiddie–schedule for affective disorders and schizophrenia for school-age children present and lifetime version
- DBDRS
Disruptive behavior disorder rating scale
- PCT
Placebo controlled titration
Author contributions
KV conceptualized and designed the study, designed the data collection instruments, collected the data, extracted the data, conducted the initial analyses and drafted the initial manuscript. ML, JMS and JO conceptualized and designed the study, designed the data collection instruments and co-wrote the manuscript. AW, MB and RS collected the data. JWRT supervised the analyses. PB advised on the medication protocol.
Funding
This study is part of the project ‘Reduce and optimize methylphenidate use in children and adolescents with ADHD’ (grant # p3119), funded by Innovation fund of health insurances (Innovatiefonds Zorgverzekeraars).
Data availability
Deidentified individual participant data (including data dictionaries) will be made available, upon publication to researchers who provide a methodologically sound proposal for use in achieving the goals of the approved proposal. Proposals should be submitted to k.vertessen@vu.nl.
Code availability
The statistical analysis plan and codes will be made available upon request. Proposals should be submitted to k.vertessen@vu.nl.
Declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
Karen Vertessen has been involved in a clinical trial sponsored by Takeda. The other authors have no conflicts of interest relevant to this article to disclose.
Ethical approval
The local ethics committee approved the study (METC VUMC, # 2016.594).
Informed consent
Parents and children older than 11 years provided signed informed consent approved by the local ethics committee.
Consent for publication
All authors reviewed and revised the manuscript and approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
References
- 1.Willcutt EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics. 2012;9(3):490–499. doi: 10.1007/s13311-012-0135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cortese S, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry. 2018;5(9):727–738. doi: 10.1016/S2215-0366(18)30269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attention deficit hyperactivity disorder: diagnosis and management NICE guideline (2018). www.nice.org.uk/guidance/ng87. Accessed 30 Jun 2021
- 4.Wolraich M.L. et al. (2019) Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. www.aappublications.org/news. Accessed 30 Jun 2021
- 5.Canadian ADHD Resource Alliance (CADDRA): Canadian ADHD Practice Guidelines, Fourth Edition, Toronto ON; CADDRA, 2018. www.caddra.ca. Accessed 30 Jun 2021
- 6.Farhat LC, et al. The effects of stimulant dose and dosing strategy on treatment outcomes in attention-deficit/hyperactivity disorder in children and adolescents: a meta-analysis. Mol Psychiatry. 2022;27(3):1562–1572. doi: 10.1038/s41380-021-01391-9. [DOI] [PubMed] [Google Scholar]
- 7.Rapport MD, Denney C. Titrating methylphenidate in children with attention-deficit/hyperactivity disorder: is body mass predictive of clinical response?”. J Am Acad Child Adolesc Psychiatry. 1997;36(4):523–530. doi: 10.1097/00004583-199704000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Newcorn JH, Stein MA, Cooper KM. Dose-response characteristics in adolescents with attention-deficit/ hyperactivity disorder treated with OROS® methylphenidate in a 4-week, open-label, dose-titration study. J Child Adolesc Psychopharmacol. 2010;20(3):187–196. doi: 10.1089/cap.2009.0102. [DOI] [PubMed] [Google Scholar]
- 9.Rapprot MD, et al. Attention deficit disorder and methylphenidate: normalization rates, clinical effectiveness, and response prediction in 76 children. J Am Acad Child Adolesc Psychiatry. 1994;33(6):882–893. doi: 10.1097/00004583-199407000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Vitiello B, et al. Methylphenidate dosage for children with ADHD over time under controlled conditions: lessons from the MTA. J Am Acad Child Adolesc Psychiatry. 2001;40(2):188–196. doi: 10.1097/00004583-200102000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Medori R, et al. A randomized, placebo-controlled trial of three fixed dosages of prolonged-release OROS methylphenidate in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63(10):981–989. doi: 10.1016/j.biopsych.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Greenhill LL, et al. Impairment and deportment responses to different methylphenidate doses in children with ADHD: the MTA titration trial. J Am Acad Child Adolesc Psychiatry. 2001;40(2):180–187. doi: 10.1097/00004583-200102000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Swanson JM, et al. Effects of stimulant medication on learning in children with ADHD. J Learn Disabil. 1991;24(4):219–230. doi: 10.1177/002221949102400406. [DOI] [PubMed] [Google Scholar]
- 14.Volkow N.D and Swanson J.M. (2003) Variables That Affect the Clinical Use and Abuse of Methylphenidate in the Treatment of ADHD. http://ajp.psychiatryonline.org [DOI] [PubMed]
- 15.Rapport MD, et al. Attention deficit disorder and methylphenidate: a multilevel analysis of dose-response effects on children’s impulsivity across settings. J Am Acad Child Adolesc Psychiatry. 1988;27(1):60–69. doi: 10.1097/00004583-198801000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Stein MA, et al. Dopamine transporter genotype and methylphenidate dose response in children with ADHD. Neuropsychopharmacology. 2005;30(7):1374–1382. doi: 10.1038/sj.npp.1300718. [DOI] [PubMed] [Google Scholar]
- 17.Faraone S.v. , et al. Placebo and nocebo responses in randomised, controlled trials of medications for ADHD: a systematic review and meta-analysis. Mol Psychiatry. 2022;27(1):212–219. doi: 10.1038/s41380-021-01134-w. [DOI] [PubMed] [Google Scholar]
- 18.The MTA Cooperative Group A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder the MTA cooperative group. Arch Gen Psyciatry. 1999;56(12):1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- 19.Greenhill LL, et al. Medication treatment strategies in the MTA study: relevance to clinicians and researchers. J Am Acad Child Adolesc Psychiatry. 1996;35(10):1304–1313. doi: 10.1097/00004583-199610000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Gray JR, Kagan J. The challenge of predicting which children with attention deficit-hyperactivity disorder will respond positively to methylphenidate. J Appl Dev Psychol. 2000;21:471–489. doi: 10.1016/S0193-3973(00)00050-2. [DOI] [Google Scholar]
- 21.Johnston BA, et al. Predicting methylphenidate response in attention deficit hyperactivity disorder: a preliminary study. J Psychopharmacol. 2015;29(1):24–30. doi: 10.1177/0269881114548438. [DOI] [PubMed] [Google Scholar]
- 22.Sandler AD, Glesne CE, J. W. Bodfish J.W. Conditioned placebo dose reduction: a new treatment in attention-deficit hyperactivity disorder? J Dev Behav Pediatr. 2010;31(5):369–375. doi: 10.1097/DBP.0b013e3181e121ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Volkow ND, et al. Effects of expectation on the brain metabolic responses to methylphenidate and to its placebo in non-drug abusing subjects. Neuroimage. 2006;32(4):1782–1792. doi: 10.1016/j.neuroimage.2006.04.192. [DOI] [PubMed] [Google Scholar]
- 24.Benedetti F. Placebo effects. Oxford: Oxford University Press; 2020. [Google Scholar]
- 25.Reichart CG, Wals M, Hillegers M. Vertaling K-sads. Utrecht: HC Rümke Groep; 2000. [Google Scholar]
- 26.Oosterlaan J, et al. Vragenlijst voor gedragsproblemen bij kinderen 6–16 jaar Handleiding. Amsterdam: Harcourt Publisher; 2008. [Google Scholar]
- 27.Luman M, Goos V, Oosterlaan J. Instrumental learning in ADHD in a context of reward: intact learning curves and performance improvement with methylphenidate. J Abnorm Child Psychol. 2015;43(4):681–691. doi: 10.1007/s10802-014-9934-1. [DOI] [PubMed] [Google Scholar]
- 28.Janssen TWP, et al. A randomized controlled trial investigating the effects of neurofeedback, methylphenidate, and physical activity on event-related potentials in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2016;26(4):344–353. doi: 10.1089/cap.2015.0144. [DOI] [PubMed] [Google Scholar]
- 29.A. Scheres et al. (2003) The Effect of Methylphenidate on Three Forms of Response Inhibition in Boys With AD/HD. [DOI] [PubMed]
- 30.J. M. Swanson et al. (2012) Categorical and Dimensional Definitions and Evaluations of Symptoms of ADHD: History of the SNAP and the SWAN Rating Scales HHS Public Access. www.ADHD.net [PMC free article] [PubMed]
- 31.Arnett AB, et al. The SWAN captures variance at the negative and positive ends of the ADHD symptom dimension. J Atten Disord. 2013;17(2):152–162. doi: 10.1177/1087054711427399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wigal T, et al. Safety and tolerability of methylphenidate in preschool children with ADHD. J Am Acad Child Adolesc Psychiatry. 2006;45(11):1294–1303. doi: 10.1097/01.chi.0000235082.63156.27. [DOI] [PubMed] [Google Scholar]
- 33.Twisk JWR. Applied mixed model analysis. Cambridge: Cambridge University Press; 2019. [Google Scholar]
- 34.Cohen J. Statistical power analysis for the behavioral sciences. Cambridge: Academic press; 2013. [Google Scholar]
- 35.Buitelaar JK, et al. Prediction of clinical response to methylphenidate in children with attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1995;34(8):1025–1032. doi: 10.1097/00004583-199508000-00012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified individual participant data (including data dictionaries) will be made available, upon publication to researchers who provide a methodologically sound proposal for use in achieving the goals of the approved proposal. Proposals should be submitted to k.vertessen@vu.nl.
The statistical analysis plan and codes will be made available upon request. Proposals should be submitted to k.vertessen@vu.nl.