Abstract
Serum albumin was shown to stimulate markedly various photoreactions in isolated bean and lettuce chloroplasts. The maximal effect was obtained when this compound was present during the homogenization step and continuously in the chloroplast preparation. The “basal” electron transport was enhanced using various acceptors and stimulation was obtained also in the presence of uncouplers. The quantum requirement for ferricyanide reduction was appreciably reduced. Serum albumin increased the rate of cyclic phosphorylation and the ratio of P/e2 in non-cyclic phosphorylation. The increase in phosphorylation is supposedly due to inhibition of the rate of decay of the high energy non-phosphorylated intermediate, XE.
It is postulated that serum albumin affects chloroplast photoreactions by binding endogenously released unsaturated fatty acids.
Full text
PDF
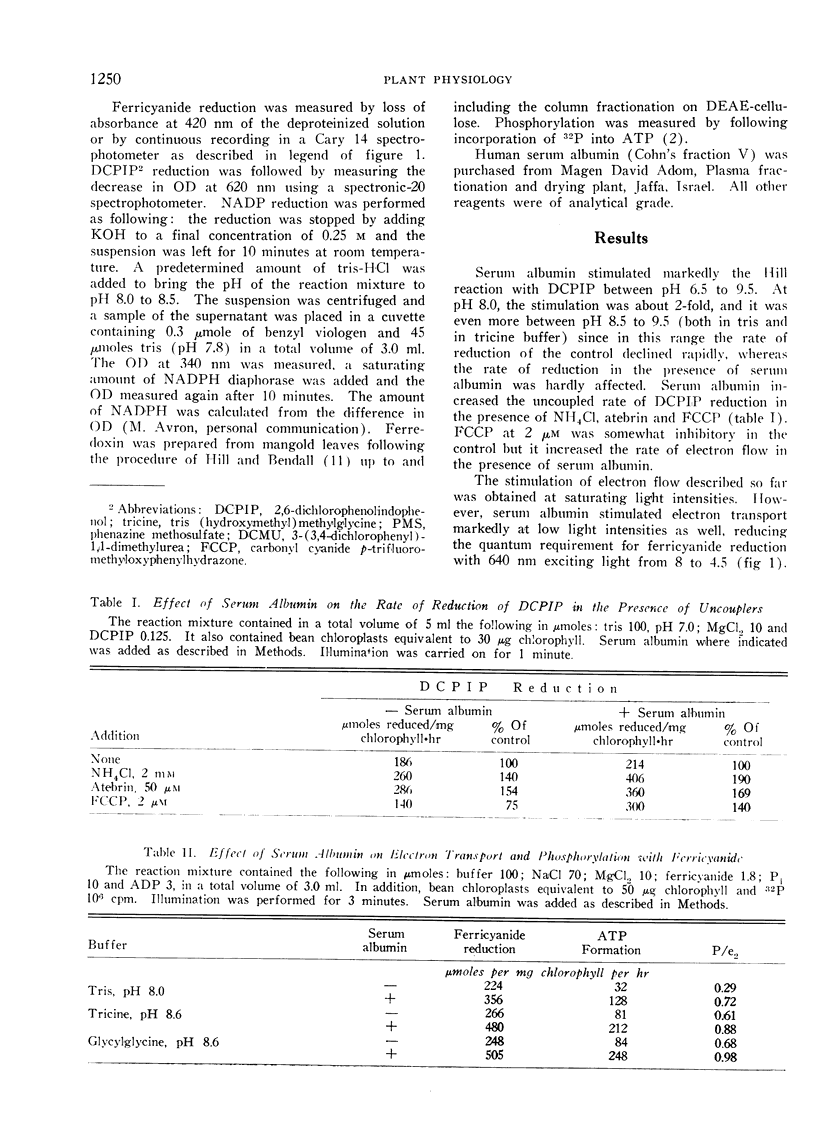
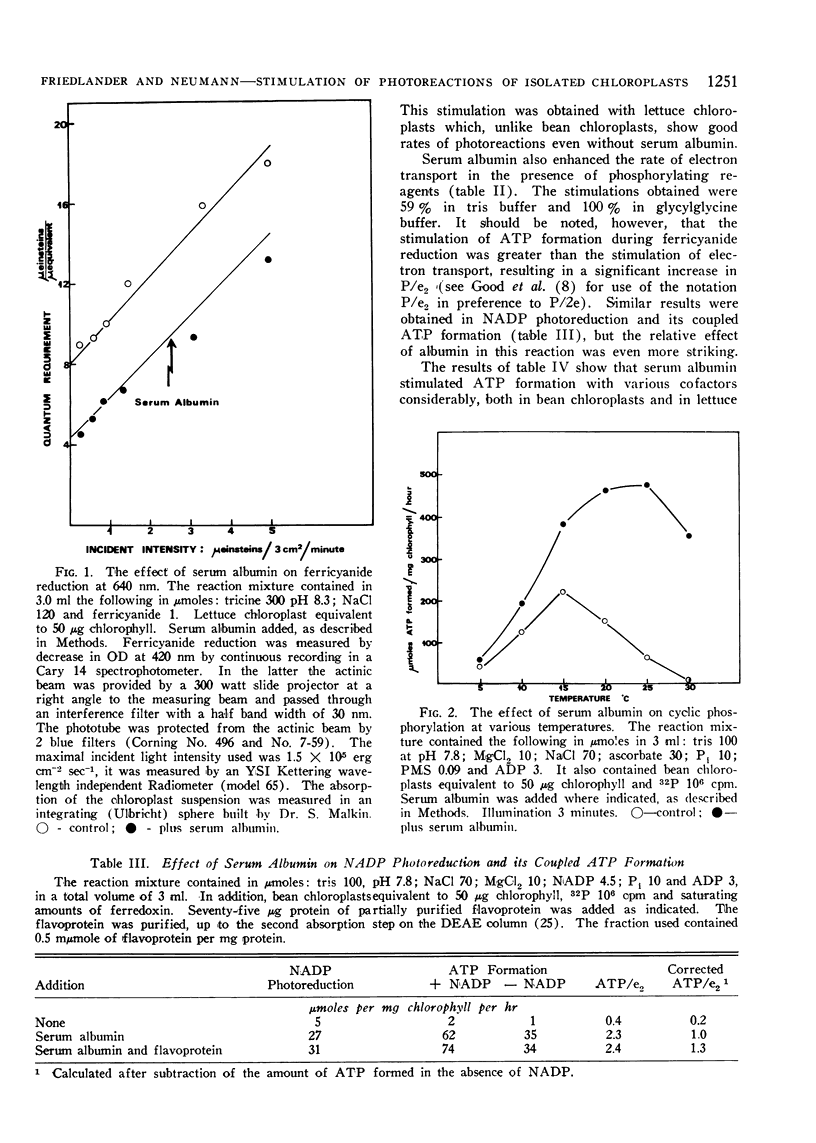
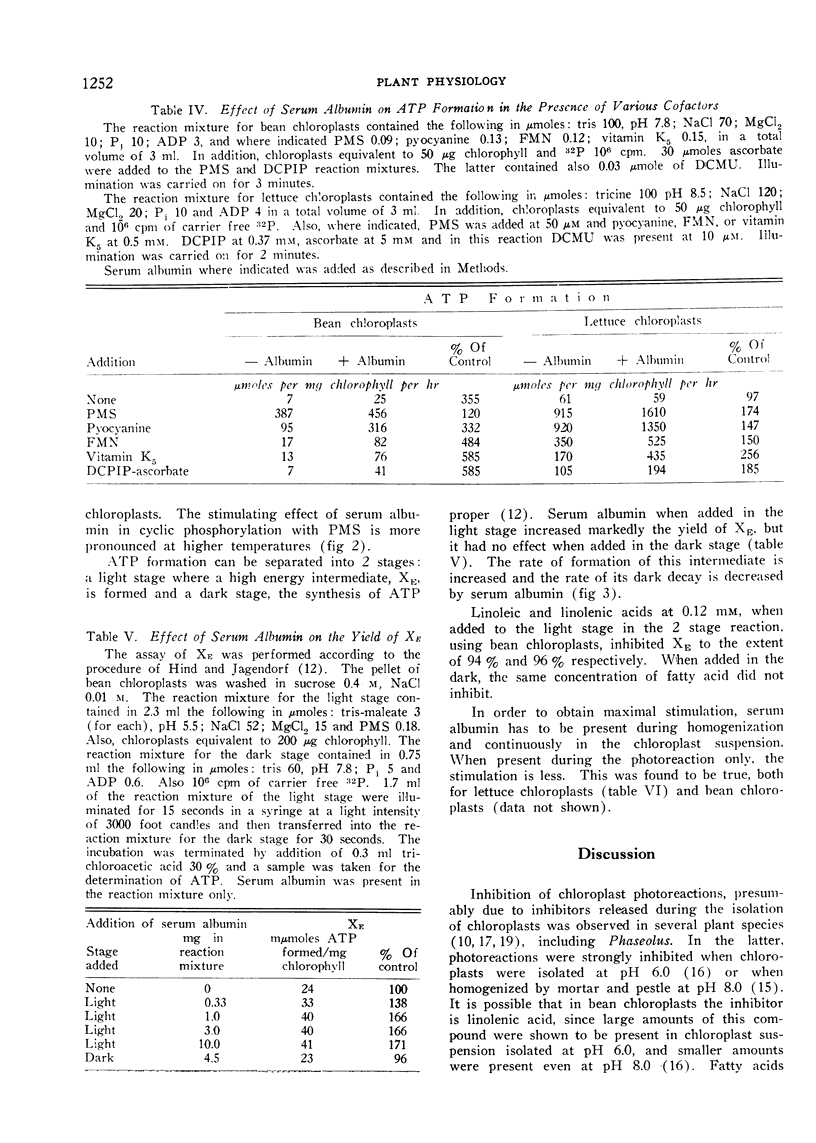
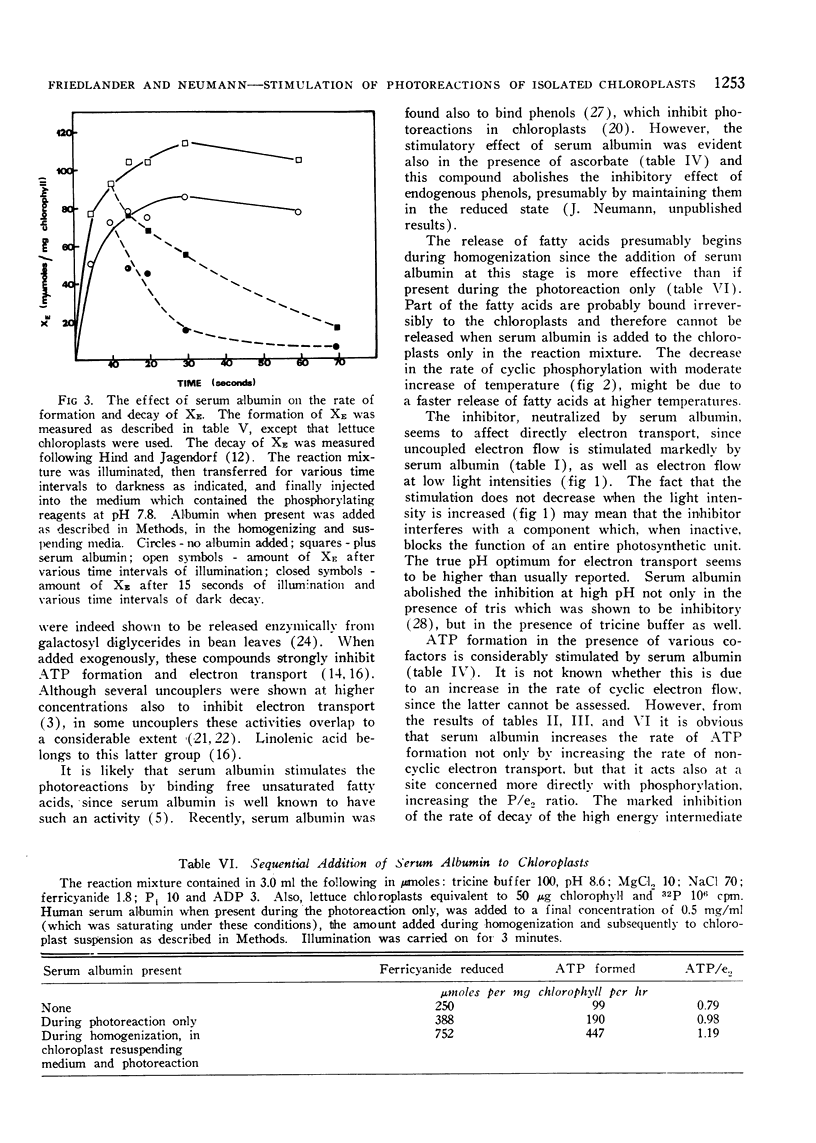

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M. Photophosphorylation by swiss-chard chloroplasts. Biochim Biophys Acta. 1960 May 20;40:257–272. doi: 10.1016/0006-3002(60)91350-0. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORST P., LOOS J. A., CHRIST E. J., SLATER E. C. Uncoupling activity of long-chain fatty acids. Biochim Biophys Acta. 1962 Aug 27;62:509–518. doi: 10.1016/0006-3002(62)90232-9. [DOI] [PubMed] [Google Scholar]
- Good N. E. Carbon Dioxide & the Hill Reaction. Plant Physiol. 1963 May;38(3):298–304. doi: 10.1104/pp.38.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromet-Elhanan Z. The relationship of cyclic and non-cyclic electron flow patterns with reduced indophenols to photophosphorylation. Biochim Biophys Acta. 1967 May 9;131(3):526–537. doi: 10.1016/0005-2728(67)90012-6. [DOI] [PubMed] [Google Scholar]
- HILL R., BENDALL F. Crystallization of a photosynthetic reductase from a green plant. Nature. 1960 Jul 30;187:417–417. doi: 10.1038/187417a0. [DOI] [PubMed] [Google Scholar]
- Hind G., Jagendorf A. T. SEPARATION OF LIGHT AND DARK STAGES IN PHOTOPHOSPHORYLATION. Proc Natl Acad Sci U S A. 1963 May;49(5):715–722. doi: 10.1073/pnas.49.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn J. S. Photophosphorylation by isolated chloroplasts of Euglena gracilis. Biochem Biophys Res Commun. 1966 Aug 12;24(3):329–333. doi: 10.1016/0006-291x(66)90159-8. [DOI] [PubMed] [Google Scholar]
- Margulies M. M. Concerning the Preparation of Chloroplasts Active in Hill and Photosynthetic Phosphorylation Activities from Leaves of Phaseolus vulgaris. Plant Physiol. 1966 Oct;41(8):1320–1322. doi: 10.1104/pp.41.8.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty R. E., Jagendorf A. T. Chloroplast damage due to enzymatic hydrolysis of endogenous lipids. Plant Physiol. 1965 Jul;40(4):725–735. doi: 10.1104/pp.40.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miflin B. J., Hageman R. H. Demonstration of Photophosphorylation by Maize Chloroplasts. Plant Physiol. 1963 Jan;38(1):66–70. doi: 10.1104/pp.38.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molotkovsky Y. G., Zhestkova I. M. Morphological and functional changes in isolated chloroplasts under the influence of oleate. Biochim Biophys Acta. 1966 Jan 4;112(1):170–172. doi: 10.1016/s0926-6585(96)90020-5. [DOI] [PubMed] [Google Scholar]
- Neumann J., Drechsler Z. Inhibition of photo-induced electron transport and related reactions in isolated chloroplasts by phenol. Plant Physiol. 1967 Apr;42(4):573–577. doi: 10.1104/pp.42.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann J., Jagendorf A. T. Dinitrophenol as an uncoupler of photosynthetic phosphorylation. Biochem Biophys Res Commun. 1964 Aug 11;16(6):562–567. doi: 10.1016/0006-291x(64)90193-7. [DOI] [PubMed] [Google Scholar]
- Neumann J., Jagendorf A. Uncoupling photophosphorylation by detergents. Biochim Biophys Acta. 1965 Nov 29;109(2):382–389. doi: 10.1016/0926-6585(65)90165-2. [DOI] [PubMed] [Google Scholar]
- SASTRY P. S., KATES M. HYDROLYSIS OF MONOGALACTOSYL AND DIGALACTOSYL DIGLYCERIDES BY SPECIFIC ENZYMES IN RUNNER-BEAN LEAVES. Biochemistry. 1964 Sep;3:1280–1287. doi: 10.1021/bi00897a016. [DOI] [PubMed] [Google Scholar]
- SHIN M., TAGAWA K., ARNON D. I. CRYSTALLIZATION OF FERREDOXIN-TPN REDUCTASE AND ITS ROLE IN THE PHOTOSYNTHETIC APPARATUS OF CHLOROPLASTS. Biochem Z. 1963;338:84–96. [PubMed] [Google Scholar]
- WEINBACH E. C., SHEFFIELD H., GARBUS J. RESTORATION OF OXIDATIVE PHOSPHORYLATION AND MORPHOLOGICAL INTEGRITY TO SWOLLEN, UNCOUPLED RAT LIVER MITOCHONDRIA. Proc Natl Acad Sci U S A. 1963 Sep;50:561–568. doi: 10.1073/pnas.50.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]


