Abstract
In this study, cytokine patterns produced by CD4+ T cells isolated from antrum or corpus gastral biopsy specimens of 10 patients with Helicobacter pylori-positive gastritis were compared. To this end, expression of intracellular cytokines (interleukin-4 [IL-4] and gamma interferon) and of CD4 was assessed by flow cytometry. Ten to 60% of the isolated CD4+ T cells produced gamma interferon upon stimulation. With the exception of one patient, IL-4-positive CD4+ cells were not detected. Therefore, CD4+ cells infiltrating antrum and corpus stomach mucosa during H. pylori infection show a Th1 phenotype. This polarized Th1-type response may contribute to the inability of the immune system to eradicate H. pylori infection.
Originally described in 1983 (26), Helicobacter pylori is now considered an important human pathogen causing gastritis and duodenal ulceration (16, 21). In addition, infection with H. pylori has been associated with gastric lymphoma (20, 27) and seems to be a risk factor for adenocarcinoma of the stomach (11). Several pathogenic factors of H. pylori, including the cytotoxin VacA and the protein CagA, may contribute to the pathogenesis of disease (3, 4). In addition, host factors may also play a pathogenic role, because autoantibodies appear during H. pylori infection in certain patients (1, 8, 18). Once a person is infected, H. pylori can persist in the stomach for decades despite a systemic immune response (23). The reasons for the failure of the immune system to control infection are not yet completely understood. It is therefore of importance to analyze systemic and local T- and B-cell responses in patients chronically infected with H. pylori. Respective immunological studies have shown that the numbers of CD4+ and CD8+ T cells are increased in the gastric mucosa of patients with H. pylori gastritis (10, 19) and that T cells isolated from the antrum region are able to produce gamma interferon (IFN-γ) (12, 13). However, these studies did not differentiate between CD4+, CD8+, and NK cells. Very recently, the technique of intracellular staining of cytokines was used to demonstrate that CD4+ and CD8+ cells from the antrum region of patients with H. pylori gastritis produce IFN-γ but nearly no interleukin-4 (IL-4) (2). Far less characterized are immune responses in the corpus region of the stomach. Because corpus-predominant H. pylori gastritis is associated with the appearance of autoantibodies and atrophic changes (8, 18), the T-cell response in the corpus region may be different.
For this reason, in this study the cytokine profiles of gastric T cells derived from the corpus and antrum regions of the stomach were compared. To this end, cytokines (IFN-γ and IL-4) were stained intracellularly in CD4+ and CD8+ cells prepared from gastric mucosa either of patients with H. pylori-positive gastritis or of patients without gastritis. The patients enrolled in this study underwent upper gastrointestinal endoscopy for diagnostic reasons. At least four biopsy specimens each were obtained from the gastric antrum and corpus regions of each patient. Two biopsy specimens from each location were processed for histological examination, and the remaining two specimens were processed for cytokine analysis of infiltrating lymphocytes. For the latter purpose, the biopsy specimens were mechanically disrupted with a mesh (Costar Netwell, 74 μm) and single-cell suspensions were prepared. Thereafter, the cells were washed once in balanced salt solution and suspended in Click’s medium, supplemented as described previously (24) and containing brefeldin A at 10 μg/ml. For activation of the cells, phorbol-12-myristate-13-acetate (50 ng/ml) and ionomycin (750 ng/ml) were added. After 4 h of incubation at 37°C, the cells were washed in phosphate-buffered saline (PBS), fixed with 2% formaldehyde solution for 20 min, and split into two aliquots. The cells were then incubated for 20 min in saponin buffer (PBS containing 0.5% saponin and 3% fetal calf serum) containing either fluorescein isothiocyanate (FITC)-conjugated mouse immunoglobulin G1 (IgG1) isotype control antibody (Ab) (2.5 μg/ml) and phycoerythrin (PE)-conjugated rat IgG1 isotype control Ab (5 μg/ml [aliquot 1]) or FITC-conjugated anti-IFN-γ Ab (2.5 μg/ml), PE-conjugated anti-IL-4 Ab (5 μg/ml), and CyChrome-conjugated anti-CD4 Ab (aliquot 2). Instead of an isotype control Ab, for two patients aliquot 1 contained FITC-conjugated anti-IFN-γ Ab, PE-conjugated anti-IL-4 Ab, and CyChrome-conjugated anti-CD8 Ab (1:100) in order to simultaneously analyze cytokine production by CD4+ and CD8+ cells. After this step, cells were washed twice in saponin buffer and once in PBS and then analyzed by fluorescence-activated cell sorting (FACS) in a FACScan (Becton Dickinson) with Lysys II software. For FACS analysis, a forward/side scatter-based gate, which did not contain larger cells (for example, macrophages), was chosen.
Biopsy specimens from a total of 43 patients were analyzed in this study. After exclusion of patients with other gastric disorders (e.g., H. pylori-negative gastritis, gastritis induced by nonsteroidal anti-inflammatory drugs, malignancies), 30 patients (12 females and 18 males; age range, 22 to 82 years) remained for evaluation in this study. Twelve of the 30 patients were positive for H. pylori, based on histological detection, and had active chronic gastritis. The 18 uninfected patients had normal gastric mucosa. Based on endoscopic examination, 11 of the 12 H. pylori-positive patients had uncomplicated chronic gastritis; only 1 patient had duodenal ulcers. CD4+ cells from 10 of the 12 H. pylori-positive patients were analyzed by FACS. Yields of viable mononuclear cells derived from the biopsy specimens of H. pylori-infected patients or noninfected control patients ranged from 102 to 105; however, cell numbers in H. pylori-infected patients usually were three to four times higher (data not shown).
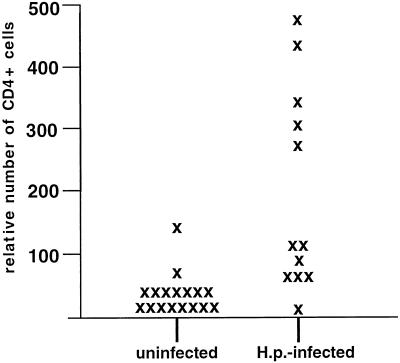
Accordingly, as depicted in Fig. 1, the average relative number of CD4+ cells present in biopsy specimens, as assessed by FACS analysis, was about seven times higher in H. pylori-positive patients than in control patients. Therefore, the majority of analyzed CD4+ lymphocytes derived from the gastric mucosa of H. pylori-infected patients are related to the infection.
FIG. 1.
Relative numbers of CD4+ T cells present in gastral biopsy specimens of H. pylori-infected patients and uninfected control patients. T cells were isolated from gastral biopsy specimens as described in the text and processed for intracellular staining of cytokines and CD4. Thereafter, the cells were resuspended in PBS and analyzed by FACS. The figure shows mean numbers of antrum and corpus CD4+ T cells present in 100 μl of PBS, as measured by FACS. H.p., H. pylori.
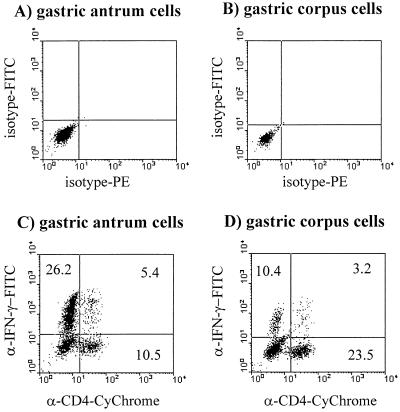
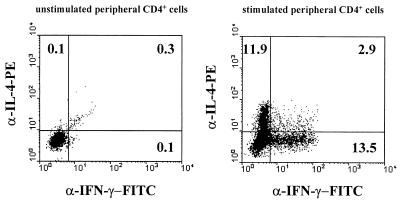
After stimulation of the cells, between 10 and 60% of the CD4+ T cells derived from H. pylori-positive patients stained positively for IFN-γ (Table 1). A representative FACS analysis of antrum and corpus CD4+ cells is shown in Fig. 2. In contrast, IL-4-positive CD4+ cells were not detected, with the exception of one H. pylori-positive patient whose CD4+ cells contained a low percentage of IL-4-positive cells (3% of the CD4+ cells [data not shown]). No major differences in the cytokine profiles of T cells isolated from the antrum and corpus regions were observed. The few CD4+ cells isolated from uninfected control patients also showed a clear Th1 phenotype (data not shown). The validity and sensitivity of the IL-4 antibody were confirmed by showing that this antibody was able to stain up to 10% of stimulated human peripheral T cells that had been cultured for 2 weeks in the presence of IL-2 and IL-4 (Fig. 3). In contrast, the antibody did not stain freshly isolated, activated peripheral blood CD4+ cells (data not shown). Specific binding of the anticytokine antibodies was further proven by using isotype-matched PE- or FITC-coupled control antibodies, which did not stain more than 0.3% of the CD4+ or CD8+ T cells (Fig. 2A and B). Due to the limiting numbers of T cells present in the specimens, in four patients T cells from only one location (corpus or antrum) could be analyzed. In all cases, CD4-negative cells also contained a high proportion of IFN-γ-positive cells. In biopsy specimens from two patients, it was demonstrated that this IFN-γ is mainly produced by CD8+ cells (data not shown). IL-4-positive CD8+ cells were not detected.
TABLE 1.
IFN-γ-positive and IL-4-positive CD4+ T cells in biopsy specimens of patients with H. pylori gastritisa
| Patient | % IFN-γ-positive CD4+ cells
|
% IL-4-positive CD4+ cells
|
||
|---|---|---|---|---|
| Antrum | Corpus | Antrum | Corpus | |
| 1 | 34 | 12 | 0 | 0 |
| 2 | 40 | 41 | 0 | 0 |
| 3 | 31 | 34 | 0 | 3 |
| 4 | 22 | 39 | 0 | 0 |
| 5 | ND | 50 | ND | 0 |
| 6 | 37 | ND | 0 | ND |
| 7 | ND | 37 | ND | 0 |
| 8 | 25 | 38 | 0 | 0 |
| 9 | 30 | 16 | 0 | 0 |
| 10 | 60 | ND | 0 | ND |
T cells were isolated from stomach biopsy specimens of the antrum and corpus regions of H. pylori-positive patients. Intracellular staining of CD4, IFN-γ, and IL-4 was performed as described in the text. Data are for all H. pylori-positive patients included in this study. ND, not determined.
FIG. 2.
IFN-γ production by T cells isolated from the stomach of a patient with H. pylori-positive gastritis. T cells were isolated from the antrum (A and C) and corpus (B and D) regions. The T cells were stimulated, and intracellular staining of CD4, IFN-γ, and IL-4 was performed as described in the text. (A and B) Staining with control Abs, which were isotype matched to the anticytokine Abs: y axes, FITC-conjugated mouse IgG1 isotype control Ab; x axes, PE-conjugated rat IgG1-PE isotype control Ab. (C and D) Staining with CyChrome-conjugated anti-CD4 Ab (x axes) and FITC-conjugated anti-IFN-γ Ab (y axes).
FIG. 3.
IFN-γ and IL-4 production by peripheral T cells. Peripheral blood mononuclear cells were isolated from heparinized blood of a healthy donor by using a Ficoll-Hypaque density gradient. The cells were cultured in Click’s medium for two weeks in the presence of recombinant human IL-2 (10 U/ml) and recombinant human IL-4 (20 U/ml). Thereafter, the cells were left untreated (left) or stimulated (right), and intracellular staining of CD4, IFN-γ, and IL-4 was performed as described in the text. Gating was restricted to CD4+ cells according to staining with anti-CD4 CyChrome. x axes, staining with FITC-conjugated anti-IFN-γ Ab; y axes, staining with PE-conjugated anti-IL-4 Ab.
These data show that CD4+ as well as CD8+ lymphocytes infiltrating the gastric mucosa during chronic H. pylori gastritis have a Th1 phenotype and that Th0 and Th2 are not present. Of note, all analyzed T cells displayed this Th1 pattern regardless of whether they were isolated from the antrum or corpus region, demonstrating that the T-cell responses against H. pylori do not differ between the antral and corpus regions.
Regarding antral T cells, our results confirm data very recently obtained by Bamford et al. (2) by a similar technique. However, in their study, CD4 was measured only on the surface of the cells. Since phorbol-12-myristate-13-acetate stimulation leads to downregulation of CD4 from the T-cell surface, only those T cells whose CD4 expression is not lost after stimulation were analyzed in that study. These T-cell populations, however, may not represent all CD4+ T cells in the stomach. For this reason, CD4 was measured intracellularly in the present study, which allowed clear discrimination between CD4+ and CD4-negative cells even after stimulation.
Analysis of CD4+ T-cell responses in human H. pylori gastritis has also been addressed by analysis of H. pylori-specific T-cell clones, which were cloned from the antral gastric mucosa of H. pylori-infected patients (5–7). In an apparent contradiction of our results, in one of these studies most of the T-cell clones from patients with uncomplicated chronic gastritis (without ulcer) displayed a Th0 phenotype (5). A possible explanation for this apparent discrepancy is that the H. pylori-specific Th0 cell clones were generated by multiple antigenic restimulation of gastric T cells in vitro. Alternatively, the frequency of H. pylori-specific Th0 cells within CD4+ cells in biopsy specimens is so low that they cannot be detected by the method applied herein. On the other hand, the observation that mRNA for IFN-γ and IL-12 is expressed in the gastric mucosa of infected patients (6, 14) would be in accordance with our results. Since IL-12 is known to shift the immune response towards Th1 (22, 25), expression of IL-12 at the local site of H. pylori infection could favor the in situ development of a Th1 response against H. pylori.
What could be the relevance of a polarized Th1-response in H. pylori gastritis? In a mouse infection model using Helicobacter felis, it was demonstrated that Th1 cells contributed to disease whereas Th2 cells were protective because they reduced the bacterial load in the stomach of mice (17). The protective effect of a Th2-type response may result from the induction of local IgA production (15). If this concept also applies for H. pylori and humans, H. pylori might have gained the ability to evoke the “wrong” (Th1) immune response during its evolutionary adaptation to the stomach mucosa. The Th1 response may block the development of a protective (Th2) immune response and induce chronic inflammation by production of IFN-γ. A possible mechanism for the detrimental effect of IFN-γ produced by Th1 cells during H. pylori gastritis, as previously suggested, is induction of major histocompatibility complex class II molecules, which may lead to enhanced adherence of H. pylori to epithelial cells and consequent apoptosis (9). Further investigations will have to investigate the mechanisms which influence the balance of the Th1/Th2 response during H. pylori infection.
Acknowledgments
We thank Barbara Bodendorfer and Claudia Gießler for perfect technical assistance.
This work was supported by the Interdisziplinäres Zentrum für Klinische Forschung of the University of Erlangen (project A4).
REFERENCES
- 1.Appelmelk B J, Negrini R, Moran A P, Kuipers E J. Molecular mimicry between Helicobacter pylori and the host. Trends Microbiol. 1997;5:70–73. doi: 10.1016/S0966-842X(96)10084-6. [DOI] [PubMed] [Google Scholar]
- 2.Bamford K B, Fan X, Crowe S E, Leary J F, Gourley W K, Luthra G K, Brooks E G, Graham D Y, Reyes V E, Ernst P B. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114:482–492. doi: 10.1016/s0016-5085(98)70531-1. [DOI] [PubMed] [Google Scholar]
- 3.Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, Massone A, Papini E, Xiang Z Y, Figura N, Rappuoli R. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci USA. 1993;90:5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cover T L, Blaser M J. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J Biol Chem. 1992;267:10570–10575. [PubMed] [Google Scholar]
- 5.D’Elios M M, Manghetti M, Almerigogna F, Amedei A, Costa F, Burroni D, Baldari C T, Romagnani S, Telford J L, Del Prete G. Different cytokine profile and antigen-specificity repertoire in Helicobacter pylori-specific T cell clones from the antrum of chronic gastritis patients with or without peptic ulcer. Eur J Immunol. 1997;27:1751–1755. doi: 10.1002/eji.1830270723. [DOI] [PubMed] [Google Scholar]
- 6.D’Elios M M, Manghetti M, De Carli M, Costa F, Baldari C T, Burroni D, Telford J L, Romagnani S, Del Prete G. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J Immunol. 1997;158:962–967. [PubMed] [Google Scholar]
- 7.Di Tommaso A, Xiang Z, Bugnoli M, Pileri P, Figura N, Bayell P F, Rappuoli R, Abrignani S, De Magistris M T. Helicobacter pylori-specific CD4+ T-cell clones from peripheral blood and gastric biopsies. Infect Immun. 1995;63:1102–1106. doi: 10.1128/iai.63.3.1102-1106.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faller G, Steininger H, Kranzlein J, Maul H, Kerkau T, Hensen J, Hahn E G, Kirchner T. Antigastric autoantibodies in Helicobacter pylori infection: implications of histological and clinical parameters of gastritis. Gut. 1997;41:619–623. doi: 10.1136/gut.41.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan X, Crowe S E, Behar S, Gunasena H, Ye G, Haeberle H, Van Houten N, Gourley W K, Ernst P B, Reyes V E. The effect of class II major histocompatibility complex expression on adherence of Helicobacter pylori and induction of apoptosis in gastric epithelial cells: a mechanism for T helper cell type 1-mediated damage. J Exp Med. 1998;187:1659–1669. doi: 10.1084/jem.187.10.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan X J, Chua A, Shahi C N, McDevitt J, Keeling P W, Kelleher D. Gastric T lymphocyte responses to Helicobacter pylori in patients with H. pylori colonisation. Gut. 1994;35:1379–1384. doi: 10.1136/gut.35.10.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forman D, Newell D G, Fullerton F, Yarnell J W, Stacey A R, Wald N, Sitas F. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. Br Med J. 1991;302:1302–1305. doi: 10.1136/bmj.302.6788.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haeberle H A, Kubin M, Bamford K B, Garofalo R, Graham D Y, El-Zaatari F, Karttunen R, Crowe S E, Reyes V E, Ernst P B. Differential stimulation of interleukin-12 (IL-12) and IL-10 by live and killed Helicobacter pylori in vitro and association of IL-12 production with gamma interferon-producing T cells in the human gastric mucosa. Infect Immun. 1997;65:4229–4235. doi: 10.1128/iai.65.10.4229-4235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karttunen R, Karttunen T, Ekre H P, MacDonald T T. Interferon gamma and interleukin 4 secreting cells in the gastric antrum in Helicobacter pylori positive and negative gastritis. Gut. 1995;36:341–345. doi: 10.1136/gut.36.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karttunen R A, Karttunen T J, Yousfi M M, el-Zimaity H M, Graham D Y, El-Zaatari F A. Expression of mRNA for interferon-gamma, interleukin-10, and interleukin-12 (p40) in normal gastric mucosa and in mucosa infected with Helicobacter pylori. Scand J Gastroenterol. 1997;32:22–27. doi: 10.3109/00365529709025058. [DOI] [PubMed] [Google Scholar]
- 15.Lee C K, Weltzin R, Thomas W D, Jr, Kleanthous H, Ermak T H, Soman G, Hill J E, Ackerman S K, Monath T P. Oral immunization with recombinant Helicobacter pylori urease induces secretory IgA antibodies and protects mice from challenge with Helicobacter felis. J Infect Dis. 1995;172:161–172. doi: 10.1093/infdis/172.1.161. [DOI] [PubMed] [Google Scholar]
- 16.Marshall B J, Warren J R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;i:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 17.Mohammadi M, Nedrud J, Redline R, Lycke N, Czinn S J. Murine CD4 T-cell response to Helicobacter infection: TH1 cells enhance gastritis and TH2 cells reduce bacterial load. Gastroenterology. 1997;113:1848–1857. doi: 10.1016/s0016-5085(97)70004-0. [DOI] [PubMed] [Google Scholar]
- 18.Negrini R, Savio A, Poiesi C, Appelmelk B J, Buffoli F, Paterlini A, Cesari P, Graffeo M, Vaira D, Franzin G. Antigenic mimicry between Helicobacter pylori and gastric mucosa in the pathogenesis of body atrophic gastritis. Gastroenterology. 1996;111:655–665. doi: 10.1053/gast.1996.v111.pm8780570. [DOI] [PubMed] [Google Scholar]
- 19.Papadimitriou C S, Ioachim-Velogianni E E, Tsianos E B, Moutsopoulos H M. Epithelial HLA-DR expression and lymphocyte subsets in gastric mucosa in type B chronic gastritis. Virchows Arch A. 1988;413:197–204. doi: 10.1007/BF00718611. [DOI] [PubMed] [Google Scholar]
- 20.Parsonnet J, Hansen S, Rodriguez L, Gelb A B, Warnke R A, Jelum E, Orentreich N, Vogelman J H, Friedman G D. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 21.Rauws E A J, Tytgat G N J. Cure of duodenal ulcer associated with eradication of Helicobacter pylori. Lancet. 1990;335:1233–1235. doi: 10.1016/0140-6736(90)91301-p. [DOI] [PubMed] [Google Scholar]
- 22.Romagnani S. Human TH1 and TH2 subsets: regulation of differentiation and role in protection and immunopathology. Int Arch Allergy Immunol. 1992;98:279–285. doi: 10.1159/000236199. [DOI] [PubMed] [Google Scholar]
- 23.Sobala G M, Crabtree J E, Dixon M F. Acute Helicobacter infection: clinical features, local and systemic immune response, gastric mucosal histology and gastric juice ascorbic acid concentration. Gut. 1991;32:1415–1418. doi: 10.1136/gut.32.11.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solbach W, Forberg K, Kammerer E, Bogdan C, Röllinghoff M. Suppressive effect of cyclosporin A on the development of Leishmania tropica-induced lesions in genetically susceptible BALB/c mice. J Immunol. 1986;137:702–707. [PubMed] [Google Scholar]
- 25.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 26.Warren J R, Marshall B. Unidentified curved bacillus on gastric epithelium in active chronic gastritis. Lancet. 1983;i:1273–1275. [PubMed] [Google Scholar]
- 27.Wotherspoon A C, Ortiz-Hidalgo C, Falzon M R, Isaacson P G. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet. 1991;338:1175–1176. doi: 10.1016/0140-6736(91)92035-z. [DOI] [PubMed] [Google Scholar]