Abstract
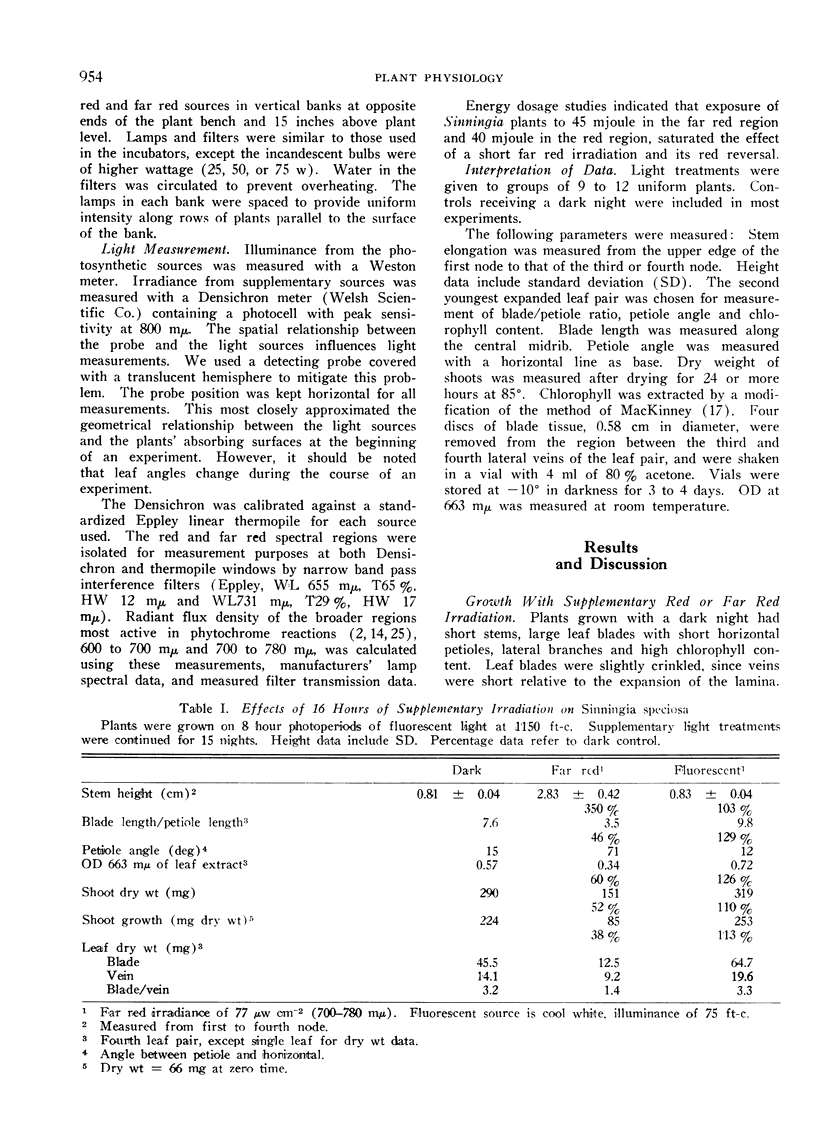
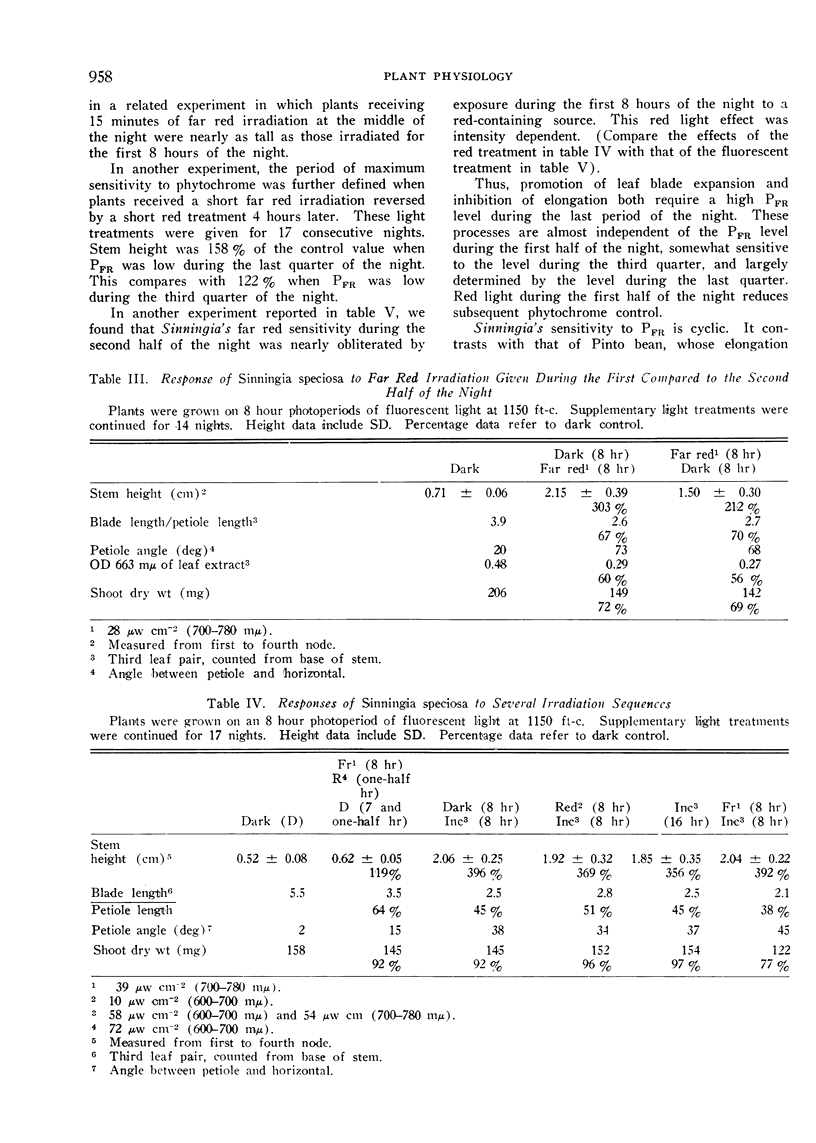
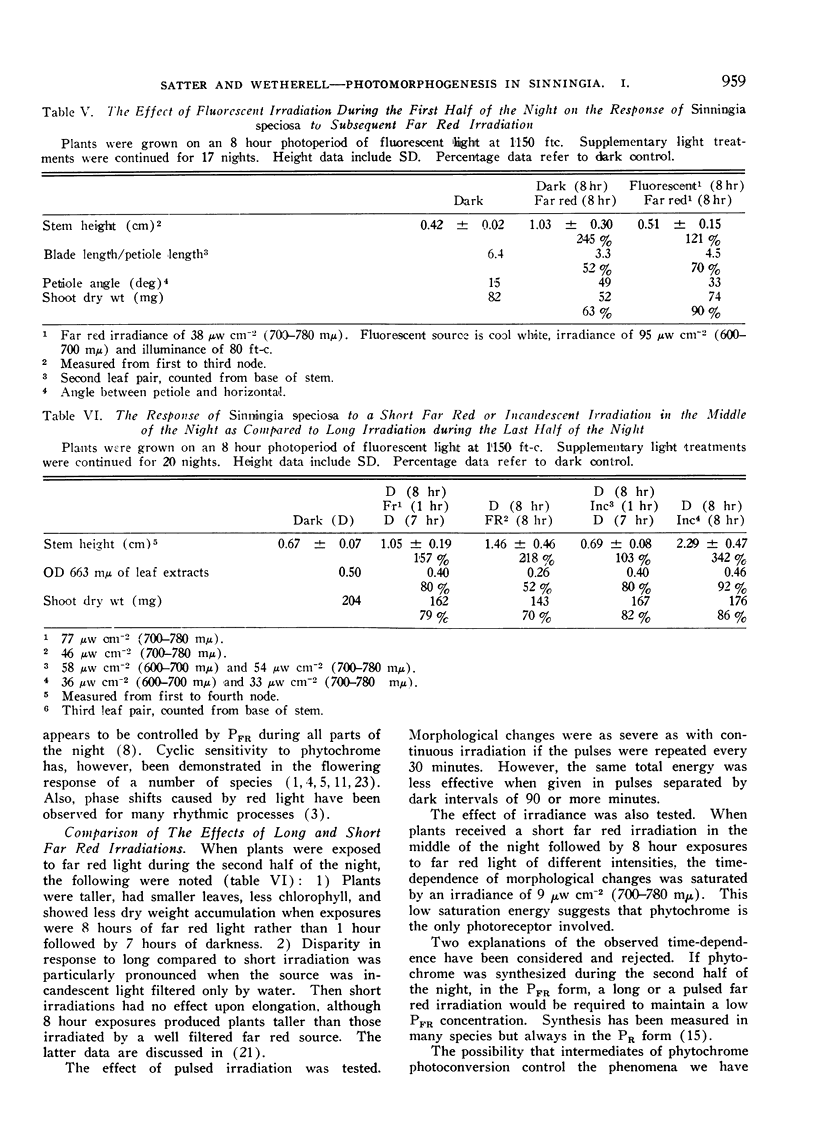
The morphological development of Sinningia speciosa plants that were exposed to supplementary far red light was very different from that of plants receiving dark nights. After several nights of such irradiation, stems and petioles were elongated, petioles were angulated, leaf blade expansion was inhibited, plants were chlorotic and the accumulation of shoot dry weight was retarded.
Red reversibility of the morphological changes potentiated by far red light indicated control by the phytochrome system. A high PFR level during the last half of the night inhibited stem elongation and promoted leaf blade expansion, but both of these processes were hardly affected by the PFR level during the first half of the night. Thus sensitivity to PFR was cyclic.
The interpretation of our experiments was complicated by quantitative morphological differences resulting from long, as compared to short, far red irradiations.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Downs R. J. Photoreversibility of Leaf and Hypocotyl Elongation of Dark Grown Red Kidney Bean Seedlings. Plant Physiol. 1955 Sep;30(5):468–473. doi: 10.1104/pp.30.5.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EILAM Y., KLEIN S. The effect of light intensity and sucrose feeding on the fine structure in chloroplasts and on the chlorophyll content of etiolated leaves. J Cell Biol. 1962 Aug;14:169–182. doi: 10.1083/jcb.14.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredericq H. Conditions Determining Effects of Far-Red and Red Irradiations on Flowering Response of Pharbitis nil. Plant Physiol. 1964 Sep;39(5):812–816. doi: 10.1104/pp.39.5.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredericq H., De Greef J. Red (R), far-red (FR) photoreversible control of growth and chlorophyll content in light-grown thalli of Marchantia polymorpha L. Naturwissenschaften. 1966 Jul;53(13):337–337. doi: 10.1007/BF00631208. [DOI] [PubMed] [Google Scholar]
- Gurnani S., Arifuddin M. Effect of visible light on amino acids. II. Histidine. Photochem Photobiol. 1966 Apr;5(4):341–345. doi: 10.1111/j.1751-1097.1966.tb05803.x. [DOI] [PubMed] [Google Scholar]
- MEGO J. L., JAGENDORF A. T. Effect of light on growth of Black Valentine bean plastids. Biochim Biophys Acta. 1961 Oct 28;53:237–254. doi: 10.1016/0006-3002(61)90437-1. [DOI] [PubMed] [Google Scholar]
- Mancinelli A. L., Yaniv Z., Smith P. Phytochrome and Seed Germination. I. Temperature Dependence and Relative P(FR) Levels in the Germination of Dark-germinating Tomato Seeds. Plant Physiol. 1967 Mar;42(3):333–337. doi: 10.1104/pp.42.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satter R. L., Wetherell D. F. Photomorphogenesis in Sinningia speciosa, cv. Queen Victoria II. Stem Elongation: Interaction of a Phytochrome Controlled Process and a Red-requiring, Energy Dependent Reaction. Plant Physiol. 1968 Jun;43(6):961–967. doi: 10.1104/pp.43.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withrow R. B., Klein W. H., Elstad V. Action Spectra of Photomorphogenic Induction and Its Photoinactivation. Plant Physiol. 1957 Sep;32(5):453–462. doi: 10.1104/pp.32.5.453. [DOI] [PMC free article] [PubMed] [Google Scholar]



