Abstract
Purpose:
To compare the role of MR for assessment of extent of disease in women newly diagnosed with breast cancer imaged with digital mammography (DM) alone versus digital breast tomosynthesis (DBT).
Methods:
Retrospective review was conducted of 401 consecutive breast MR exams (10/1/2013–7/31/2015) from women who underwent preoperative MR for newly diagnosed breast cancer by either DM or DBT, leaving 388 exams (201 DM and 187 DBT). MR detection of additional, otherwise occult, disease was stratified by modality, breast density, and background parenchymal enhancement. A true-positive finding was defined as malignancy in the ipsilateral-breast >2 cm away from the index-lesion or in the contralateral breast.
Results:
50 additional malignancies were detected in 388 exams (12.9%), 37 ipsilateral and 13 contralateral. There was no difference in the MR detection of additional disease in women imaged by either DM versus DBT (p = 0.53). In patients with DM, there was no significant difference in the rate of MR additional cancer detection in dense versus non-dense breasts (p = 0.790). However, in patients with DBT, MR detected significantly more additional sites of malignancy in dense compared to non-dense breasts (p = 0.017). There was no difference in false-positive MR exams (p = 0.470) for DM versus DBT. For both DM and DBT cohorts, higher MR background parenchymal enhancement was associated with higher false-positive (p = 0.040) but no significant difference in true-positive exams.
Conclusions:
Among patients with DBT imaging at cancer diagnosis, women with dense breasts appear to benefit more from preoperative MR than non-dense women. In women imaged only with DM, MR finds additional malignancy across all breast densities.
Keywords: Breast cancer, Tomosynthesis, Breast MRI, Staging, Dense breast
1. Introduction
The detection of breast cancer has evolved considerably in the past decade with the introduction of digital breast tomosynthesis (DBT) and an increased use of breast MR. The addition of the quasi 3-dimensional technique of DBT improves not only cancer detection by increasing lesion conspicuity but also specificity compared to imaging with digital mammography (DM) alone [[2–7]]. Despite improvements achieved with DBT imaging, once a breast cancer is diagnosed, accurate preoperative assessment of tumor extent and assessment of the contralateral breast for synchronous cancer remains challenging. Additional ipsilateral disease is especially important if the patient is considering breast conservation and identifying contralateral disease allows concomitant treatment. Detection of additional disease may also identify different tumor subtypes, which could change therapy decisions.
In addition to finding more cancers, DBT better defines disease extent than imaging with DM alone [8,9]. Fontaine et al., recently showed improved diagnostic accuracy for additional ipsilateral and contralateral breast cancer when using combination of DM with DBT compared to DM alone in women with non-dense breasts [9]. Mariscotti et al., demonstrated that the combination of DM and ultrasound with DBT performed as part of mammographic imaging in patients newly diagnosed with breast cancer yielded a sensitivity of 97.7% in the preoperative assessment of disease extent, which was not significantly improved by the addition of MR [8]. Routine use of MR to determine disease extent remains controversial since it may prompt more extensive surgical interventions, specifically mastectomies, with no impact on long term outcomes such as survival [10–12]. As DBT imaging increases, the benefit, if any, of preoperative breast MR in newly diagnosed cancer diagnosis is an area of clinical equipoise.
Kim et al., compared the diagnostic performance of DBT with breast MR in assessing extent of disease in newly diagnosed cancer patients and concluded that DBT provided lower diagnostic performance than MR as an adjunctive to DM [13]. The outcomes of MR in determining the extent of disease after cancer detected with either DBT or DM alone has been previously evaluated in screen detected cancers [14]. MR detected additional, mammographically occult cancer in both the DBT and DM populations; however, there were fewer additional sites of malignancy detected with MR in patients who had cancers detected after routine screening evaluation with DBT compared to those screened with DM alone.
We hypothesized that addition of MR to both DBT and DM would improve evaluation of the disease extent in patients with known malignancy. The purpose of this study was to compare the role of preoperative MR in women newly diagnosed with screen-detected or symptomatically presenting cancers imaged with either DBT or DM alone. In addition, imaging outcomes were assessed by mammographic breast density and MR background parenchymal enhancement (BPE).
2. Subjects and methods
2.1. Study design and patient characteristics
This study was approved by the institutional review board, and the requirement for informed consent was waived. This was a retrospective analysis of consecutive breast MRs for newly diagnosed breast cancer from 10/1/2013 to 7/31/2015, including patients with biopsy-proven breast cancer detected on either screening or diagnostic mammography (DM or combination of DM and DBT) with mammography performed within 12-weeks prior to breast MR. At the time of the study, our University had two screening locations – one used only DM (DM group) and the other used only combination of DM and DBT (DBT group). There was no difference in the patient populations seeking care at the two centers. All patients were imaged with DBT if they presented to the latter center regardless of ability to pay for the additional testing. Patients were excluded if they had neoadjuvant chemotherapy prior to surgery.
The medical records of each patient were reviewed to determine the initial date and mode of cancer presentation, tumor subtype, grade, hormone receptor status and staging. The mammographic modality (DM vs. DBT), indication (screening vs. diagnostic), breast density (in accordance with Breast Imaging-Reporting and Data System (BI-RADS) Atlas 4th edition categories [15]), and mammographic finding (calcification, mass, asymmetry/focal asymmetry, or architectural distortion) were extracted from mammography reports. MR reports were reviewed and the global assessment of BPE (BPE- categorized as minimal, mild, moderate or marked [15]) and any suspicious additional lesions were recorded. Pathology records were reviewed for final tissue diagnosis of the additional MR detected lesions. Patients with additional lesions (BI-RADS 4 or 5) detected on MR were reviewed by two fellowship-trained breast imagers, EC with 27 years and EM with 95 years of experience and radiologic-pathologic concordance was determined by matching the lesions on MR with the pathology reports. Distance of any suspicious lesion from the index cancer in each case was also recorded. Additional malignant lesions were considered as true-positives if they were in the ipsilateral breast and separated from the index lesion by at least 2 cm of intervening normal-appearing tissue or, in the contralateral, uninvolved breast. Studies were excluded if final pathologic disposition of an additional BI-RADS 4 or 5 lesion was unclear.
2.2. MR and mammographic imaging
Full-field DM examinations were performed on a DM system (GE Healthcare, Chalfont St-Giles, UK). All DBT imaging was performed using Selenia Dimensions (Hologic, Bedford, MA) and these exams were interpreted with additional acquired DM images. Dynamic contrast–enhanced MR examinations were performed using a 1.5-T scanner (Siemens Espree) using a dedicated surface breast coil array (matrix size, 256 × 256; slice thickness, 2–3.5 mm; flip angle, 20°) with the patient placed in the prone position. Bilateral, fat-suppressed, T2-weighted and slab interleaved, 3D, fat-suppressed, spoiled gradient echo sequences were acquired. Sequential post-contrast MR series were acquired for approximately 6 min after contrast injection (Omniscan; GE Healthcare) per standard clinical protocol at our institution at the time.
MR and mammographic findings were considered discordant if there were additional suspicious lesions on MR not identified on preoperative mammographic (DM or DBT) imaging or, if suspicious lesions were detected on mammographic imaging and subsequently characterized as negative or benign on MR.
MR detected BI-RADS category 4 or 5 lesions that were pathologically proven additional sites of malignancy (ductal carcinoma in-situ (DCIS) or invasive disease) were considered as true-positives. The MR findings of additional lesions were considered false-positives if subsequent biopsy yielded a benign finding or a high-risk lesion. MR performance was stratified by mammographic modality (DM versus DBT), mammographic breast density, and BPE.
2.3. Statistical analysis
Differences between the groups were compared using two-sample t-test for continuous variables and Chi-square test for categorical variables. A p-value of less than 0.05 was considered statistically significant and 95% confidence intervals were reported where applicable. Breast density and BPE were dichotomized for analysis; breast density was divided into non-dense (category 1 or 2) and dense (category 3 or 4) and BPE was divided into low BPE (minimal or mild) and high BPE (moderate or marked). The positive predictive values (PPVs) of MR plus DM and MR plus DBT for detection of breast cancers were calculated on a per-lesion basis.
3. Results
3.1. Patients’ characteristics
A total of 401 consecutive MR exams performed for preoperative assessment inpatients with biopsy proven primary breast cancer were reviewed. Thirteen patients were excluded as they underwent total mastectomy without localization of possible additional disease and therefore, the final pathologic disposition of the lesions was unknown. The final cohort was 388 patients with a mean age of 55.4 years (range, 24–87 years). The majority of patients (n = 291, 75%) had invasive ductal cancer (IDC), 33 (8.5%) had invasive lobular cancer (ILC) and, 64 (16.5%) had DCIS.
Mammographic imaging at the time of cancer diagnosis was DM in 201 (52%) patients and DBT in 187 (48%) patients. There were no significant differences in breast density, T-stage, N-stage, histological grade, and hormone receptor status between women with DM versus DBT (Table 1).
Table 1.
Patients’ characteristics comparing patients with DM detected cancer with those with DBT detected cancers.
| DM cohort (n = 201) | DBT cohort (n = 187) | P-Value | |
|---|---|---|---|
| Age (mean) | 55.26 | 55.55 | 0.817 |
| Mammographic findings | 0.321 | ||
| Asymmetry | 24 (12%) | 17 (9%) | |
| Mass | 125 (62.1%) | 106 (57%) | |
| Architectural distortion | 14 (7%) | 17 (9%) | |
| Calcifications | 38 (18.9%) | 47 (25%) | |
| Tumor subtype | 0.070 | ||
| DCIS | 27 (13.4%) | 37 (19.7%) | |
| IDC | 152 (75.6%) | 139 (74.3%) | |
| ILC | 22 (10.9%) | 11 (5.8%) | |
| Tumor grade | 0.835 | ||
| I | 38 (18.9%) | 38 (20.3%) | |
| II | 92 (45.7%) | 80 (42.7%) | |
| III | 71 (35.3%) | 69 (36.9%) | |
| T-stage | 0.065 | ||
| T1 | 125 (62.2%) | 140 (74.8%) | |
| T2 | 61 (30.3%) | 38 (20.3%) | |
| T3 | 13 (6.4%) | 8 (4.3%) | |
| T4 | 2 (1%) | 1 (0.5%) | |
| N-stage | 0.512 | ||
| N0 | 146 (73%) | 148 (79.1%) | |
| N1 | 38 (18.9%) | 26 (13.9%) | |
| N2 | 12 (5.9%) | 9 (4.8%) | |
| N3 | 5 (2.5%) | 4 (2.1%) | |
| Estrogen-receptor | 0.991 | ||
| Positive | 156 (77.6%) | 137 (73.3%) | |
| Negative | 45 (22.4%) | 50 (26.7%) | |
| Progesterone-receptor | 0.793 | ||
| Positive | 139 (69.2%) | 127 (67.9%) | |
| Negative | 62 (30.8%) | 60 (32.1%) | |
| HER2 | 0.549 | ||
| Positive | 47 (23.4%) | 39 (20.9%) | |
| Negative | 154 (76.6%) | 148 (79.1%) |
The mammographic findings prompting biopsy yielding malignancy were classified as the following: 41 (10.5%) asymmetries; 231 (59.5%) masses, 31 (8%) architectural distortions, and 85 (22%) calcifications, demonstrating no significant difference when comparing DM vs. DBT group (Table 1). Cancer was unifocal in 327 cases (84.3%) and multifocal in 61 (15.7%).
3.2. Characteristics of occult malignant lesions detected at MR
In 303 patients (78.1%), MR did not find any additional lesions. In the 81 patients (20.8%) with additional MR lesions, biopsy yielded malignancy in 50 cases (12.9%) (true-positive) and 31 patients (8%) had benign biopsy results (false-positives). Four patients (1%) had additional mammographic findings (asymmetry in 2 and focal asymmetry in 2 cases without any calcifications or sonographic correlate) on conventional imaging (DM or DBT) that were subsequently characterized as benign on MR.
In 81 patients with additional MR findings, 128 additional lesions were identified by MR, including 50 malignant lesions (in 50 patients), 10 high-risk lesions (in 10 patients), and 68 benign lesions (in 34 patients; 50% fibrocystic changes). Twelve patients had both additional malignant and benign lesions and 1 case had both benign and high-risk additional lesions. Of the 50 additional malignant lesions detected by MR, 37 were ipsilateral (74%) and 13 were contralateral (26%%) cancers, including 22 invasive cancer (44%) and 28 (56%) DCIS. Table 2 summarizes the characterizations of the additional malignancies detected on MRI.
Table 2.
Characterization of additional malignancy detected on MRI.
| Additional malignancies (n = 50) | |
|---|---|
| Tumor subtype | |
| DCIS | 28 (56%) |
| IDC | 18 (36%) |
| ILC | 4 (8%) |
| Tumor side | |
| Ipsilateral | 37 (74%) |
| Contralateral | 13 (26%) |
| MRI finding | |
| Mass | 37 (74%) |
| Non-mass enhancement | 13 (26%) |
| Histological diagnosis | |
| MR guided biopsy | 23 (46%) |
| US guided biopsy | 12 (24%) |
| Localization for excision | 7 (14%) |
| Mastectomy | 8 (16%) |
3.3. Performance of MR based on the mammographic modality
There was no significant difference in discordant MR findings (p = 0.33), cancer detection (p = 0.53), or false-positive exams (p = 0.47) in women who underwent DM versus DBT (Table 3). The performance of MR to detect additional disease was similar in patients initially imaged with DM alone versus DBT with comparable PPVs (62.9%; 95% CI 44.9–78.0% and 60.9%; 95% CI 45.4–74.5%), respectively. Figs. 1–4 show a true-positive and false-positive case from each imaging modality.
Table 3.
MR detected additional malignancy based on modality, mammographic breast density and MR breast parenchymal enhancement.
| Discordant results (%) | True positive (%) | False positive (%) | No additional disease (%) | |
|---|---|---|---|---|
| DM cohort (n = 201) | 48 (23%) | 28 (14%) | 18 (9%) | 155 (77%) |
| DBT cohort (n = 187) | 37 (20%) p = 0.330 |
22 (12%) p = 0.530 |
13 (7%) p = 0.470 |
152 (81%) p = 0.313 |
| Non-dense (n = 195) | 31 (16%) | 19 (10%) | 11(6%) | 165 (85%) |
| Dense (n = 193) | 54 (28%) p = 0.004 |
31 (16%) p = 0.062 |
20 (10%) p = 0.086 |
142 (73%) p = 0.007 |
| Low BPE (n = 275) | 52 (19%) | 32 (12%) | 17 (6%) | 226 (88%) |
| High BPE (n = 113) | 33 (30%) p = 0.020 |
18 (16%) p = 0.250 |
14 (13%) p = 0.040 |
81 (72%) p = 0.020 |
DM: digital mammography.
DBT: digital breast tomosynthesis.
BPE: background parenchymal enhancement.
No additional disease: defined as a negative result by MR.
The p-values of less than 0.05 are marked as bold.
Fig. 1.
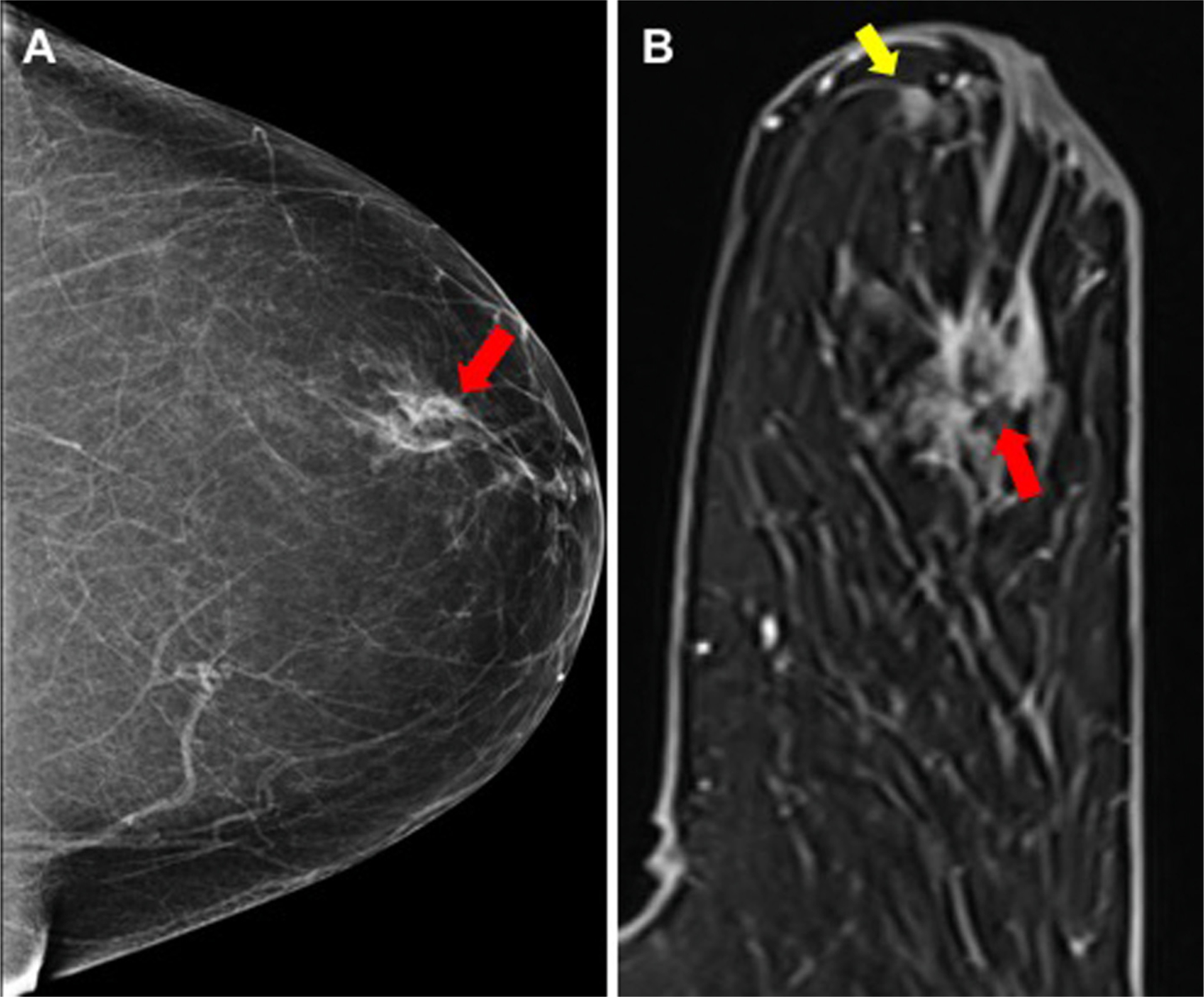
True-positive MR finding not seen on DM imaging: (A) DM showed irregular mass with indistinct margins in the retroareolar left breast (red arrow) in 64-year-old woman with pathology revealing invasive ductal carcinoma, (B) MR showed additional mass (yellow arrow) more than 2 cm anterior to the index cancer not appreciated on DM or physical exam, histopathology confirms that the additional focus is malignant. There was also DCIS involving the nipple-areolar complex.
Fig. 4.
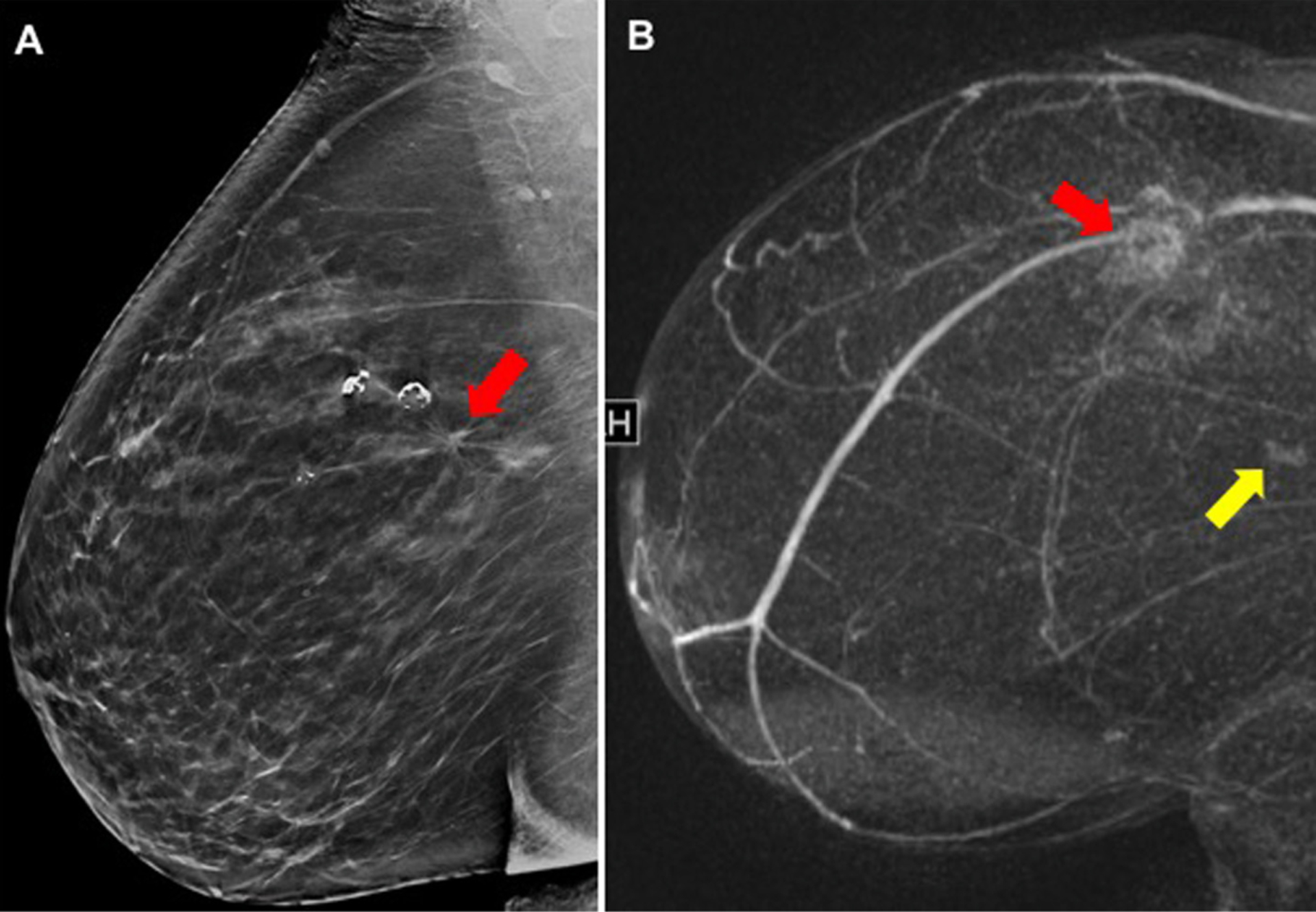
False-positive MR finding after DBT imaging: (A) DBT shows an asymmetry with architectural distortion in the right breast (red arrow) in 58-year-old woman. Biopsy yields invasive ductal carcinoma. (B) On MR, additional finding was a mass detected in the posterior (yellow arrow) and inferior right breast; biopsy yields a sclerosed fibroadenoma, benign results.
3.4. Performance of MR based on the breast density
Performance of MR was further stratified by mammographic breast density in the entire cohort (Table 3) and separately for DM and DBT (Table 4). The performance of MR to detect additional disease was similar in dense and non-dense breasts, however, there were significantly higher discordant MR findings in patients with dense breasts (p = 0.004) with a trend toward significance for true-positive exams (p = 0.06; Table 3). In patients imaged with DBT, those with non-dense breasts had significantly fewer additional cancers detected by MR (7% vs 18%, p = 0.017) and also had less false-positive findings (p = 0.014). However, in patients imaged with DM, there was no significant difference in the MR detection of additional cancer (p = 0.79) or false positive rates (p = 0.94) when comparing patients based on breast density.
Table 4.
MR detected additional malignancy stratified by breast mammographic density.
| Density | Total | True positives (%) | False positive (%) | No add. disease (%) | |
|---|---|---|---|---|---|
| DM | Non-dense | 91 | 12 (13%) | 8 (9%) | 71 (78%) |
| Dense | 110 | 16 (14%) p = 0.790 |
10 (10%) p = 0.941 |
84 (76%) p = 0.781 |
|
| DBT | Non-dense | 104 | 7 (7%) | 3 (3%) | 94 (90%) |
| Dense | 83 | 15 (18%) p = 0.017 |
10 (12%) p = 0.014 |
58 (70%) p = 0.001 |
DM: digital mammography.
DBT: digital breast tomosynthesis.
No additional disease: defined as a negative result by MR including both TN and FN.
The p-values of less than 0.05 are marked as bold.
3.5. Performance of MR based on BPE
Background parenchymal enhancement on MR was significantly higher in patients with mammographically dense breasts (p = 0.001). Overall, higher BPE was associated with higher discordant MR findings and higher false-positive rates (p = 0.02 and 0.04, respectively) but no significant difference in true-positive exams (p = 0.25). When stratifying MR detection rates in patients with high BPE by DM versus DBT imaging, there was a trend toward higher true-positive MR findings in patients that had DM compared to DBT (21% vs. 10%), however the difference was not significant (p = 0.160; Table 5).
Table 5.
MR detected additional malignancy stratified by BPE.
| Density | Total | True positives (%) | False positive (%) | No add. disease (%) | |
|---|---|---|---|---|---|
| Low | DM | 143 | 16 (11%) | 12 (8%) | 115 (81%) |
| BPE | DBT | 132 | 16 (12%) p = 0.810 |
5 (4%) p = 0.113 |
111 (85%) p = 0.427 |
| High | DM | 58 | 12 (21%) | 6 (11%) | 40 (68%) |
| BPE | DBT | 55 | 6 (10%) p = 0.160 |
8 (14%) p = 0.498 |
41(74%) p = 0.511 |
DM: digital mammography.
DBT: digital breast tomosynthesis.
BPE: background parenchymal enhancement.
No additional disease: defined as a negative result by MR including both TN and FN.
4. Discussion
This study compared the role of preoperative breast MR to assess the extent of disease in women newly diagnosed with breast cancer, imaged with DM versus DBT at diagnosis. MR detected additional foci of cancer, regardless of the imaging modality used at diagnosis.
It is well known that increased breast density is associated with both a decrease in mammographic sensitivity as well as an increase in the risk of developing breast cancer. Approximately 43% of women aged 40 to 74 years in the United States have dense breasts [16] and a growing body of evidence suggests that some of them may benefit from supplemental screening. However, after a breast cancer is diagnosed, there are no specific guidelines for recommending staging MR based on breast density alone. In our study, we found a trend of increasing detection by MR of additional sites of malignancies in patients with dense breasts compared to those with non-dense breasts, irrespective of initial imaging modality – DM versus DBT.
Digital breast tomosynthesis has higher cancer detection in both dense and non-dense breasts [6], however, little data is available comparing the performance of DBT to MR in assessing the extent of cancer based on breast density. Prior studies have demonstrated that MR is more sensitive and less specific for malignancy than conventional mammography [21], matching our dataset. To optimize the use of breast MR in pre-surgical planning, it is necessary to identify a subset of patients who will benefit most from this exam. Girardi et al. suggested that MR can detect synchronous lesions in both dense and fatty breasts without statistically significant differences [23]. In our study, women with lower breast density and available DBT imaging had fewer additional sites of malignancy detected on MR compared to women with dense breasts (7% versus 18%, respectively, p = 0.017). However, in women imaged with DM, MR found additional malignancy across all breast densities. These findings suggest that in women imaged with DBT, MR may have less value for determining extent of disease in women with non-dense breasts. These data complement a recent prospective study comparing diagnostic accuracy of mammography (DM vs. DM/DBT) in 166 women with breast cancer showing that the added diagnostic value of DBT was limited to the group of women with lower breast density [9]. Despite increased detection of breast cancer on DBT, this modality is still an x-ray-based, anatomic imaging technique and studies have reported fewer improvements in cancer detection with DBT in extremely dense breast tissue compared to other density subgroups [17]. Rafferty et al., demonstrated that DBT did not improve the breast cancer detection rate in extremely dense breasts, when compared to DM alone screening [17]. Fat–fibroglandular tissue interface is required in order to identify small masses and architectural distortions on DBT slabs, otherwise the overlapping tissue can still obscure such subtle findings in dense breasts []. This may explain why, in our cohort, MR still found a significant number of additional cancers in women with dense breasts, even if imaged with DBT.
Although it has been shown that BPE is influenced by hormonal changes and breast cancer risk increases with higher BPE [25,26], there are controversial reports in regards to the correlation of BPE and mammographic breast density. Our results show significantly higher BPE in patients with dense breasts. In addition, higher BPE was associated with a higher rate of false-positive MR findings but was not associated with a decrease in cancer detection, as found in prior studies [27,28].
Our study has several limitations. While we did include consecutive cases, this was retrospective at a single, academic center and selection bias may have existed. Another limitation is that some patients were excluded when pathology did not report the entire mastectomy specimen and lesions identified as suspicious on MR could not be localized for evaluation. Finally, in 4 cases, there were subtle mammographic asymmetries/focal asymmetries with no sonographic correlate and no enhancement on MR that were assumed to represent benign parenchyma without biopsy. We also performed subgroup analyses of our data to evaluate role of density and BPE and in some subgroups, the comparisons may be affected due to a relatively small size. For this reason, the conclusions are based predominantly on analyses of all lesions in all patients and there is a need for further prospective studies to evaluate the findings.
In conclusion, among patients with DBT imaging at cancer diagnosis, women with dense breasts appear to benefit more from preoperative MR than non-dense women. In women imaged only with DM, MR finds additional malignancy in both dense and non-dense breasts.
Fig. 2.
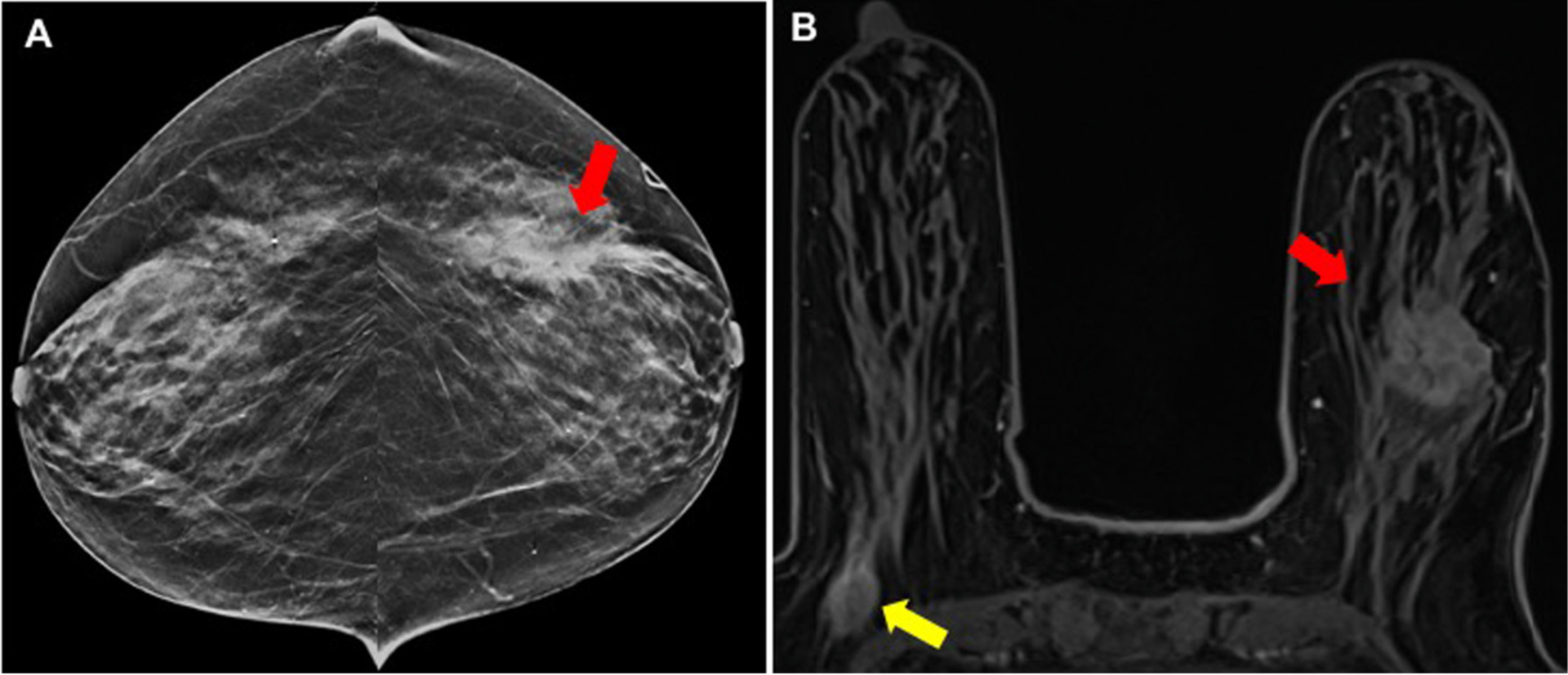
True-positive MR finding not seen on DBT imaging: An 80-year-old woman presented with a palpable lump in the left breast. (A) DBT demonstrates a 2.6 cm, irregular mass with biopsy yielding invasive ductal carcinoma (red arrow). (B) MR shows the biopsy proven cancer and additional 1.5 cm enhancing mass in the axillary tail of the contralateral, right breast (yellow arrow). Biopsy confirms invasive mammary carcinoma in the right breast.
Fig. 3.
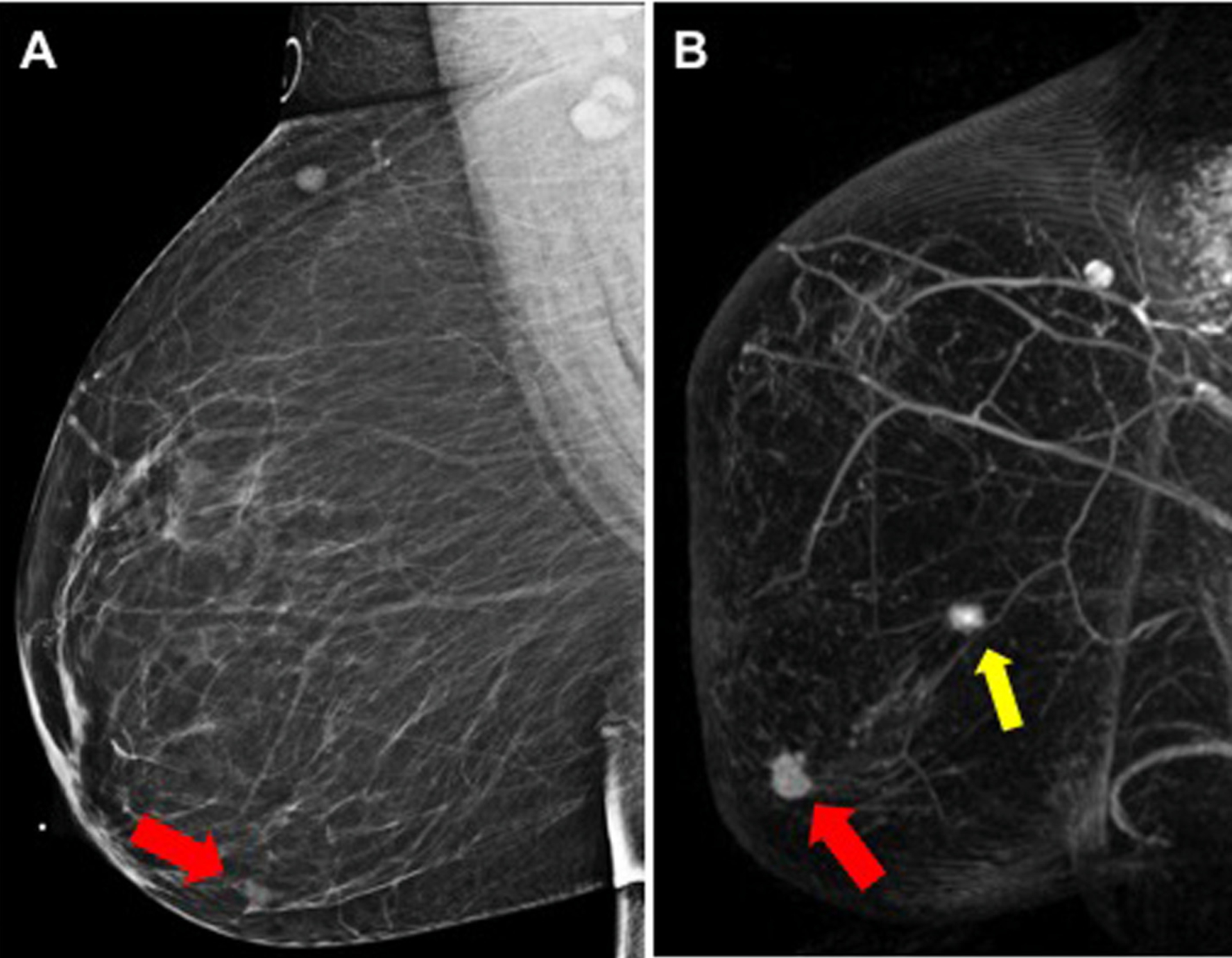
False-positive MR finding after DM-alone imaging: (A) DM shows an obscured mass in the lower-outer right breast (red arrow), in 51-year-old woman with biopsy yielding invasive ductal carcinoma, (B) MR showed an additional enhancing mass (yellow arrow), posterior to the index cancer; biopsy yields benign fibrocystic changes.
Funding
This work was supported by NIH/NCI grant P30CA016520 to Dr. Qi Long. Dr. McDonald received salary support from the Susan G. Komen Foundation CCR16376362.
References
- [2].Rafferty EA, Park JM, Philpotts LE, Poplack SP, Sumkin JH, Halpern EF, et al. Assessing radiologist performance using combined digital mammography and breast tomosynthesis compared with digital mammography alone: results of a multicenter, multireader trial. Radiology 2013;266(1):104–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gur D, Abrams GS, Chough DM, Ganott MA, Hakim CM, Perrin RL, et al. Digital breast tomosynthesis: observer performance study. AJR Am J Roentgenol 2009;193(2):586–91. [DOI] [PubMed] [Google Scholar]
- [4].Park JM, Franken EA Jr, Garg M, Fajardo LL, Niklason LT. Breast tomosynthesis: present considerations and future applications. Radiographics 2007;27(Suppl. 1):S231–40. [DOI] [PubMed] [Google Scholar]
- [5].McDonald ES, Oustimov A, Weinstein SP, Synnestvedt MB, Schnall M, Conant EF. Effectiveness of digital breast tomosynthesis compared with digital mammography: outcomes analysis from 3 years of breast cancer screening. JAMA Oncol 2016;2(6):737–43. [DOI] [PubMed] [Google Scholar]
- [6].Conant EF, Barlow WE, Herschorn SD, Weaver DL, Beaber EF, Tosteson ANA, et al. Association of digital breast tomosynthesis vs digital mammography with cancer detection and recall rates by age and breast density. JAMA Oncol 2019;5(5):635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bahl M, Mercaldo S, Dang PA, McCarthy AM, Lowry KP, Lehman CD. Breast cancer screening with digital breast tomosynthesis: are initial benefits sustained? Radiology 2020:191030. [DOI] [PubMed] [Google Scholar]
- [8].Mariscotti G, Houssami N, Durando M, Bergamasco L, Campanino PP, Ruggieri C, et al. Accuracy of mammography, digital breast tomosynthesis, ultrasound and MR imaging in preoperative assessment of breast cancer. Anticancer Res 2014;34(3):1219–25. [PubMed] [Google Scholar]
- [9].Fontaine M, Tourasse C, Pages E, Laurent N, Laffargue G, Millet I, et al. Local tumor staging of breast cancer: digital mammography versus digital mammography plus tomosynthesis. Radiology 2019;291(3):594–603. [DOI] [PubMed] [Google Scholar]
- [10].Houssami N, Turner R, Morrow M. Preoperative magnetic resonance imaging in breast cancer: meta-analysis of surgical outcomes. Ann Surg 2013;257(2):249–55. [DOI] [PubMed] [Google Scholar]
- [11].Mariscotti G, Durando M, Tagliafico A, Campanino PP, Bosco D, Casella C, et al. Preoperative breast cancer staging with multi-modality imaging and surgical outcomes. Eur J Radiol 2020;122:108766. [DOI] [PubMed] [Google Scholar]
- [12].Keymeulen K, Geurts SME, Lobbes MBI, Heuts EM, Duijm LEM, Kooreman LFS, et al. Population-based study of the effect of preoperative breast MRI on the surgical management of ductal carcinoma in situ. Br J Surg 2019;106(11):1488–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kim WH, Chang JM, Moon HG, Yi A, Koo HR, Gweon HM, et al. Comparison of the diagnostic performance of digital breast tomosynthesis and magnetic resonance imaging added to digital mammography in women with known breast cancers. Eur Radiol 2016;26(6):1556–64. [DOI] [PubMed] [Google Scholar]
- [14].Chudgar AV, Conant EF, Weinstein SP, Keller BM, Synnestvedt M, Yamartino P, et al. Assessment of disease extent on contrast-enhanced MRI in breast cancer detected at digital breast tomosynthesis versus digital mammography alone. Clin Radiol 2017;72(7):573–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].D’Orsi CJ, Sickles EA, Mendelson EB, Morris EA. BI-RADS® Atlas, breast imaging reporting and data system. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- [16].Sprague BL, Gangnon RE, Burt V, Trentham-Dietz A, Hampton JM, Wellman RD, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst 2014;106(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rafferty EA, Durand MA, Conant EF, Copit DS, Friedewald SM, Plecha DM, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA 2016;315(16):1784–6. [DOI] [PubMed] [Google Scholar]
- [21].Sardanelli F, Giuseppetti GM, Panizza P, Bazzocchi M, Fausto A, Simonetti G, et al. Sensitivity of MRI versus mammography for detecting foci of multifocal, multicentric breast cancer in fatty and dense breasts using the whole-breast pathologic examination as a gold standard. AJR Am J Roentgenol 2004;183(4):1149–57. [DOI] [PubMed] [Google Scholar]
- [23].Girardi V, Carbognin G, Camera L, Baglio I, Bucci A, Bonetti F, et al. Multifocal, multicentric and contralateral breast cancers: breast MR imaging in the preoperative evaluation of patients with newly diagnosed breast cancer. Radiol Med 2011;116(8):1226–38. [DOI] [PubMed] [Google Scholar]
- [25].Delille JP, Slanetz PJ, Yeh ED, Kopans DB, Garrido L. Physiologic changes in breast magnetic resonance imaging during the menstrual cycle: perfusion imaging, signal enhancement, and influence of the T1 relaxation time of breast tissue. Breast J 2005;11(4):236–41. [DOI] [PubMed] [Google Scholar]
- [26].King V, Brooks JD, Bernstein JL, Reiner AS, Pike MC, Morris EA. Background parenchymal enhancement at breast MR imaging and breast cancer risk. Radiology 2011;260(1):50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ray KM, Kerlikowske K, Lobach IV, Hofmann MB, Greenwood HI, Arasu VA, et al. Effect of background parenchymal enhancement on breast MR imaging interpretive performance in community-based practices. Radiology 2018;286(3):822–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].DeMartini WB, Liu F, Peacock S, Eby PR, Gutierrez RL, Lehman CD. Background parenchymal enhancement on breast MRI: impact on diagnostic performance. AJR Am J Roentgenol 2012;198(4):W373–80. [DOI] [PubMed] [Google Scholar]


