Abstract
Post-Covid-19 Condition (PCC) is a syndrome comprised of symptoms persisting 3 months or more beyond SARS-CoV-2 primary infection. It is typically characterized by fatigue, cognitive problems and psychiatric symptoms, as well as cardiac symptoms that contribute to exercise intolerance in many. Despite the high prevalence of PCC among those with a prior SARS-CoV-2 infection, there is currently no widely accepted rehabilitation strategy, and many conventional modalities are movement-based. Non-invasive brain stimulation methods such as repetitive transcranial magnetic stimulation (rTMS) may have some potential to alleviate the cognitive and affective symptoms of PCC without reliance on exercise. The purpose of the present study was to explore the feasibility and tolerability of using rTMS to treat symptoms of “brain fog” and affective disturbance among those living with PCC, using a case series design. We enrolled four individuals with PCC following a confirmed SARS-CoV-2 infection, at least 3 months after the resolution of the primary infection. Participants were randomized to 4 sessions of active and 2 sessions of sham intermittent theta-burst stimulation (iTBS); two intensities of iTBS were evaluated: iTBS-300 and iTBS-600. No adverse events occurred in active or sham stimulation; 2 participants reported tingling sensation on the scalp but no other tolerability issues. Trends in symptoms suggested improvements in cognitive interference, quality of life, and anxiety in the majority of participants. In summary, in this case series iTBS was well tolerated among 4 individuals with PCC; active stimulation was associated with positive trends in some primary symptom clusters as compared with sham stimulation. Future studies should examine the effects of iTBS on PCC symptoms in the context of experimental studies and randomized controlled trials.
Keywords: Long-COVID, PCC, rTMS, COVID-19, Brain stimulation, Case series
1. Introduction
Post-COVID-19 condition (PCC) affects approximately 10–12% of individuals who have had a prior symptomatic SARS-CoV-2 infection (Nalbandian et al., 2022; Thaweethai et al., 2023). Among the long list of symptoms of PCC, those that appear to interfere most with daily functioning across domains are fatigue and cognitive dysfunction, the latter being colloquially known as “brain fog” (Asadi-Pooya et al., 2022; Lam et al., 2023). Among those presenting with PCC, post-exertion malaise syndrome (PEMS) is a common feature (Twomey et al., 2022), affecting more than half of patients. For this reason, rehabilitation of PCC may need to emphasize treatment modalities beyond movement and exercise in order to be viable for all individuals affected.
Repetitive transcranial magnetic stimulation (rTMS) has been used to treat neuropsychiatric conditions involving challenges with concentration, attention and fatigue, including depression (McClintock et al., 2018) and concussion (Mollica et al., 2021). Meta-analytic reviews have revealed that a single dose of intermittent theta burst stimulation (iTBS) can result in acute improvements in cognitive performance, particularly executive function and attention (Lowe et al., 2018). One prior case study showed that direct current stimulation reduced severe anxiety symptoms in a patient with PCC (Shinjo et al., 2020), and prior studies have shown that cognitive and psychiatric symptoms in PCC patients may be closely linked to prefrontal hypoxia (Hall et al., 2023). To date, two uncontrolled case series have probed the effects of rTMS for PCC. Sasaki et al. (2023) applied conventional high-frequency rTMS to the occipital and frontal lobe midline of 12 patients with PCC, observing a reduction in fatigue, improvement in spontaneity, cognitive function, and hypoperfusion. Noda et al. (2023), on the other hand, administered a combined protocol involving excitatory (intermittent theta burst stimulation [iTBS]) and inhibitory (low frequency) rTMS applied to the left dlPFC and right lateral orbitofrontal cortex (LOFC), demonstrating improvements in neuropsychiatric symptoms among patients with PCC.
As such, rTMS provides promise as a viable treatment modality with respect to PCC symptoms. However, no prior studies have compared different variants of iTBS (iTBS-300 and iTBS-600) or used a sham control condition. Also, among possible targets for rTMS within the cortex, it is not clear which might be best suited for rTMS application. Based on our prior work summarizing the cognitive impact of iTBS (Lowe et al., 2018), we anticipated that the left dorsolateral prefrontal cortex would be the most appropriate target, given its link to executive function, hypo-frontality in affective disorders, and responsivity to rTMS stimulation (Kawabata et al., 2022).
The objective of the current study was to examine the feasibility and tolerability of several iTBS variants (iTBS-300, iTBS-600) and sham stimulation in PCC patients. Secondarily, we aimed to examine signs of improvement in cognitive, affective and functional symptoms following each iTBS variant.
2. Methods
2.1. Participants
We recruited 4 patients who presented with symptoms of PCC at the time of study enrollment, and at least one prior SARS-CoV-2 infection confirmed by RAT or PCR. Patient characteristics are presented in Table 1. The inclusion criteria for the study were 18–60 years of age, a prior positive COVID-19 test (RAT or PCR) and persisting PCC symptoms for at least 3 months beyond their acute COVID-19 illness phase. Exclusion criteria encompassed any medical condition or neurological disorders contraindicating the rTMS protocol, such as the presence of metal in the cranium, history of seizures, among others (Rossi et al., 2011). Among those enrolled in the study, 2 were young adults (ages 18-29 years) and two were adults (ages 30-55 years); mean age was 34 years. All cases (4 of 4) reported cognitive symptoms at baseline; 3 of 4 cases reported fatigue at baseline. Two reported their primary COVID-19 symptoms at the time of infection as "moderately" or "very" severe, while two reported thier infection symptoms as "slightly" severe. Mean GAD-7 score at baseline was 1.67; mean BDFS score at baseline was 2.0. All prospective patients were vetted by AB. The study protocol was reviewed and approved by the Office of Research Ethics at the University of Waterloo (Approval Number: 45082). All participants provided informed consent before participating in the study.
Table 1.
Demographic and baseline characteristics of study participants.
| Characteristics | Participant 1 | Participant 2 | Participant 3 | Participant 4 |
|---|---|---|---|---|
| Age | Young adult (18–29) | Young adult (18–29) | Adult (30–55) | Adult (30–55) |
| Sex | Male | Female | Female | Female |
| Resting motor threshold | 47 | 46 | 34 | 63 |
| Stimulation intensity | 38 | 37 | 27 | 50 |
| Stimulation order | iTBS300 → iTBS600 → Sham | iTBS600 → Sham → iTBS300 | Sham →iTBS300 → iTBS600 | iTBS → Sham → iTBS600 |
2.2. Procedure
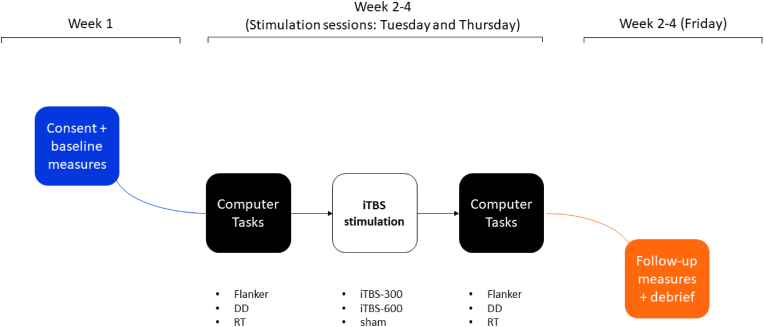
Following a 7-day lead-in period, participants were pseudo-randomized to three stimulation conditions using Latin square (two active conditions: iTBS-600, iTBS-300) and one sham condition (iTBS-600 with coil rotated 180°). At the end of each week self-reported symptoms of anxiety, quality of life, functional abilities were completed online by each participant. A battery of three cognitive tasks were administered in the following fixed order pre-to post-stimulation: 1) a visual reaction time (RT) task, 2) a cognitive interference task (i.e., a modified version of the Flanker task), and 3) a delay discounting (DD) task. These three cognitive tasks were chosen because of their ability to assess two major dimensions of executive control (i.e., behavioral inhibition, Flanker; complex decision making, DD), as well as simple information processing speed. The post-stimulation battery in each case was delayed by 8 min following the conclusion of stimulation in order to allow for the accumulation of long-term potentiation (LTP) effects, which are typically delayed by 5+ minutes (Huang et al., 2005). Cognitive tasks were delivered by desktop computer using Inquisit Lab 5 software in a temperature- and sound -controlled room with static ambient lighting, adjacent to the stimulation room.
Brain Stimulation. Stimulation was delivered at 80% of resting motor threshold (RMT) using the burst patterns and frequencies recommended by Huang et al. for iTBS-300 and iTBS-600 (Huang et al., 2005). Theta burst protocols involve delivery of triplet 50Hz pulses repeating at 5 Hz. Intermittent theta burst stimulation (iTBS) involves interleaving 2 s of active stimulation with 8 s rest periods, with a total pulse delivery of 600. Two variants of iTBS used; the first was the standard version described above (iTBS-600). The second (iTBS-300) was an abbreviated variant, involving only 300 pulses. Each participant received brain stimulation twice per week (Tuesdays and Thursdays) at the same time of day on each occasion; stimulation was delivered using a MagVenture MagPro x100 biphasic stimulator, coupled with a MCF-B70 stimulation coil. Coil placement was guided via an EEG cap using the international 10–20 system, with the F3 electrode site indicating the point of stimulation. Stimulation intensity was calibrated in the first stimulation session using RMT, which was assessed using electromyography via the right abductor pollicis brevis (APB) muscle. Single pulses of stimulation were applied to contralateral motor cortex. RMT was defined as the minimum stimulation intensity required to evoke a 50 μV peak-to-peak amplitude in 5 of 10 consecutive single pulses.
2.3. Measures
Response Inhibition. Response inhibition was assessed using a modified version of the Eriksen Flanker task (Eriksen and Eriksen, 1974), administered by desktop computer. In the task, participants were presented with a series of trials involving stimuli comprising an array of seven letters; they were instructed to respond to the middle letter in each array (the target) by pressing a corresponding keyboard key. If the target letter was 'H' or 'K', participants were asked to press the 'A' keyboard key; however, if the middle letter was 'S' or 'C', participants were required to press the 'D' keyboard key. Participants were presented with two types of trials corresponding with two conditions: (i) the congruent noise condition and (ii) the incongruent noise condition. In the congruent noise condition, the target letter always matched the surrounding letters (e.g., HHHHHHH or CCCCCCC), whereas in the incongruent noise condition, the target letter was flanked by different letters (e.g., CCCHCCC or HHHSHHH). Participants were instructed to respond to the target letter as quickly and accurately as possible. The task included a total of five blocks, starting with a block of 60 mixed trials. This was followed by four test blocks in the following order: 50 congruent, 50 incongruent, 50 congruent, and 50 incongruent. Flanker interference score was calculated by subtracting the average response time in the congruent condition from that in the incongruent condition, with a higher score indicating poorer task performance, and consequently, indicating poor response inhibition.
Reaction time. Reaction time was assessed using a computer-administered psychomotor vigilance task (Murray et al., 2001). Participants were instructed to focus on a blank white screen showing a central fixation cross. Following this, a visual stimulus, represented by a red circle, appeared within a time window ranging from 2 to 8 s. Participants were then required to respond promptly by pressing the spacebar on a computer keyboard. The task consisted of 20 trials within a single block; reaction times were recorded for each trial.
Complex decision making. Delay discounting was assessed using a modified version of the DD paradigm by Koffarnus and Bickel (2014). DD represents the tendency of individuals to devalue or discount rewards based on the time delay before receiving them. Individuals showing strong delay discounting tend to choose smaller, immediate rewards over larger, delayed ones. In the task, participants were presented with various monetary choices, such as “Would you rather have $500 now or $1000 in 3 weeks?” The questions continued, keeping the monetary values constant but altering the duration of the delay to receive the larger sum (e.g., 1 day, 2 years). Responses were converted to “k” values, with a higher number signifying a stronger inclination to devalue rewards relative to the time delay before receiving them.
Executive dysfunction. Symptoms of executive dysfunction were assessed using the Barkeley Deficits in Executive Function Scale (BDEFS) (Barkley, 2011). We used four items from the “self-restraint” subscale of BDEFS: (1) “I am unable to inhibit my reactions or responses to events or to other people”, (2) “I act without thinking”, (3) “I make impulsive comments to others”, and (4)“I am likely to do things without considering the consequences for doing them”. Responses were indicated on a numerical scale where 1 = never or rarely, 2 = sometimes, 3 = often, and 4 = very often.
QOL measure: The QOL measure was derived from the WHO Quality of Life scale (The Whoqol Group, 1998). The following seven items were selected to measure quality of life: (1) “How would you rate your quality of life?”, (2) “How satisfied are you with your health?”, (3) “How much do you enjoy life?”, (4) “To what extent do you feel your life to be meaningful?”, (5) “How well are you able to concentrate?”, (6) “How satisfied are you with yourself?”, and (7) “How satisfied are you with your personal relationships?”. Responses were indicated on a numerical scale where 1 = low/poor, 3 = indifferent and 5 = high/excellent; all item responses were averaged together to yield a total score, with higher values indicating better quality of life.
Functional abilities. Functional abilities were assessed using a custom-designed scale called the post-COVID-19 syndrome Functional Impacts Scale (PCS-FI). The scale was partially derived from the Sheehan Disability Scale (2008) (Sheehan and Sheehan, 2008), the Katz ADL Scale (1983) (Katz, 1983), and the Lawton-Brody Scale (1969) (Lawton and Brody, 1969). Participants were asked to respond to 11 items on a scale of 0–7. A copy of the measure can be found in Appendix 1.
Anxiety. Anxiety was assessed using the Generalized Anxiety Disorder Scale-7 item version (GAD-7) (Spitzer et al., 2006). The items included in this scale are a variety of symptoms of anxiety: (1) feeling nervous, anxious or on edge, (2) not being able to stop or control worrying, (3) worrying too much about different things, (4) trouble relaxing, (5) being so restless that it's hard to sit still, (6) becoming easily annoyed or irritable, and (7) feeling afraid as if something awful might happen. Responses are given on a 4-point scale, where 0 = “not at all”, 1 = “two days or less days”, 3 = “three or more days”, and 4 = “nearly everyday”. Higher scores on the GAD-7 are indicative of higher levels of generalized anxiety symptoms.
Subjective measures. We used a custom-made questionnaire to assess the subjective impressions of the participants regarding brain stimulation and tolerability issues. Refer to Table 2 for the question descriptions and participants' responses.
Table 2.
Tolerability of stimulation.
| Disagree | Neutral | Agree | |
|---|---|---|---|
| Recommendation for brain stimulation to others with Long COVID-19 | 0 | 0 | 3 |
| The brain stimulation sessions were comfortable and well-tolerated | 0 | 0 | 3 |
| Experiencing discomfort or side effects after the brain stimulation sessions | 2 | 1 | 0 |
| No additional COVID-19 infection or other respiratory illnesses, such as a cold or flu, occurred during the study. | 0 | 0 | 3 |
| Tingling at scalp | Other | None | |
| Sensations during and after brain stimulation | 2 | 0 | 1 |
3. Results
The 4 participants ranged in age from 24 to 49 years and 3 (75%) were female (Table 1). One of the three participants did not complete the tolerability/side effect survey, and so responses were based on the remaining three. All three participants (3/3) agreed with the statement that the procedure was well-tolerated and comfortable, with no discomfort or side effects following the brain stimulation. Two out of three participants reported experiencing only tingling at the scalp during the procedure, among all the proposed sensations. No other tolerability issues were reported. All three participants (3/3) agreed with the statement that they would recommend brain stimulation to others with Long COVID-19.
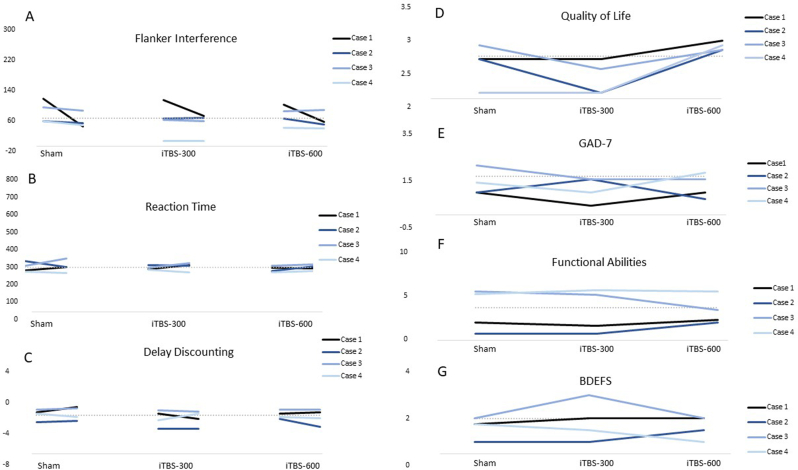
Changes in cognitive, affective and functional outcomes in each stimulation condition are presented in Fig. 1. Flanker interference improved relative to baseline in 3 of 4 cases (Fig. 1, panel A) following iTBS-600 stimulation. Likewise, quality of life scores increased relative to baseline in 3 of 4 cases following iTBS-600 stimulation (Fig. 1, panel D). Finally, GAD-7 symptom scores decreased relative to baseline in 3 of 4 cases following iTBS-300 stimulation (Fig. 1, panel E). Two participants reported improved concentration and thinking during the week of active stimulation.
Fig. 1.
Changes in cognitive task performance, quality of life, anxiety, functional abilities and self-reported executive dysfunction as a function of stimulation condition.
Flanker interference scores, simple reaction time scores, and delay discounting are coded such that lower scores equate with better performance. GAD-7 = Generalized Anxiety Disorder scale, 7-item version; BDEFS=Berkeley Deficits in Executive Function Scale. For cognitive tests, baseline performance is estimated from performance on the cognitive task during the lead-in period averaged together with each pre-stimulation baseline score; for self-reported outcomes, baseline performance is estimated based on self-reported symptoms during the lead-in period only.
4. Discussion
The purpose of this study was to further examine the potential of non-invasive brain stimulation for improving symptoms of PCC. Given the prevalence of exercise intolerance (>50%) among those living with PCC, non-movement rehabilitation modalities are important to explore. As a brain stimulation modality, rTMS is particularly important to evaluate because of its previously documented benefits for many cognitive and affective symptoms that comprise the PCC syndrome (Begemann et al., 2020; Lowe et al., 2018; Parikh et al., 2022; Valiengo et al., 2022). It was hypothesized that rTMS would be well-tolerated and safe in this population, and that positive trends in cognitive, psychiatric, functional and quality of life domains would be evident following excitatory stimulation of the left dorsolateral prefrontal cortex. Two prior case series (Sasaki et al., 2023; Noda et al., 2023) showed positive effects, but—as is typical of case series—they did not include a sham condition; likewise, only one variant of iTBS was examined in one of the two studies.
Findings from the current study indicated that rTMS targeting the left dlPFC was safe and well-tolerated among participants. All follow-up respondents stated that they would recommend brain stimulation to others with PCC. The pattern of findings with respect to PCC symptom measures were also promising. Results indicated that for iTBS-600, there were reductions in cognitive interference scores relative to baseline for 3 out of 4 cases, and improvement in quality of life compared to baseline for 3 of 4 cases. In 3 of 4 cases, improvements in anxiety were noted following iTBS-300. These findings were evident based on pairs of stimulation sessions per iTBS modality, and prior studies using more stimulation sessions (≥10) showed more robust effects (Noda et al., 2023; Sasaki et al., 2023). The trends here and in prior studies (Noda et al., 2023; Sasaki et al., 2023) justify more rigorous evaluation of rTMS for PCC symptoms in the context of a randomized controlled trial wherein statistical significance and effect sizes can be calculated in order to draw conclusions about the relative efficacy of iTBS and other stimulation variants.
It is not fully clear why there appear to be differences between the two stimulation types (iTBS-300 vs. iTBS-600) in relation to their effects on different outcomes (GAD-7 versus QOL). Given the case series format, future studies comparing the two stimulation types for different outcomes should be undertaken. If such studies replicate the same observed patterns under more rigorous experimental conditions, further interpretation would be warranted.
In the context of future trials, a greater stimulation volume should be considered in order to more fully evaluate the limits of tolerability and trajectories of symptom improvement. For instance, stimulation regimens could be comprised of higher number of stimulation sessions, more stimulation days, and higher stimulation intensity. It is not currently known what the appropriate dosage might be for PCC, but other trials treating affective symptoms among those meeting the criteria for major depressive disorder would typically involve 15-20 sessions (5 per week), at 100% of RMT or higher (Fitzgerald et al., 2009). Increasing the number, frequency, and intensity of stimulation may be warranted in order to achieve therapeutic effect of rTMS on the symptom outcomes of interest. Although sustained change was beyond the intent of the current translational study, the ultimate goal of rTMS for improving PCC symptoms would be foster sustained symptom remission. The potential of rTMS to induce sustained remission must be evaluated in future research.
4.1. Mechanisms of neuromodulation
It is of increasing interest to consider subtypes of PCC (Lam et al., 2023), as well as premorbid psychiatric and chronic pain symptomology. A recent prospective study of 25,114 French individuals measured affective symptoms between April 6, 2020 and May 4, 2020 (early pandemic) and assessed incident PCC symptoms between December, 2020 and January, 2021 (mid-pandemic). Findings revealed that early pandemic depressive symptoms were reliable predictors of later incident PCC symptoms among those who were subsequently infected with serologically confirmed SARS-CoV-2 (Matta et al., 2023). This pattern matched the findings of an early short-term prospective study involving American adults which found that baseline affective symptoms predicted follow-up PCC symptoms at a shorter interval (Tenforde et al., 2020). In all cases, there is potential for rTMS to resolve both lingering depressive symptoms and physical symptom reporting that might give rise to higher rates of PCC diagnosed among those with premorbid psychiatric co-morbidities. Symptom relief and restoration of function may likewise occur when rTMS is improving affective mediators of purely physical PCC symptoms.
Finally, placebo effects of neuromodulation interventions are important to consider, particularly in light of the fact that there is substantial overlap in areas of functional activation associated with placebo and brain stimulation targets for depression, including the left dlPFC (Burke et al., 2022). These findings advance the importance of using sham control conditions in neuromodulation studies; given that prior case series involving PCC have not included sham, the inclusion of the feature in the current study is an important strength. However, even in this case it must be noted that placebo may still account for some of the patterns observed.
4.2. Strengths and limitations
The current case series had several methodological strengths including the use of a sham control, standardized cognitive testing and counterbalancing of stimulation conditions across participants so as to eliminate contemporaneous time-related effects on outcomes. This case series study also has several notable limitations. Firstly, the small sample size, with only 4 participants, may restrict the applicability of the findings to the larger population. Secondly, the absence of multi-participant groups with randomization limits our ability to definitively establish the effectiveness, tolerability or safety of TBS in a comprehensive manner. Thirdly, our application of brain stimulation was limited to only two sessions of active iTBS 300 and two sessions of active iTBS 600. However, to induce optimal and lasting effects, patients typically require repeated and prolonged stimulation to promote synaptic plasticity. Furthermore, the absence of long-term outcome data hinders our understanding of how long the positive effects of brain stimulation might last. Finally, given that this is a case series and each participant is considered separately, no inferential statistics are possible to compute.
5. Conclusion
This study demonstrated that the iTBS was well tolerated among 4 participants with PCC, with no reported adverse events. Some evidence of improved cognitive interference, quality of life and anxiety were observed. The current findings provide further evidence that iTBS could be a feasible and well-tolerated treatment for patients experiencing PCC. Given the prevalence of PEMS in this population, a non-exercise modality targeting cognitive, affective and functional symptoms warrants further investigation in the context of randomized preclinical experiments and randomized controlled trials.
Funding
Supported by funding to PAH from the Canadian Institutes for Health Research (CIHR; GA3-177733) and the Natural Sciences and Engineering Council of Canada (NSERC; RGPIN-2019-04606).
CRediT authorship contribution statement
Mohammad Nazmus Sakib: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. Ashish Saragadam: Investigation, Methodology, Writing – review & editing. Mariella C. Santagata: Investigation, Methodology, Writing – review & editing. Marie Jolicoeur-Becotte: Investigation, Methodology, Writing – review & editing. Lena Kozyr: Investigation, Methodology, Writing – review & editing. Amer M. Burhan: Investigation, Methodology, Writing – review & editing. Peter A. Hall: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Writing – original draft, Writing – review & editing.
Declaration of competing interest
Authors declare no competing interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2024.100736.
Appendix.
Fig. 1.
Procedure flowchart
Table 1.
Data collection and brain stimulation schedule
| Week 1 | Monday | Tuesday | Wednesday | Thursday | Friday | Saturday | Sunday |
|---|---|---|---|---|---|---|---|
| Questionnaires/Baseline | Yes | Yes | Yes | Yes | Yes | – | – |
| Stimulation | – | – | – | – | – | – | – |
| Week 2 | Monday | Tuesday | Wednesday | Thursday | Friday | Saturday | Sunday |
| Questionnaires | Yes | Yes | Yes | Yes | Yes | ||
| Stimulation | |||||||
| Participant 1 | – | iTBS 300 | – | iTBS 300 | – | – | – |
| Participant 2 | – | iTBS 600 | – | iTBS 600 | – | – | – |
| Participant 3 | – | Sham | – | Sham | – | – | – |
| Participant 4 | – | iTBS 300 | – | iTBS 300 | – | – | – |
| Week 3 | Monday | Tuesday | Wednesday | Thursday | Friday | Saturday | Sunday |
| Questionnaires | Yes | Yes | Yes | Yes | Yes | ||
| Stimulation | |||||||
| Participant 1 | – | iTBS 600 | – | iTBS 600 | – | – | – |
| Participant 2 | – | Sham | – | Sham | – | – | – |
| Participant 3 | – | iTBS 300 | – | iTBS 300 | – | – | – |
| Participant 4 | – | sham | – | sham | – | – | – |
| Week 4 | Monday | Tuesday | Wednesday | Thursday | Friday | Saturday | Sunday |
| Questionnaires | Yes | Yes | Yes | Yes | Yes | ||
| Stimulation | |||||||
| Participant 1 | – | Sham | – | Sham | – | – | – |
| Participant 2 | – | iTBS 300 | – | iTBS 300 | – | – | – |
| Participant 3 | – | iTBS 600 | – | iTBS 600 | – | – | – |
| Participant 4 | – | iTBS 600 | – | iTBS 600 | – | – | – |
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Asadi-Pooya A.A., Akbari A., Emami A., Lotfi M., Rostamihosseinkhani M., Nemati H.…Shahisavandi M. Long COVID syndrome-associated brain fog. J. Med. Virol. 2022;94(3):979–984. doi: 10.1002/jmv.27404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley R. Guilford Press; New York: 2011. Barkley Deficits in Executive Functioning Scale (BDEFS) [Google Scholar] [Google Scholar]
- Begemann M.J., Brand B.A., Ćurčić-Blake B., Aleman A., Sommer I.E. Efficacy of non-invasive brain stimulation on cognitive functioning in brain disorders: a meta-analysis. Psychol. Med. 2020;50(15):2465–2486. doi: 10.1017/S0033291720003670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke M.J., Romanella S.M., Mencarelli L., Greben R., Fox M.D., Kaptchuk T.J., et al. Placebo effects and neuromodulation for depression: a meta-analysis and evaluation of shared mechanisms. Mol. Psychiatr. 2022;27(3):1658–1666. doi: 10.1038/s41380-021-01397-3. [DOI] [PubMed] [Google Scholar]
- Eriksen B.A., Eriksen C.W. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychophys. 1974;16(1):143–149. doi: 10.3758/BF03203267. [DOI] [Google Scholar]
- Fitzgerald P.B., Hoy K., McQueen S., Maller J.J., Herring S., Segrave R.…Daskalakis Z.J. A randomized trial of rTMS targeted with MRI based neuro-navigation in treatment-resistant depression. Neuropsychopharmacology. 2009;34(5):1255–1262. doi: 10.1038/npp.2008.233. [DOI] [PubMed] [Google Scholar]
- Hall P.A., Ayaz H., Meng G., Hudson A., Sakib M.N., Quah A.C.K.…Fong G.T. Neurocognitive and psychiatric symptoms following infection with COVID-19: evidence from laboratory and population studies. Brain Behav Immun Health. 2023;28 doi: 10.1016/j.bbih.2023.100595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.-Z., Edwards M.J., Rounis E., Bhatia K.P., Rothwell J.C. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J. Am. Geriatr. Soc. 1983;31(12):721–727. doi: 10.1111/j.1532-5415.1983.tb03391.x. [DOI] [PubMed] [Google Scholar]
- Kawabata Y., Imazu S.I., Matsumoto K., Toyoda K., Kawano M., Kubo Y.…Kanazawa T. rTMS therapy reduces hypofrontality in patients with depression as measured by fNIRS. Front. Psychiatr. 2022;13 doi: 10.3389/fpsyt.2022.814611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus M.N., Bickel W.K. A 5-trial adjusting delay discounting task: accurate discount rates in less than one minute. Exp. Clin. Psychopharmacol. 2014;22(3):222–228. doi: 10.1037/a0035973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam G.Y., Damant R.W., Ferrara G., Lim R.K., Stickland M.K., Ogando N.S.…Smith M.P. Characterizing long-COVID brain fog: a retrospective cohort study. J. Neurol. 2023;270(10):4640–4646. doi: 10.1007/s00415-023-11913-w. [DOI] [PubMed] [Google Scholar]
- Lawton M.P., Brody E.M. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontol. 1969;9(3):179–186. [PubMed] [Google Scholar]
- Lowe C.J., Manocchio F., Safati A.B., Hall P.A. The effects of theta burst stimulation (TBS) targeting the prefrontal cortex on executive functioning: a systematic review and meta-analysis. Neuropsychologia. 2018;111:344–359. doi: 10.1016/j.neuropsychologia.2018.02.004. [DOI] [PubMed] [Google Scholar]
- Matta J., Robineau O., Wiernik E., Carrat F., Severi G., Touvier M., et al. Depression and anxiety before and at the beginning of the COVID-19 pandemic and incident persistent symptoms: a prospective population-based cohort study. Mol. Psychiatr. 2023:1–11. doi: 10.1038/s41380-023-02179-9. [DOI] [PubMed] [Google Scholar]
- McClintock S.M., Reti I.M., Carpenter L.L., McDonald W.M., Dubin M., Taylor S.F.…Treatments Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. J. Clin. Psychiatr. 2018;79(1) doi: 10.4088/JCP.16cs10905. 16cs10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollica A., Safavifar F., Fralick M., Giacobbe P., Lipsman N., Burke M.J. Transcranial magnetic stimulation for the treatment of concussion: a systematic review. Neuromodulation. 2021;24(5):803–812. doi: 10.1111/ner.13319. [DOI] [PubMed] [Google Scholar]
- Murray M.M., Foxe J.J., Higgins B.A., Javitt D.C., Schroeder C.E. Visuo-spatial neural response interactions in early cortical processing during a simple reaction time task: a high-density electrical mapping study. Neuropsychologia. 2001;39(8):828–844. doi: 10.1016/s0028-3932(01)00004-5. [DOI] [PubMed] [Google Scholar]
- Nalbandian A., Desai A.D., Wan E.Y. Post-COVID-19 condition. Annu. Rev. Med. 2022 doi: 10.1146/annurev-med-043021-030635. [DOI] [PubMed] [Google Scholar]
- Noda Y., Sato A., Shichi M., Sato A., Fujii K., Iwasa M.…Osawa R. Real world research on transcranial magnetic stimulation treatment strategies for neuropsychiatric symptoms with long-COVID in Japan. Asian Journal of Psychiatry. 2023;81 doi: 10.1016/j.ajp.2022.103438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh T.K., Strawn J.R., Walkup J.T., Croarkin P.E. Repetitive transcranial magnetic stimulation for generalized anxiety disorder: a systematic literature review and meta-analysis. Int. J. Neuropsychopharmacol. 2022;25(2):144–146. doi: 10.1093/ijnp/pyab077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S., Hallett M., Rossini P.M., Pascual-Leone A. Screening questionnaire before TMS: an update. Clin. Neurophysiol. 2011;122(8):1686. doi: 10.1016/j.clinph.2010.12.037. [DOI] [PubMed] [Google Scholar]
- Sasaki N., Yamatoku M., Tsuchida T., Sato H., Yamaguchi K. Effect of repetitive transcranial magnetic stimulation on long coronavirus disease 2019 with fatigue and cognitive dysfunction. Prog Rehabil Med. 2023;8 doi: 10.2490/prm.20230004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan K.H., Sheehan D.V. Assessing treatment effects in clinical trials with the discan metric of the Sheehan Disability Scale. Int. Clin. Psychopharmacol. 2008;23(2):70–83. doi: 10.1097/YIC.0b013e3282f2b4d6. [DOI] [PubMed] [Google Scholar]
- Shinjo S.K., Brunoni A.R., Okano A.H., Tanaka C., Baptista A.F. Transcranial direct current stimulation relieves the severe anxiety of a patient with COVID-19. Brain Stimul. 2020;13(5):1352–1353. doi: 10.1016/j.brs.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer R.L., Kroenke K., Williams J.B., Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch. Intern. Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- Tenforde M.W., Kim S.S., Lindsell C.J., Rose E.B., Shapiro N.I., Files D.C., et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network—United States, March–June 2020. Morb. Mortal. Wkly. Rep. 2020;69(30):993. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaweethai T., Jolley S.E., Karlson E.W., Levitan E.B., Levy B., McComsey G.A.…Zisis S. Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA. 2023 doi: 10.1001/jama.2023.8823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Whoqol Group The world health organization quality of life assessment (WHOQOL): development and general psychometric properties. Soc. Sci. Med. 1998;46(12):1569–1585. doi: 10.1016/s0277-9536(98)00009-4. [DOI] [PubMed] [Google Scholar]
- Twomey R., DeMars J., Franklin K., Culos-Reed S.N., Weatherald J., Wrightson J.G. Chronic fatigue and postexertional malaise in people living with long COVID: an observational study. Phys. Ther. 2022;102(4) doi: 10.1093/ptj/pzac005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiengo L., Maia A., Cotovio G., Gordon P.C., Brunoni A.R., Forlenza O.V., Oliveira-Maia A.J. Repetitive transcranial magnetic stimulation for major depressive disorder in older adults: systematic review and meta-analysis. J. Gerontol.: Series A. 2022;77(4):851–860. doi: 10.1093/gerona/glab235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.