Abstract
Aim
Spectral Flux (SF), which is based on common algorithms in the audio processing field, was applied to quantitatively analyze ECG signals to optimize the timing of defibrillation. With the aim of proving the performance in optimizing the timing of defibrillation, SF was compared with Amplitude Spectrum Area (AMSA) in a porcine model of ventricular fibrillation (VF) in a retrospective analysis experiment.
Methods
A total of 56 male domestic pigs, weighing 40 ± 5 kg, were induced to undergo VF. Animals were then left untreated for 10 min, and after 6 min of cardiopulmonary resuscitation (CPR) defibrillation was performed. The respective SF and AMSA values were calculated every minute during VF and CPR. Comparisons were made through receiver operating characteristic (ROC) curves, one-way analyses of variance (one-way ANOVA), and scatterplots for the successful initial defibrillation sample (positive samples, Group R) and the failed initial defibrillation sample (negative samples, Group N) to illustrate the performance in optimizing the timing of defibrillation for the AMSA and SF methods.
Result
Values of SF and AMSA gradually decreased during the 10 min VF period and increased in during the 6 min CPR period. The scatterplots showed that both metrics had the ability to distinguish positive and negative samples (p < .001). Meanwhile, ROC curves showed that SF (area under the curve, AUC = 0.798, p < .001) had the same ability as AMSA (AUC = 0.737, p < .001) to predict the successful defibrillation (Z = 1.35, p = 0.177). Moreover, when comparing the values for AMSA and SF between the successful initial defibrillation samples (Group R) and the failed initial defibrillation samples (Group N), the results showed that the values of both AMSA and SF in Group R were significantly higher than those in Group N (p < .001).
Conclusion
In the present study, SF method had the same ability as AMSA to predict successful defibrillation with significantly higher values in cases of successful defibrillation than the instances in which defibrillation failed. Additionally, SF method might be more stable than AMSA for filtering out the higher frequency interference signals due to the narrower frequency range and had higher specificity and predictive accuracy than AMSA. So SF method had high clinical potential to optimize the timing of defibrillation. Nevertheless, further animal and clinical studies are still needed to confirm the effectiveness and practicality of SF as a predictive module for defibrillators in clinical practice.
Keywords: Spectral Flux, Amplitude Spectrum Area, ECG signals, Defibrillation
Introduction
Out-of-hospital cardiac arrests (OHCA), of which 19.5% are caused by ventricular fibrillation (VF), are a leading cause of death in the United States.1, 2 According to the guidance of the American Heart Association (AHA), electrical defibrillation is the best treatment after attack of VF.3, 4 However, the failure of defibrillation might result in post-resuscitation myocardial dysfunction and failure of resuscitation,5, 6, 7 therefore, optimizing defibrillation timing is very significant to improve the performance of electrical shocks.
Previous investigations used coronary perfusion pressure (CPP), VF wavelet amplitude and median frequency to optimize the timing of the electrical shock.5 However, CPP is difficult to apply in out-of-hospital settings due to the invasive measurements.5, 6 Heitor et al. had proposed use of the Amplitude Spectrum Area (AMSA), which was defined as the sum of the products of each frequency and its corresponding amplitude in the frequency domain.6 Besides that, there are various other methods based on the time domain or frequency domain.4, 5, 8 Nevertheless, due to the wide frequency range, some other interference factors like electromyogram will be involved in their calculation which led to low accuracy, no analysis methods had been widely used in clinical practice up until now.8, 9
Recently, Some researchers found that either improved methods and algorithms, or combined methods that were used to analyze the ECG signal during VF, could increase the prediction accuracy, sensitivity and specificity, such as Spectral Centroid (SC), Spectral Energy (SE) and Optimal Amplitude Spectrum Area (Opt-AMSA).10, 11, 12, 13, 14, 15 Spectral Flux (SF) is a new, more stable algorithm from the audio processing field that uses the Euclidean distance between two spectra to measure the spectral shape variation.12 In this algorithm SF measures the rate of change of spectral content of the ECG signal.16 Furthermore, as the special feature of SF, the captured frequency range of 10–30 Hz (AMSA 4–48 Hz) will effectively filter out the higher frequency interference signals, such as electromyogram and so on.
In the animal and clinical studies, It has been demonstrated that higher AMSA values are associated with higher shock success and ROSC and resulted in less post-resuscitation myocardial dysfunction and better survival with epinephrine administration. AMSA was proposed as a tool to guide defibrillation in adults, because AMSA may predict if defibrillation could terminate VF with concurrent ROSC.17, 18, 19 Therefore, in the present study, we sought to retrospectively analyze the extent of changes in ECG signals as quantified by SF, compared with AMSA, in order to propose a reliable threshold to predict the timing of defibrillation.
Methods
In this retrospective study, a total of 56 male domestic pigs weighing 40 ± 5 kg were included. All the animals were from the previous mature VF induced cardiac arrest porcine model protocols which were approved by the Institutional Animal Care and Use Committee of Tang Wanchun laboratories of emergency and critical care medicine, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China20, 21 and received humane care in compliance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of the People's Republic of China. Animals were maintained on laboratory chow and housed in a specific pathogen-free room at a constant temperature (20–22 °C) with 10 h of light and 14 h of dark exposure. During the experiment, all animals were given sedation and analgesic treatment to alleviate pain. All animal experiments were performed in accordance with the ARRIVE guidelines.
Animal preparation
The animals were fasted overnight, while remaining free to drink water. Ketamine (20 mg/kg) was first injected into the animals by intramuscular injection for anesthesia. This was followed by injection of sodium pentobarbital (30 mg/kg) into the cardiovascular system. We injected the animals with an added dose of sodium pentobarbital (8 mg/kg) if necessary, or at intervals of about 1 hour, to retain anesthesia in case animals awakened or showed restlessness. With the assistance of a VELA ventilator (CareFusion California, USA), we provided a tidal volume of 10 mL/kg, a peak flow below 40 L/min and 0.21 FiO₂ to animals by a cuffed endotracheal tube which was introduced into the trachea. A BeneView T5 patient monitor (Mindray, Shenzhen, China) was used to measure the end-tidal carbon dioxide pressure (ETCO2) and the ETCO2 was maintained between 35 and 45 mmHg. With the assistance of a cooling/warm blanket (HGT-200II, Hokai, Zhuhai, China), the animals’ body temperatures were retained at 37.5 ± 0.5 °C during the entire experiment.
For the measurement of aortic pressure (AP) and collection of blood samples, a 7F pentalumen thermodilution-tip catheter (Abbott Critical Care 41216; North Chicago, IL) was advanced from the right femoral artery into the thoracic aorta. For the measurements of right atrial pressure (RAP), core blood temperature (Tc), and venous blood samples, another 7F pentalumen thermodilution-tip catheter was advanced from the right femoral vein and directed into the pulmonary artery. For inducing ventricular fibrillation (VF), a 5F pacing catheter (EP Technologies Inc; Mountain View, CA) was advanced from the right external jugular vein into the right ventricle. Hard gel defibrillation pads (stat-padz, Zoll Medical Corporation, Chelmsford, MA, USA) were applied with an anterior to lateral placement. An accelerometer-base handheld CPR device (CPR-D-padz, Zoll Medical Corporation, Chelmsford, MA, USA) was positioned on the surface of each pig’s sternum and underneath the rescuer’s hands during chest compressions. The positions of all catheters were confirmed by characteristic pressure morphology and with fluoroscopy, if necessary. The piston of the compressor was positioned in the midline at the level of the fifth interspace. To measure the ECG signal, three adhesive electrodes were applied to the shaved skin of the right upper and left upper-and-lower limbs.
Experimental procedures
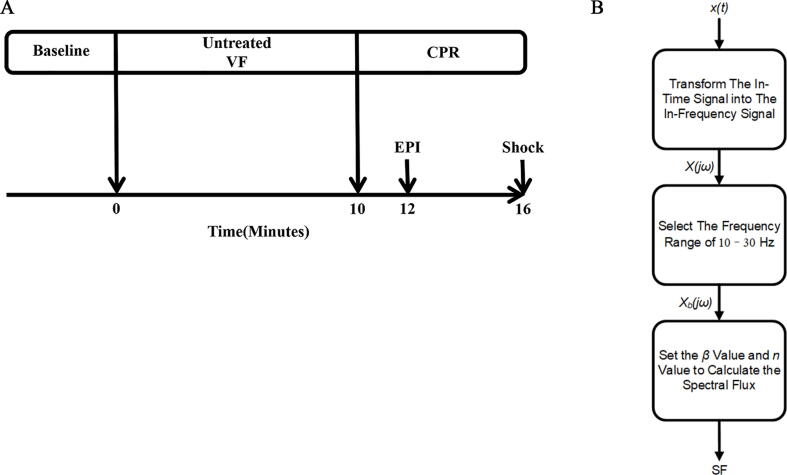
Before using 2 mA alternating current to induce VF through the pacing catheter, we measured the baseline (BL) signal for fifteen minutes in the right ventricular endocardium. After inducing the VF, mechanical ventilation was discontinued. Before the resuscitation procedure commenced, in order to avoid injury to the heart, the pacing catheter was removed. After 10 consecutive minutes of untreated VF, the two-person CPR for basic life support that was recommended by the 2015 AHA guidelines were initiated by two researchers.22 Chest compression was maintained for 6 minutes. After two minutes of CPR, the animals were injected with epinephrine at a dose of 20 µg/kg. In order to terminate the VF, a single 120 J biphasic shock (M-Series, Zoll, Chelmsford, USA) was applied (Fig. 1A).
Fig. 1.
(A) Experimental timeline. Baseline, baseline measurements; VF, ventricular fibrillation; CPR, cardiopulmonary resuscitation; EPI, administration of epinephrine; Shock, defibrillation. (B) A flowchart of the procedure for calculating SF.
The animals were regarded as exhibiting return of spontaneous circulation (ROSC) if they had a mean aortic pressure of over than 50 mmHg that persisted for an interval of 5 min or more. If the animals achieved ROSC, mechanical ventilation was initiated with 100% oxygen and continued for thirty minutes. For the following thirty minutes the oxygen was reduced to 50%; it was further reduced to 21% thereafter. All of the animals were monitored for the first 2 hours.
General measurements
A data acquisition system based on Windaq hardware/software (Dataq, Akron, USA) was used to continuously record the hemodynamic data. The CPP was computed by a digital system through the differences between diastolic arterial pressure and the contemporaneous right atrium pressure.
SF and AMSA methods
We retrospectively analyzed the ECG signals and the success rate of the first defibrillation in 56 animals. The MATLAB 2015a Signal Processing Toolbox was used to carry out the analyses. The one-second waveforms from ECG lead II before the initial defibrillation were analyzed for each sample of data, and the results of the defibrillation were assessed. A 4–48 Hz band-pass filter was used to remove low-frequency noise caused by chest compression and high-frequency interference from the power-line interference and electromyography. Thereafter the one-second time segments were transformed from the time domain to the frequency domain with a Fast Fourier Transform (FFT).
AMSA and SF were calculated by the following equations.
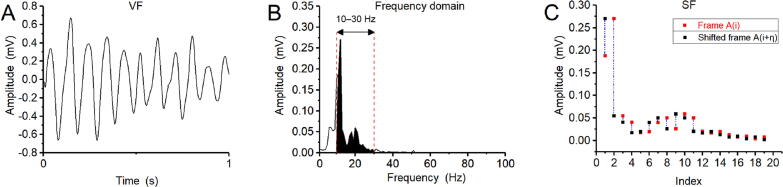
In these formulae is the amplitude corresponding to the frequency and denotes the length of the dataset; the values n and represent the time interval between two peaks. If , SF stands for Euclidean distance. Therefore in the present work was set to the typical value and was set to the typical value . AMSA used a frequency range of 4–48 Hz for calculation while SF used a frequency range of 10–30 Hz. Figs. 1B and 2 showed the process of calculating SF.
Fig. 2.
The calculation process for the SF method. (A) A one-second VF signal in the time domain was transformed to the frequency domain. (B) The amplitude of the calculated frequency range (10–30 Hz) for SF was selected, and the obtained amplitude data were divided into two frames according to requirements. (C) A diagram of SF calculation.
Statistical analyses
One-way ANOVA and scatterplots were used to analyze the differences between the successful initial defibrillation sample (Group R) and the failed initial defibrillation sample (Group N) for the AMSA and SF methods. The values were presented as “mean ± standard deviation” and 95% confidence interval (CI). We plotted the receiver operating characteristic (ROC) curves of both methods for the result of initial defibrillation and compared their area under the curve (AUC) values with a Z-test. In the Confusion Matrix (Table 2), TP represents the number of samples whose true value is positive and the model classifies them as positive (True-Positive). FP represents the number of samples with negative true values and positive model classification (False-Positive). TN represents the number of samples whose true value is negative and the model classifies them as negative (True-Negative). FN represents the number of samples where the true value is positive and the model is classified as negative (False-Negative). True Positive Rate (TPR) represents sensitivity, TPR = TP / TP + FN. True Negative Rate (TNR) represents specificity, TNR = TN / TN + FP. Accuracy = TP + TN / TP + TN + FP + FN. For all statistical analyses, a value of p < .05 was considered to be statistically significant.
Table 2.
Confusion matrix.
| Confusion Matrix | True Value |
||
|---|---|---|---|
| Positive | Negative | ||
| Prediction | Positive | TP (True-Positive) | FP (False-Positive) |
| Negative | FN (False-Negative) | TN (True-Negative) | |
Results
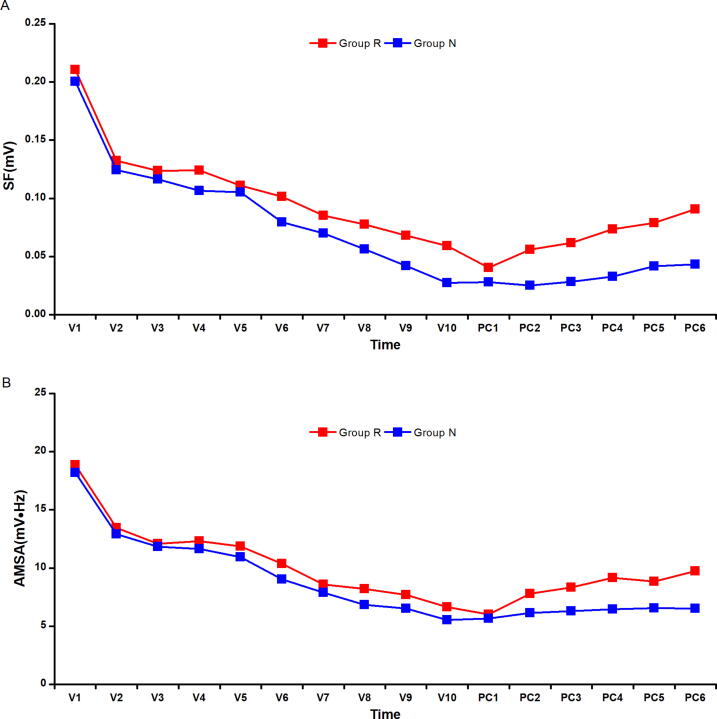
A total of 27 pigs were successful ROSC by the first defibrillation, while 29 pigs were unsuccessful ROSC. The success ratio for the initial defibrillation was therefore 48%. The values of SF and AMSA subsequently gradually decreased over time during the 10 min period of untreated VF, while they increased during the 6 min period of chest compression (Fig. 3).
Fig. 3.
The trend of SF and ASMA during 10 min untreated VF (V1–V10) and 6 min chest compression between Group R and Group N (PC1–PC6).
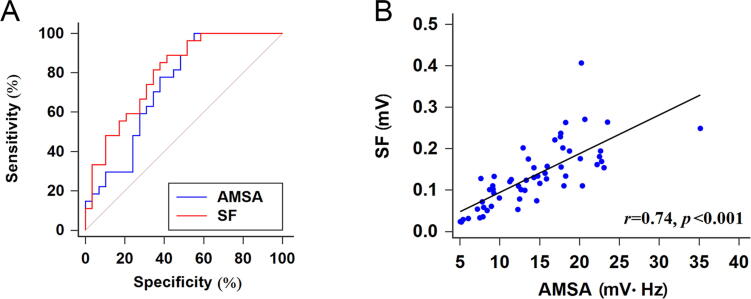
Furthermore, the ROC curves showed that the AUC for SF (0.798, p < .001, Fig. 4A) was higher than the AUC for AMSA (0.737, p < .001, Fig. 4A); meanwhile, the comparison of the diagnosed efficiency between them indicated no statistically significant difference (Z = 1.35, p = 0.177, Fig. 4A). The AMSA method with a cut-off value of 8.94 yielded a sensitivity of 100.00% and a specificity of 48.39%, while the positive predictive value (PPV) was 61.36%, the negative predictive value (NPV) was 100.00%, and the accuracy was 71.43% (Table 1). For the SF method with a cut-off value of 10.21 × 10-2 the sensitivity and specificity were 88.89% and 58.62%, while PPV, NPV and accuracy were respectively 66.67%, 85.00% and 73.21% (Table 1). Additionally, the scatterplot depicting the correlation between SF and ASMA (Fig. 4B) showed that SF was correlation with AMSA (p < .001 SF vs ASMA).
Fig. 4.
Analysis of correlation between SF and ASMA. (A) ROC curves for the SF and AMSA methods; p = 0.177 SF vs ASMA. (B) Scatterplot of SF against AMSA data; p < 0.001 SF vs ASMA.
Table 1.
Performance of predictions from AMSA and SF with different cut-off value selection criteria.
| Method | Criterion | Cut-off value | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|
| AMSA | Yuden Index | 8.94 (mV·Hz) | 100 | 48.39 | 61.36 | 100 | 71.43 |
| Maximum of Sensitivity Specificity | 12.65 (mV·Hz) | 77.78 | 62.07 | 71.05 | 75.00 | 69.64 | |
| Minimum of distance to corner | 12.65 (mV·Hz) | 77.78 | 62.07 | 71.05 | 75.00 | 69.64 | |
| SF | Yuden Index | 10.21 (×10−2 mV) | 88.89 | 58.62 | 66.67 | 85.00 | 73.21 |
| Maximum of Sensitivity Specificity | 10.21 (×10−2 mV) | 88.89 | 58.62 | 66.67 | 85.00 | 73.21 | |
| Minimum of distance to corner | 10.21 (×10−2 mV) | 88.89 | 58.62 | 66.67 | 85.00 | 73.21 | |
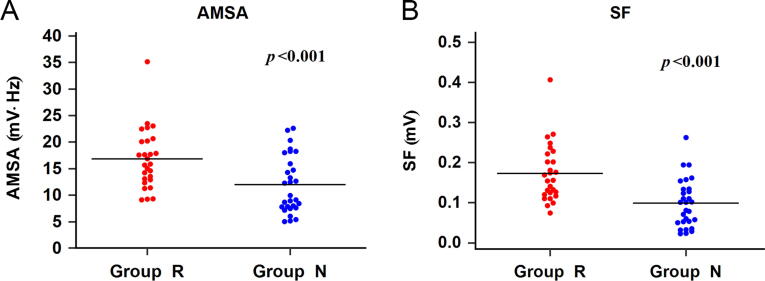
We also compared the values of AMSA and SF between Group R and Group N. The results showed that the mean AMSA values of Group R were 16.07 5.07 mV·Hz (CI: 14.06–18.07 mV·Hz) and the mean Group N values were 10.98 4.54 mV·Hz (CI: 9.25–12.71 mV·Hz). The AMSA values in Group R were higher than those in Group N (p < .001, Fig. 5A). Meanwhile, the mean values of SF were 17.14 7.10 × 10-2 mV (CI: 14.33–19.95 × 10-2 mV) for Group R and 9.6 5.87 × 10-2 mV (CI: 7.38–11.85 × 10-2 mV) for Group N. The mean values of SF in Group R were also higher than those in Group N (p < .001, Fig. 5B).
Fig. 5.
Scatterplots of values for the SF and AMSA methods in Group R and Group N. (A) Comparison of AMSA values between Group R and Group N (p < 0.001). (B) Comparison of SF values between Group R and Group N (p < 0.001).
Discussion
In this study, we found that the values of SF and AMSA maintained the same trend during the 10 min period of untreated VF and the 6 min period of chest compression. In addition, the analysis of the ROC curves indicated that the SF method had the same ability as AMSA to predict successful defibrillation, and the scatterplot demonstrated that the metrics of the SF method were correlated to the AMSA. Furthermore, we found that the values from AMSA and SF were significantly higher in cases of successful defibrillation than the instances in which defibrillation failed. Finally, the specificity and predictive accuracy of SF (58.62% and 73.21%) were higher than those of AMSA (48.39% and 71.43%).
A number of studies indicated that higher AMSA reflects higher myocardial energy and higher probability of successful defibrillation.6, 14 Recently, two animal studies found that AMSA driven-shock resulted in less post-resuscitation myocardial dysfunction and better survival, attributed to attaining ROSC with less electrical and adrenergic myocardial burdens.17, 23 Additionally, other clinical studies demonstrated that AMSA values are associated with the probability of low-energy shock success so that they could guide energy optimization in shockable cardiac arrest patients, thus increasing ROSC achievement in the out-of-hospital cardiac arrest.24, 25 In our study we retrospectively studied the AMSA method. The AMSA method is a weighted sum of the effective frequency components of the ECG signal, and a value that could indirectly reflect the degree of activity of the heart. The results of the initial defibrillation timing were basically consistent with previous studies, which validated the effectiveness of the AMSA method.
SF is an acoustic method that represents an important type of method for analyzing features in the frequency domain, and is able to measure changes from one spectrum to another spectrum.12, 16, 26 It can measure the spectral change between two successive frames and was computed as the squared difference between the normalized magnitudes of the spectra of the two successive short-term windows, so we are able to use SF to measure the rate of change in the spectral content of the ECG signal with time.16, 27 On the other hand, the calculated results from the SF method were in the range [0, M], where M denotes the maximum value of spectral amplitude of ECG signals. Lower values of SF indicated that the amplitude spectrum had hardly change or the amplitude was small, while the higher values demonstrated the larger change in the amplitude spectrum,12 that demonstrated that SF method was more stable. Moreover, the frequency range of SF (10–30 Hz) compared to AMSA (4–48 Hz), SF could avoid the interference by the higher frequency, especially the electromyogram from heart. This feature of SF made the prediction timing of defibrillation more precise. Herein SF was calculated in our study to analyze the ECG signals during the VF period in the porcine model of cardiac arrest.
According to the analysis of the data, via comparing our study’s results from the AMSA and SF methods, we found that the SF values were similar to the AMSA values which were both decreased during the 10 min VF period, and then increased during the 6 min chest compression period. Similarly, the scatterplots and ROC curves showed that the ability of the SF method to predict the timing of defibrillation was as good as that of the AMSA method, in particular, the AUC for SF was larger than the AUC for AMSA, and the two metrics were correlated.
When considering the balance between the sensitivity and specificity of the SF method, different choices of cut-off criterion would lead to different cut-off values, thus hence sensitivities and specificities.28 The confusion matrix corresponding to the optimal threshold point on the ROC curve will be the basis for calculating indicators such as sensitivity, specificity, and accuracy. So the point on the ROC curve corresponds to the optimal threshold point based on the Youden index. In our study, we selected the optimal cut-off value with an amplitude threshold by the Youden index method; another two we selected the cut-off value as the point on the ROC curve where the sensitivity was closest to specificity and the cut-off value as the point on the curve that was closest to the upper-left corner.29, 30, 31, 32, 33 We found that the cut-off value, sensitivity, specificity and accuracy by all three criteria were coincidentally equal (10.21 × 10-2, 88.89%, 58.62% and 73.21%). Performances of predictions including PPV and NPV with different cut-off value selection criteria are shown in Table 1.
In addition, it could be seen from Fig. 5 that SF had the same effect as AMSA in predicting defibrillation, so SF can be regarded as a method that is supplementary or alternative to AMSA in order to improve the outcome and survival following VF-related cardiac arrest.
Inevitably, some limitations were existed in this study. Firstly, in order to avoid the interference to the performance of the predictors caused by chest compression, all the waveforms of VF in our study did not include the chest compression period. Secondly, just only 1 s ECG records before defibrillation were used, the closer the ECG before defibrillation was, the more it truly reflected the energy of the myocardium which was important for successful defibrillation and resuscitation. Besides, current animal and clinical studies proved that 1 s ECG segments could achieved the possibility of an AHA compliant shock decision, as well as improved the success of defibrillation and the OHCA survival.14, 34, 35, 36 For the future work, we plan to validate the effect of SF under other data lengths. Finally, this paper was retrospective study and the predictive performance of the algorithms had not been validated in clinical applications, nor verified in human trials. We expect to collect more samples, to model the methodologies and extract the diagnostic features by deep learning methods, artificial intelligence and big database, in order to integrate SF method into real defibrillators and bring it into perspective human trials and clinical applications.
Conclusions
In the present study, SF method had the same ability as AMSA to predict successful defibrillation with significantly higher values in cases of successful defibrillation than the instances in which defibrillation failed. Additionally, SF method might be more stable than AMSA for filtering out the higher frequency interference signals due to the narrower frequency range and had higher specificity and predictive accuracy than AMSA. So SF method had high clinical potential to optimize the timing of defibrillation. Nevertheless, further animal and clinical studies are still needed to confirm the effectiveness and practicality of SF as a predictive module for defibrillators in clinical practice.
Funding
Funding for this project were provided by the Basic and Applied Basic Research Program of Guangzhou (No. 2023A03J0714) and the Basic and Applied Basic Research Program of Guangdong (No. 2022A1515140033).
Ethics approval
All of the experiments carried out on these animals were approved by the Institutional Animal Care and Use Committee of the Tang Wanchun Laboratories of Emergency & Critical Care Medicine at Sun Yat-sen Memorial Hospital, Sun Yat-sen University.
CRediT authorship contribution statement
Yuanshan Li: Writing – original draft, Software, Methodology, Formal analysis. Tianen Zhou: Writing – original draft, Methodology, Investigation, Formal analysis. Qiyu Yang: Software, Methodology, Investigation, Formal analysis, Conceptualization. Yujing Lu: Software, Methodology, Investigation, Formal analysis. Zhengfei Yang: Writing – review & editing, Supervision, Conceptualization. Jun Jiang: Writing – review & editing, Supervision, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Zhengfei Yang, Email: yangzhf@mail.sysu.edu.cn, yangzhengfei@vip.163.com.
Jun Jiang, Email: jiangjungd@163.com.
References
- 1.Schoene P., Coult J., Murphy L., et al. Course of quantitative ventricular fibrillation waveform measure and outcome following out-of-hospital cardiac arrest. Heart Rhythm. 2014;11:230–236. doi: 10.1016/j.hrthm.2013.10.049. [DOI] [PubMed] [Google Scholar]
- 2.Soar J., Donnino M.W., Maconochie I., et al. 2018 International consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations summary. Resuscitation. 2018;133:194–206. doi: 10.1016/j.resuscitation.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Mozaffarian D., Benjamin E.J., Go A.S., et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131 doi: 10.1161/CIR.0000000000000152. e29-322. [DOI] [PubMed] [Google Scholar]
- 4.Wu X., Bisera J., Tang W. Signal integral for optimizing the timing of defibrillation. Resuscitation. 2013;84:1704–1707. doi: 10.1016/j.resuscitation.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Li Y., Tang W. Optimizing the timing of defibrillation: the role of ventricular fibrillation waveform analysis during cardiopulmonary resuscitation. Crit Care Clin. 2012;28:199–210. doi: 10.1016/j.ccc.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Povoas H.P., Bisera J. Electrocardiographic waveform analysis for predicting the success of defibrillation. Crit Care Med. 2000;28:N210–N211. doi: 10.1097/00003246-200011001-00010. [DOI] [PubMed] [Google Scholar]
- 7.Sun S., Weng Y., Wu X., et al. Optimizing the duration of CPR prior to defibrillation improves the outcome of CPR in a rat model of prolonged cardiac arrest. Resuscitation. 2011;82:S3–S7. doi: 10.1016/S0300-9572(11)70144-7. [DOI] [PubMed] [Google Scholar]
- 8.Gong Y., Lu Y., Zhang L., Zhang H., Li Y. Predict defibrillation outcome using stepping increment of poincare plot for out-of-hospital ventricular fibrillation cardiac arrest. Biomed Res Int. 2015;2015 doi: 10.1155/2015/493472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu G., Brittain J.S., Holland P., et al. Removing ECG noise from surface EMG signals using adaptive filtering. Neurosci Lett. 2009;462:14–19. doi: 10.1016/j.neulet.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 10.He M., Gong Y., Li Y., et al. Combining multiple ECG features does not improve prediction of defibrillation outcome compared to single features in a large population of out-of-hospital cardiac arrests. Critical care (London, England) 2015;19:425. doi: 10.1186/s13054-015-1142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howe A., Escalona O.J., Di Maio R., et al. A support vector machine for predicting defibrillation outcomes from waveform metrics. Resuscitation. 2014;85:343–349. doi: 10.1016/j.resuscitation.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 12.An L.A. Wiley-IEEE Press; 2012. Introduction to Audio Content Analysis: Applications in Signal Processing and Music Informatics. [Google Scholar]
- 13.Shandilya S., Ward K., Kurz M., Najarian K. Non-linear dynamical signal characterization for prediction of defibrillation success through machine learning. BMC Med Informat Decision Making. 2012;12:116. doi: 10.1186/1472-6947-12-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie Z., Yang Q., Li M., et al. Amplitude screening improves performance of AMSA method for predicting success of defibrillation in swine model. Am J Emerg Med. 2019;37:1224–1229. doi: 10.1016/j.ajem.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Yang Q., Li M., Huang Z., et al. Validation of spectral energy for the quantitative analysis of ventricular fibrillation waveform to guide defibrillation in a porcine model of cardiac arrest and resuscitation. J Thoracic Dis. 2019;11:3853–3863. doi: 10.21037/jtd.2019.09.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chowdhury M.E.H., Alzoubi K., Khandakar A., et al. Wearable real-time heart attack detection and warning system to reduce road accidents. Sensors (Basel, Switzerland) 2019;19 doi: 10.3390/s19122780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aiello S.R., Mendelson J.B., Baetiong A., Radhakrishnan J., Gazmuri R.J. Targeted delivery of electrical shocks and epinephrine, guided by ventricular fibrillation amplitude spectral area, reduces electrical and adrenergic myocardial burden, improving survival in swine. J Am Heart Assoc. 2021;10:e023956. doi: 10.1161/JAHA.121.023956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gentile F.R., Wik L., Aramendi E., et al. aMplitude spectral area of ventricular fibrillation and amiOdarone Study in patients with out-of-hospital cArdIaC arrest. The MOSAIC study. Front Cardiovasc Med. 2023;10:1179815. doi: 10.3389/fcvm.2023.1179815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ristagno G., Li Y., Fumagalli F., Finzi A., Quan W. Amplitude spectrum area to guide resuscitation-a retrospective analysis during out-of-hospital cardiopulmonary resuscitation in 609 patients with ventricular fibrillation cardiac arrest. Resuscitation. 2013;84:1697–1703. doi: 10.1016/j.resuscitation.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 20.Li H., Yang Z., Liu Y., et al. Is esophageal temperature better to estimate brain temperature during target temperature management in a porcine model of cardiopulmonary resuscitation? BioMed research international. 2017;2017:1279307. doi: 10.1155/2017/1279307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y., Wang P., Wen C., et al. Endovascular hypothermia improves post-resuscitation myocardial dysfunction by increasing mitochondrial biogenesis in a pig model of cardiac arrest. Cryobiology. 2019;89:6–13. doi: 10.1016/j.cryobiol.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Travers A.H., Perkins G.D., Berg R.A., et al. Part 3: Adult basic life support and automated external defibrillation: 2015 International consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation. 2015;132:S51–S83. doi: 10.1161/CIR.0000000000000272. [DOI] [PubMed] [Google Scholar]
- 23.Aiello S., Perez M., Cogan C., et al. Real-time ventricular fibrillation amplitude-spectral area analysis to guide timing of shock delivery improves defibrillation efficacy during cardiopulmonary resuscitation in swine. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.006749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frigerio L., Baldi E., Aramendi E., et al. End-tidal carbon dioxide (ETCO(2)) and ventricular fibrillation amplitude spectral area (AMSA) for shock outcome prediction in out-of-hospital cardiac arrest. Are they two sides of the same coin? Resuscitation. 2021;160:142–149. doi: 10.1016/j.resuscitation.2020.10.032. [DOI] [PubMed] [Google Scholar]
- 25.Gentile F.R., Wik L., Isasi I., et al. Amplitude spectral area of ventricular fibrillation and defibrillation success at low energy in out-of-hospital cardiac arrest. Internal Emerg Med. 2023;18:2397–2405. doi: 10.1007/s11739-023-03386-6. [DOI] [PubMed] [Google Scholar]
- 26.Le P.N., Ambikairajah E., Epps J., Sethu V., Choi E.H.C. Investigation of spectral centroid features for cognitive load classification. Speech Commun. 2011;53:540–551. [Google Scholar]
- 27.Giannakopoulos T, Pikrakis A. Introduction to audio analysis. 2014.
- 28.Habibzadeh F., Habibzadeh P., Yadollahie M. On determining the most appropriate test cut-off value: the case of tests with continuous results. Biochem Med. 2016;26:297–307. doi: 10.11613/BM.2016.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doi S.A.R. Springer; Berlin Heidelberg: 2013. Using and Interpreting Diagnostic Tests with Quantitative Results. [Google Scholar]
- 30.McNeil B.J., Keller E., Adelstein S.J. Primer on certain elements of medical decision making. New England J Med. 1975;293:211–215. doi: 10.1056/NEJM197507312930501. [DOI] [PubMed] [Google Scholar]
- 31.Metz C.E. Basic principles of ROC analysis. Sem Nucl Med. 1978;8:283–298. doi: 10.1016/s0001-2998(78)80014-2. [DOI] [PubMed] [Google Scholar]
- 32.Metz C.E., Goodenough D.J., Rossmann K. Evaluation of receiver operating characteristic curve data in terms of information theory, with applications in radiography. Radiology. 1973;109:297–303. doi: 10.1148/109.2.297. [DOI] [PubMed] [Google Scholar]
- 33.Youden W.J. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 34.Endoh H., Hida S., Oohashi S., et al. Prompt prediction of successful defibrillation from 1-s ventricular fibrillation waveform in patients with out-of-hospital sudden cardiac arrest. J Anesthes. 2011;25:34–41. doi: 10.1007/s00540-010-1043-x. [DOI] [PubMed] [Google Scholar]
- 35.Jaureguibeitia X., Zubia G., Irusta U., Aramendi E., Corcuera C. Shock decision algorithms for automated external defibrillators based on convolutional networks. IEEE Access. 2020:1. [Google Scholar]
- 36.Yang Z., Yang Z., Lu W., et al. A probabilistic neural network as the predictive classifier of out-of-hospital defibrillation outcomes. Resuscitation. 2005;64:31–36. doi: 10.1016/j.resuscitation.2004.07.002. [DOI] [PubMed] [Google Scholar]