Abstract
Background:
Although radical cystectomy (RC) is the standard of care for patients with bacillus Calmette-Guérin (BCG)-unresponsive high-risk non–muscle-invasive bladder cancer (NMIBC), many patients are ineligible for surgery or elect bladder preservation.
Objective:
To evaluate the efficacy and safety of atezolizumab in BCG-unresponsive high-risk NMIBC.
Design, setting, and participants:
This was a single-arm phase 2 trial in patients with BCG-unresponsive high-risk NMIBC who were ineligible for or declined RC.
Intervention:
Intravenous atezolizumab every 3 wk for 1 yr.
Outcome measurements and statistical analysis:
The primary endpoint was the pathological complete response (CR) rate for patients with carcinoma in situ (CIS) determined via mandatory biopsy at 6 mo. Event-free survival (EFS) at 18 mo for patients with non-CIS tumors and treatment-related adverse events (TRAEs) were key secondary endpoints.
Results and limitations:
Of 172 patients enrolled in the trial, 166 received at least one dose of atezolizumab (safety analysis) and 129 were eligible (efficacy analysis). Of the 74 patients with CIS, 20 (27%) experienced a CR at 6 mo. The median duration of response was 17 mo, and 56% (95% confidence interval [CI] 34–77%) of the responses were durable to at least 12 mo. The 18-mo actuarial EFS rate among 55 patients with Ta/T1 disease was 49% (90% CI 38–60%). Twelve of 129 eligible patients experienced progression to muscle-invasive or metastatic disease. Grade 3–5 TRAEs occurred in 26 patients (16%), including three treatment-related deaths. The study was limited by the small sample size and a high rate of patient ineligibility.
Conclusions:
The efficacy of atezolizumab observed among patients with BCG-unresponsive NMIBC is similar to results from similar trials with other agents, but did not meet the prespecified efficacy threshold. Modest efficacy needs to be balanced with a significant rate of TRAEs and the risk of disease progression when considering systemic immunotherapy in early-stage bladder cancer.
Patient summary:
We tested intravenous immunotherapy (atezolizumab) in patients with high-risk non–muscle-invasive bladder cancer that recurred after BCG (bacillus Calmette-Guérin) treatment. Although we found similar outcomes to previous trials, the benefit of this therapy is modest and needs to be carefully balanced with the significant risk of side effects.
Keywords: Bladder cancer, Bacillus Calmette-Guérin–unresponsive, Immune checkpoint inhibitor
1. Introduction
High-risk non–muscle-invasive bladder cancer (NMIBC) is defined as high-grade Ta (noninvasive) or T1 (invasion into the lamina propria) papillary tumor or carcinoma in situ (CIS). Intravesical bacillus Calmette-Guérin (BCG) effectively eradicates CIS in approximately 80% of cases, and reduces the risk of recurrence and progression to muscle-invasive bladder cancer (MIBC) for NMIBC cases [1,2]. Nonetheless, recurrence is common, and high-grade recurrence is associated with a significant risk of progression. Approximately 40% of patients with CIS or high-grade T1 tumor will experience recur, and 15–20% will have progression at 5 yr [3,4]. More than half of patients with high-risk NMIBC ultimately proceed to radical cystectomy (RC) or die from their disease during median follow-up of 15 yr [5]. Since survival probability declines significantly after progression to MIBC, patients with high-grade recurrence after adequate BCG therapy are typically advised to undergo RC. However, RC is associated with a high morbidity rate, a perioperative mortality rate of approximately 5% within 90 d [6], and a need to adapt to the long-term implications of urinary diversion [7,8]. Many patients are medically ineligible for RC, and many others decline RC in favor of alternative therapies with marginal or poorly defined efficacy [9,10]. There is therefore a large unmet need for novel bladder-preserving therapies for patients with high-grade recurrence after adequate BCG therapy.
The clinical trial landscape for second-line bladder-preserving therapy in patients who are unresponsive to or relapse after BCG has advanced rapidly [11–15] since the development of a consensus definition of BCG-unresponsive high-risk NMIBC and standardization of optimal clinical trial design [16–18]. A joint panel of the American Urological Association (AUA) and the US Food and Drug Administration (FDA) determined that a single-arm phase 2 or 3 trial assessing investigational agents in BCG-unresponsive CIS would be adequate to demonstrate evidence of patient benefit to justify introduction of new therapies into clinical practice [16]. Importantly, it is believed that CIS cannot be completely resected at the time of diagnosis, so that the complete response (CR) rate to the experimental treatment can be interpreted as a demonstration of drug efficacy. Without an effective standard bladder-preserving therapy available for these patients, there is no appropriate comparator arm for a randomized trial, and the single-arm trial design has been considered the most feasible option. Outcomes after second-line single-agent intravesical chemotherapy have been poor [9,19], although a regimen of sequential gemcitabine and docetaxel has more recently been adopted in routine practice on the basis of results from a retrospective multicenter series [20].
With the advent of systemic immune checkpoint blockade for metastatic and locally advanced urothelial carcinoma [21] and evidence of PD-L1 expression in bladder tumors recurring after BCG [20], we hypothesized that systemic atezolizumab (anti-PD-L1) would provide an efficacious bladder-preserving treatment with adequate safety in patients with BCG-unresponsive high-risk NMIBC. It has since been demonstrated that pembrolizumab is efficacious in this disease state [11] and the FDA have approved its use in this setting.
2. Patients and methods
2.1. Patients
Eligible participants had, within 60 d of trial registration, histologically confirmed urothelial carcinoma of the bladder that met the criteria for BCG-unresponsive high-risk NMIBC according to the FDA guidance statement [22]. BCG-unresponsive NMIBC was defined as CIS (±Ta/T1 tumor) recurring within 12 mo, or high-grade Ta/T1tumor (without CIS) recurring within 6 mo of the last dose of adequate BCG. Adequate BCG was defined as at least induction BCG (≥5 doses) and the first round of maintenance or a second induction course of BCG (≥2 doses) for patients with CIS/Ta disease at the time of recurrence, and at least induction BCG (≥5 doses) for patients with T1 tumors at the time of recurrence. No limitations were set on possible dose reductions for previously administered BCG. Patients were stratified as CIS ± Ta/T1 tumors versus high-grade Ta/T1 tumors without CIS. For participation in the trial, patients had to be ineligible for or decline RC.
Ta and/or T1 tumors required visual complete transurethral resection of bladder tumor (TURBT), and patients with T1 tumors required a restaging TURBT. For patients with T1 tumors, muscularis propria had to be present in the specimen from at least one of the two TURBTs. The most recent TURBT had to be within 60 d of registration, and a cystoscopy confirming the absence of visible papillary disease was required within 21 d. Histologic subtypes other than glandular or squamous differentiation were excluded. Patients with urothelial carcinoma of the upper urinary tract or prostatic urethra within 24 mo before registration were excluded. White-light and blue-light cystoscopy could both be used, but investigators were encouraged to use the same technique throughout the trial in any one patient.
Initially, a high number of ineligible patients were enrolled in the trial primarily because of misinterpretation of the disease criteria. Ineligible patients were allowed to continue on therapy once started if deemed appropriate by the treating investigator. They were followed for disease assessment and follow-up according to the trial protocol. After the introduction of mandatory screening of eligibility by the study chair, the subsequent ineligibility rate decreased.
Patients enrolled in the trial who received at least one dose of atezolizumab regardless of eligibility status were included in the safety analysis. Eligible patients who received at least one dose of atezolizumab were considered evaluable and were included in the efficacy analysis.
2.2. Study design and treatment
SWOG S1605 was an open-label, single-arm, phase 2 clinical trial registered on ClinicalTrials.gov (NCT02844816). The protocol is included in the Supplementary material. The trial was conducted according to the Declaration of Helsinki in compliance with good clinical practice guidelines through the National Clinical Trials Network (NCTN) under the leadership of SWOG and received approval from the National Cancer Institute central institutional review board. Patients provided written, informed consent.
Patients received 1200 mg of atezolizumab intravenously every 3 wk for up to 17 cycles (51 wk) in the absence of disease recurrence or unacceptable toxicity.
2.3. Endpoints and assessments
Patients without recurrence were monitored using cystoscopy and cytology every 3 mo for 24 mo, and then every 6 mo for the following 36 mo. All patients with CIS at study entry underwent mandatory biopsy at 6 mo. Treatment and surveillance after high-grade recurrence were left to the discretion of the investigator.
The trial was designed with two co-primary endpoints: (1) the pathological CR rate at 6 mo in patients with CIS (±Ta/T1 tumor), and (2) event-free survival (EFS) at 18 mo in all evaluable patients. CR was defined as the absence of high-grade urothelial carcinoma on bladder biopsy and the absence of evidence of upper tract or urethral recurrence.
For EFS, an event was defined as the first occurrence of any of the following: biopsy-proven high-grade bladder cancer (including persistent CIS at 3 and/or 6 mo); high-grade upper tract urothelial carcinoma; high-grade urothelial carcinoma of the prostatic urethra; muscle-invasive disease; clinical evidence of metastatic disease; or death due to any cause.
The duration of response was defined from the date of biopsy identifying CR to the first high-grade recurrence or death, with censoring at the last cystoscopy. A study amendment after enrollment of 54 patients with CIS and 51 patients with Ta/T1 without CIS enabled patients with persistent CIS or high-grade Ta tumor at 3 mo to continue on atezolizumab with a mandatory biopsy 3 mo later. Enrollment after this amendment included 47 patients with CIS and 20 patients with Ta/T1 without CIS. Treatment was discontinued for all T1 or MIBC recurrences.
Secondary outcome measures included progression-free survival, defined as the time from registration to first evidence of biopsy-proven MIBC (≥T2), nodal or distant metastasis, or death from any cause. Patients without an event were censored at the date of their last cystoscopy. Other secondary outcomes included overall survival, assessment of adverse events using the National Cancer Institute Common Terminology Criteria for Adverse Events v4.0, and the incidence and timing of cystectomy, MIBC, and metastasis. Clinical CR at 3 mo for the group with CIS was added post hoc to facilitate comparisons to other contemporary trials. Clinical CR was defined as either (1) biopsy-proven CR at 3 mo or (2) in the absence of a biopsy, both cystoscopy that was not suspicious for cancer and the absence of high-grade cytology.
2.4. Statistical analysis
The plan was to evaluate two co-primary objectives sequentially. The EFS in all patients would only be evaluated if the CR rate in evaluable patients in the CIS subset was statistically significant.
The sample size was calculated on the assumption of a CR rate of 30% at 6 mo in the CIS group as the null hypothesis, and a CR rate of 50% as the alternative hypothesis on the basis of the early AUA/FDA guidance [16]. With a one-sided α level of 0.05 and 96% power, the target accrual was 70 evaluable patients with CIS. We aimed to enroll 65 evaluable patients with Ta/T1 disease. Assuming a null hypothesis of an EFS rate of 20% at 18 mo, this provided 71% power for an alternative hypothesis of and EFS rate of 30% at 18 mo (one-sided α = 0.05). We specified that the regimen would significantly improve EFS relative to historical trial data if the lower bound of the 90% confidence interval (CI) for EFS at 18 mo excluded 20% [16]. To enroll 135 evaluable patients, the total target sample size was 148 to allow for 10% ineligibility and drop out. The total sample size accrual goal was amended twice during the trial to account for the high rate of ineligibility of registered patients resulting in a final target sample size of 202 patients.
Because there were no safety or efficacy data available for atezolizumab in patients with NMIBC when the trial was initiated, we planned an interim futility analysis of the CR rate at 6 mo among the first 25 evaluable patients with CIS. A favorable evaluation allowing continuation to full accrual required at least seven CRs among the first 25 patients, which was equivalent to testing the alternative hypothesis of a 50% CR rate with a one-sided α = 0.02. Patient accrual was allowed to continue beyond 25 patients in the first stage while the 6-mo biopsy data matured.
3. Results
3.1. Patients
Between February 7, 2017 and July 5, 2019, 172 patients were enrolled in the trial at 68 NCTN group sites in the USA and Canada. The Consolidated Standards of Reporting Trials (CONSORT) flow diagram in Figure 1 shows patient enrollment, eligibility, and treatment disposition, stratified by histology (CIS ± Ta/T1 vs Ta/T1 without CIS). After exclusion of six patients who did not receive any atezolizumab, 166 patients were available for the safety analysis. It was later found that 37 patients did not meet the inclusion criteria, so the final eligible and evaluable population consisted of 74 patients with CIS (±Ta/T1), including 43 (58%) with CIS only and 55 with Ta/T1 tumor. The baseline characteristics of the CIS (±Ta/T1) and Ta/T1 cohorts are summarized in Table 1. The median survival follow-up for all 129 eligible, alive patients was 41 mo.
Fig. 1 –
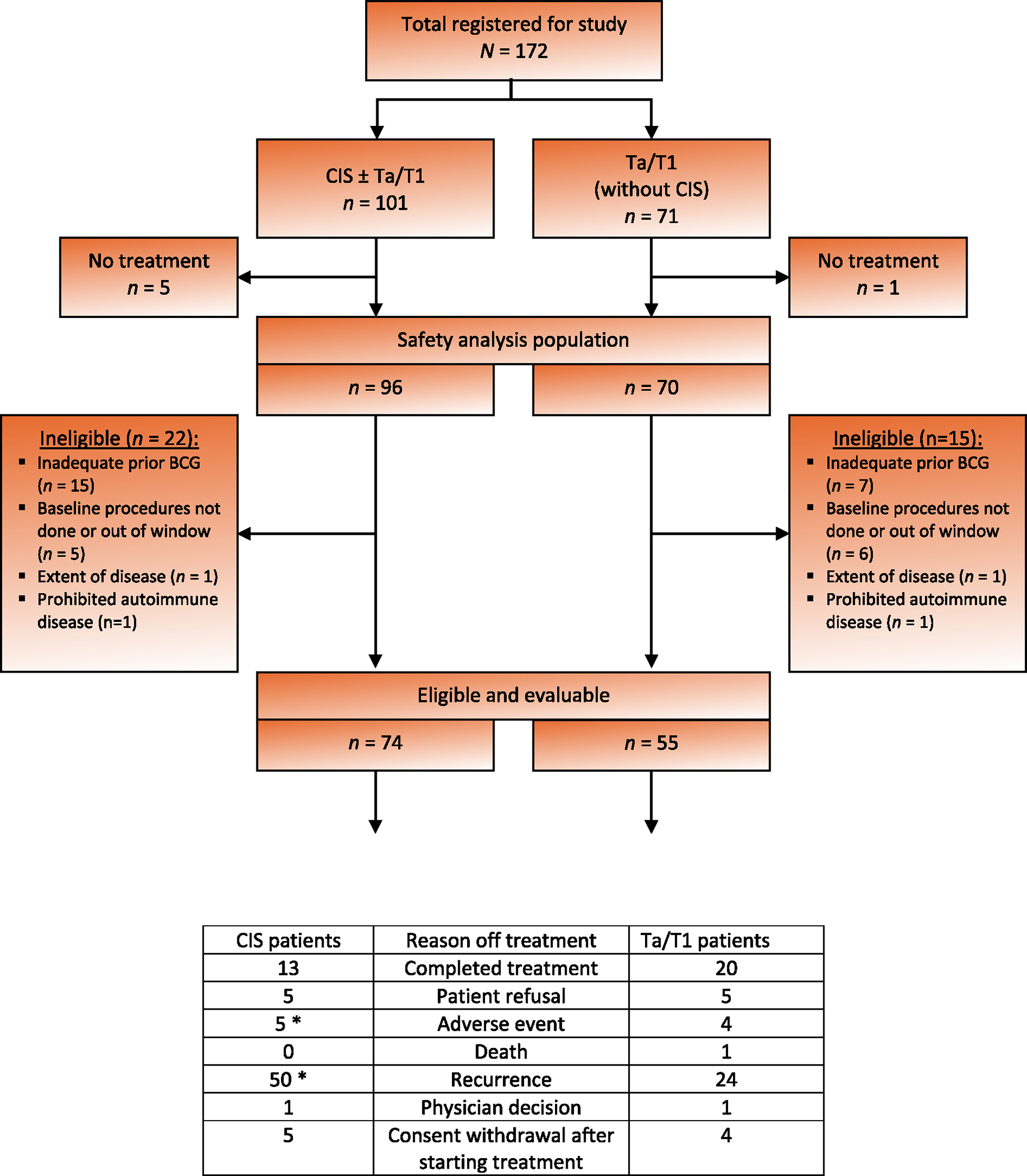
Consolidated Standards of Reporting Trials (CONSORT) diagram. *Two treatment-related deaths occurred in patients with carcinoma in situ (CIS) who came off treatment for other reasons. BCG = bacillus Calmette-Guérin.
Table 1.
Patient characteristics stratified by histology at study entrya
| CIS ± Ta/T1 (n = 74) | Ta/T1 only (n = 55) | |
|---|---|---|
|
| ||
| Male, n (%) | 62 (84) | 46 (84) |
| Median age, yr (range) | 73 (47–90) | 74 (38–97) |
| Race, n (%) | ||
| White | 70 (95) | 49 (89) |
| Other, unknown | 4 | 6 |
| Zubrod performance status, n (%) | ||
| 0 | 57 (77) | 36 (65) |
| 1 | 17 (23) | 17 (31) |
| 2 | 0 (0) | 2 (4) |
| Histology, n (%) | ||
| CIS only | 43 (58) | |
| CIS + Ta | 14 (19) | |
| CIS + T1 | 13 (18) | |
| CIS + Ta + T1 | 4 (5) | |
| Ta only | 30 (55) | |
| T1 only | 22 (40) | |
| Ta + T1 | 3 (5) | |
| Median prior BCG instillations, n (range) | 12 (6–29) | 12 (6–31) |
| Median time since last BCG, d (range) | 154 (5–346) | 127 (67–273) |
| Reason for not undergoing cystectomy | ||
| Ineligible | 5 | 7 |
| Patient choice | 69 (93%) | 48 (87%) |
BCG = bacillus Calmette-Guérin; CIS = carcinoma in situ.
Only eligible patients who received some protocol treatment are included.
3.2. Complete response in the CIS cohort
We observed only five CRs among the initial 25 patients (≥7 required to proceed), so the trial was closed to accrual because of futility on July 5, 2019. The 6-mo evaluation of the 25th patient occurred in March 2019, and all source documents for all patients were available for review in June 2019. This time interval allowed for accrual of 74 evaluable patients to the CIS cohort at the time of trial closure, so that the target sample size was achieved. The median follow-up (defined as the date of last cystoscopy) was 41 mo for CIS patients who were alive and without high-grade recurrence.
The primary endpoint of the study was the CR rate at 6 mo assessed via mandatory biopsy in patients with CIS (±Ta/T1). A CR was observed in 20/74 patients (27%, 95% CI 17–38%). In an unplanned analysis to allow comparison to other similar trials, the clinical CR rate was also calculated at the 3-mo time point: 32/74 patients had a clinical CR (43%, 95% CI 32–55%). Of nine patients with persistent or recurrent CIS and/or Ta tumor at 3 mo who continued with atezolizumab treatment, two were disease-free at 6 mo.
The median duration of response beyond the 6-mo CR among the 20 patients who responded (Fig. 2A) was 17 mo (95% CI 7–not reached). The actuarial durable CR rates for the CIS cohort at 6, 12, and 18 mo after obtaining a CR were 90% (95% CI 76–100%), 56% (95% CI 34–77%), and 45% (95% CI 23–66%), respectively.
Fig. 2 –
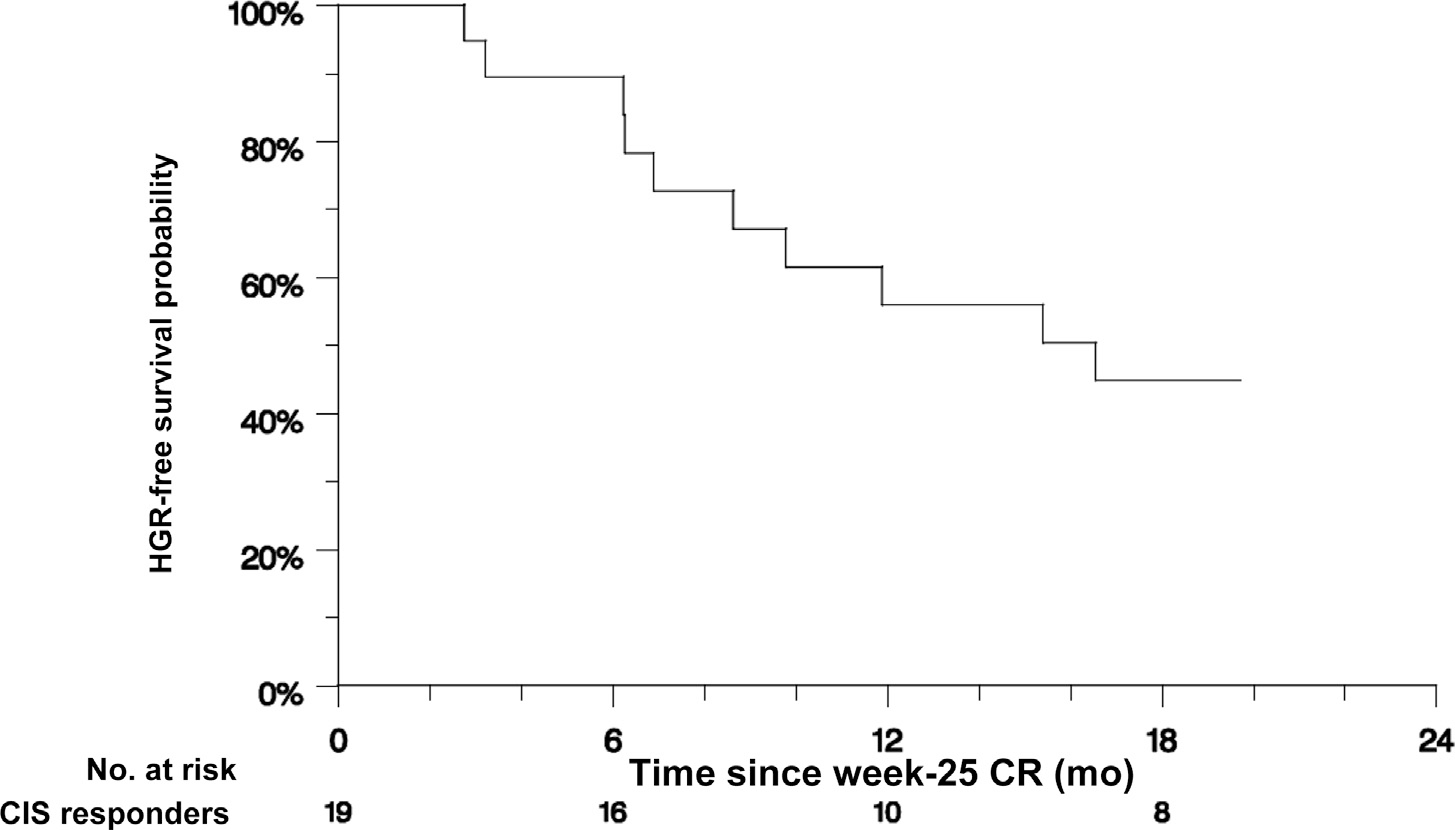
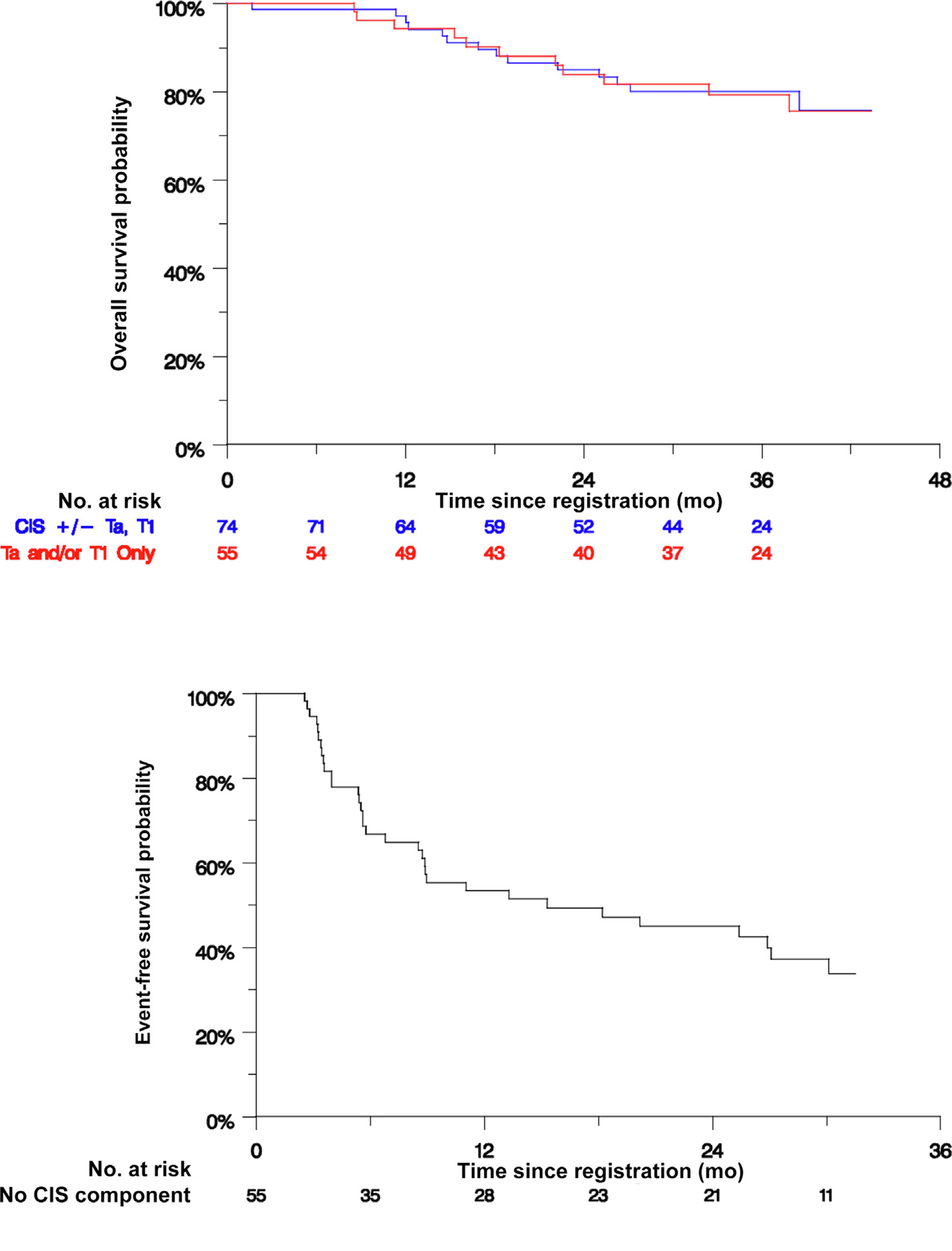
Response and survival estimates. (A) Duration of response after 6-mo biopsy in 20 evaluable patients with CIS ± Ta/T1 who experienced a CR. One patient with CIS experienced a CR but withdrew consent immediately after the 6-mo biopsy, leaving 19 patients for analysis. Eleven patients experienced HGR after a median of 17 mo. The HGR-free survival rates were 90% (95% CI 76–100%) at 6 mo, 56% (95% CI 24–77%) at 12 mo, and 45% (95% CI 23–66%) at 12 mo. (B) Event-free survival in all 55 evaluable patients with Ta/T1 disease without CIS. Thirty-three patients experienced HGR after a median of 15 mo. The event-free survival rates were 67% (95% CI 54–79%) at 6 mo, 53% (95% CI 40–67%) at 12 mo, and 49% (95% CI 36–63%) at 18 mo. (C) Overall survival for all evaluable patients stratified by the presence (n = 74) or absence (n = 55) of CIS. There were 14 deaths in the group with CIS and 11 deaths in the group without CIS, with 3-yr overall survival rates of 80% and 79%, respectively. CI = confidence interval; CIS = carcinoma in situ; CR = complete response; HGR = high-grade recurrence.
The stage of recurrent or persistent disease on atezolizumab is summarized in Supplementary Table 1 for patients with CIS at baseline. A for-cause biopsy was performed at 3 mo in 41 CIS patients, of whom 34 (83%) had high-grade bladder cancer and seven (17%) had benign findings. In the CIS cohort, 46 patients, including all seven with negative biopsy at 3 mo, underwent mandatory biopsy at 6 mo, which was positive for high-grade malignancy in 26 cases (57%). All but one patient with high-grade disease at 6 mo had positive cytology and/or suspicious cystoscopy at that time point. The sensitivity and specificity of cytology and cystoscopy at 3 and 6 mo are summarized in Supplementary Table 2.
In the CIS cohort, the sole site of recurrence was the upper tract in two patients and the prostatic urethra in one patient after 9–12 mo. The CR rate at 3 and 6 mo was therefore not affected by recurrences outside the bladder. One patient in the non-CIS cohort had an isolated prostatic urethral recurrence at 6 mo.
Because the primary endpoint of CR at 6 mo for patients with CIS did not meet the prespecified criteria, we did not conduct EFS analysis for all patients.
3.3. Other outcomes
Fifty-five of the planned 65 eligible patients were enrolled in the Ta/T1 cohort. The 18-mo EFS rate for these patients, which was a prespecified secondary endpoint, was 49% (90% CI 34–57%; Fig. 2B). The lower confidence bound notably excluded 20%. A high-grade recurrence was observed in 32 and three died without recurrence, representing a total of 35 events in 55 patients (64%), with median EFS of 15 mo.
Among all 129 eligible patients, seven experienced progression to MIBC (Supplementary Table 3). All but one of these patients underwent RC, of whom four had pelvic lymph node metastasis. Two of these patients were found to have MIBC while still on treatment with atezolizumab. Another five patients developed metastatic (M1) urothelial carcinoma without preceding evidence of localized MIBC (Supplementary Table 4). Of the 12 patients with progression to MIBC or metastatic disease, the baseline histology was CIS ± Ta/T1 in seven and Ta/T1 without CIS in five. Thirty-three patients (26%), including 27/74 with CIS (±Ta/T1) and 6/55 with Ta/T1 tumor at baseline, underwent RC at a median of 106 d (range 33–1233) after discontinuing atezolizumab (Table 2). Overall survival for all eligible patients stratified by CIS status is depicted in Figure 2C.
Table 2.
Pathological staging at the time of RC for patients who underwent RC
| Bladder tumor T stage | All RC patients (n = 34)a | CIS ± Ta/T1at study entry (n = 27) | Ta/T1at study entry (n = 7) |
|---|---|---|---|
|
| |||
| pT0 N0 | 2 | 0 | 2 |
| pTa N0 | 4 | 2 | 2 |
| pTis N0b | 18 | 16 | 2 |
| pT1 N0 | 3 | 2 | 1 |
| pT2 N0 | 2 | 2 | 0 |
| pTany N1–3 | 4c | 4 | 0 |
| Awaiting documentation | 1 | 1 | 0 |
| Concomitant high-grade extravesical carcinoma | |||
| pT1 in the renal pelvisd | 1 | 0 | 1 |
| Incidental CIS in the ureters | 9 | 8 | 1 |
| pT2 N1 in the prostatic urethrad | 1 | 1 | 0 |
| Incidental CIS in the urethra | 5 | 5 | 0 |
| Median atezolizumab doses, n (IQR) | 6 (5–8) | - | - |
| {range} | {1–17} | ||
| Median time from last atezolizumab dose to RC, d (IQR) | 87 (57–201) | - | - |
| {range} | {33–1546} | ||
CIS = carcinoma in situ; IQR = interquartile range; RC = radical cystectomy.
One patient received neoadjuvant chemotherapy, resulting in ypT4bN1 tumor.
One patient had pNx disease.
One pT1 with concomitant pT2 in the prostatic urethra; two pT3; and one ypT4b.
Patient had concurrent pT1 tumor in the bladder.
3.4. Safety
An adverse event of any grade was observed in 162/166 patients (98%) in the safety population, and this was treatment-related in 142 cases (86%; Table 3). Grade 3–4 treatment-related adverse events (TRAEs) were observed in 23 patients (14%). Another three patients (1.8%) died, one due to immune-related myasthenia gravis followed by respiratory failure (after the 1st cycle of atezolizumab), one due to immune-related myositis (10 mo after discontinuing atezolizumab [4 cycles] because of high-grade recurrence), and one due to sepsis (after the 13th cycle of atezolizumab). TRAEs observed in 5% or more of the safety population at any grade are listed in Table 4, and all TRAEs observed are listed in Supplementary Table 5.
Table 3.
Summary of adverse events in safety-evaluable patientsa
| Adverse event | Patients with at least one AE, n (%) | ||
|---|---|---|---|
|
| |||
| CIS cohort (n = 96) | Non-CIS cohort (n = 70) | All patients (n = 166) | |
|
| |||
| Any adverse event | |||
| Any cause | 93 (97%) | 69 (99%) | 162 (98%) |
| Treatment-related | 81 (84%) | 61 (87%) | 142 (86%) |
| Grade 3–4 AEs | |||
| Any cause | 36 (38%) | 26 (37%) | 57 (34.3%) |
| Treatment-related | 13 (14%) | 10 (14.3%) | 23 (14%) |
| Grade 5 AEs | |||
| Any cause | 2 (2%) | 1 (1%) | 3 (2%) |
| Treatment-related | 2 (2%) | 1 (1%) | 3 (2%) |
| Serious AEs | |||
| Any cause | 18 (19%) | 15 (21%) | 33 (20%) |
| Treatment-related | 9 (9%) | 8 (11%) | 17 (10%) |
| AE leading to ATZ discontinuation | 9 (9%) | 6 (9%) | 15 (9%) |
| AE leading to ATZ dose interruption/delay | 19 (20%) | 18 (26%) | 37 (22%) |
AE = adverse event; ATZ = atezolizumab; CIS = carcinoma in situ.
Includes AEs occurring on or after the start of treatment and up to 90 d after the date on which the last ATZ dose was administered.
Table 4.
TRAEs in safety-evaluable patients (n = 166)
| TRAEsa | Patients, n (%) | ||
|---|---|---|---|
|
| |||
| Grade 1–2 | Grade 3 | Grade 4 | |
|
| |||
| Highest grade for those with any TRAE | 116 (70) | 19 (11) | 5 (3) |
| Fatigue | 70 (42) | 2 (1) | 0 |
| Diarrhea | 31 (19) | 3 (2) | 0 |
| Aspartate aminotransferase increased | 22 (13) | 2 (1) | 1 (1) |
| Alanine aminotransferase increased | 20 (12) | 1 (1) | 2 (1) |
| Rash - maculopapular | 15 (9) | 4 (2) | 0 |
| Anemia | 17 (10) | 1 (1) | 0 |
| Pruritus | 17 (10) | 1 (1) | 0 |
| Nausea | 16 (10) | 2 (1) | 0 |
| Hypothyroidism | 15 (9) | 0 | 0 |
| Creatinine increased | 12 (7) | 1 (1) | 0 |
| Alkaline phosphatase increased | 11 (7) | 1 (1) | 0 |
| Hyperthyroidism | 11 (7) | 0 | 0 |
| Headache | 10 (6) | 0 | 0 |
| Anorexia | 9 (5) | 1 (1) | 0 |
| Hyponatremia | 7 (4) | 2 (1) | 1 (1) |
| Skin/subcutaneous tissue disorder (other) | 10 (6) | 0 | 0 |
| Arthralgia | 9 (5) | 0 | 0 |
| Dyspnea | 7 (4) | 1 (1) | 1 (1) |
TRAE = treatment-related adverse events.
Adverse events were excluded if considered unlikely to be or not related to treatment. Adverse events observed in ≥5% of patients at any grade were included. Sup**plementary Ta**ble 5 provides a full list of all TRAEs.
4. Discussion
This trial demonstrated the efficacy of atezolizumab in the treatment of BCG-unresponsive high-risk NMIBC. The CR rate assessed via mandatory biopsy in patients with CIS was 27% at 6 mo and 13.5% at 18 mo, and the 18-mo EFS rate among patients with Ta/T1 tumor was 49%. Although these results are similar to those reported from other single-arm trials in patients with BCG-unresponsive high-risk NMIBC, they did not meet the prespecified thresholds and SWOG S1605 must therefore be considered a negative trial.
Results have recently been reported from several single-arm trials in patients with BCG-unresponsive CIS. None of the other trials incorporated a mandatory biopsy at 6 mo, and not all reported CR rates at 6 mo. Unevaluable patients were included as events in our analysis but have generally been excluded from the efficacy analysis in other trials. The CR rate at 3 and 12 mo was 43% and 20% with atezolizumab, 41% and 19% with pembrolizumab (n = 96) [11], 53% and 24% with nadofaragene firadenovec (mandatory biopsy at 12 mo; n = 103) [12], 40% and 17% with oportuzumab monatox (n = 93) [13], and 55% and 40% with nogapendekin alfa inbakicept (N-803; n = 80) [14], respectively. The median duration of response was 16.5 mo for atezolizumab, 16.2 mo for pembrolizumab [11], 9.7 mo for nadofaragene [12], 9.4 mo for oportuzumab [13], and 24.1 mo for nogapendekin [14]. On the basis of these results, the FDA has approved pembrolizumab and nadofaragene firadenovec for use in patients with BCG-unresponsive CIS and is currently reviewing nogapendekin for the same indication.
Results for patients with BCG-unresponsive Ta/T1 tumors have been reported for nadofaragene (EFS rate 44% at 12 mo) [12], nogapendekin (disease-free survival rate 57% at 12 mo and 48% at 24 mo) [14], and pembrolizumab (EFS rate 42% at 12 mo and 33% at 24 mo) [23], all of which are comparable to the EFS rate of 49% at 18 mo we observed with atezolizumab. The FDA has indicated that drug approval for BCG-unresponsive Ta/T1 disease will require a randomized trial.
Our results highlight that the potential benefit of these novel agents needs to be balanced carefully with the potential risks, especially in relation to disease progression and drug toxicity. Rates of progression with bladder-preserving strategies will vary according to the baseline features of the patient population, the rate and timing of subsequent cystectomy, follow-up duration. Most progression events occur many months to several years after discontinuation of the study drug and reflect the natural history of BCG-unresponsive CIS that is not adequately eradicated. Similarly, our results highlight the risks of significant toxicity and even death with systemic immunotherapy.
We implemented a prespecified futility analysis after enrollment of the first 25 eligible patients with CIS on the basis of guidelines available at the time of protocol implementation in 2016. Even though the CR rate of 20% observed did not meet the futility boundary (28%) and patient recruitment was stopped early, the target number of patients with CIS (n ≥ 70) was achieved. In retrospect, as more data have been reported in the interim, testing against the alternative hypothesis of CR = 50% (with one-sided α of 0.02) at 6 mo, which was considered a clinically meaningful benchmark according to the 2014 FDA-AUA workshop [16], may have been too optimistic, especially with the use of mandatory biopsy.
The impact of the mandatory biopsy at 6 mo in the CIS cohort is uncertain. Since all patients proceeded to biopsy without a preceding cystoscopy, it is impossible to determine what proportion of these patients would have had a biopsy prompted by abnormal cystoscopy. Many patients had subtle erythematous changes noted at the time of biopsy that might not have required biopsy, depending on the treating urologist’s judgment. However, this reinforces the potential value of mandatory biopsy in trials where the cystoscopic appearance is subjective. Only one patient with high-grade recurrence on mandatory biopsy had no abnormality on cystoscopy or cytology. The nadofaragene trial included a mandatory biopsy at 12 mo for all patients (including Ta/T1 without CIS) [12]. It was estimated that 5/151 patients (3.3%) had a high-grade recurrence that would have been missed without the mandatory biopsy.
The results from SWOG S1605 are limited by the small sample size. The wide CI for the CR rate suggests some uncertainty with respect to the magnitude of the efficacy. The high rate of ineligibility in the CIS arm is an additional limitation, although these exclusions ensure maximal homogeneity of the study population. Most investigators chose not to keep patients on atezolizumab if there was persistent CIS or recurrent high-grade Ta at 3 mo, which limited our ability to assess possible delayed responses. We had inadequate tissue for systematic immunohistochemical assessment of PD-L1 expression.
5. Conclusions
The efficacy of systemic atezolizumab in patients with BCG-unresponsive high-risk NMIBC was similar to that reported in recent trials with other agents, but did not meet the prespecified efficacy threshold in our trial. Although this trial did not reveal any unexpected toxicity, the adverse events observed highlight the risks associated with systemic immunotherapy in this population. The trial results underscore the need to carefully balance treatment-related toxicity and the risk of progression against the modest efficacy of systemic immunotherapy observed in patients with BCG-unresponsive CIS and Ta and T1 tumors who are ineligible for or decline cystectomy.
Supplementary Material
Acknowledgement:
We gratefully acknowledge all the patients and their caregivers who made this trial possible. In addition, we would like to thank the study investigators, research nurses and coordinators, data managers, and other staff at each study site and in the offices of the cooperative groups, including the Data Safety Monitoring Committee in SWOG. The trial was conducted with participation from SWOG, the Alliance for Clinical Trials, ECOG/ACRIN, NRG, and the Canadian Clinical Trials Group.
Funding/Support and role of the sponsor:
This work was supported by NIH/NCI grants CA180888, CA180819, CA180820, CA180821, and CA180863, and in part by Genentech. The sponsors played a role in the design and conduct of the study and approval of the manuscript.
Footnotes
Peer Review Summary overview
Peer Review Summary and Supplementary data to this article can be found online at https://doi.org/10.1016/j.eururo.2023.08.004.
This trial is registered on ClinicalTrials.gov as NCT02844816.
Data from this study were previously presented at the 2020 and 2021 American Society of Clinical Oncology annual meetings.
Financial disclosures: Peter C. Black certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Peter C. Black has consulted for AbbVie, Astellas Pharma, AstraZeneca, EMD-Serono, Ferring, Pfizer, Janssen Oncology, Bayer, Merck, Sanofi Canada, Biosyent, Roche Canada, MDxHealth, Urogen Pharma, Bristol-Myers Squibb, Prokarium, Protara, QED, STIMIT, and Verity; has received research funding from iProgen; and shares a patent with Decipher Biosciences. Parminder Singh has served on advisory boards for Aveo Pharmaceuticals, Bayer Healthcare Pharmaceuticals, EMD Serono Inc, and Janssen Research & Development, LLC. Vadim S. Koshkin reports consultant or advisory fees from Clovis Oncology, Pfizer, EMD Serono, Seagen, AstraZeneca, Janssen, Astellas, Seattle Genetics, Dendreon, Guidepoint, GLG, and ExpertConnect; and research funding from Janssen, Clovis Oncology, Nektar, Taiho, Merck, and Advanced Accelerator Applications (Novartis). Kelly L. Stratton has received individual research funding from Merck & Company, Inc. and Janssen Pharmaceuticals LP. Wassim Kassouf has consulted for Sesen Bio, Ferring, Roche, BMS, Merck, Janssen, Bayer, Astellas, Seagen, Pfizer/EMD Serono, and Photocure. Seth P. Lerner has participated in clinical trials run by Aura Bioscience, Endo, FKD, JBL (SWOG), Genentech (SWOG), Merck (Alliance), QED Therapeutics, Vaxiion, and Viventia; is a consultant/advisory board member for Aura Bioscience, BMS, C2i Genomics, Ferring, Incyte, Pfizer/EMD Serono, Protara, Stimit, Vaxiion, and Verity; holds a patent for a TCGA classifier; and has received honoraria from Grand Rounds Urology and UroToday. The remaining authors have nothing to disclose.
References
- [1].Chang SS, Bochner BH, Chou R, et al. Treatment of non-metastatic muscle-invasive bladder cancer: AUA/ASCO/ASTRO/SUO guideline. J Urol 2017;198:552–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol 2000;163:1124–9. [PubMed] [Google Scholar]
- [3].Lobo N, Hensley PJ, Bree KK, et al. Updated European Association of Urology (EAU) prognostic factor risk groups overestimate the risk of progression in patients with non-muscle–invasive bladder cancer treated with bacillus Calmette-Guerin. Eur Urol Oncol 2022;5:84–91. [DOI] [PubMed] [Google Scholar]
- [4].Martin-Doyle W, Leow JJ, Orsola A, Chang SL, Bellmunt J. Improving selection criteria for early cystectomy in high-grade T1 bladder cancer: a meta-analysis of 15,215 patients. J Clin Oncol 2015;33:643–50. [DOI] [PubMed] [Google Scholar]
- [5].Cookson MS, Herr HW, Zhang ZF, Soloway S, Sogani PC, Fair WR. The treated natural history of high risk superficial bladder cancer: 15-year outcome. J Urol 1997;158:62–7. [DOI] [PubMed] [Google Scholar]
- [6].Korkes F, Timoteo F, Baccaglini W, et al. Postoperative mortality rate after radical cystectomy: a systematic review of epidemiologic series. Urol Int 2023;107:96–104. [DOI] [PubMed] [Google Scholar]
- [7].Mitra AP, Cai J, Miranda G, et al. Management trends and outcomes of patients undergoing radical cystectomy for urothelial carcinoma of the bladder: evolution of the University of Southern California experience over 3,347 cases. J Urol 2022;207:302–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shabsigh A, Korets R, Vora KC, et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol 2009;55:164–74. [DOI] [PubMed] [Google Scholar]
- [9].Skinner EC, Goldman B, Sakr WA, et al. SWOG S0353: phase II trial of intravesical gemcitabine in patients with nonmuscle invasive bladder cancer and recurrence after 2 prior courses of intravesical bacillus Calmette-Guerin. J Urol 2013;190:1200–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Steinberg RL, Thomas LJ, Brooks N, et al. Multi-institution evaluation of sequential gemcitabine and docetaxel as rescue therapy for nonmuscle invasive bladder cancer. J Urol 2020;203:902–9. [DOI] [PubMed] [Google Scholar]
- [11].Balar AV, Kamat AM, Kulkarni GS, et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): an open-label, single-arm, multicentre, phase 2 study. Lancet Oncol 2021;22:919–30. [DOI] [PubMed] [Google Scholar]
- [12].Boorjian SA, Alemozaffar M, Konety BR, et al. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: a single-arm, open-label, repeat-dose clinical trial. Lancet Oncol 2021;22:107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bio Sesen. Presentation at the Jefferies virtual healthcare conference, June 4, 2021. [Google Scholar]
- [14].Chamie K, Chang SS, Kramolowsky E, et al. IL-15 superagonist NAI in BCG-unresponsive non-muscle-invasive bladder cancer. NEJM Evid 2022;2:EVIDoa2200167. [DOI] [PubMed] [Google Scholar]
- [15].Packiam VT, Lamm DL, Barocas DA, et al. An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non-muscle-invasive bladder cancer: Interim results. Urol Oncol 2018;36:440–7. [DOI] [PubMed] [Google Scholar]
- [16].Jarow JP, Lerner SP, Kluetz PG, et al. Clinical trial design for the development of new therapies for nonmuscle-invasive bladder cancer: report of a Food and Drug Administration and American Urological Association public workshop. Urology 2014;83:262–4. [DOI] [PubMed] [Google Scholar]
- [17].Jarow JP, Thompson IM, Kluetz PG, et al. Drug and device development for localized prostate cancer: report of a Food and Drug Administration/American Urological Association public workshop. Urology 2014;83:975–8. [DOI] [PubMed] [Google Scholar]
- [18].Lerner SP, Dinney C, Kamat A, et al. Clarification of bladder cancer disease states following treatment of patients with intravesical BCG. Bladder Cancer 2015;1:29–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dinney CP, Greenberg RE, Steinberg GD. Intravesical valrubicin in patients with bladder carcinoma in situ and contraindication to or failure after bacillus Calmette-Guerin. Urol Oncol 2013;31:1635–42. [DOI] [PubMed] [Google Scholar]
- [20].Inman BA, Sebo TJ, Frigola X, et al. PD-L1 (B7–H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer 2007;109:1499–505. [DOI] [PubMed] [Google Scholar]
- [21].Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016;387:1909–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].US Department of Health and Human Services, Food and Drug Administration. BCG-unresponsive nonmuscle invasive bladder cancer: developing drugs and biologics for treatment. Guidance for industry. Silver Spring, MD: FDA; 2018. https://www.fda.gov/media/101468/download. [Google Scholar]
- [23].Necchi A, Roumiguié M, Esen AA, et al. Pembrolizumab (pembro) monotherapy for patients (pts) with high-risk non–muscle-invasive bladder cancer (HR NMIBC) unresponsive to bacillus Calmette-Guérin (BCG): results from cohort B of the phase 2 KEYNOTE-057 trial. J Clin Oncol 2023;41(Suppl 6):LBA442. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


