Abstract
Bartonella henselae is an emerging pathogen causing cat scratch disease, bacillary angiomatosis, and peliosis hepatis. Progress in understanding the pathogenesis of and the immune response to these infections has been limited by the lack of an animal model. Following intraperitoneal infection of C57BL/6 mice with B. henselae, organs were cleared of cultivatable bacteria within 6 days. In contrast, B. henselae DNA could be detected in liver tissue for at least 3 months. Liver tissue showed granulomatous inflammation reaching its highest degree of intensity during the fourth week of infection and resolving within 12 weeks postinfection. This mouse model is applicable to the study of the pathogenesis of B. henselae and the immune response to this pathogen in the immunocompetent host.
Bartonella henselae, a gram-negative bacterium in the family Bartonellaceae, was recently recognized to cause cat scratch disease (CSD), bacillary angiomatosis (BA), peliosis hepatis, and persistent bacteremia (13, 16, 17). CSD is a common cause of subacute regional granulomatous lymphadenitis in immunocompetent persons (7). In contrast, BA and peliosis hepatis, as well as bacteremia, occur most commonly in individuals infected with the human immunodeficiency virus (HIV). BA refers to a vasculoproliferative disease that often involves the skin in the form of nodular lesions, but may disseminate to other organs. Peliosis hepatis is characterized by vascular proliferation in liver tissue resulting in blood-filled spaces and may be associated with BA in HIV-infected patients (9). Progress in understanding the pathogenesis of and the immune response to these infections has been limited by the lack of a suitable animal model.
Recently, cats experimentally infected with B. henselae by intravenous inoculation were shown to develop histopathologic lesions in multiple organs, including granulomas in liver and lymph nodes (6). Therefore cats might be used for studying infection with B. henselae. However, because of the advantages rodents offer for immunological investigation, we have developed a murine model of B. henselae infection.
B. henselae Houston 1 (ATCC 49882) recovered from the bloodstream of an HIV-positive patient in 1992 (12) was used throughout. To increase and maintain virulence in mice, initial in vivo passages were performed as follows. Bacteria from a log-phase culture in brucella broth (BBL Microbiology Systems, Cockeysville, Md.) supplemented with hemin (250 μg/ml) and 8% Fildes solution (14) and incubated at 37°C in 7% CO2 in air were pelleted by centrifugation (5,000 × g, 30 min), washed three times in phosphate-buffered saline, and administered intraperitoneally (i.p.) to C57BL/6 mice in doses ranging from 5 × 107 to 5 × 108 CFU. After 3 days, spleens were removed, homogenized, and inoculated into supplemented brucella broth. On day 5 of incubation, the bacteria were harvested and injected i.p. again. After completing a minimum of four in vivo passages, bacteria grown from spleen homogenates were used for infection experiments without prior freezing to ensure optimal viability.
Female C57BL/6 and BALB/c mice raised in our breeding facilities were used at the age of 10 to 12 weeks. All inoculations were performed i.p. in a volume of 0.5 ml of phosphate-buffered saline. Inocula were determined by plating of 10-fold serial dilutions on Columbia agar (Oxoid, Basingstoke, United Kingdom) supplemented with 5% human blood.
Clearance of bacteria from infected organs.
To determine the course of infection in C57BL/6 mice, 9.1 × 107 CFU of viable bacteria were inoculated i.p. Groups of five animals each were killed 6 h postinfection; daily from day 1 to day 6; and on days 10, 21, 36, 64, and 94. From all mice, bacterial loads in liver, spleen, lung, kidney, and brain were determined by plating of 10-fold serial dilutions of organ homogenates on Columbia agar supplemented with 5% human blood. In addition, quantitative cultures of blood specimens were performed at the time of necropsy. Before being plated on Columbia agar, erythrocytes were lysed either by freezing and thawing or by being mixed vigorously with distilled water. The detection limits of these procedures were 10 bartonellae per organ and 1 bartonella per 0.25 ml of blood, respectively. All cultures were incubated at 37°C in 7% CO2 in air for at least 3 weeks before being coded as negative for growth. Colonies were identified as B. henselae by colony morphology and Gram stain.
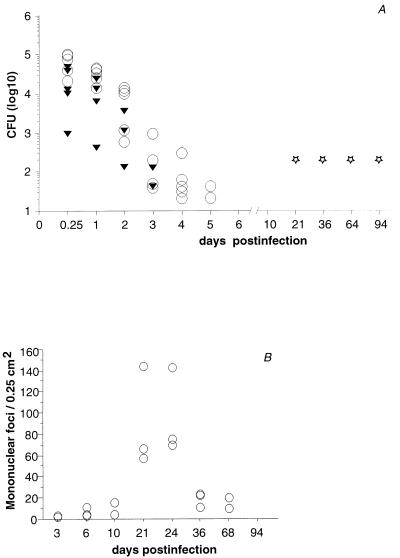
Infected mice did not show any signs of morbidity. Quantitative spleen and liver cultures showed clearance of cultivatable organisms within the first 6 days of infection (Fig. 1A). From blood and from brain, low numbers of organisms could be recovered only at 6 h postinfection in three of five mice (12, 20, and 8 organisms/ml of blood) and in one of five animals (10 organisms/brain), respectively. Kidney and lung remained sterile throughout this experiment. Subsequent experiments with inocula ranging from 3.5 × 107 to 2.0 × 108 CFU yielded comparable results. Rapid clearance of cultivatable organisms seemed to indicate resistance of immunocompetent C57BL/6 mice to B. henselae. We therefore also investigated the course of infection for a period of 10 days in BALB/c mice, which are known to be more susceptible than C57BL/6 mice to several pathogens, such as Listeria, Yersinia, and Leishmania species. However, no significant differences were found (data not shown).
FIG. 1.
C57BL/6 mice were infected i.p. with B. henselae. To determine the course of infection, bacterial loads in livers and spleens were determined by culture. Nested PCR was performed with samples of B. henselae DNA from culture-negative livers. The cellular inflammatory reaction in liver tissue was quantified by counting of mononuclear foci. (A) Following i.p. inoculation with 9.1 × 107 CFU of B. henselae, cultivatable organisms were cleared from livers (▾) and spleens (○) of C57BL/6 mice within 4 and 6 days, respectively (five animals per group, with each triangle or circle representing a positive culture from a single organ). DNA from B. henselae (⋆) could be detected in liver tissue for at least 3 months (with each star representing three positive results from three mice). (B) Kinetics of cellular inflammatory reaction in liver tissue following i.p. inoculation with 1.2 × 108 CFU of B. henselae (three mice per group, with each circle representing a single mouse). Mononuclear foci were counted from hematoxylin-and-eosin-stained sections.
Detection of B. henselae DNA in liver tissue.
In CSD, isolation of B. henselae from patients has been rare (4). In contrast, detection of B. henselae DNA by PCR could be achieved in clinical specimens from the majority of patients (1, 15). To determine the presence of B. henselae DNA in mouse tissue, we developed a nested PCR assay to amplify part of the B. henselae gltA gene (11). The outer primer sequences were 5′ GGT CCC AAC TCT TGC CGC TAT G 3′ and 5′ CAG CCG ACA CTG CGT GCT AAT G 3′. The sequences of the inner primers were 5′ ATG CCT AAA AAT GTT ACA AGA 3′ and 5′ CGT GCT AAT GCA AAA AGA AC 3′. The sensitivity of PCR was 10 CFU per organ, which equals the sensitivity of culture. Liver homogenates of C57BL/6 mice infected with 9.1 × 107 viable B. henselae organisms were analyzed by PCR. Mice were sacrificed at days 21, 36, 64, and 94. DNA from the livers of three animals each was extracted with a QIAamp tissue kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Extracts were stored at −70°C. PCR was performed with Taq polymerase (InViTek, Berlin, Germany) in a volume of 25 μl in two sequential tubes. Following initial denaturation for 4 min at 95°C, amplification with outer primers was performed with 30 cycles of 1 min at 94°C, 1 min at 70°C, and 2 min at 72°C. Amplification with inner primers consisted of 30 cycles of 1 min at 94°C, 10 s at 30°C, and 1 min at 72°C. The final extension step was extended to 10 min in both rounds of PCR. Positive controls were obtained by spiking of liver tissue homogenate from an uninfected mouse with B. henselae prior to DNA extraction. DNA from the liver of an uninfected mouse was used as a negative control. Numerous negative controls were included to exclude cross-contamination. The reaction product was visualized by electrophoresis in an agarose gel and by staining with ethidium bromide. The expected product size was 354 bp. PCR was repeated twice for two separately prepared DNA samples. Nested PCR for the gltA gene revealed the presence of B. henselae DNA for at least 3 months in the livers of all mice examined. This result indicates the presence of B. henselae in liver tissue.
Histopathology.
Bartonella-induced histopathological alterations were determined for C57BL/6 mice infected with inocula ranging from 5 × 105 to 8.5 × 108 viable B. henselae organisms. Animals were killed at different times up to day 94 postinfection. From three animals per group, sections of formalin-fixed liver, spleen, lung, kidney, and brain tissue were stained with hematoxylin and eosin. Because granulomas characteristically contain mainly CD4+ T cells and monocyte-derived CD11b+ cells, tissue sections showing accumulations of mononuclear cells were analyzed immunohistochemically. Frozen liver sections were stained with rat anti-mouse anti-CD4 (10), anti-CD8 (10), or anti-CD11b (3) monoclonal antibodies as the primary antibodies and peroxidase-conjugated goat anti-rat immunoglobulin G (Dianova, Hamburg, Germany) as the secondary antibody. Color was developed with diaminobenzidine and hydrogen peroxide as a substrate. Negative controls were free of artifacts due to endogenous peroxidase activity.
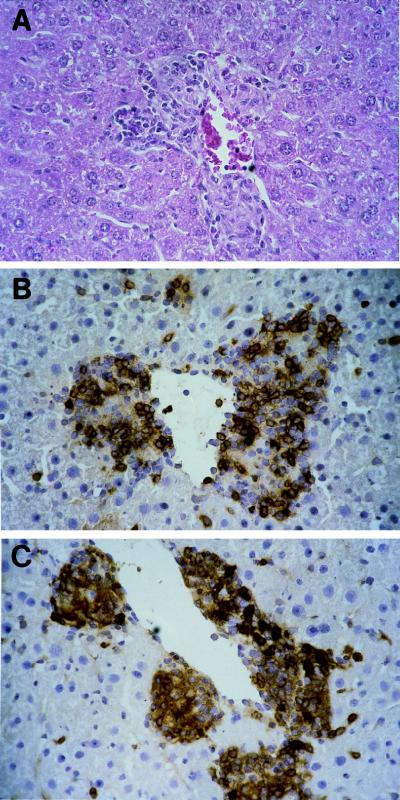
Inoculation with at least 5 × 107 organisms reproducibly showed the following course of tissue reaction. Beginning at day 3 postinfection, liver tissue showed a few small aggregates of lymphocytes and monocytes that expanded in size and number over the next few days. During the second week, granulomatous lesions, consisting of lymphocytes, monocytes, and epithelioid cells, became more obvious. Granulomatous lesions expanded in size and number until reaching maximal density during the fourth week of infection, when up to 130 randomly distributed mononuclear foci were counted per 0.25 cm2 in liver sections (Fig. 2A). Mononuclear cells were predominantly CD4+ lymphocytes (Fig. 2B) or CD11b+ monocytes (Fig. 2C). CD8+ lymphocytes were found in low numbers. They were located mainly in the periphery (not shown). Two weeks later, a marked reduction of inflammatory lesions was observed, and at the end of the third month postinfection, liver tissue was completely devoid of inflammatory lesions (Fig. 1B). The other organs investigated did not show significant lesions at any time. Inocula from 5 × 106 to 5 × 107 CFU caused a similar course of inflammation in liver tissue. The density of infiltrates, however, was markedly lower, and there was more variation in numbers of infiltrates between mice of a group than in mice infected with higher inocula. Inocula lower than 5 × 106 CFU did not cause significant tissue reactions (data not shown).
FIG. 2.
Granulomatous mononuclear cell infiltration with lymphocytes and epithelioid cells in liver tissue of C57BL/6 mice at day 21 postinfection with 1.2 × 108 B. henselae organisms. Sections were stained with hematoxylin and eosin (A), anti-CD4 antibody (B), and anti-CD11b antibody (C).
To exclude nonspecific cellular reactions stimulated by a high antigen burden, mice were inoculated i.p. with either 8.4 × 107 viable organisms or the identical dose of bacteria killed by 60 min of incubation in a 60°C water bath. At day 24 postinfection, sections from liver tissue stained with hematoxylin and eosin showed numerous granulomatous lesions in 7 of 10 mice infected with viable bacteria. In the other animals of this group, aggregates of lymphocytes and monocytes were noted. In contrast, no granuloma formation was observed in liver sections of 10 mice inoculated with heat-killed B. henselae. Eight mice of this group showed single small accumulations of mononuclear cells; the others were devoid of histopathological lesions.
Our results show that B. henselae causes granulomatous inflammation in livers of C57BL/6 mice after high-dose i.p. infection. In contrast to CSD, which is histologically characterized by necrotizing granulomas (2), we did not find necrosis in mouse liver granulomas. However, it has to be mentioned that granulomas in murine infection generally do not undergo necrosis, even in murine tuberculosis (5). While cultures from liver tissue were negative after day 3 postinfection, DNA from B. henselae could be detected for at least 3 months. Since the sensitivities of PCR and culture do not differ significantly, the most probable reason might be a switch of organisms to a dormant state within the first week postinfection. This could also explain the presence of numerous inflammatory foci in culture-negative livers. However, the fate of B. henselae in its host is largely unknown. A key element in establishing this infection model was probably the passage of bacteria through mice prior to experimental infection. Enhancement of virulence by mouse passage has been shown for instance in murine listeriosis (8).
In conclusion, we have established a murine model of i.p. B. henselae infection. Potential applications of this animal model include the feasibility of studying the pathogenesis of B. henselae infection in the immunocompetent host, investigating the tissue persistence of B. henselae, and analyzing the immune response to B. henselae infection. Further investigation should also focus on the course of infection in immunosuppressed mice, because in human B. henselae infection, vasculoproliferative diseases occur in immunocompromised but not in immunocompetent patients.
REFERENCES
- 1.Avidor B, Kletter Y, Abulafia S, Golan Y, Ephros M, Giladi M. Molecular diagnosis of cat scratch disease: a two-step approach. J Clin Microbiol. 1997;35:1924–1930. doi: 10.1128/jcm.35.8.1924-1930.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cockerell J C, Connor D H. Cat scratch disease. In: Connor D H, Chandler F W, Schwartz D A, Manz H J, Lack E E, editors. Pathology of infectious diseases. Stamford, Conn: Appleton & Lange; 1979. pp. 461–468. [Google Scholar]
- 3.Dobrick P, Miksits K, Hahn H. L3T4(CD4)-, Lyt-2(CD8)- and Mac-1(CD11b)-phenotypic leukocytes in murine cryptococcal meningoencephalitis. Mycopathologia. 1995;131:159–166. doi: 10.1007/BF01102895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dolan M J, Wong M T, Regnery R L, Jorgensen J H, Garcia M, Peters J, Drehner D. Syndrome of Rochalimaea henselae adenitis suggesting cat scratch disease. Ann Intern Med. 1993;118:331–336. doi: 10.7326/0003-4819-118-5-199303010-00002. [DOI] [PubMed] [Google Scholar]
- 5.Dunn P L, North R J. Virulence ranking of some Mycobacterium tuberculosis and Mycobacterium bovis strains according to their ability to multiply in the lungs, induce lung pathology, and cause mortality in mice. Infect Immun. 1995;63:3428–3437. doi: 10.1128/iai.63.9.3428-3437.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guptill L, Slater L, Wu C C, Lin T L, Glickman L T, Welch D F, HogenEsch H. Experimental infection of young specific pathogen-free cats with Bartonella henselae. J Infect Dis. 1997;176:206–216. doi: 10.1086/514026. [DOI] [PubMed] [Google Scholar]
- 7.Jackson L A, Perkins B A, Wenger J D. Cat scratch disease in the United States: an analysis of three national databases. Am J Public Health. 1993;83:1707–1711. doi: 10.2105/ajph.83.12.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackaness G B. Cellular resistance to infection. J Exp Med. 1962;116:381–406. [PubMed] [Google Scholar]
- 9.Maurin M, Birtles R, Raoult D. Current knowledge of Bartonella species. Eur J Clin Microbiol Infect Dis. 1997;16:487–506. doi: 10.1007/BF01708232. [DOI] [PubMed] [Google Scholar]
- 10.Mielke M E, Ehlers S, Hahn H. T-cell subsets in delayed-type hypersensitivity, protection, and granuloma formation in primary and secondary Listeria infection in mice: superior role of Lyt-2+ cells in acquired immunity. Infect Immun. 1988;56:1920–1925. doi: 10.1128/iai.56.8.1920-1925.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norman A F, Regnery R, Jameson P, Greene C, Krause D C. Differentiation of Bartonella-like isolates at the species level by PCR-restriction fragment length polymorphism in the citrate synthase gene. J Clin Microbiol. 1995;33:1797–1803. doi: 10.1128/jcm.33.7.1797-1803.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Regnery R L, Anderson B E, Clarridge III J E, Rodriguez-Barradas M C, Jones D C, Carr J H. Characterization of a novel Rochalimaea species, R. henselae sp. nov., isolated from blood of a febrile, human immunodeficiency virus-positive patient. J Clin Microbiol. 1992;30:265–274. doi: 10.1128/jcm.30.2.265-274.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Regnery R L, Olson J G, Perkins B A, Bibb W. Serological response to “Rochalimaea henselae” antigen in suspected cat-scratch disease. Lancet. 1992;339:1443–1445. doi: 10.1016/0140-6736(92)92032-b. [DOI] [PubMed] [Google Scholar]
- 14.Schwartzman W A, Nesbit C A, Baron E J. Development and evaluation of a blood-free medium for determining growth curves and optimizing growth of Rochalimaea henselae. J Clin Microbiol. 1993;31:1882–1885. doi: 10.1128/jcm.31.7.1882-1885.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott M A, McCurley T L, Vnencak J C, Hager C, McCoy J A, Anderson B, Collins R D, Edwards K M. Cat scratch disease: detection of Bartonella henselae DNA in archival biopsies from patients with clinically, serologically, and histologically defined disease. Am J Pathol. 1996;149:2161–2167. [PMC free article] [PubMed] [Google Scholar]
- 16.Slater L N, Welch D F, Min K W. Rochalimaea henselae causes bacillary angiomatosis and peliosis hepatis. Arch Intern Med. 1992;152:602–606. [PubMed] [Google Scholar]
- 17.Zangwill K M, Hamilton D H, Perkins B A, Regnery R L, Plikaytis B D, Hadler J L, Cartter M L, Wenger J D. Cat scratch disease in Connecticut. Epidemiology, risk factors, and evaluation of a new diagnostic test. N Engl J Med. 1993;329:8–13. doi: 10.1056/NEJM199307013290102. [DOI] [PubMed] [Google Scholar]