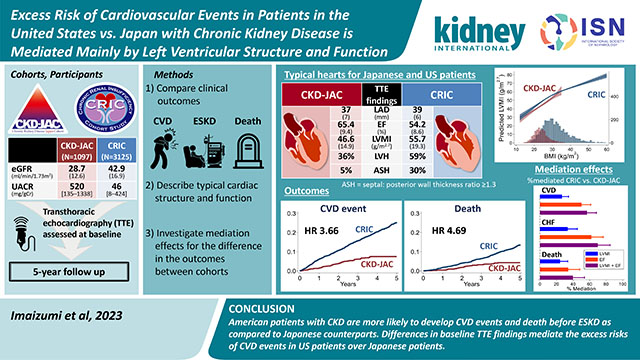
Abstract
While patients receiving dialysis therapy in the United States are more likely to develop cardiovascular disease (CVD) than those in Japan, direct comparisons of patients with predialysis chronic kidney disease (CKD) are rare. To study this, we compared various outcomes in patients with predialysis CKD using data from the Chronic Renal Insufficiency Cohort (CRIC) and CKD Japan Cohort (CKD-JAC) studies and determine the mediators of any differences. Candidate mediators included left ventricular (LV) indices assessed by echocardiography. Among 3125 CRIC and 1097 CKD-JAC participants, the mean LV mass index (LVMI) and ejection fraction (EF) were 55.7 and 46.6 g/m2.7 and 54% and 65%, respectively (P<0.001, both). The difference in body mass index (32 and 24 kg/m2, respectively) largely accounted for the differences in LVMI and C-reactive protein (CRP) across cohorts. Low EF and high LVMI were significantly associated with subsequent CVD in both cohorts (P interaction >0.05). During a median follow-up of 5 years, CRIC participants were at higher risk for CVD (adjusted hazard ratio [95% confidence interval]: 3.66 [2.74–4.89]) and death (4.69 [3.05–7.19]). A threefold higher CRP concentration and higher phosphate levels in the US cohort were moderately strong mediators of the differences in CVD. However, echocardiographic parameters were stronger mediators than these laboratory measures. LVMI, EF, and their combination mediated the observed difference in CVD (27%, 50%, and 57%, respectively) and congestive heart failure (33%, 62%, and 70%, respectively). Thus, higher LV mass and lower EF, even in the normal range, were found to be predictive of CVD in CKD.
Keywords: Chronic kidney disease; Cardiovascular disease; Left ventricular hypertrophy, Mediation analysis
Graphical Abstract:
Lay Summary
Cardiovascular disease is very common in patients with chronic kidney disease (CKD). Although US hemodialysis patients are more likely to develop heart disease compared with Japanese patients, no direct comparison exists for non-dialysis-dependent CKD. Using patient data from the US and Japan, we found that US patients with CKD had higher rates of heart failure and heart attack than Japanese patients. We then aimed to clarify how this happened. Our results revealed that the enlarged hearts of US patients and their weak contractility play important roles. These features explain most of the differences in cardiovascular outcomes between the US and Japan. Checking the heart with echocardiography may help identify high-risk patients. In addition, obesity and inflammation, which were related to each other, were associated with the enlargement of the heart. Therefore, countermeasures against obesity can protect patients with CKD from heart disease.
Introduction
Cardiovascular disease (CVD) is an important outcome for patients with chronic kidney disease (CKD).1–6 Studies have shown that dialysis patients in the United States (US) are more likely to die or to develop CVD events than their Japanese counterparts.7,8 However, little is known about patients with predialysis CKD due to a dearth of direct comparisons. Reports on the incidence of CVD from East Asia,9–11 the US, and European countries12 suggest that the incidence of CVD is lower in East Asian countries than in the US and European countries. However, patient characteristics such as baseline kidney function, urinary protein, and history of CVD or diabetes mellitus (DM) varied among studies. Thus, whether there is a difference in the incidence of major clinical events between Japanese and American patients with CKD, and if so, what factors contribute to this difference, remains to be elucidated. To address this question, we need to harmonize patient-level data from each country rather than to compare aggregated data.
Among the various types of CVD, a growing number of patients develop congestive heart failure (CHF) as kidney function declines.13 Left ventricular (LV) structure and function are important clinical measures that are predictive of future outcomes. Several observational studies have shown that LV mass index (LVMI) or LV hypertrophy (LVH) is associated with CVD events in patients with CKD.14–16 Similarly, reduced ejection fraction (EF), even in the absence of clinical heart failure, was also shown to be associated with CVD and all-cause mortality in patients with CKD.17 Therefore, we highlighted the LV indices as proxies of subclinical or preclinical disease that could mediate subsequent cardiovascular events and explain the differences in outcomes between the US and Japan.
We aimed to investigate the distributions of LVMI and EF in individuals with CKD residing in Japan and the US, the differences in the association of these LV measures with subsequent major clinical events, and how much these measures can account for the differences between these countries using individual data from well-established CKD cohorts: the Chronic Renal Insufficiency Cohort (CRIC) study and Chronic Kidney Disease Japan Cohort (CKD-JAC) study.
Methods
Participants
The CRIC study is an ongoing multicenter, prospective observational cohort study of participants with CKD recruited from seven clinical centers in the US. The details of the cohort are described elsewhere.18,19 Of the 3939 participants from the Phase I CRIC study recruited between 2003 and 2008, we excluded those who did not undergo echocardiography (n = 434), those whose information was not sufficient for further analyses (n = 322), and those who developed CVD events before undergoing echocardiography (n = 58). Thus, we used data from 3125 participants with baseline echocardiography data.
The CKD-JAC study is a multicenter, prospective observational cohort study across Japan. The participants were enrolled between April 2007 and December 2008.20 Data from one site (#2) with 153 participants were excluded because of the limited number of participants for whom echocardiographic results were available. Participants underwent echocardiography six months before and three months after study enrollment according to the protocol, and 1171 participants underwent baseline echocardiography. Baseline characteristics were compared between those with and without echocardiography. We excluded 74 of 1171 participants due to a lack of relevant information. Ultimately, we included data from 1097 participants from the CKD-JAC study and 3125 participants from the CRIC study with baseline echocardiography data (Figure 1). Only data obtained within the first five years in both cohorts were used in this study.
Figure 1.
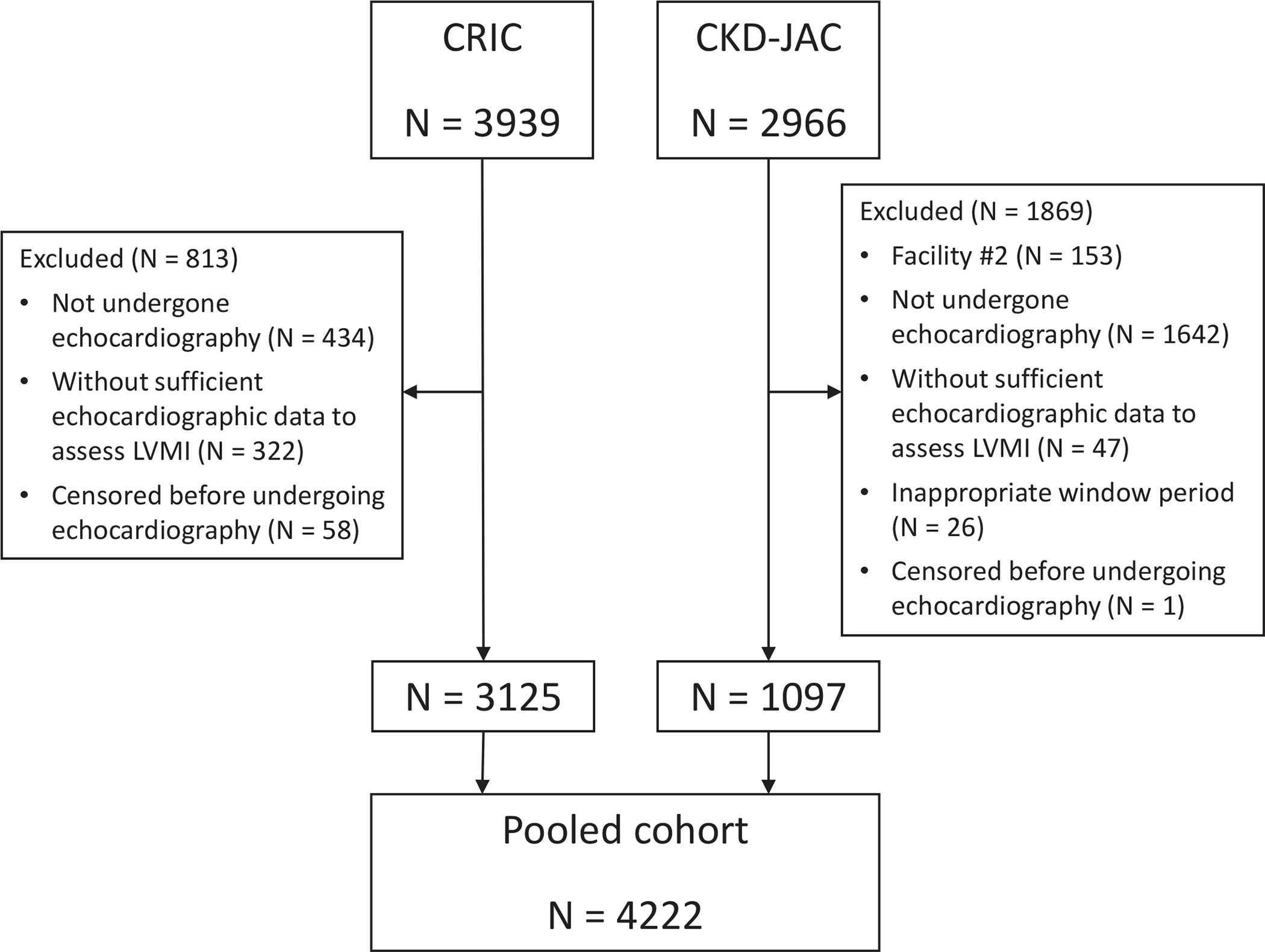
Flow diagram of participant selection. CRIC, chronic renal insufficiency cohort; CKD-JAC, chronic kidney disease Japan cohort; LVMI, left ventricular mass index.
The protocol was approved by the ethics committee of each participating institution, and the study was conducted following the Declaration of Helsinki. All patients provided written informed consent. The combined secondary analysis was approved by the institutional review board at the University of Pennsylvania.
Measurement of left ventricular indices
In the CRIC study, transthoracic echocardiography was performed 1 year after enrollment using protocols established by the American Society of Echocardiography. Images were transferred to the core echocardiography laboratory (University of Pennsylvania), where they were read by a registered diagnostic cardiac sonographer. Readers were blinded to the baseline estimated glomerular filtration rate (eGFR) and other clinical characteristics of the participants.
In the CKD-JAC study, at each clinical site, participants underwent echocardiography, and the images were assessed by trained technicians in accordance with the guidelines of echocardiographic measurement procedures.21 The details are provided in the Supplementary Methods.
Outcomes
We examined the following outcomes: CVD events, atherosclerotic CVD (ASCVD), CHF, death, and kidney failure. We defined a CVD event as a composite of the following events: any fatal cardiovascular event, myocardial infarction, stroke, interventions for peripheral artery disease (PAD), and hospitalization for CHF. We also defined ASCVD as a composite of myocardial infarction, ischemic stroke, and PAD. CHF and kidney failure were defined as CHF requiring hospitalization and the initiation of kidney replacement therapy, respectively. Follow-up was censored at kidney failure. All CVD events were adjudicated by an independent committee in each cohort. Details of each outcome event are described in the Supplementary Methods.
Covariates
In both studies, clinical data, including medical history and anthropometric measurements, were collected at enrollment, and laboratory parameters were measured centrally from blood and urine samples using standardized assays, as reported previously (Supplementary methods).19,22 The eGFR in the CRIC study was calculated using an equation derived from CRIC study participants based on serum creatinine and cystatin C levels, age, sex, and race,23 while the eGFR in the CKD-JAC study was calculated using the Japanese formula derived in Japanese patients with CKD: eGFR (ml/min/1.73 m2) = 194 × age−0.287 × [serum creatinine (mg/mL)]−1.094 × [0.739 if female].24 Other covariates are defined in the Supplementary Methods.
Causal mediation and bias analysis
We implemented mediation analysis to estimate the causal role of laboratory and echocardiographic parameters in the differences between the CRIC and CKD-JAC studies with respect to cardiovascular outcomes. We used directed acyclic graphs to guide the assessment of the strength of mediators using a counterfactual approach (Supplementary Figure S1).25 We estimated the proportion of mediated effects on the association between region (US vs. Japan) and outcomes. Potential mediators included echocardiographic parameters (LVMI and LVEF) and laboratory parameters (phosphate, iFGF23, and CRP). Details are provided in the Supplementary Methods.
Statistical analyses
Descriptive data are expressed as the mean (standard deviation) and median [interquartile range (IQR)] for normally and nonnormally distributed continuous variables, respectively. Multiple comparisons were performed for LV indices, body mass index, SBP, and levels of serum phosphate and hemoglobin across levels of eGFR using Dunnett’s test.
In survival analyses, we employed the Kaplan–Meier method and log-rank test to compare CVD events, ASCVD, CHF, death, and kidney failure between the cohorts, as well as among four ethnic groups. We also drew adjusted event-free survival curves for patients with average demographic characteristics, adjusted for age, sex, baseline eGFR, urinary albumin-creatinine ratio (UACR), and DM using separate Cox models for cohort and ethnic group. Multivariable Cox proportional hazards models were employed to examine the association between LV indices (i.e., EF, LVMI, LVH, and LV geometry) and CVD events, adjusted for age, sex, baseline smoking status, eGFR, UACR, DM, a history of any CVD, and CRP (Model 1). To independently assess the effect of hypertrophic changes or reduced contractility in the LV on the outcomes, we also adjusted for EF when examining the association of LVMI, LVH, and LV geometry with the outcomes. When the exposure of interest was EF, we also adjusted for LVMI in each model. For further modeling, we added systolic blood pressure, obesity category, the number of classes of antihypertensive agents, and hemoglobin to Model 1 (Model 2). We then added corrected calcium, phosphate, total parathyroid hormone (PTH), intact fibroblast growth factor-23 (iFGF23), active vitamin D supplementation, and 25(OH) vitamin D to Model 2 (Model 3). The proportional hazard assumption was examined using Schoenfeld residuals. We also examined the association between region and outcomes in the subset of those with no prior CVD and those with eGFR 20–60 mL/min/1.73 m2.
We used multiple imputation to handle missing data in the multivariable analysis. The covariates included in our imputation models were baseline demographics, as shown in Table 1, and outcomes.26 We performed chained equations with 20 imputations and then combined the results across the 20 imputed datasets using Rubin’s formula.27
Table 1.
Baseline characteristics of the CRIC and CKD-JAC studies
| Total (N = 4222) | CKD-JAC (n = 1097) | CRIC (n = 3125) | |
|---|---|---|---|
|
| |||
| Age, years | 59 (11) | 61 (11) | 59 (11) |
| Sex (male) | 2421 (57) | 710 (65) | 1711 (55) |
| Race/ethnicity | |||
| Non-Hispanic White | 1330 (32) | 0 (0) | 1330 (43) |
| Non-Hispanic Black | 1280 (30) | 0 (0) | 1280 (41) |
| Hispanic | 392 (9) | 0 (0) | 392 (13) |
| Asian | 1185 (28) | 1097 (100) | 88 (3) |
| Other | 35 (1) | 0 (0) | 35 (1) |
| eGFR, mL/min/1.73 m2 | 39.2 (17.1) | 28.7 (12.6) | 42.9 (16.9) |
| UACR, mg/g | 109 (13–733) | 520 (135–1388) | 46 (8–424) |
| Diabetes | 1990 (47) | 461 (42) | 1529 (49) |
| Atrial fibrillation | 597 (14) | 28 (3) | 569 (18) |
| BMI (kg/m2) | 30 (8) | 24 (4) | 32 (8) |
| Smoking status (current smoker) | 554 (14) | 171 (17) | 383 (12) |
| Alcohol consumption (yes) | 816 (20) | 195 (20) | 621 (20) |
| Obesity category | |||
| Underweight | 96 (2) | 72 (7) | 24 (1) |
| Normal | 908 (22) | 442 (40) | 466 (15) |
| Overweight | 1203 (28) | 234 (21) | 969 (31) |
| Obese I | 1094 (26) | 277 (25) | 817 (26) |
| Obese II or higher | 921 (22) | 72 (7) | 849 (27) |
| Systolic BP (mmHg) | 129 (21) | 132 (18) | 127 (22) |
| Diastolic BP (mmHg) | 72 (13) | 76 (12) | 70 (13) |
| Medications | |||
| Number of antihypertensive agents | 3 (2–4) | 3 (2–4) | 3 (2–4) |
| ACEi/ARB | 3087 (73) | 913 (83) | 2174 (70) |
| β-blockers | 1769 (42) | 223 (20) | 1546 (50) |
| α-blockers | 610 (14) | 131 (12) | 479 (15) |
| Calcium channel blockers | 1915 (45) | 634 (58) | 1281 (41) |
| Diuretics | 2209 (52) | 378 (34) | 1831 (59) |
| Active vitamin D | 266 (6) | 83 (8) | 183 (6) |
| Phosphate binders | 251 (6) | 27 (2) | 224 (7) |
| Antiplatelets | 1771 (42) | 245 (22) | 1526 (49) |
| Statins | 2,305 (55) | 476 (43) | 1829 (59) |
| Any CVD history | 1367 (32) | 302 (28) | 1065 (34) |
| CAD history | 850 (20) | 159 (14) | 691 (22) |
| CHF history | 370 (9) | 63 (6) | 307 (10) |
| Stroke history | 449 (11) | 123 (11) | 326 (10) |
| PAD history | 257 (6) | 46 (4) | 211 (7) |
| Laboratory data | |||
| Alb, g/dL | 4.0 (0.5) | 4.0 (0.4) | 4.0 (0.5) |
| Hb, g/dL | 12.6 (1.8) | 12.2 (1.9) | 12.8 (1.8) |
| Total cholesterol, mg/dL | 185 (45) | 193 (45) | 183 (44) |
| HbA1c (NGSP), % | 6.4 (1.4) | 6.0 (1.0) | 6.5 (1.5) |
| CRP*, mg/L | 1.78 [0.78–4.72] | 0.82 [0.46–1.78] | 2.40 [1.01–5.77] |
| Corrected calcium, mg/dL | 9.4 (0.5) | 9.2 (0.5) | 9.5 (0.5) |
| Phosphate*, mg/dL | 3.7 (0.9) | 3.3 (0.6) | 3.8 (0.9) |
| 25(OH) vitamin D, ng/mL | 20.2 [11.8–31.7] | 14.1 [8.0–22.1] | 23.5 [14.0–34.7] |
| Total PTH*, pg/mL | 71 [45–12] | 110 [75–177] | 61 [39–97] |
| Intact FGF23†, pg/mL | 55 [41–83] | 58 [42–92] | 53 [40–73] |
| Echocardiographic findings | |||
| LVMI, g/m2.7 | 53.4 (18.7) | 46.6 (14.9) | 55.7 (19.3) |
| LVH | 2245 (53) | 395 (36) | 1850 (59) |
| Relative wall thickness | 0.5 (0.1) | 0.4 (0.1) | 0.6 (0.2) |
| Left ventricular geometry | |||
| Normal | 904 (21) | 473 (43) | 431 (14) |
| Concentric remodeling | 1068 (25) | 224 (21) | 844 (27) |
| Eccentric LVH | 472 (11) | 159 (15) | 313 (10) |
| Concentric LVH | 1773 (42) | 236 (22) | 1537 (49) |
| Septal-to-posterior wall thickness ratio | 1.1 (1.0–1.3) | 1.0 (1.0–1.1) | 1.2 (1.0–1.3) |
| Asymmetric septal hypertrophy | 985 (23) | 50 (5) | 935 (30) |
| Ejection fraction, % | 56.9 (10.0) | 65.4 (9.4) | 54.2 (8.6) |
| LAD, mm | 39 (7) | 37 (7) | 39 (6) |
Data are expressed as N (%) for categorical values and mean (S.D.) or median (interquartile range) for continuous values.
Converted into values equivalent to those obtained with CRIC assays
Values measured in 1955 individuals (1058 from CKD-JAC and 897 from CRIC).
eGFR, estimated glomerular filtration rate; UACR, urinary albumin-creatinine ratio; BMI, body mass index; BP, blood pressure; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CVD, cardiovascular disease; CAD, coronary artery disease; CHF, congestive heart failure; PAD, peripheral artery disease; NGSP, National Glycohemoglobin Standardization Program; PTH, parathyroid hormone; FGF, fibroblast growth factor; LVMI, left ventricular mass index; LVH, left ventricular hypertrophy; LAD, left atrial dimension.
Assuming nonlinear associations of LVMI and EF with subsequent CVD events, we used restricted cubic spline (RCS) analyses with three knots (at the 10th, 50th, and 90th percentiles of the distribution of the combined population of both cohorts) and performed multivariable Cox proportional hazards models, including an interaction term of cohort-by-LVMI or cohort-by-EF. The references were set at 47 g/m2.7 for LVMI and 60% for EF.
To examine the association of BMI with LVMI, EF, CRP, and asymmetric septal hypertrophy (ASH), we used RCS analyses with three knots and performed multivariable linear regression models to predict the levels of LVMI, EF, and CRP and multivariable modified Poisson models28 to examine the probability of ASH in the combined cohort.
All continuous variables with right-skewed distributions were logarithmically transformed (UACR, CRP, intact PTH, and FGF23). Two-sided p values less than 0.05 were considered indicative of statistical significance. Statistical analyses were performed using Stata/MP 17.0 (Stata Corp., College Station, TX, USA) and R 4.1.3 for causal mediation analysis.
Results
Baseline characteristics of the study subjects
The characteristics of the study participants are shown in Table 1. Overall, CRIC participants were younger and more likely to be female than CKD-JAC participants. Due to the different inclusion criteria, the mean eGFR levels in the CRIC study were higher than those in the CKD-JAC study (42.9 ± 16.9 and 28.7 ± 12.6 mL/min/1.73 m2 in the CRIC and CKD-JAC studies, respectively). CRIC participants were more likely to have DM and were more obese than CKD-JAC participants, as shown by the average BMI of 32 and 24 kg/m2, respectively. However, the UACR was lower in CRIC participants than in CKD-JAC participants (46 mg/g and 520 mg/g, respectively). Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers and calcium channel blockers were more commonly prescribed among participants in the CKD-JAC study, while diuretics and β-blockers were more commonly prescribed to participants in the CRIC study. The prevalence of any CVD, CAD, CHF, and PAD was higher in the CRIC study. In the CKD-JAC study, those who underwent echocardiography were older, had a higher UACR, had a higher prevalence of DM, and more often had a history of CAD (Supplementary Table S1).
Echocardiographic findings showed that CRIC participants were characterized by concentric LVH with ASH, reduced ventricular contraction, and mildly dilated left atrial dimension compared to CKD-JAC participants (Figure 2). Factors relevant to increased LV mass are shown across levels of eGFR in Supplementary Figure S2. No substantial differences in SBP or hemoglobin levels were observed between the CRIC and CKD-JAC participants, while CRIC participants had significantly higher BMI and serum phosphate levels than CKD-JAC participants.
Figure 2.
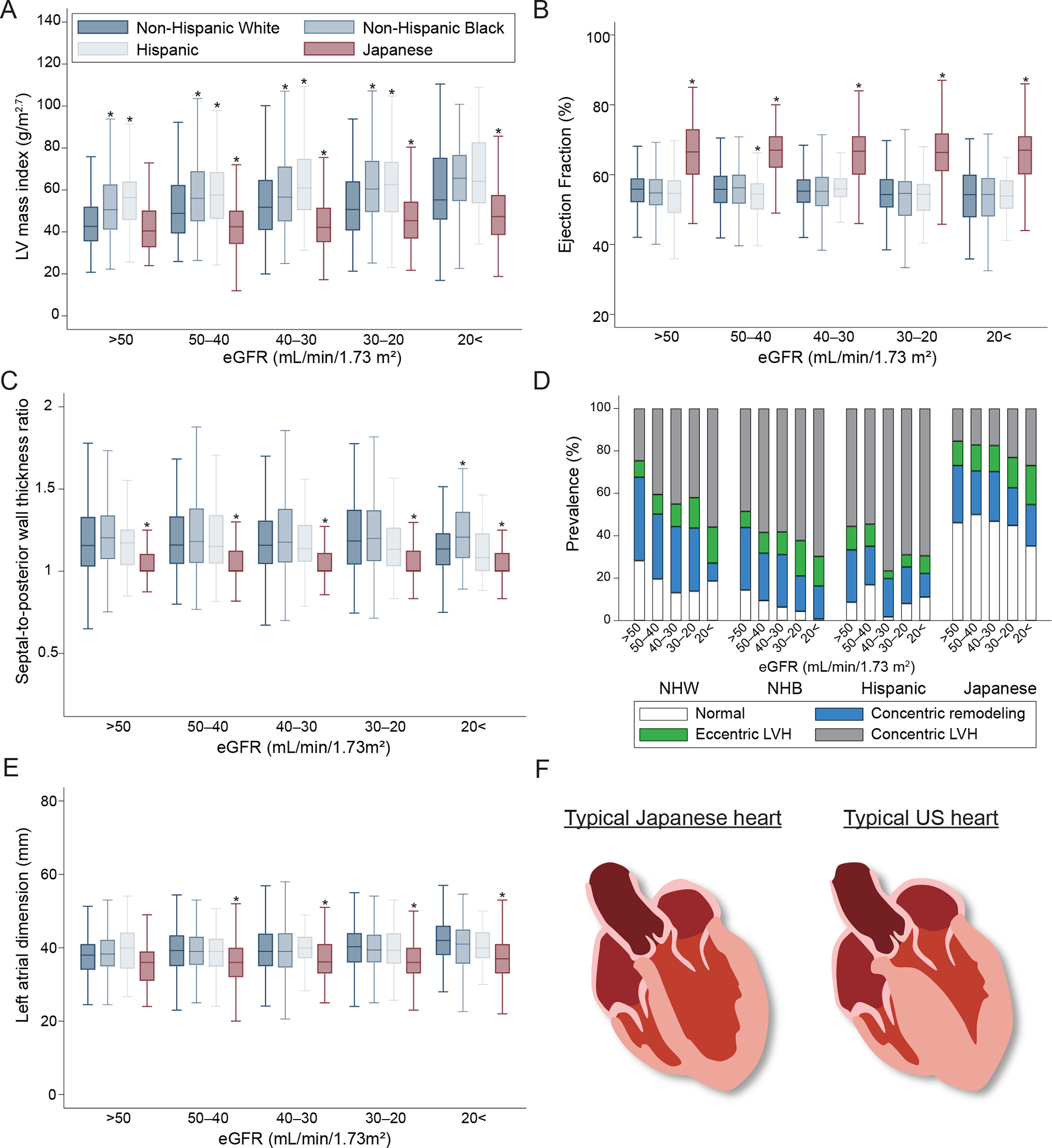
Comparison of echocardiographic measurements over eGFR levels across ethnic groups. A. Left ventricular mass index. LVM (g) = 0.8 × {1.04 × [([LVEDD + IVSd + PWd]3 - LVEDD3)]} + 0.6. LVMI (g/m2.7) = LVM/(height [m])2.7. B. Ejection fraction. {([end-diastolic volume] - [end-systolic volume])/[end-systolic volume]} × 100%. C. Septal-to-posterior wall thickness ratio. D. Left ventricular geometry. LVH: LVMI >50 g/m2.7 in males and >47 g/m2.7 in females. RWT: (IVSd + PWd)/LVEDD ≥0.45. (1) normal (normal LVMI and RWT), (2) concentric remodeling (normal LVMI and high RWT), (3) eccentric hypertrophy (increased LVMI and normal RWT), or (4) concentric hypertrophy (high LVMI and RWT). E. Left atrial dimension. F. Typical hearts in the CRIC and CKD-JAC studies. Comparisons between ethnic groups were performed with the Dunnett test using non-Hispanic white as the reference (*P <0.05). LV, left ventricular; LVMI, LV mass index; LVH, LV hypertrophy; RWT, relative wall thickness; IVSd, interventricular septum thickness at end-diastole; PWd, posterior wall thickness in diastolic phase; LVEDD, LV internal end-diastolic diameter; eGFR, estimated glomerular filtration rate; LV, left ventricular; NHW, non-Hispanic white; NHB, non-Hispanic black; LVH, left ventricular hypertrophy.
LV indices, inflammation, and obesity
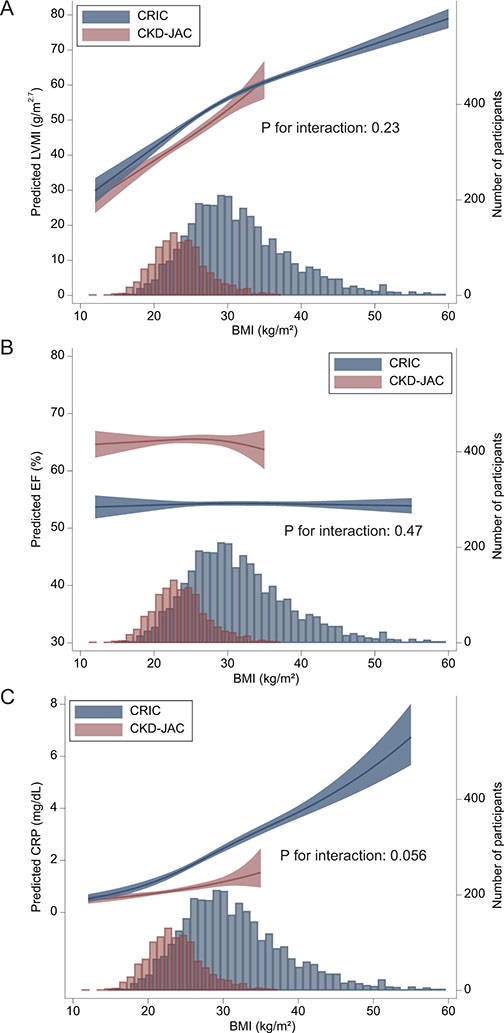
In both cohorts, a linear association between LVMI and BMI was observed by spline analysis, although obesity did not have any effect on EF (Figure 3). At a given level of BMI, the predicted value of LVMI was equivalent in both cohorts. Similarly, the probability of ASH increased as BMI increased in the combined cohort (Supplementary Figure S3). Factors associated with LVMI and EF were explored by conducting multiple linear regression models in the combined cohort (Supplementary Tables S2 and S3). Patients with a prior history of CAD and CHF had a higher LVMI by 2.63 (1.38–3.88) and 7.88 (6.15–9.61) and a lower EF by 3.48 (2.76–4.19) and 6.51 (5.53–7.49), respectively. Patients receiving diuretics also had a higher LVMI by 1.67 (0.65–2.70) and a lower EF by 0.77 (0.18–1.36). The difference in LVMI between the cohorts was substantially attenuated by adding the obesity category and antihypertensive medications. However, the difference in EF between the cohorts did not change by including potential confounders.
Figure 3.
Association of BMI with predicted LVMI, EF, and CRP. Association of BMI with LVMI (A), EF (B), and CRP (C), which were predicted using a multivariable linear regression model adjusted for age, sex, baseline smoking status, eGFR, ln(UACR), diabetes, history of CVD, atrial fibrillation, hemoglobin, and systolic blood pressure. The P values for the interaction between BMI and cohort were 0.23, 0.47, and 0.056.
There was also a linear association between CRP levels and BMI in both cohorts. At a given level of BMI, the predicted level of CRP was higher in CRIC participants than in CKD-JAC participants.
Differences in the outcomes between the CRIC and CKD-JAC studies
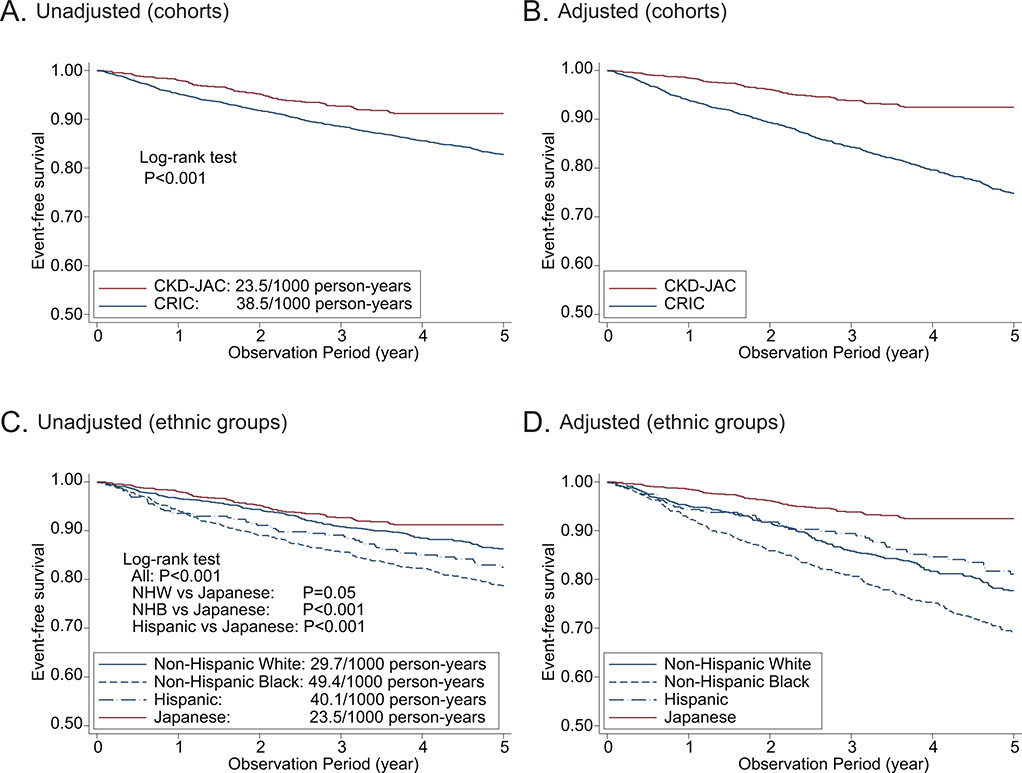
The median observation periods were 5.0 years [IQR, 4.0–5.0] in the CRIC study and 3.8 years [IQR, 2.3–4.0] in the CKD-JAC study. CKD-JAC participants had a significantly lower CVD risk than CRIC participants (Supplementary Table S4), and in a comparison by ethnicity, Japanese patients had the lowest CVD risk, followed by non-Hispanic white, Hispanic, and non-Hispanic black patients. Adjustment for important confounders did not change these results (Figure 4). The same was true for ASCVD, CHF, and death (Supplementary Figures S4 and S5). Regarding ASCVD, ischemic stroke was dominant in the CKD-JAC study (55.6% vs. 23.8%), whereas acute myocardial infarction (AMI) was dominant in the CRIC study (7.4% vs. 51.7%).
Figure 4.
Unadjusted and adjusted CVD event-free survival between the CRIC and CKD-JAC. A. Unadjusted CVD event-free survival between cohorts. B. Adjusted CVD event-free survival between cohorts. C. Unadjusted CVD event-free survival across ethnic groups. D. Adjusted CVD event-free survival across ethnic groups. CVD, cardiovascular disease; CKD-JAC, chronic kidney disease Japan cohort; CRIC, chronic renal insufficiency cohort; NHW, non-Hispanic white; NHB, non-Hispanic black.
CKD-JAC participants were more likely to develop kidney failure in the unadjusted Kaplan–Meier analysis; however, when adjusted for age, sex, eGFR, UACR, and DM, the incidence of kidney failure was lower among CKD-JAC participants than among CRIC participants.
Echocardiographic parameters and outcome events
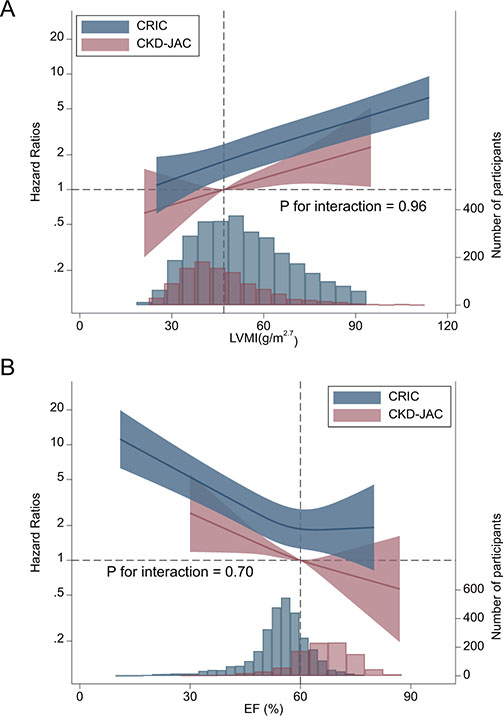
LVEF, LVMI, and LVH were significantly associated with CVD events in both cohorts, without a significant interaction (P interaction= 0.68, 0.72, and 0.89, respectively; Table 2). Spline analyses showed that the risk of CVD events increased almost linearly as LVMI increased or EF decreased, without a significant interaction between these indices and the study group, i.e., the CRIC study vs. the CKD-JAC study (P interaction = 0.96 and 0.70, respectively) (Figure 5).
Table 2.
Cox proportional hazards models for assessing the association of left ventricular indices with the incidence of CVD events
| Left ventricular indices | Events | Subjects | Incidence rate* | Model 1 HR (95% CI) | Model 2 HR (95% CI) | Model 3 HR (95% CI) |
|---|---|---|---|---|---|---|
|
| ||||||
| LVEF (per 10%) | ||||||
| All (CRIC+CKD-JAC) | 551 | 4222 | 35.3 | 0.72 (0.66–0.78) | 0.72 (0.67–0.79) | 0.72 (0.66–0.78) |
| CRIC | 472 | 3125 | 38.5 | 0.71 (0.65–0.77) | 0.71 (0.65–0.78) | 0.71 (0.65–0.78) |
| CKD-JAC | 79 | 1097 | 23.5 | 0.79 (0.65–0.96) | 0.79 (0.64–0.97) | 0.77 (0.62–0.96) |
| P for interaction | 0.51 | 0.56 | 0.68 | |||
|
| ||||||
| LVMI (continuous, per 10 g/m2.7) | ||||||
| All (CRIC+CKD-JAC) | 551 | 4222 | 35.3 | 1.19 (1.14–1.24) | 1.21 (1.15–1.26) | 1.19 (1.14–1.24) |
| CRIC | 472 | 3125 | 38.5 | 1.19 (1.14–1.24) | 1.21 (1.15–1.26) | 1.19 (1.14–1.25) |
| CKD-JAC | 79 | 1097 | 23.5 | 1.17 (1.02–1.34) | 1.22 (1.05–1.41) | 1.17 (1.00–1.37) |
| P for interaction | 0.72 | 0.86 | 0.72 | |||
|
| ||||||
| LVH | ||||||
| All (CRIC+CKD-JAC) | ||||||
| without LVH | 124 | 1976 | 15.9 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| with LVH | 427 | 2246 | 54.6 | 1.60 (1.29–2.00) | 1.64 (1.30–2.06) | 1.56 (1.24–1.97) |
| CRIC | ||||||
| without LVH | 90 | 1275 | 16.3 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| with LVH | 382 | 1850 | 56.8 | 1.60 (1.25–2.04) | 1.61 (1.25–2.09) | 1.55 (1.19–2.01) |
| CKD-JAC | ||||||
| without LVH | 34 | 701 | 14.9 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| with LVH | 45 | 396 | 41.4 | 1.72 (1.07–2.76) | 1.84 (1.10–3.08) | 1.69 (1.00–2.86) |
| P for interaction | 0.79 | 0.81 | 0.89 | |||
|
| ||||||
| Geometry | ||||||
| All (CRIC+CKD-JAC) | ||||||
| Normal | 46 | 790 | 15.6 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Concentric remodeling | 78 | 1186 | 16.1 | 0.91 (0.63–1.32) | 0.91 (0.63–1.32) | 0.87 (0.60–1.26) |
| Eccentric LVH | 79 | 379 | 63.7 | 1.44 (1.00–2.06) | 1.50 (1.04–2.17) | 1.39 (0.96–2.02) |
| Concentric LVH | 348 | 1867 | 52.9 | 1.54 (1.12–2.11) | 1.56 (1.13–2.17) | 1.45 (1.04–2.01) |
| CRIC | ||||||
| Normal | 25 | 340 | 17.0 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Concentric remodeling | 65 | 935 | 16.0 | 0.96 (0.62–1.51) | 0.97 (0.62–1.52) | 0.94 (0.60–1.48) |
| Eccentric LVH | 61 | 236 | 72.3 | 1.50 (0.96–2.34) | 1.56 (0.99–2.46) | 1.46 (0.92–2.30) |
| Concentric LVH | 321 | 1614 | 54.5 | 1.57 (1.06–2.33) | 1.59 (1.06–2.38) | 1.49 (1.00–2.24) |
| CKD-JAC | ||||||
| Normal | 21 | 450 | 14.2 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Concentric remodeling | 13 | 251 | 16.3 | 0.66 (0.31–1.40) | 0.69 (0.32–1.49) | 0.68 (0.31–1.46) |
| Eccentric LVH | 18 | 143 | 45.5 | 1.26 (0.64–2.48) | 1.37 (0.68–2.77) | 1.29 (0.63–2.63) |
| Concentric LVH | 27 | 253 | 39.1 | 1.64 (0.92–2.91) | 1.79 (0.96–3.35) | 1.60 (0.84–3.02) |
| P for interaction | 0.73 | 0.70 | 0.85 | |||
Multivariable models adjusted for Model 1: age, sex, baseline smoking status, eGFR, ln(UACR), diabetes mellitus, history of any CVD, CRP, CRIC (vs. CKD-JAC), and EF (or LVMI); Model 2: model 1 + systolic blood pressure, obesity category, number of classes of antihypertensive agents, and hemoglobin; Model 3: model 2 + corrected calcium, phosphate, ln(total PTH), ln(intact FGF23), active vitamin D supplementation, and ln(25(OH) vitamin D). CVD, cardiovascular disease; LVMI, left ventricular mass index; LVH, left ventricular hypertrophy; UACR, urinary albumin-creatinine ratio; PTH, parathyroid hormone; FGF, fibroblast growth factor.
Number of events per 1000 person-years
Emboldened values were statistically significant (P < 0.05).
Figure 5.
Restricted cubic spline analysis to compare the associations of LVMI and EF with subsequent CVD events between the CKD-JAC and CRIC populations. A. LVMI. The reference was set at 47 g/m2.7. There was no statistically significant interaction between LVMI and cohort (P interaction = 0.96). B. EF. The reference was set at 60%. There was no statistically significant interaction between EF and cohort (P interaction = 0.70). Multivariable model adjusted for age, sex, baseline smoking status, eGFR, ln(UACR), diabetes, history of any CVD, systolic blood pressure, obesity category, number of classes of antihypertensive agents, and hemoglobin. CVD, cardiovascular disease; LVMI, left ventricular mass index; EF, ejection fraction; CKD-JAC, chronic kidney disease Japan cohort; CRIC, chronic renal insufficiency cohort; eGFR, estimated glomerular filtration rate; UACR, urinary albumin-creatinine ratio.
Multivariable models demonstrated that high serum phosphate and low 25(OH) vitamin D levels were associated with subsequent CVD events (Supplementary Table S5). After adjustment for LVMI, obesity was found to be associated with a lower risk of CVD events. Regarding other outcomes, while EF and LVMI were not significantly associated with ASCVD (Supplementary Table S6), both were significantly associated with CHF (HR, 0.61 (95% CI, 0.55–0.68) for EF and 1.26 (95% CI, 1.19–1.33) for LVMI) (Supplementary Table S7) and with all-cause death (HR, 0.86 (95% CI, 0.77–0.97) for EF and 1.20 (95% CI, 1.13–1.28) for LVMI) (Supplementary Table S8). In addition, while LVMI was associated with kidney failure (HR, 1.06 (95% CI, 1.01–1.10), EF was not (HR, 0.94 (95% CI, 0.87–1.02)) (Supplementary Table S9).
LAD was also significantly associated with CVD events, CHF, and death (HR, 1.22 (1.06–1.42), 1.48 (1.22–1.80), and 1.26 (1.03–1.53), respectively) (Supplementary Table S10)
Mediation effects of echocardiographic and laboratory parameters on the association between region and outcomes
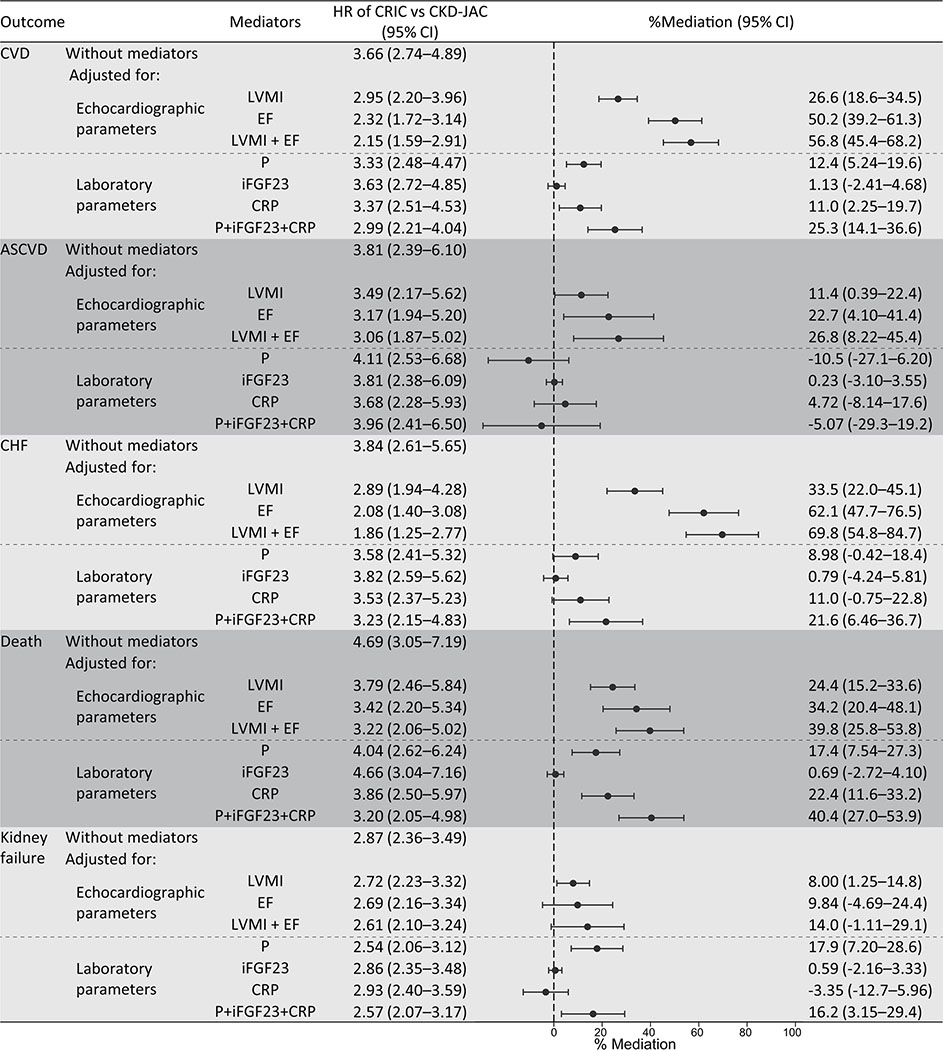
Echocardiographic parameters predominantly mediated the difference in CVD events between the study groups by 57% (LVMI: 27% and EF: 50%), while laboratory parameters moderately mediated the difference by 25% (phosphate: 12% and CRP: 11%) (Figure 6). The mediation effects of echocardiographic parameters were more prominent on CHF than in the other outcome events. Regarding laboratory parameters, the mediation effects of phosphate were significant on CVD, death, and kidney failure (12.4% (5.24–19.7%), 17.4 (7.54–27.3%), and 17.9 (7.20–28.6%), respectively), while iFGF23 showed no significant mediation effects on any outcome. The mediation effects of CRP were comparable to those of serum phosphate on CVD, CHF, and death.
Figure 6.
The proportion of the mediating effects of LVMI, EF, phosphate, intact FGF23, and CRP on the difference between the cohorts The total effect is the HR as the difference between the CKD-JAC and the CRIC studies (reference category is the CKD-JAC), and the direct effect is the HR of the same effect after excluding the effect of mediators. We adjusted for age, sex, baseline smoking status, eGFR, urinary albumin-creatinine ratio, DM, history of any CVD, CRP, systolic blood pressure, obesity category, the number of classes of antihypertensive agents, hemoglobin, corrected calcium, phosphate, total parathyroid hormone, intact fibroblast growth factor-23, active vitamin D supplementation, and 25(OH) vitamin D (factors in Model 3 except for LVMI, EF, phosphate, iFGF23, and CRP). LVMI, left ventricular mass index; EF, ejection fraction; CKD-JAC, chronic kidney disease Japan cohort; CRIC, chronic renal insufficiency cohort; CVD, cardiovascular disease; CHF, congestive heart failure.
Sensitivity Analysis
Those without a CVD history (N=2851) showed higher HRs for CVD event and CHF (2.32 [1.32–4.09] and 2.02 [0.87–4.65], respectively) (Supplementary Table S11).
In the mediation analysis, similar results were obtained under the counterfactual approach using CMAverse (Supplementary Table S12). Regarding unmeasured confounders between exposure and outcome, given the HR of 1.88 [1.36–2.56] in the association between region and CVD events (Supplementary Table S3), the E-value of this HR was calculated to be 2.10–3.17.
Discussion
Our international comparative study of patients with predialysis CKD showed that CRIC participants had higher risks of CVD events, death before kidney failure, and kidney failure than CKD-JAC participants. These international differences in mortality were much larger, and those in CVD events were slightly higher between Japanese and US patients with predialysis CKD than those reported in studies of hemodialysis patients.8,16 US patients had a higher LVMI, a higher percentage of concentric LVH and ASH, and a lower EF than Japanese patients. Regarding laboratory parameters, US patients had threefold higher CRP concentrations and higher phosphate levels, which were moderate mediators of the difference in CVD between US and Japanese counterparts. The observed differences in the incidences of CVD, CHF, and ASCVD between the cohorts were even more strongly mediated by baseline echocardiographic parameters. These results suggest that cardiac structural and functional characteristics assessed by echocardiography play a crucial role in the development of outcome events in patients with CKD.
This study found that the association between echocardiographic parameters and outcomes was comparable across cohorts, with no significant interaction with cohort. Although the prognostic impact of these parameters was comparable, the prevalence of abnormal parameters was clearly different, as shown in Figure 2. Thus, echocardiographic parameters are worth evaluating as a milestone toward subsequent events.
The present study was of great value in that it was an international comparison using individual patient-level data from large CKD cohorts. In previous studies, the reported prevalence of LVH was between 13% and 60%.29–34 However, international comparisons cannot be made because of the differences in determinant factors such as levels of BP, eGFR, and hemoglobin. Similarly, few international studies have directly compared the distribution of EF in patients with CKD across countries or ethnic groups. A previous epidemiological study showed that the prevalence of heart failure with preserved EF was higher in Japan than in other countries.35 However, to the best of our knowledge, there have been no comparative studies on CKD patient populations.
Regarding LV geometry, a previous study showed that eccentric and concentric LVH were associated with CVD events, death, and kidney failure. Their effect sizes on these outcomes were reported to be greater than in this study. This discrepancy could be explained by the fact that they did not adjust for CRP, markers for mineral bone disorder, or LVEF.36
We showed that obesity, which is often observed in US patients with CKD, was associated with high LVMI, especially ASH, but not with decreased EF. This is in line with previous studies in general populations showing that increased BMI was associated with high LVMI37 and that adiposity was especially associated with concentric rather than eccentric hypertrophy.38 Morbid obesity and obstructive sleep apnea syndrome were associated with hypertrophic cardiomyopathy-like ASH39,40, and reverse remodeling was reported to occur after weight-reducing surgery.41 The possible explanations proposed were increased insulin resistance due to obesity42 and obesity-related systemic inflammation.43
CRIC participants had lower EF, which, even within the normal range, was reported to have a strong association with CVD outcomes.44 Based on our finding that patients with a history of CHF and those with a history of CAD had 6.5% and 3.5% lower EF than patients without, respectively (Supplementary Table S3), the much higher prevalence of CHF and CAD among US patients (Table 1) partly explains the difference in EF between the cohorts. We hypothesized that the temporary drop in contractility through cardiac remodeling due to CHF may lead to a decrease in EF in CRIC participants. Our previous report revealed the predominant use of diuretics in the CRIC study,45 suggesting that CRIC participants had more congestion than CKD-JAC participants. Moreover, the CRIC study showed that pulmonary hypertension is common in CKD patients and is strongly associated with CVD outcomes.46 Congestion and pulmonary hypertension are possible mediators between lower EF and higher incidence of CHF. These parameters were unfortunately not measured in the CKD-JAC study.
Serum phosphate was shown to be a moderate mediator of kidney failure. Our previous report showed a substantial difference in serum phosphate levels between the cohorts47, and the present study added longitudinal evidence. CRP, which was associated with high LVMI and decreased EF (Supplementary Tables S1, S2), was as strong a mediator as phosphate regarding the differences in CVD, CHF, and death. Given our finding that CRP was associated with obesity, inflammation might be modifiable by weight loss in US patients.
The present study had several methodological advantages over past research. First, we were able to compare the baseline demographics and outcome events using individual data from well-established CKD cohorts in the US and Japan. Second, in the multivariable analysis, we used harmonized laboratory data, including CRP, phosphate, iFGF23, and PTH, which allowed us to standardize important covariates for comparing LV indices. Finally, our study is the first to use mediation analyses focusing on LV indices to explain the differences in the outcome events across countries.
This study had several limitations. First, only one-third of the participants in the CKD-JAC group underwent echocardiography at baseline, even though the protocol stated that all participants would undergo echocardiography. Clinical indications, such as a history of CVD or DM, should have served as an incentive to perform echocardiography. Therefore, CKD-JAC participants who underwent echocardiography may have been more likely to develop subsequent CVD events than those who did not. Second, parameters for diastolic dysfunction, congestion, pulmonary hypertension, and valvular diseases, were not included in the data from Japan. Third, some of the variables were not standardized, including CVD history.45 However, each cohort had an event adjudication process by an independent committee, which allowed us to compare the outcome events precisely. Finally, in the causal mediation framework, there may be unmeasured confounders. Given that the association between region and CVD events had an HR of 1.88, the E-value was calculated to be 2.10–3.17. This means that the observed HR could be explained away by an unmeasured confounder that is associated with both exposure and outcome by 2.10- to 3.17-fold each. Commonly reported confounders such as high salt intake, Mediterranean diet, omega-3 fatty acids, and physical activity, do not have an impact that alone could explain away the association between region and CVD events.48–55 In addition, adjustment for numerous measured confounders related to the unmeasured variable may attenuate the residual confounding associations.56 Therefore, we consider the effects of unmeasured confounders on the association between region and CVD events to be limited. Furthermore, given the greater association of region with CVD events and CHF in those without a history of CVD, it would be even more difficult to find unmeasured confounders that would have enough impact to explain away this association.
In conclusion, US patients most often had concentric hypertrophic cardiomyopathy with a lower EF and a higher LVMI than Japanese patients. These differences in LV indices and the higher CRP levels in the US explained the higher incidence of CVD among US patients. Given the association of BMI with LVMI and CRP, intervening against obesity may reduce the CVD incidence. Screening for echocardiographic LV indices may be useful to identify patients with CKD who are at highest risk of CVD.
Disclosure
T.I. reports receiving a lecture fee from Kyowa Kirin Company (KKC). T.H., N.F., S.M. and M.F. report receiving consulting and lecture fees from KKC. H.F. serves as a consultant to KKC and received a grant from the National Institutes of Health (NIH). A.G. reports receiving a grant from the NIH. W.Y. reports receiving a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. All remaining authors have nothing to disclose.
Supplementary Material
Supplementary Table S1. Baseline characteristics of those with or without echocardiography in the Chronic Kidney Disease Japan Cohort (CKD-JAC) study.
Supplementary Table S2. Factors associated with left ventricular mass index (LVMI) in the combined cohort.
Supplementary Table S3. Factors associated with left ventricular ejection fraction (LVEF) in the combined cohort
Supplementary Table S4. Description of outcome events.
Supplementary Table S5. Factors associated with the incidence of cardiovascular disease (CVD) events.
Supplementary Table S6. Association of left ventricular indices with the incidence of atherosclerotic cardiovascular disease (ASCVD) events.
Supplementary Table S7. Association of left ventricular indices with the incidence of congestive heart failure (CHF).
Supplementary Table S8. Association of left ventricular indices with the incidence of all-cause death.
Supplementary Table S9. Association of left ventricular indices with the incidence of kidney failure.
Supplementary Table S10. Association of left atrial dimension (LAD: /10 mm) with subsequent cardiovascular disease (CVD) events.
Supplementary Table S11. Subgroup analyses of the association between region and outcomes (no cardiovascular disease [CVD] history and estimated glomerular filtration rate [eGFR] 20–60 mL/min/173 m2).
Supplementary Table S12. Proportion mediated of parameters associated with outcomes using mediation analysis.
Supplementary Figure S1. Causal diagram.
Supplementary Figure S2. Distribution of factors associated with increased left ventricular mass index (LVMI) compared among ethnic groups across levels of estimated glomerular filtration rate (eGFR).
Supplementary Figure S3. Association between body mass index (BMI) and asymmetric septal hypertrophy.
Supplementary Figure S4. Unadjusted and adjusted event-free survival of adverse events (Chronic Renal Insufficiency Cohort [CRIC] vs. Chronic Kidney Disease Japan Cohort [CKD-JAC]).
Supplementary Figure S5. Unadjusted and adjusted event-free survival of adverse events (4 different race-ethnic groups).
Acknowledgments
We thank all the investigators and participants of the CRIC study and the CKD-JAC study for their contributions. The CKD-JAC study was conducted by principal investigators at the following medical centers: Japan Community Health Care Organization Sendai Hospital (Miyagi), JA Toride Medical Center (Ibaraki), Jichi Medical University (Tochigi), Tokyo Women’s Medical University Hospital (Tokyo), St. Luke’s International Hospital (Tokyo), Showa University Hospital (Tokyo), Showa University Yokohama Northern Hospital (Kanagawa), Showa University Fujigaoka Hospital (Kanagawa), Gifu Prefectural General Medical Center (Gifu), Kasugai Municipal Hospital (Aichi), Tosei General Hospital (Aichi), Osaka University Hospital (Osaka), Osaka General Medical Center (Osaka), Osaka City General Hospital (Osaka), Kurashiki Central Hospital (Okayama), Fukuoka Red Cross Hospital (Fukuoka), and Iizuka Hospital (Fukuoka).
Footnotes
Support for this work is described in the Supplementary material.
References
- 1.de Jager DJ, Grootendorst DC, Jager KJ, et al. Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA. 2009;302(16):1782–1789. [DOI] [PubMed] [Google Scholar]
- 2.Ninomiya T, Kiyohara Y, Kubo M, et al. Chronic kidney disease and cardiovascular disease in a general Japanese population: The Hisayama Study. Kidney Int. 2005;68(1):228–236. [DOI] [PubMed] [Google Scholar]
- 3.Manjunath G, Tighiouart H, Ibrahim H, et al. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol. 2003;41(1):47–55. [DOI] [PubMed] [Google Scholar]
- 4.Muntner P, He J, Hamm L, Loria C, Whelton PK. Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol. 2002;13(3):745–753. [DOI] [PubMed] [Google Scholar]
- 5.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(5 Suppl 3):S112–119. [DOI] [PubMed] [Google Scholar]
- 6.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. [DOI] [PubMed] [Google Scholar]
- 7.Stirnadel-Farrant HA, Karaboyas A, Cizman B, et al. Cardiovascular Event Rates Among Hemodialysis Patients Across Geographical Regions—A Snapshot From The Dialysis Outcomes and Practice Patterns Study (DOPPS). Kidney Int Reports. 2019;4(6):864–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodkin DA, Bragg-Gresham JL, Koenig KG, et al. Association of Comorbid Conditions and Mortality in Hemodialysis Patients in Europe, Japan, and the United States: The Dialysis Outcomes and Practice Patterns Study (DOPPS). J Am Soc Nephrol. 2003;14(12):3270–3277. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka K, Watanabe T, Takeuchi A, et al. Cardiovascular events and death in Japanese patients with chronic kidney disease. Kidney Int. 2017;91(1):227–234. [DOI] [PubMed] [Google Scholar]
- 10.Yuan Q, Xie Y, Peng Z, et al. Urinary magnesium predicts risk of cardiovascular disease in Chronic Kidney Disease stage 1–4 patients. Clin Nutr. 2021;40(4):2394–2400. [DOI] [PubMed] [Google Scholar]
- 11.Ryu H, Kim J, Kang E, et al. Incidence of cardiovascular events and mortality in Korean patients with chronic kidney disease. Sci Rep. 2021;11(1):1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grams ME, Sang Y, Ballew SH, et al. Predicting timing of clinical outcomes in patients with chronic kidney disease and severely decreased glomerular filtration rate. Kidney Int. 2018;93(6):1442–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schefold JC, Filippatos G, Hasenfuss G, Anker SD, Von Haehling S. Heart failure and kidney dysfunction: Epidemiology, mechanisms and management. Nat Rev Nephrol. 2016;12(10):610–623. [DOI] [PubMed] [Google Scholar]
- 14.Shlipak MG, Fried LF, Cushman M, et al. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA. 2005;293(14):1737–1745. [DOI] [PubMed] [Google Scholar]
- 15.Chen S, Chang J, Liu W, et al. Echocardiographic parameters are independently associated with increased cardiovascular events in patients with chronic kidney disease. Nephrol Dial Transplant. 2012;27(3):1064–1070. [DOI] [PubMed] [Google Scholar]
- 16.Dubin RF, Deo R, Bansal N, et al. Associations of Conventional Echocardiographic Measures with Incident Heart Failure and Mortality: The Chronic Renal Insufficiency Cohort. Clin J Am Soc Nephrol. 2017;12(1):60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu IW, Hung MJ, Chen YC, et al. Ventricular function and all-cause mortality in chronic kidney disease patients with angiographic coronary artery disease. J Nephrol. 2010;23(2):181–188. [PubMed] [Google Scholar]
- 18.Lash JP, Go AS, Appel LJ, et al. Chronic renal insufficiency cohort (CRIC) study: Baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4(8):1302–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldman HI. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol. 2003;14(7 supple 2):S148–53. [DOI] [PubMed] [Google Scholar]
- 20.Imai E, Matsuo S, Makino H, et al. Chronic Kidney Disease Japan Cohort study: Baseline characteristics and factors associated with causative diseases and renal function. Clin Exp Nephrol. 2010;14(6):558–570. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka S, Hayashi T, Kihara Y, et al. Standard measurement of cardiac function indexes. J Med Ultrason. 2006;33(2):123–127. [DOI] [PubMed] [Google Scholar]
- 22.Imai E, Matsuo S, Makino H, et al. Chronic Kidney Disease Japan Cohort (CKD-JAC) study: design and methods. Hypertens Res. 2008;31(6):1101–1107. [DOI] [PubMed] [Google Scholar]
- 23.Anderson AH, Yang W, Hsu C, et al. Estimating GFR Among Participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2012;60(2):250–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–992. [DOI] [PubMed] [Google Scholar]
- 25.Van Der weele T, Vansteelandt S. Mediation analysis with multiple mediators. Epidemiol Method. 2014;2(1):95–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18(6):681–694. [DOI] [PubMed] [Google Scholar]
- 27.Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev Sci. 2007;8(3):206–213. [DOI] [PubMed] [Google Scholar]
- 28.Zou G A Modified Poisson Regression Approach to Prospective Studies with Binary Data. Am J Epidemiol. 2004;159(7):702–706. [DOI] [PubMed] [Google Scholar]
- 29.Matsushita K, Kwak L, Sang Y, et al. Kidney Disease Measures and Left Ventricular Structure and Function: The Atherosclerosis Risk in Communities Study. J Am Heart Assoc. 2017;6(9): e006259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou C, Wang F, Wang J-W, Zhang L-X, Zhao M-H. Mineral and Bone Disorder and Its Association with Cardiovascular Parameters in Chinese Patients with Chronic Kidney Disease. Chin Med J (Engl). 2016;129(19):2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HJ, Kang E, Oh YK, et al. The association between soluble klotho and cardiovascular parameters in chronic kidney disease: Results from the KNOW-CKD study. BMC Nephrol. 2018;19(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nitta K, Iimuro S, Imai E, et al. Risk factors for increased left ventricular hypertrophy in patients with chronic kidney disease: findings from the CKD-JAC study. Clin Exp Nephrol. 2019;23(1):85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park M, Hsu C, Li Y, et al. Associations between Kidney Function and Subclinical Cardiac Abnormalities in CKD. J Am Soc Nephrol. 2012;23(10):1725–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peterson GE, De Backer T, Contreras G, et al. Relationship of left ventricular hypertrophy and diastolic function with cardiovascular and renal outcomes in African Americans with hypertensive chronic kidney disease. Hypertension. 2013;62(3):518–525. [DOI] [PubMed] [Google Scholar]
- 35.Shiba N, Nochioka K, Miura M, Kohno H, Shimokawa H. Trend of westernization of etiology and clinical characteristics of heart failure patients in Japan: First report from the CHART-2 Study. Circ J. 2011;75(4):823–833. [DOI] [PubMed] [Google Scholar]
- 36.Paoletti E, De Nicola L, Gabbai FB, et al. Associations of Left Ventricular Hypertrophy and Geometry with Adverse Outcomes in Patients with CKD and Hypertension. Clin J Am Soc Nephrol. 2016;11(2):271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cai S, Dong J, Cheng B, et al. Relationship of a new anthropometric index with left ventricular hypertrophy in hypertensive patients among the Han Chinese. BMC Cardiovasc Disord. 2022;22(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woodiwiss AJ, Libhaber CD, Majane OHI, Libhaber E, Maseko M, Norton GR. Obesity promotes left ventricular concentric rather than eccentric geometric remodeling and hypertrophy independent of blood pressure. Am J Hypertens. 2008;21(10):1144–1151. [DOI] [PubMed] [Google Scholar]
- 39.Wong RCC, Tan KB. Asymmetric left ventricular hypertrophy associated with morbid obesity mimicking familial hypertrophic cardiomyopathy. Singapore Med J. 2014;55(12):e201–e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nerbass FB, Pedrosa RP, Danzi-Soares NJ, Drager LF, Arteaga-Fernández E, Lorenzi-Filho G. Obstructive sleep apnea and hypertrophic cardiomyopathy: A common and potential harmful combination. Sleep Med Rev. 2013;17(3):201–206. [DOI] [PubMed] [Google Scholar]
- 41.Frea S, Andreis A, Scarlatta V, et al. Subclinical left ventricular dysfunction in severe obesity and reverse cardiac remodeling after bariatric surgery. J Cardiovasc Echogr. 2020;30(1):22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang CJ, Bao XR, Du GW, et al. Effects of insulin resistance on left ventricular hypertrophy in patients with CKD stage 1–3. Int Urol Nephrol. 2014;46(8):1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirano H, Kanaji Y, Sugiyama T, et al. Impact of pericoronary adipose tissue inflammation on left ventricular hypertrophy and regional physiological indices in stable coronary artery disease patients with preserved systolic function. Heart Vessels. 2021;36(1):24–37. [DOI] [PubMed] [Google Scholar]
- 44.Stewart S, Playford D, Scalia GM, et al. Ejection fraction and mortality: a nationwide register-based cohort study of 499 153 women and men. Eur J Heart Fail. 2021;23(3):406–416. [DOI] [PubMed] [Google Scholar]
- 45.Imaizumi T, Hamano T, Fujii N, et al. Cardiovascular disease history and β-blocker prescription patterns among Japanese and American patients with CKD: a cross-sectional study of the CRIC and CKD-JAC studies. Hypertens Res. 2021;44(6):700–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Navaneethan SD, Roy J, Tao K, et al. Prevalence, predictors, and outcomes of pulmonary hypertension in CKD. J Am Soc Nephrol. 2016;27(3):877–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fujii N, Hamano T, Hsu JY, et al. A Comparative Study of Serum Phosphate and Related Parameters in Chronic Kidney Disease between the USA and Japan. Am J Nephrol. 2022; 53(2–3):226–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abdelhamid AS, Brown TJ, Brainard JS, et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2018;11(11): CD003177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Estruch R, Ros E, Salas-Salvadó J, et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N Engl J Med. 2018;378(25):e34. [DOI] [PubMed] [Google Scholar]
- 50.Cheng W, Zhang Z, Cheng W, Yang C, Diao L, Liu W. Associations of leisure-time physical activity with cardiovascular mortality: A systematic review and meta-analysis of 44 prospective cohort studies. Eur J Prev Cardiol. 2018;25(17):1864–1872. [DOI] [PubMed] [Google Scholar]
- 51.O’Donnell M, Mente A, Rangarajan S, et al. Urinary Sodium and Potassium Excretion, Mortality, and Cardiovascular Events. N Engl J Med. 2014;371(7):612–623. [DOI] [PubMed] [Google Scholar]
- 52.Schnohr P, O’Keefe JH, Lange P, Jensen GB, Marott JL. Impact of persistence and non-persistence in leisure time physical activity on coronary heart disease and all-cause mortality: The Copenhagen City Heart Study. Eur J Prev Cardiol. 2017;24(15):1615–1623. [DOI] [PubMed] [Google Scholar]
- 53.Sezaki A, Imai T, Miyamoto K, Kawase F, Shimokata H. Mediterranean diet score and incidence of IHD: A global comparative study. Public Health Nutr. 2019;22(8):1444–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Micha R, Khatibzadeh S, Shi P, et al. Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: A systematic analysis including 266 country-specific nutrition surveys. BMJ. 2014;348:g2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Althoff T, Sosič R, Hicks JL, King AC, Delp SL, Leskovec J. Large-scale physical activity data reveal worldwide activity inequality. Nature. 2017;547(7663):336–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vanderweele TJ, Mathur MB. Commentary: Developing best-practice guidelines for the reporting of E-values. Int J Epidemiol. 2020;49(5):1495–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Baseline characteristics of those with or without echocardiography in the Chronic Kidney Disease Japan Cohort (CKD-JAC) study.
Supplementary Table S2. Factors associated with left ventricular mass index (LVMI) in the combined cohort.
Supplementary Table S3. Factors associated with left ventricular ejection fraction (LVEF) in the combined cohort
Supplementary Table S4. Description of outcome events.
Supplementary Table S5. Factors associated with the incidence of cardiovascular disease (CVD) events.
Supplementary Table S6. Association of left ventricular indices with the incidence of atherosclerotic cardiovascular disease (ASCVD) events.
Supplementary Table S7. Association of left ventricular indices with the incidence of congestive heart failure (CHF).
Supplementary Table S8. Association of left ventricular indices with the incidence of all-cause death.
Supplementary Table S9. Association of left ventricular indices with the incidence of kidney failure.
Supplementary Table S10. Association of left atrial dimension (LAD: /10 mm) with subsequent cardiovascular disease (CVD) events.
Supplementary Table S11. Subgroup analyses of the association between region and outcomes (no cardiovascular disease [CVD] history and estimated glomerular filtration rate [eGFR] 20–60 mL/min/173 m2).
Supplementary Table S12. Proportion mediated of parameters associated with outcomes using mediation analysis.
Supplementary Figure S1. Causal diagram.
Supplementary Figure S2. Distribution of factors associated with increased left ventricular mass index (LVMI) compared among ethnic groups across levels of estimated glomerular filtration rate (eGFR).
Supplementary Figure S3. Association between body mass index (BMI) and asymmetric septal hypertrophy.
Supplementary Figure S4. Unadjusted and adjusted event-free survival of adverse events (Chronic Renal Insufficiency Cohort [CRIC] vs. Chronic Kidney Disease Japan Cohort [CKD-JAC]).
Supplementary Figure S5. Unadjusted and adjusted event-free survival of adverse events (4 different race-ethnic groups).