Abstract
The Helicobacter pylori cytotoxin is proteolytically cleaved at a flexible hydrophilic loop into two subunits. Deletion of the loop sequences had no effect on biological activity of the toxin in the HeLa cell vacuolation assay but favored the organization of the protein into hexameric rather than heptameric structures.
Pathogenic strains of Helicobacter pylori produce a potent cytotoxin, VacA, which causes massive vacuolar degeneration in target cells in vitro (6). It has been suggested (7, 12) that this toxin is a member of the A-B family of bacterial toxins which consists of two distinct moieties, A and B, involved in enzymatic activity and membrane translocation, respectively (9). VacA purified from the supernatant of H. pylori cultures is found as high-molecular-weight oligomeric structures with either six- or sevenfold radial symmetry (7, 12). Each oligomer is formed from monomers of approximately 90 kDa. Each monomer in the oligomer is clearly structured in two distinct subunits. After release from the bacteria, the 90-kDa monomer can undergo specific proteolytic cleavage at a hydrophilic loop to generate 37- and 58-kDa fragments which remain associated in the oligomeric structure (12). These fragments likely represent the subunits observed in the oligomeric structure. In this case, we may speculate that the hydrophilic loop forms a hinge region between the two subunits and proteolysis at this site may be important for activity of the toxin.
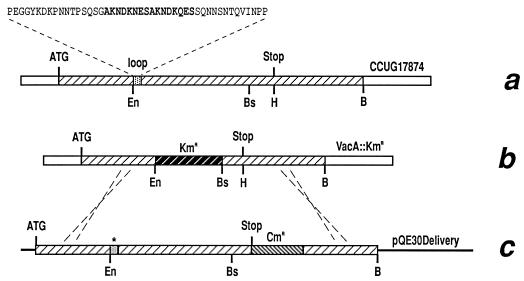
To test this hypothesis, we have engineered the gene coding for VacA in H. pylori in order to produce mutant forms of the protein with deletions or substitutions in the loop region. The strategy used to generate the mutants is shown in Fig. 1. We made three constructs. In the first, sequences within the loop coding for the 8-amino-acid (8-aa) repeat containing the major site of proteolytic cleavage (5) were deleted by PCR synthesis of a fragment coding for aa 34 to 343 and skipping aa 344 to 359 by using primers A and C (Table 1). This fragment was then introduced into a delivery plasmid containing the entire vacA gene and 3′ flanking sequences plus a Cmr cassette (13, 14) inserted in the HindIII site located 80 bp after the stop codon of the vacA coding sequences (Fig. 1c). In the second case, a larger deletion of sequences coding for 46 aa between positions 327 and 372 was created by using the Quickchange kit (Stratagene) and primers D and E (Table 1). The 46 aa deleted correspond to the entire hydrophilic loop as predicted by the Hydropathy (Kyte-Doolittle) program of the DNA Strider sequence analysis package. Finally, using primers A and B, we made a construct in which sequences coding for the 8-aa repeat were replaced with sequences coding for the 16 aa of an analogous cleavage loop of diphtheria toxin (CAGNRVRRSVGSSLSC), in order to determine whether a loop with similar properties but with a different amino acid sequence could be substituted.
FIG. 1.
Schematic representation of the strategy used for mutant construction. (a) Structure of the vacA gene in H. pylori CCUG17874. The sequence of the 46-aa hydrophilic loop containing the 8-aa repeat is shown. (b) Structure of the H. pylori VacA::Kmr strain in which the region between restriction sites EcoNI (En) and BsaBI (Bs) has been replaced with a kanamycin resistance cassette. (c) Structure of the delivery plasmid containing mutated vacA genes. Dotted lines indicate the region of recombination to replace the vacA gene. H, HindIII, B, BamHI.
TABLE 1.
Primers used
| Primera | Nucleotide sequence |
|---|---|
| A | GAATGGGATCCGCCTTTTTCACAACCGTGAT |
| B | TTTTTTGCGCACTATTGGGTGGGTTAATGACCTGAGTGTTACTATTATTTTGACTGCATGACAATGAGCTACCTACTGATCGCCTGACACGATTTCCTGCACAACCACTTTGAGAAGGGGT |
| C | TTTTTTGCGCACTATTGGGTGGGTTAATGACCTGAGTGTTACTATTATTTTGACTACCACTTTGAGAAGGGGT |
| D | GCTCCTAATAGTGCGCAAAAAACAGA |
| E | ACTATTAGGAGCGATAATGTTTAACC |
Primer A, forward primer for amplification of loop fragment; primer B, reverse primer for amplification of diphtheria toxin loop fragment; primer C, reverse primer for amplification of deletion fragment; primer D, forward primer for amplification of the whole construct with large deletion of the loop; primer E, reverse primer for amplification of the whole construct with large deletion of the loop.
Each of the constructs was introduced, by natural transformation, into an H. pylori recipient strain in which a large central region of 2.4 kbp of the vacA gene (GenBank accession no. S72494) between the EcoNI and BsaBI restriction sites had been replaced with a Kmr cassette (4). This recipient strain did not produce VacA (Fig. 2a, lane 2) and did not have any cytotoxic activity in vitro (Fig. 2b). Transformants were selected by resistance to chloramphenicol and sensitivity to kanamycin. The gene replacements were confirmed by genomic DNA sequencing.
FIG. 2.
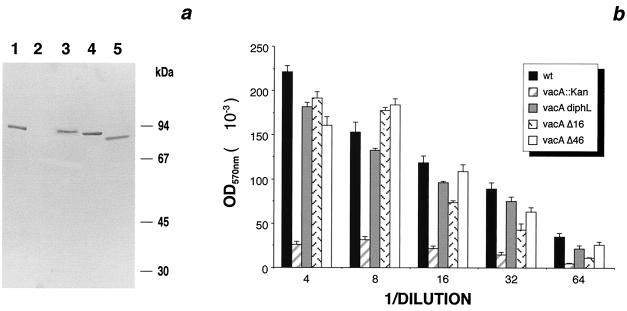
(a) Immunoblot of water extract of H. pylori expressing wild-type and mutant VacA proteins. Lane 1, wild-type VacA; lane 2, VacA::Kmr; lane 3, VacA with diphtheria toxin loop substitution; lane 4, VacA with 16-aa deletion; lane 5, VacA with 46-AA deletion. (b) Neutral red uptake of the HeLa cells treated with water extracts shown in panel a. wt, wild type.
Neither deletion nor substitution of the loop had any effect on the synthesis or stability of the protein. As can be seen in Fig. 2a, extracts of bacteria expressing each form of the toxin, obtained by vortexing bacterial pellets in water, contained similar quantities of the mature polypeptide but with slightly different electrophoretic mobilities which corresponded to the reduced size. Figure 2b shows the vacuolating activities of the water extracts quantitated by uptake of neutral red dye (2, 10). Surprisingly, all three of the altered proteins had vacuolating activity on HeLa cells similar to that of the wild-type protein. The mutated proteins were also equally well exported into the culture supernatant and could be purified by size exclusion chromatography as previously described (8) or by using an affinity matrix made with a monoclonal antibody which recognizes the native oligomeric structure (11). Extensive processing of the protein into the 37- and 58-kDa subunits was, however, observed only in the protein in which the loop had been replaced with the diphtheria toxin loop sequences (data not shown).
These data indicate that the specific cleavage site in the 8-aa repeat is not essential for activity of the toxin. It cannot be excluded, however, that alternative cleavage sites may be used by proteases in the cell culture medium or associated with the cell. Recently, Giannelli et al. have demonstrated that mutation of the specific cleavage site in the analogous loop of E. coli heat-labile toxin reduced the sensitivity to proteases but did not affect toxicity, although the kinetics of intoxication were significantly slowed (3). It is likely that, in this case and perhaps also for VacA, the proteolysis depends only on the presence of an extended hydrophilic linker sequence between the two subunits. Interestingly, even deletion of 46 aa in the VacA protein had no effect on activity. Hence, the fact that the cleavage into the 37- and 58-kDa fragments may not be essential for toxic activity must be considered.
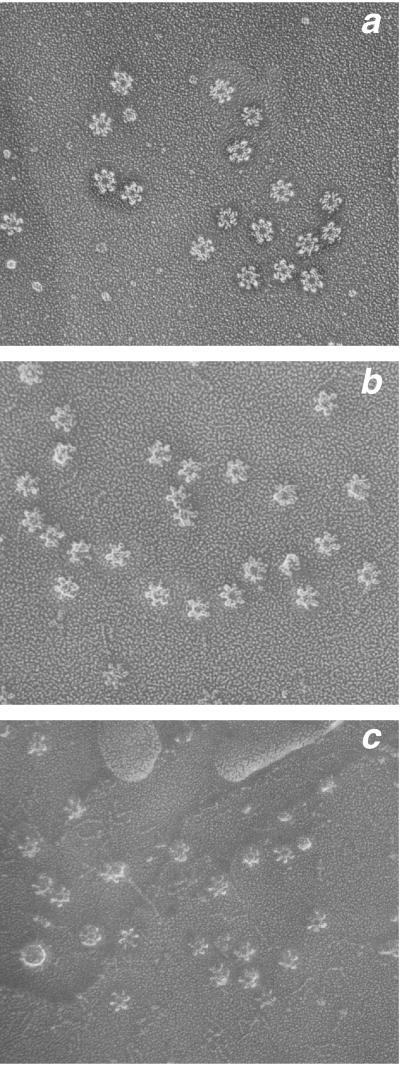
Deletion of the loop sequences did, however, cause observable differences in the oligomeric structure of the toxin. Quick-frozen, deep-etched preparations of wild-type toxin revealed a mixture of flower-shaped oligomeric structures with either six- or sevenfold radial symmetry (7). Our interpretation of these structures based on a three-dimensional reconstruction from tilted pairs of micrographs (5) is that each oligomer contains six or seven copies of the 90-kDa monomer. In preparations of wild-type toxin, approximately 70% of the oligomeric structures had sevenfold radial symmetry (7). Visualization of the mutated toxins by the same technique (Fig. 3) revealed that they formed indistinguishable oligomeric structures; however, the ratio of molecules with sixfold symmetry to those with sevenfold symmetry was altered. The toxin deleted of 16 aa formed predominantly structures with sixfold symmetry, but approximately 21% of the structures had sevenfold symmetry; in preparations of the molecule lacking 46 aa, only oligomers with sixfold symmetry were observed (Table 2). Hence, deletion of amino acids in the loop region appears to constrain the oligomeric structure to sixfold radial symmetry.
FIG. 3.
Electron micrographs of purified VacA molecules after quick-freeze deep-etch preparation. (a) Wild-type VacA; (b) VacA with 16-aa deletion; (c) VacA with 46-aa deletion. Magnification, ×52,000.
TABLE 2.
Symmetry of oligomers resulting from amino acid deletion
| VacA form | No. of oligomers with indicated symmetry/total no. (%)
|
|
|---|---|---|
| Sixfold | Sevenfold | |
| Native | 83/275 (30) | 192/275 (70) |
| 16-aa deletion | 245/312 (79) | 66/312 (21) |
| 46-aa deletion | >200 (100) | 0 (0) |
One explanation of the results described above may be that in the VacA oligomeric structure, each monomer must have two independent sites of interaction, one binding to the monomer on its right and the other binding to that on its left. The number of molecules which can enter the structure will depend on the angle of interaction and the length of the region connecting the two sites of interaction. The constriction of the structures in the large loop deletion to sixfold radial symmetry may indicate that both the 37- and 58-kDa subunits are involved in maintaining the oligomeric structure. Thus, reduction of the length of the connecting loop would pull the two subunits, and hence the sites of interaction, closer together and favor formation of hexamers.
Cover et al. (1) have recently observed VacA oligomers which appear to contain up to 12 monomeric units and have suggested a model in which the toxin can form double oligomers with P2 symmetry in addition to sixfold radial symmetry. In their preparations, the majority of oligomers had sixfold radial symmetry and molecules with sevenfold symmetry were interpreted as partially denatured structures. It is notable that the VacA from the strain used in these studies lacks one of the 8-aa repeats at the cleavage site and hence has a loop correspondingly shorter than that of the strain used here and in previous studies (5, 7). Hence we conclude from the data presented here that this interpretation is incorrect, that both hexamers and heptamers are intact molecules, and that the ratio of heptameric to hexameric structures depends on the length and flexibility of the loop connecting the 37- and 58-kDa subunits.
Acknowledgments
We gratefully acknowledge Girgio Corsi for the excellent technical assistance with the artwork.
REFERENCES
- 1.Cover T L, Hanson P I, Heuser J E. Acid-induced dissociation of VacA, the Helicobacter pylori vacuolating cytotoxin, reveals its pattern of assembly. J Cell Biol. 1997;138:759–769. doi: 10.1083/jcb.138.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Bernard M, Papini E, de Filippis V, Gottardi E, Telford J L, Manetti R, Fontana A, Rappuoli R, Montecucco C. Low pH activates the vacuolating toxin of Helicobacter pylori, which becomes acid and pepsin resistant. J Biol Chem. 1995;270:23937–23940. doi: 10.1074/jbc.270.41.23937. [DOI] [PubMed] [Google Scholar]
- 3.Giannelli V, Fontana M R, Giuliani M M, Guangcai D, Rappuoli R, Pizza M. Protease susceptibility and toxicity of heat-labile enterotoxins with a mutation in the active site or in the protease-sensitive loop. Infect Immun. 1997;65:331–334. doi: 10.1128/iai.65.1.331-334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Labigne-Roussel A, Courcoux P, Tompkins L. Gene disruption and replacement as a feasible approach for mutagenesis of Campylobacter jejuni. J Bacteriol. 1988;170:1704–1708. doi: 10.1128/jb.170.4.1704-1708.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanzavecchia S, Lupetti P, Bellon P L, Dallai R, Rappuoli R, Telford J L. Three dimensional reconstruction of metal replicas of the Helicobacter pylori vacuolating cytotoxin. J Struct Biol. 1998;121:9–18. doi: 10.1006/jsbi.1997.3941. [DOI] [PubMed] [Google Scholar]
- 6.Leunk R D, Johnson P T, David B C, Kraft W G, Morgan D R. Cytotoxic activity in broth-culture filtrates of Campylobacter pylori. J Med Microbiol. 1988;26:93–99. doi: 10.1099/00222615-26-2-93. [DOI] [PubMed] [Google Scholar]
- 7.Lupetti P, Heuser J E, Manetti R, Massari P, Lanzavecchia S, Bellon P L, Dallai R, Rappuoli R, Telford J L. Oligomeric and subunit structure of the Helicobacter pylori vacuolating cytotoxin. J Cell Biol. 1996;133:801–807. doi: 10.1083/jcb.133.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manetti R, Massari P, Burroni D, de Bernard M, Marchini A, Olivieri R, Papini E, Montecucco C, Rappuoli R, Telford J L. Helicobacter pylori cytotoxin: importance of native conformation for induction of neutralizing antibodies. Infect Immun. 1995;63:4476–4480. doi: 10.1128/iai.63.11.4476-4480.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montecucco C, Papini E, Schiavo G. Molecular models of toxin membrane translocation. In: Alouf J E, Freer J H, editors. Sourcebook of bacterial protein toxins. New York, N.Y: Academic Press, Inc.; 1991. pp. 45–56. [Google Scholar]
- 10.Papini E, Bugnoli M, de Bernard M, Figura N, Rappuoli R, Montecucco C. Bafylomicin A1 inhibits Helicobacter pylori-induced vacuolization of HeLa cells. Mol Microbiol. 1993;7:323–327. doi: 10.1111/j.1365-2958.1993.tb01123.x. [DOI] [PubMed] [Google Scholar]
- 11.Reyrat J M, Charrel M, Pagliaccia C, Burroni D, Lupetti P, de Bernard M, Ji X, Norais N, Papini E, Dallai R, Rappuoli R, Telford J L. Characterisation of a monoclonal antibody and its use to purify the cytotoxin of Helicobacter pylori. FEMS Microbiol Lett. 1998;165:79–84. doi: 10.1111/j.1574-6968.1998.tb13130.x. [DOI] [PubMed] [Google Scholar]
- 12.Telford J L, Ghiara P, dell’Orco M, Comanducci M, Burroni D, Bugnoli M, Tecce M F, Censini S, Covacci A, Xiang Z, Papini E, Montecucco C, Parente L, Rappuoli R. Gene structure of Helicobacter pylori cytotoxin and evidence of its key role in gastric disease. J Exp Med. 1994;179:1653–1658. doi: 10.1084/jem.179.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Taylor D E. Chloramphenicol resistance in Campylobacter coli: nucleotide sequence, expression, and cloning vector construction. Gene. 1990;94:23–28. doi: 10.1016/0378-1119(90)90463-2. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Roos K P, Taylor D E. Transformation of Helicobacter pylori by chromosomal metronidazole resistance and by a plasmid with a selectable chloramphenicol resistance marker. J Gen Microbiol. 1993;139:2485–2493. doi: 10.1099/00221287-139-10-2485. [DOI] [PubMed] [Google Scholar]