Abstract
Purpose:
To evaluate a triplet regimen combining immune checkpoint blockade, AKT pathway inhibition, and (nab-) paclitaxel as first-line therapy for locally advanced/metastatic triple-negative breast cancer (mTNBC).
Patients and Methods:
The single-arm CO40151 phase Ib study (NCT03800836), the single-arm signal-seeking cohort of IPATunity130 (NCT03337724), and the randomized phase III IPATunity170 trial (NCT04177108) enrolled patients with previously untreated mTNBC. Triplet therapy comprised intravenous atezolizumab 840 mg (days 1 and 15), oral ipatasertib 400 mg/day (days 1–21), and intravenous paclitaxel 80 mg/m2 (or nab-paclitaxel 100 mg/m2; days 1, 8, and 15) every 28 days. Exploratory translational research aimed to elucidate mechanisms and molecular markers of sensitivity and resistance.
Results:
Among 317 patients treated with the triplet, efficacy ranged across studies as follows: median progression-free survival (PFS) 5.4 to 7.4 months, objective response rate 44% to 63%, median duration of response 5.6 to 11.1 months, and median overall survival 15.7 to 28.3 months. The safety profile was consistent with the known toxicities of each agent. Grade ≥3 adverse events were more frequent with the triplet than with doublets or single-agent paclitaxel. Patients with PFS >10 months were characterized by NF1, CCND3, and PIK3CA alterations and increased immune pathway activity. PFS <5 months was associated with CDKN2A/CDKN2B/MTAP alterations and lower predicted phosphorylated AKT-S473 levels.
Conclusions:
In patients with mTNBC receiving an ipatasertib/atezolizumab/taxane triplet regimen, molecular characteristics may identify those with particularly favorable or unfavorable outcomes, potentially guiding future research efforts.
Translational Relevance.
Combined immune checkpoint blockade, AKT pathway inhibition, and taxane therapy is a rational therapeutic strategy aimed at improving the efficacy of immunotherapy in triple-negative breast cancer. Although overall clinical outcomes were modest, translational studies provide valuable insight into the molecular characteristics and mechanisms associated with sensitivity and resistance. Longer (>10 months) progression-free survival (PFS) was characterized by NF1, CCND3, and PIK3CA alterations; increased baseline stromal tumor-infiltrating lymphocytes and CD8 T cells; and increased baseline immune pathway activity, whereas shorter (<5 months) PFS was associated with CDKN2A/CDKN2B/MTAP alterations and lower predicted phosphorylated AKT-S473 levels. These biomarker findings may guide future trial design and patient selection, including a priori identification of patients with highly resistant disease or disease that is likely to progress rapidly, for whom better treatment options are urgently required.
Introduction
Triple-negative breast cancer (TNBC) represents a heterogeneous group of cancers, some of which are associated with an aggressive course and a dismal prognosis. As biological understanding deepens and new targets are identified, the implications of genomic and proteomic diversity have an increasingly profound impact on prognosis and treatment strategies (1, 2). Among the most important developments in the management of metastatic TNBC in recent years has been the incorporation of immune checkpoint inhibitors targeting programmed death-1 (PD-1) or programmed death ligand-1 (PD-L1) into first-line therapy for PD-L1–positive metastatic TNBC. Two randomized phase III trials [KEYNOTE-355 evaluating the addition of pembrolizumab to standard chemotherapy (3) and IMpassion130 evaluating the addition of atezolizumab to nanoparticle albumin-bound (nab-) paclitaxel (4)] demonstrated improved progression-free survival (PFS) compared with chemotherapy alone in patients with PD-L1–positive tumors. Both trials also demonstrated a clinically meaningful improvement in overall survival (OS) in PD-L1–positive TNBC (5, 6). In contrast, IMpassion131 showed no benefit from the addition of atezolizumab to conventional paclitaxel (7).
Although immunotherapy has transformed the management of metastatic TNBC for patients with PD-L1–positive tumors, outcomes remain poor with a median OS of approximately 2 years (5, 6). For the 60% of patients with PD-L1–negative TNBC (3, 4), treatment options are even more limited and novel therapeutic strategies are needed. A potential mechanism for resistance to checkpoint inhibitors is loss of PTEN, a negative regulator of AKT. The phosphatidylinositol 3-kinase (PI3K)/AKT signaling pathway is frequently activated in TNBC (8) and represents a promising target. Genetic alterations in phosphatidylinositol-4, 5-bisphosphate 3-kinase, catalytic subunit, alpha (PIK3CA), AKT1, and/or PTEN were previously reported in 44% of almost 800 patients with metastatic TNBC (9). Preclinical models suggested that sensitivity to AKT inhibitors was associated with activation of PI3K and/or PTEN deletions (10, 11). In the clinical setting, two randomized phase II trials [LOTUS evaluating ipatasertib (12); PAKT evaluating capivasertib (13)] demonstrated improved PFS when an AKT inhibitor was combined with paclitaxel as first-line therapy for metastatic TNBC. Both trials assessed the doublet combination in an unselected population, but the effect on PFS was more pronounced in predefined populations of patients with PIK3CA/AKT1/PTEN-altered tumors. These observations supported phase III evaluation of ipatasertib in a biomarker-selected population of patients, and the IPATunity130 randomized phase III trial (NCT03337724) was initiated to evaluate a doublet of ipatasertib and paclitaxel in patients with PIK3CA/AKT1/PTEN-altered tumors. However, final OS results from LOTUS suggested an effect of ipatasertib irrespective of PIK3CA/AKT1/PTEN alteration (14), perhaps explained by the heterogeneity of the population and the relatively small sample size. Therefore, interest remained to explore ipatasertib in a broader population of patients.
Inhibiting the PI3K/AKT pathway with a PI3K-beta inhibitor has been shown to reverse resistance to T-cell–mediated immunotherapy and the combination of PI3K-beta inhibition and PD-(L)1 axis blockade showed synergistic antitumor responses (15). AKT inhibitors may restore or enhance physiologic function of T cells in the tumor microenvironment and enhance expansion of tumor-specific subpopulations with a stem-like memory cell phenotype (16, 17). Concurrent ipatasertib therapy may enhance checkpoint inhibitor efficacy by retaining a stem-like phenotype in memory T cells, preventing exhaustion and enabling a long-term response in patients (18, 19). Thus, by targeting both the AKT pathway and immune checkpoints simultaneously, it was anticipated that adaptive resistance mechanisms would be less likely to emerge. There is also strong preclinical evidence that AKT inhibition would sensitize tumors to treatment with PD-L1 inhibition (20, 21). These results, as well as the tolerability and limited overlapping toxicity of atezolizumab, ipatasertib, and (nab-)paclitaxel, provided the rationale for evaluating a triplet regimen combining these three agents.
A multicenter phase Ib study (CO40151) was initiated to assess the feasibility, tolerability, and early efficacy signals of a triplet combining atezolizumab, ipatasertib, and a taxane as first-line therapy for locally advanced/metastatic TNBC. Preliminary results from the first 26 patients after a median follow-up of 6.1 months demonstrated a 73% confirmed objective response rate (ORR; ref. 22). Responses were seen irrespective of PD-L1 status or PIK3CA/AKT1/PTEN alteration status. These initial results reported in early 2019 led to evaluation of the triplet regimen in Cohort C of the IPATunity130 trial in patients with TNBC whose tumors did not have PIK3CA/AKT1/PTEN alterations, and later in the global randomized phase III IPATunity170 trial, which compared the triplet regimen against doublets or paclitaxel alone in two independently enrolled cohorts according to PD-L1 status.
We report final results from the phase Ib CO40151 study, together with data generated for the triplet regimen in the two subsequent phase III trials, IPATunity170 and Cohort C of IPATunity130. CO40151 included in-depth translational studies, aiming to elucidate mechanisms of sensitivity and resistance to the combination. Clinical and key translational results are presented together in this report to provide a comprehensive picture detailing the clinical evaluation of a triplet combining immune checkpoint blockade, AKT pathway inhibition, and taxane therapy for TNBC.
Patients and Methods
Patient populations
Results are reported from three separate trials: CO40151 (NCT03800836), IPATunity130 (NCT03337724; CO40016) Cohort C, and IPATunity170 (NCT04177108; CO41101). The cohorts and treatment arms included in this report are based on patients sharing the following eligibility criteria: women or men with unresectable locally advanced/metastatic TNBC, measurable disease according to RECIST version 1.1, no prior systemic therapy for locally advanced/metastatic TNBC, Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1, and no prior AKT inhibitor therapy. Tumors were assessed every 8 weeks according to RECIST version 1.1.
In all three trials, the protocol, informed consent form, patient materials, and relevant supporting information were approved by an Institutional Review Board/Ethics Committee before study initiation. Patients in all three trials provided signed informed consent before undergoing any study-specific procedures. The trials were conducted in full conformance with the International Conference on Harmonisation E6 guideline for Good Clinical Practice and the principles of the Declaration of Helsinki, or the applicable laws and regulations of the country in which the research was conducted, whichever afforded the greater protection to the individual.
Study designs
The designs of the three studies are summarized in Table 1 and presented in more detail in Supplementary Fig. S1. CO40151 was a multicenter phase Ib study evaluating a triplet of ipatasertib, atezolizumab, and a taxane (either paclitaxel or nab-paclitaxel) in Cohort 1. The co-primary efficacy endpoints were investigator-assessed confirmed ORR according to RECIST version 1.1 and DoR. There was no formal statistical hypothesis testing in this study.
Table 1.
Summary of trial designs.
| CO40151 | IPATunity130, Cohort C | IPATunity170 | |
|---|---|---|---|
| Design | Phase Ib | Open-label single-arm cohort within phase III | Two-cohort placebo-controlled randomized phase III |
| Geographic locations | Australia, France, Spain, UK, USA | Europe, North and South America, Asia | Europe, Asia, Australia, North and South America |
| Taxane backbone | Paclitaxel or nab-paclitaxel | Paclitaxel | Paclitaxel |
| Comparator arms | None | None | PD-L1–positive cohort: atezolizumab + paclitaxel |
| PD-L1–negative/unknown cohort: paclitaxel alone or paclitaxel + ipatasertib | |||
| PIK3CA/AKT1/PTEN mutation status | Unselected | Not altered | Unselected |
| Known BRCA1/2 mutation eligible | Yes | Yes | No (unless ineligible for PARP inhibitor) |
| Primary endpoint | Confirmed ORR and DoR | PFS | PFS and OS |
| Secondary efficacy endpoints | PFS, confirmed CBR, OS | Confirmed ORR (RECIST version 1.1), DoR, CBR, OS, 1-year PFS rate, 1-year OS rate | All nonprimary predefined endpoints were exploratory |
Abbreviations: CBR, clinical benefit rate (stable disease for ≥24 weeks or confirmed complete/partial response); DoR, duration of response (interval between confirmed complete/partial response and disease progression or death).
The phase III IPATunity130 trial included two independent randomized cohorts each comparing the doublet of ipatasertib plus paclitaxel versus placebo plus paclitaxel in patients with measurable locally advanced or metastatic breast cancer [Cohort A in TNBC (23) and Cohort B in hormone receptor–positive HER2-negative breast cancer not suitable for endocrine therapy (24), both enrolling only patients with PIK3CA/AKT1/PTEN alterations]. Neither of these two randomized cohorts demonstrated significantly improved efficacy with the addition of ipatasertib to paclitaxel (23, 24). Here we focus on Cohort C, an open-label single-arm signal-seeking cohort in patients with locally advanced/metastatic TNBC who were screened for Cohort A but found to have no PIK3CA/AKT1/PTEN alteration. Cohort C evaluated a triplet regimen of ipatasertib, atezolizumab, and paclitaxel as first-line therapy. The primary endpoint was PFS, defined as the interval between enrollment and investigator-assessed disease progression or death.
The phase III IPATunity170 trial included two independent cohorts evaluating the efficacy and safety of a triplet combining ipatasertib, atezolizumab, and paclitaxel as first-line therapy for locally advanced/metastatic TNBC. Patients with known germline BRCA1/2 mutation were ineligible unless they were not a candidate for a PARP inhibitor. Cohort 1 enrolled patients with PD-L1–nonpositive tumors (negative or unknown status) and compared the triplet versus a doublet of ipatasertib plus paclitaxel, and ipatasertib plus paclitaxel versus paclitaxel alone. Cohort 2 enrolled patients with PD-L1–positive tumors and compared the triplet versus a doublet of atezolizumab and paclitaxel. Patients were stratified according to geographic region, prior (neo)adjuvant taxane, and prior (neo)adjuvant cancer immunotherapy; patients were randomized in a 1:1:1 ratio in Cohort 1 and a 1:1 ratio in Cohort 2. The co-primary endpoints were investigator-assessed PFS (according to RECIST version 1.1) and OS. Investigator-assessed confirmed ORR, DoR, and CBR were exploratory endpoints, with efficacy evaluation in subgroups with PIK3CA/AKT1/PTEN-altered tumors predefined. Initially it was planned to enroll up to 1,155 patients from 350 sites globally. Enrollment to Cohort 2 (PD-L1–positive TNBC) was closed on August 6, 2020, following the readout of the IMpassion131 trial (NCT03125902) indicating that atezolizumab plus paclitaxel was not an appropriate control arm in PD-L1–positive TNBC (7). Enrollment to Cohort 1 (PD-L1–nonpositive TNBC) was closed on September 18, 2020, after unblinding of the TNBC portion of the IPATunity130 trial showing no benefit from the addition of ipatasertib to paclitaxel (23).
Treatment regimen
In all three trials, ipatasertib was administered orally at a fixed dose of 400 mg/day on days 1 to 21. Atezolizumab was administered intravenously at 840 mg on days 1 and 15. Taxane chemotherapy consisted of paclitaxel 80 mg/m2 (or nab-paclitaxel 100 mg/m2 in some cohorts of CO40151) administered intravenously on days 1, 8, and 15. Cycles were repeated every 28 days until loss of clinical benefit, unacceptable toxicity, withdrawal of consent, or study completion or termination. Given the risk of rash, patients were to receive ≥10 mg prednisone (or equivalent) as premedication before atezolizumab administration during cycle 1, followed by a fixed 10 mg prednisone dose for 2 to 4 days thereafter, unless contraindicated. In Arms C and D of the phase Ib CO40151 study, one drug was omitted for the first 2 weeks of the cycle (day 1 atezolizumab in Arm C, days 1–14 ipatasertib in Arm D) to enable additional translational research.
Translational research
Formalin-fixed paraffin-embedded (FFPE) tissue blocks or freshly cut unstained serial tumor slides from the most recently collected tumor tissue sample were mandatory in all trials for central molecular analysis. PD-L1 status was determined using the Ventana SP142 IHC assay (Ventana Medical Systems), with PD-L1–positive status defined as immune cell expression in ≥1% of the tumor area. PD-L1 status was also determined (retrospectively) by pathologists at HistoGeneX (now CellCarta NV) using the 22C3 pharmDx assay (Agilent). PIK3CA/AKT1/PTEN alteration status was assessed using the FoundationOne CDx (Foundation Medicine) in CO40151 and the Foundation Medicine Clinical Trial Algorithm in IPATunity130 and IPATunity170. CD8 expression was assessed by IHC (VR-454 Ventana clone C8/144B). Tumor and stromal tumor-infiltrating lymphocytes (TIL) were assessed by certified pathologists at HistoGeneX using hematoxylin and eosin–stained slides to calculate the percentage of viable tumor area occupied by immune cells and the percentage of the desmoplastic stroma area occupied by immune cells. Gene expression was assessed by RNA sequencing. A combination of differential gene expression (http://bioconductor.org/packages/release/bioc/html/limma.html), gene set enrichment analysis (GSEA; https://github.com/GSEA-MSigDB/GSEA_R and https://bioconductor.org/packages/release/bioc/html/fgsea.html) performed as described previously (25), and machine-learning (random forest; https://cran.r-project.org/web/packages/randomForest/index.html) approaches were used to study the effect of molecular pathways on responses and resistance to the combination of ipatasertib with atezolizumab and paclitaxel or nab-paclitaxel. Genomically adjacent and co-mutated genes were grouped and analyzed together (e.g., CDKN2A, CDKN2B, and MTAP).
To explore molecular characteristics of patients with primary refractory disease or exceptionally long disease stabilization within the phase Ib study, patients with particularly short or long PFS were identified. For these exploratory biomarker analyses, the biomarker-evaluable patients were categorized according to the duration of PFS: <5 versus ≥5 to ≤10 versus >10 months. A cut-off of 10 months was selected to define patients with particularly long PFS based on the median PFS of 7.2 months with atezolizumab plus nab-paclitaxel in IMpassion130 (4) and 6.0 months with atezolizumab plus paclitaxel in IMpassion131 (7).
Additional analyses explored the potential role of AKT signaling on PFS with the triplet. Activation of AKT signaling is one of the hallmarks of immune exclusion and poor response to immunotherapy (15). It was previously reported that treatment benefit from immunotherapy is reduced in tumors with low immune infiltration compared with immune-infiltrated tumors (26). Therefore, we hypothesized that patients harboring tumors with activated AKT signaling may have low immune infiltration and may benefit from a combination of ipatasertib and atezolizumab. AKT signaling can be activated via genetic and nongenetic mechanisms. Therefore, to test our hypothesis, we first developed a random forest model to predict levels of phosphorylated (p)AKT-S473 using gene expression data. We trained the model using reverse-phase protein microarray and gene expression data (n = 108) using baseline (placebo plus paclitaxel or ipatasertib plus paclitaxel) optimal cutting temperature compound samples from FAIRLANE (a randomized phase II trial evaluating the addition of ipatasertib to neoadjuvant chemotherapy for TNBC; refs. 27, 28). The final model consisted of 14 protein-coding genes. As shown in Supplementary Fig. S2, the model predicted high (≥75th percentile) and low (<25th percentile) pAKT-S473 in the unseen test dataset (n = 50) treated with placebo plus paclitaxel (cycle 1, day 8) with an AUC of 0.96.
Data availability
For up-to-date details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see: https://go.roche.com/data_sharing. Anonymized records for individual patients across more than one data source external to Roche cannot, and should not, be linked due to a potential increase in risk of patient re-identification.
Role of the funding source
Authors from F. Hoffmann-La Roche/Genentech were involved in data analysis and interpretation.
Results
Patient populations and treatment exposure
Across the three trials, 317 patients received the triplet regimen. Study CO40151 enrolled 114 female patients between February 13, 2018 and February 21, 2020. IPATunity130 Cohort C enrolled 102 patients between March 25, 2019 and February 19, 2020. IPATunity170 enrolled 242 patients from November 25, 2019 until September 17, 2020 for Cohort 1 (PD-L1–negative/unknown; n = 127) and until August 6, 2020 for Cohort 2 (PD-L1–positive TNBC, n = 115). No male patients were enrolled in any of the trials, although they were eligible. There were minor differences between the trials with respect to racial diversity, reflecting the countries participating in each of the studies (Supplementary Tables S1 and S2).
Efficacy
Efficacy results from all three trials are summarized in Table 2 and shown in more detail in Supplementary Figs. S3 to S5. With the triplet, median PFS ranged from 5.4 to 7.4 months, ORR ranged from 44% to 63%, median DoR ranged from 5.6 to 11.1 months, and median OS ranged from 15.7 to 28.3 months. Across all three trials, approximately 30% of patients receiving the triplet remained alive and progression-free at 10 months.
Table 2.
Summary of efficacy (triplet regimens shown in bold).
| Characteristic | CO40151 | IPATunity130, Cohort C | IPATunity170 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PD-L1 status | + | − | Unknown | + | − | Unknown | + | − or unknown | |||
| PIK3CA/AKT1/PTEN status | Unselected | Not altered | Unselected | ||||||||
| Treatment | Atezo + ipat + pac/nab-pac | Atezo + ipat + pac | Atezo + pac | Atezo + ipat + pac | Pac | Ipat + pac | Atezo + ipat + pac | ||||
| Number of patients | 51 | 45 | 18 | 40 | 25 | 37 | 57 | 58 | 41 | 43 | 43 |
| Median duration of follow-up, months (range) | 20.7 (1.0–43.6) | 18.0 (1.8–28.4) | 11.6 (0.3–20.5) | ||||||||
| Number of PFS events (%) | 44 (86) | 39 (87) | 16 (89) | 28 (70) | 22 (88) | 28 (76) | 38 (67) | 41 (71) | 31 (76) | 31 (72) | 29 (67) |
| Median PFS, months (95% CI) | 7.3 (5.4–9.0) | 7.2 (5.4–9.1) | 6.3 (4.9–11.0) | 6.2 (5.4–9.2) | 5.4 (3.7–8.8) | 7.4 (5.5–12.8) | 5.7 (4.0–9.1) | 5.6 (5.4–9.2) | 3.7 (3.6–5.4) | 5.6 (3.7–8.2) | 7.1 (5.1–9.3) |
| ORR, n (%) [95% CI] | 32 (63) | 20 (44) | 9 (50) | 22 (55) | 13 (52) | 19 (51) | 22 (39) | 27 (47) | 6 (15) | 13 (30) | 22 (51) |
| [49–75] | [30–59] | [26–74] | [38–71] | [31–72] | [34–68] | ||||||
| Median DoR, months (95% CI) | 7.4 (5.0–12.9) | 5.6 (3.9–7.8) | 11.1 (7.6–16.7) | 8.7 (5.7–12.7) | 9.2 (5.6–NE) | 9.8 (7.4–14.5) | 7.6 (5.7–NE) | 12.6 (4.2–13.2) | 11.1 (7.4–NE) | ||
| CBR, n (%) [95% CI] | 33 (65) | 27 (60) | 10 (56) | 22 (55) | 13 (52) | 21 (57) | 27 (47) | (n = 57) 28 (49) | 9 (22) | 16 (37) | 23 (53) |
| [51–77] | [45–74] | [32–76] | [38–71] | [31–72] | [39–73] | [34–61] | [36–63] | [11–38] | [23–53] | [38–69] | |
| Deaths, n (%) | 29 (57) | 24 (53) | 6 (33) | 15 (38) | 16 (64) | 18 (49) | 17 (30) | 17 (29) | 13 (32) | 15 (35) | 16 (37) |
| Median OS, months (95% CI) | 23.3 (16.1–39.9) | 28.3 (14.2–42.2) | NE (21.3–NE) | 25.6 (17.9–NE) | 16.7 (10.4–NE) | 22.8 (19.9–NE) | 17.2 (13.4–NE) | NE (14.1–NE) | 16.6 (9.6– NE) | 15.3 (15.3–NE) | 15.7 (12.5–NE) |
Abbreviations: atezo, atezolizumab; ipat, ipatasertib; NE, not estimable; pac, paclitaxel.
In CO40151, PFS was similar between patients receiving nab-paclitaxel [median 6.6 (95% confidence interval (CI), 3.4–9.2) months] and paclitaxel [median 7.2 (95% CI, 5.3–7.4) months; Supplementary Fig. S3A]. Median OS was 20.1 (95% CI, 11.1–30.8) months with nab-paclitaxel and 28.3 (95% CI, 17.2–43.0) months with paclitaxel, although there was extensive censoring before the median in the paclitaxel group (Supplementary Fig. S3B). There were no clear differences in PFS and OS with the triplet according to PD-L1 status (Supplementary Figs. S3C and S3D). Likewise, subgroup analyses according to PIK3CA/AKT/PTEN alteration status showed no clear differences. Median PFS was 7.4 months in patients with PIK3CA/AKT/PTEN alterations versus 6.6 months in patients without, and 6.0 months in patients with unknown PIK3CA/AKT/PTEN status; corresponding values for ORR were 53%, 62%, and 42%, respectively.
In IPATunity130 Cohort C, median PFS was 6.2 (95% CI, 5.4–9.2) months in the PD-L1–positive population, 5.4 (95% CI, 3.7–8.8) months in the PD-L1–negative population, and 7.4 (95% CI, 5.5–12.8) months in the population with unknown PD-L1 status (Supplementary Fig. S4). The 1-year PFS rate in the overall population was 31% (95% CI, 22–41%) and the 1-year OS rate was 79% (95% CI, 71–87%).
In the PD-L1–positive cohort of IPATunity170, median PFS was 5.6 (95% CI, 5.4–9.2) months with the triplet and 5.7 (95% CI, 4.0–9.1) months with atezolizumab plus paclitaxel. Median OS was not estimable for the triplet; median OS was 17.2 (95% CI, 13.4–not estimable) months with atezolizumab plus paclitaxel. PFS and OS results showed no relevant difference between the doublet and triplet treatment arms, suggesting that adding ipatasertib to atezolizumab and paclitaxel did not improve efficacy versus the doublet alone (Supplementary Figs. S5A and S5B). In the PD-L1–negative/unknown cohort of IPATunity170, there was a suggestion of longer PFS with the doublet and triplet regimens compared with paclitaxel alone [median PFS, 7.1 (95% CI, 5.1–9.3) months with the triplet; 5.6 (95% CI, 3.7–8.2) months with ipatasertib plus paclitaxel; and 3.7 (95% CI, 3.6–5.4) months with paclitaxel alone; Supplementary Fig. S5C]. However, this was not observed for OS [median OS, 15.7 (95% CI, 12.5–not estimable) months with the triplet; 15.3 (95% CI, 15.3–not estimable) months with ipatasertib plus paclitaxel; and 16.6 (95% CI, 9.6–not estimable) months with paclitaxel alone], although there was extensive censoring before the medians (Supplementary Fig. S5D).
Safety
Overall, there were no relevant differences in taxane exposure with the triplet between cohorts/arms within each study or between studies (Supplementary Table S3). In IPATunity170, overall treatment exposure was generally longer with the triplet regimen than with the control arms (doublet or single-agent regimens). In addition, atezolizumab and ipatasertib exposure with the triplet was longer in CO40151 than in either IPATunity130 or IPATunity170.
Table 3 provides an overview of safety across the three trials. In general, the frequency of grade ≥3 adverse events (AE) was higher with the triplet than with the doublet or single-agent paclitaxel. Among all 317 patients receiving the triplet, AEs led to discontinuation of ipatasertib in 31 patients (10%) and atezolizumab in 27 patients (9%), but were less common than AEs leading to discontinuation of taxane therapy (76 patients; 24%). Safety outcomes according to PD-L1 status showed no obvious differences (data not shown).
Table 3.
Summary of safety (triplet regimens shown in bold).a
| Number of patients with AE (%) | CO40151 | IPATunity130, Cohort C | IPATunity170 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PD-L1 status | + | − | Unknown | + | − | Unknown | + | − or unknown | |||
| PIK3CA/AKT1/PTEN status | Unselected | Not altered | Unselected | ||||||||
| Treatment | Atezo + ipat + pac/nab-pac | Atezo + ipat + pac | Atezo + pac | Atezo + ipat + pac | Pac | Ipat + pac | Atezo + ipat + pac | ||||
| Number of patients | 51 | 45 | 18 | 40 | 25 | 37 | 57 | 58 | 41 | 43 | 43 |
| Grade ≥3 AE | 33 (65) | 23 (51) | 9 (50) | 21 (53) | 14 (56) | 27 (73) | 20 (35) | 39 (67) | 15 (37) | 23 (53) | 29 (67) |
| Grade 5 AE | 2 (4) | 0 | 0 | 0 | 2 (8) | 2 (5) | 0 | 3 (5) | 1 (2) | 1 (2) | 3 (7) |
| Serious AE | 24 (47) | 17 (38) | 3 (17) | 8 (20) | 10 (40) | 11 (30) | 9 (16) | 15 (26) | 7 (17) | 7 (16) | 14 (33) |
| AE leading to treatment discontinuation | |||||||||||
| Ipatasertib/placebo | 1 (2) | 4 (9) | 2 (11) | 4 (10) | 1 (4) | 6 (16) | 1 (2) | 8 (14) | 1 (2) | 3 (7) | 5 (12) |
| Atezolizumab/placebo | 1 (2) | 3 (7) | 1 (6) | 4 (10) | 1 (4) | 8 (22) | 0 | 7 (12) | 1 (2) | 2 (5) | 2 (5) |
| Taxane | Pac: 15 (29) | Pac: 13 (29) | Pac: 5 (28) | 6 (15) | 3 (12) | 13 (35) | 3 (5) | 13 (22) | 4 (10) | 6 (14) | 4 (9) |
| Nab-pac: 3 (6) | Nab-pac: 1 (2) | Nab-pac: 0 | |||||||||
aAEs graded according to NCI CTCAE version 4.0 for CO40151 and IPATunity130 and version 5.0 for IPATunity170 and coded using MedDRA version 24.1.
Abbreviations: atezo, atezolizumab; ipat, ipatasertib; MedDRA, Medical Dictionary for Regulatory Activities; NCI CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events; pac, paclitaxel.
Across all arms of the phase Ib CO40151 study, the most common all-grade AEs (≥30% of patients) were diarrhea (82%), nausea (55%), alopecia (44%), fatigue (41%), rash (38%), constipation (36%), and peripheral neuropathy (32%). Hyperglycemia occurred in <10% of patients. There were two fatal AEs in CO40151 (one case each of pneumonia and ischemic stroke). The most common grade 3/4 AEs across all treatment arms were diarrhea (12%), neutropenia (11%), and rash (10%; Supplementary Table S4).
In IPATunity130 Cohort C, the most common all-grade AEs were diarrhea (84%), nausea (41%), alopecia (41%), anemia (32%), and rash (30%). Hyperglycemia was reported in 22% of patients (4% grade 3, no grade 4). The only grade 3/4 AE in >10% of patients was diarrhea (17%). Fatal AEs were reported in 4 patients in IPATunity130 Cohort C (one case each of pneumonia, suspected COVID-19, cardiac arrest, and pulmonary embolism).
In IPATunity170, the most common AEs were diarrhea (54%), alopecia (35%), constipation (35%), and nausea (31%). Hyperglycemia occurred in 13% (grade 3/4 in 1%). Among patients receiving the triplet, 14% experienced grade 3/4 diarrhea. Fatal AEs with the triplet comprised two cases of septic shock and one case each of cardiac arrest, cardiac failure, COVID-19 pneumonia, and unexplained death.
Biomarker analyses
Biomarker analyses reported here are from the CO40151 dataset. Among the 114 patients treated across the four treatment arms, biomarker samples were available for IHC evaluation from 95 patients for tumor and stromal TILs, 76 patients for determination of PD-L1 status by SP142, 61 patients for determination of PD-L1 status by 22C3, and 82 patients for CD8 evaluation. Baseline FFPE tissue was available from 87 patients for bulk RNA sequencing and 76 patients for Foundation Medicine next-generation sequencing. For all biomarker analysis datasets, approximately 60% of samples were from primary tumors and 40% from metastatic sites.
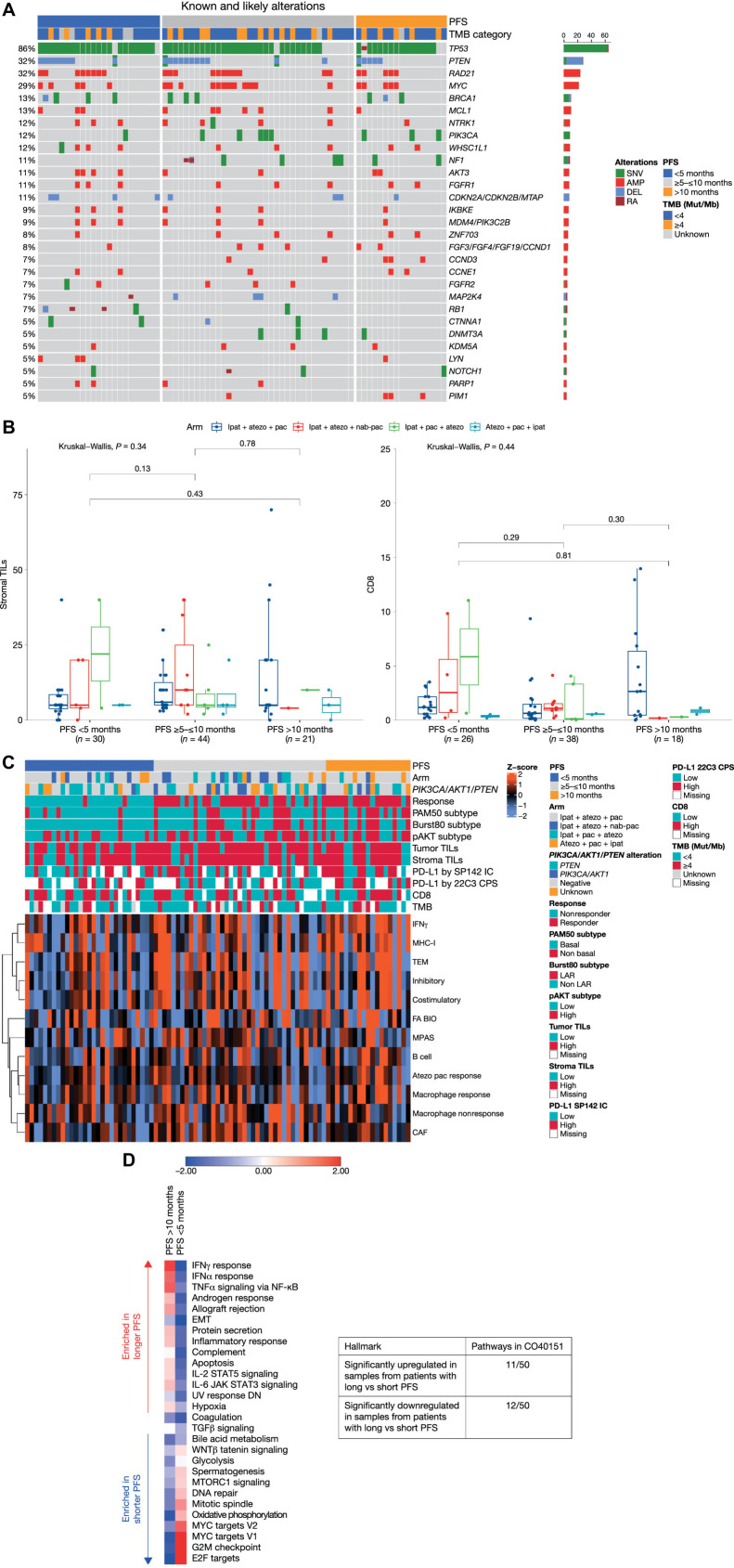
To determine whether the study population in CO40151 was representative of a typical trial population of patients with TNBC, we explored the prevalence of genetic alterations (Fig. 1A). The most frequent alterations were in TP53 (86%; predominantly short variations), PTEN (32%; predominantly deletions), RAD21 (32%; exclusively amplifications), and MYC (29%; exclusively amplifications). BRCA1 mutations were observed in 13% of samples (short variations or deletions). Overall, the distribution and prevalence were similar to observations in the randomized phase III IMpassion130 trial (29), in which the most common alterations were in TP53 (85%), MYC (22%), PIK3CA (21%), and PTEN (19%). However, PIK3CA mutations were less common in CO40151 (12% vs. 21% in IMpassion130), as were RB1 mutations (7% vs. 14%, respectively), whereas PTEN alterations were more common in CO40151 (32% vs. 19% in IMpassion130).
Figure 1.
Biomarker characterization. A, Genomic landscape. B, Stromal TILs and CD8 T cells according to PFS. The lower and upper bounds of the rectangles represent the first and third quartiles, the horizontal line represents the median, the whiskers extend to the highest and lowest values within 1.5× the interquartile range, and data beyond the end of the whiskers are outliers and are plotted as points. C, Baseline RNA-sequencing profile. Atezolizumab plus paclitaxel response represents a gene signature based on CD8-CXCL13 T cells that has previously been shown to predict better response to atezolizumab plus paclitaxel in TNBC (20). Similarly, the B-cell gene signature comprising CD19 and CXCR5 is predictive of better response to atezolizumab plus paclitaxel in TNBC. D, GSEA analysis on hallmark gene sets according to PFS. The scoring is averaged Z score of individual samples from gene set variation analysis in the subgroups with PFS <5 months and >10 months. AMP, amplification; atezo, atezolizumab; CAF, cancer-associated fibroblasts; CPS, combined positive score; DEL, deletion; EMT, epithelial–mesenchymal transition; FA BIO, fatty acid biosynthesis; IC, immune cell; ipat, ipatasertib; JAK, Janus kinase; LAR, luminal androgen receptor; MPAS, MAPK pathway activity score (30); MHC-I, major histocompatibility complex-I; Mut/Mb, mutations/megabase; NF-κB, nuclear factor kappa-B; pac, paclitaxel; RA, rearrangement; SNV, single-nucleotide variation; TEM, T effector memory.
Although none of the alterations showed a clear clustering pattern among the subgroup with shortest or longest PFS, there were some notable findings. NF1 and CCND3 alterations were absent among the small population of patients with PFS <5 months, but were more common in patients with longer PFS; likewise, PIK3CA alterations were detected in only 1 patient with PFS <5 months but in a larger proportion of patients with intermediate or long PFS. Of note, 4 of the 8 patients with CDKN2A/CDKN2B/MTAP alterations had PFS <5 months. There was no clear association between PFS and tumor mutational burden (TMB).
IHC analysis revealed no significant differences in baseline stromal TILs and CD8 T cells between patients with PFS >10 months and those with shorter (<5 months) PFS (Fig. 1B). However, patients with increased activity of immune pathways inferred from gene expression at baseline appeared to have longer PFS with the triplet (Fig. 1C; refs. 20, 30). For example, high IFNγ and MHC-I were observed in most patients with PFS >10 months, but in relatively few of those with PFS <5 months.
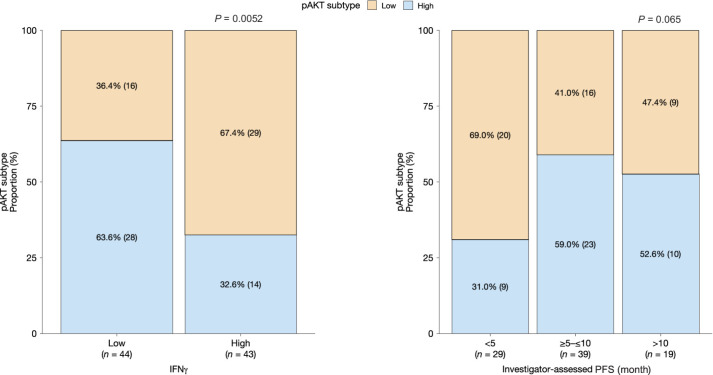
The pAKT model developed from the FAIRLANE dataset (described above) was leveraged to understand whether AKT signaling was associated with response to ipatasertib and its combination with atezolizumab. First, the potential association between the pAKT model and clinical response to ipatasertib was tested in the LOTUS randomized phase II trial in locally advanced/metastatic TNBC (12). Baseline tumor samples with high predicted pAKT-S473 levels were associated with enhanced PFS with ipatasertib plus paclitaxel but not with placebo plus paclitaxel (Supplementary Fig. S2F). In CO40151, tumors with high pAKT-S473 as predicted by a random forest model had lower immune infiltrate, as indicated by lower IFNγ signaling (Fig. 2, left panel). Among 19 patients with PFS >10 months, there was no difference in the proportion of patients with a high pAKT-S473 score (53%) versus low pAKT-S473 score (47%), whereas among 29 patients with PFS <5 months, 31% had high pAKT-S473 and 69% had low pAKT-S473 (Fig. 2, right panel). The Fisher exact test P value of 0.065 indicates a trend toward worse PFS with lower pAKT. Further exploratory analyses revealed no significant difference in PD-L1 (as measured by the 22C3 assay) between patients with high pAKT (irrespective of response) and nonresponders with low pAKT. Responding patients with low pAKT at baseline had significantly higher PD-L1 than either nonresponding patients with low pAKT or patients with high pAKT irrespective of response (Supplementary Fig. S6). Similar effects were seen when PD-L1 levels were measured by SP142, although the differences were less striking.
Figure 2.
Application of pAKT model to the CO40151 dataset.
Finally, GSEA analysis was performed on the hallmark gene sets and compared between samples from 19 patients with PFS >10 months and 29 patients with PFS <5 months. Of the 50 hallmark gene sets, 11 pathways, including immune signaling, epithelial-to-mesenchymal transition, apoptosis, and hypoxia, were significantly upregulated in patients with longer PFS, whereas upregulation of 12 different hallmark gene sets, including those involved in Wnt signaling, cell cycle, oxidative phosphorylation, and DNA repair, was associated with shorter PFS (Fig. 1D; Supplementary Table S5).
Discussion
Final results from the CO40151 phase Ib study evaluating a triplet of ipatasertib, atezolizumab, and a taxane show that the initial promising confirmed ORR of 73% observed at the preliminary analysis (22) was not maintained in subsequent analyses after expansion of the study. Furthermore, the activity was not replicated in the signal-seeking arm of IPATunity130, nor in the randomized phase III IPATunity170 trial. Consequently, in the context of the evolving treatment landscape for locally advanced/metastatic TNBC, the IPATunity170 trial was discontinued prematurely. In this report of more than 300 patients treated with the triplet in three trials, median PFS ranged from 5.4 to 7.4 months. Comparison with previous randomized phase III data in TNBC suggests that the activity of the triplet was similar to that achieved with a doublet of anti-PD-(L)1 therapy and chemotherapy alone. For example, in unselected populations, median PFS was 7.2 months with atezolizumab plus nab-paclitaxel in IMpassion130 (4), 7.5 months with pembrolizumab plus chemotherapy in KEYNOTE-355 (3), and 5.7 months with atezolizumab plus paclitaxel in IMpassion131 (7). Median OS was <24 months in most of the cohorts and treatment arms evaluating the triplet, although interpretation is limited by low event numbers and relatively short follow-up. These indirect findings suggest that the triplet does not improve clinical outcomes compared with the current standard therapies used in TNBC. The discrepancy between the early phase Ib results and the subsequent expanded cohorts could be due to chance and patient selection bias inherent to early-phase trials. It is also possible that varying patterns of steroid use during treatment, including for rash prophylaxis, may have influenced the results over time as emerging data suggest that steroid coadministration with atezolizumab may reduce proliferation pathways across immune cells (31).
As a result of the early termination of IPATunity170, analyses are only descriptive, with relatively low event numbers (particularly for OS) and short follow-up. However, results suggest that adding ipatasertib to atezolizumab plus paclitaxel does not improve PFS or OS in patients with PD-L1–positive TNBC. Interestingly, within the PD-L1–negative/unknown cohort of IPATunity170, there appeared to be numerical differences between the treatment arms (median PFS of 3.7 months with paclitaxel alone, 5.6 months with an ipatasertib plus paclitaxel doublet, and 7.1 months with the triplet). ORRs showed a similar pattern (15% vs. 30% vs. 51%, respectively) but no pattern was seen in the (relatively immature) OS results. Median PFS of 3.7 months and ORR of 15% with single-agent paclitaxel is considerably lower than typically observed and consistent with enrollment of a population with particularly aggressive and treatment-resistant disease, as expected given the exclusion of patients with PD-L1–positive and/or known BRCA-mutated tumors. This population of patients has a high unmet need, as they do not benefit from immune checkpoint blockade in the metastatic setting. For patients with BRCA1/2 mutations, the PARP inhibitors olaparib or talazoparib are indicated based on results of the OlympiAD (32, 33) and EMBRACA (34, 35) trials, respectively. In later lines, the antibody–drug conjugate sacituzumab govitecan is an active treatment for taxane-pretreated locally advanced/metastatic TNBC, as demonstrated in the phase III ASCENT trial (36). The evolving treatment landscape and availability of new agents for the treatment of TNBC may have affected OS results in the trials reported in this article.
Comparison of outcomes across cohorts with PD-L1–positive locally advanced/metastatic TNBC or cohorts with PD-L1–negative locally advanced/metastatic TNBC consistently showed shorter median PFS in IPATunity170 and IPATunity130 than in CO40151. This may reflect the shorter duration of follow-up in the phase III trials, which started after CO40151. The heterogeneity of the enrolled patient populations, the potential impact of selection bias, and subsequent therapy are also likely to contribute.
Exploratory biomarker analysis revealed that patients with high baseline predicted pAKT-S473 levels had low immune infiltrates, consistent with previous reports of an association between activated AKT signaling and immune exclusion in patients with melanoma (15). We also observed a trend toward longer PFS with high baseline predicted pAKT-S473, potentially suggesting that at least for a subset of patients whose tumors were immune excluded and had low PD-L1 levels, ipatasertib may have improved immune infiltration and synergized with atezolizumab. Ongoing analysis of samples from the biopsy cohort may characterize further the underlying mechanism of synergy between ipatasertib and atezolizumab. Although the fidelity of markers between primary and metastatic samples was not explored, representing a caveat of the study conclusions, from a genomic perspective, patients in CO40151 appeared to be representative of TNBC populations. PIK3CA mutations, which are typically associated with a worse prognosis (37), were less common in CO40151 (12% vs. 21% in IMpassion130), whereas PTEN alterations, which are associated with shorter PFS and OS among patients treated with anti-PD-(L)1 therapies (38), were more common in CO40151 (32% vs. 19% in IMpassion130). In patients with longer PFS, we observed enrichment of alterations in PIK3CA and NF1, whereas alterations in these genes are typically associated with poor prognosis (39, 40).
The totality of the results from these trials emphasizes the need for alternative therapeutic approaches for patients with PD-L1–negative locally advanced/metastatic TNBC, and for patients who are not candidates for immune checkpoint blockade. Furthermore, the outcomes illustrate the heterogeneity of the populations enrolled and the challenges of developing combination regimens for TNBC and there remains keen interest in agents targeting the PI3K/AKT pathway. Further work is required to understand mechanisms of resistance to the combination of AKT inhibition and immunotherapy.
Supplementary Material
Table S1: Representativeness of study participants Table S2: Baseline characteristics Table S3: Treatment exposure Table S4: Grade ≥3 AEs Table S5: GSEA analysis Fig S1: Trial designs Fig S2: pAKT model Figs S3–S5: Efficacy by trial Fig S6: PD-L1, pAKT and response
Acknowledgments
We thank the patients, their families, the investigators, and study staff at participating sites, and members of the independent data monitoring committees. F. Hoffmann-La Roche Ltd. sponsored all three trials reported here and funded third-party medical writing support for this manuscript, provided by Jennifer Kelly (Medi-Kelsey Ltd.).
Participating Countries
CO40151
Spain: M.J. De Miguel/V. Boni, E. Ciruelos, M. Benavent Viñales, E. Espinosa, A. Barnadas Molins, J.A. Garcia Saenz, B. Doger de Spéville Uribe
France: M.-P. Sablin, A. Italiano, F. Ghiringhelli, A. Geraud, M. Campione
UK: P. Schmid
Australia: P. Savas, R. Dear, S. White
USA: S. Sarkissian
IPATunity130 Cohort C
Brazil: R. Freitas-Junior, L. Andrade, R. Hegg, C. Barrios, S. Costa, V. Liutti, P. Xavier Santi
Ukraine: I. Bondarenko, Y. Shparyk, Y. Ostapenko
USA: J. O'Shaughnessy, G. Vidal, M. Alattar, H. Chew, A. Cuesta Fernandez, D. Riseberg, J. Suga, N. Unni
Peru: C. Torres Mattos, M. Philco, C. Castañeda, H.W. Tejada Benavides, C. Vallejos Sologuren
Poland: Z. Nowecki, M. Jarzab, E. Kalinka
UK: S. Dubey, S. Slater, N. Turner, S. Waters
France: C. Jouannaud, I. Desmoulins, W. Jacot
Korea: S.-B. Kim, J.H. Kim, K.S. Lee, M.-H. Lee, J.H. Sohn
Australia: J. Lombard, N. Woodward, P. Dinh
Taiwan: C.-S. Huang, L.-M. Tseng
Singapore: R. Dent
IPATunity170
South Korea: Y.S. Chae, J. Lee, M.H. Kim, S.-B. Kim, K.S. Lee, Y.H. Park
Spain: B. Rojas, B. Bermejo De Las Heras, B. Cantos, J.A. Garcia Saenz, E. Lopez Miranda, E.M. Perez Lopez, E. Martinez De Dueñas, B. Jiminez, M. Muñoz Mateu, M. Oliveira, S. Lopez-Tarruella Cobo
Australia: W. Xu, C. Oakman, M. Okera, D. Sabanathan, P. Savas, A. Menzies
Italy: M. De Laurentiis, V. Guarneri, M. Mansutti, A. Santoro, L. Coltelli, R. Pedersini, U. De Giorgi
Mexico: J.L. Martinez Rodriguez, J. Ceja Garcia, F. Martinez
Argentina: G. Lerzo, D. Kaen, G. Aguil, V. Caceres, E. Korbenfeld
Ukraine: O. Dudnichenko, A. Kurochkin, S. Hrybach, H. Adamchuk, O. Berzoy, I. Bondarenko, S. Polenkov
Peru: H. Gomez-Moreno, R. Alvarez, M. Philco, L. Ventura, H. Fuentes, J. Revilla Lopez, C. Torres Mattos
Poland: Z. Nowecki, M. Jarzab, R. Szoszkiewicz, E. Kalinka, P. Wysocki
Thailand: N. Parinyanitikul, P. Sunpaweravong, A. Somwangprasert, P. Tienchaianada, N. Soparattanapaisarn, P. Phongthai
Taiwan: H.-C. Wang, C.-S. Huang, L.-M. Tseng, Y.-C. Lin
Turkey: U. Demirci, A. Bilici, S. Aksoy
Russia: R. Safin, V. Chubenko, M. Frolova
USA: L.N. Durna, G. Vidal, J.T. Beck, D. Riseberg, G.B. Saylors, M. Taylor
Japan: H. Doihara, H. Iwata, R. Nishimura, M. Okada, T. Yamashita, Y. Yanagita
Hong Kong (SAR): R. Leung, T.-Y. Ng
UK: S. Slater, S. Khan, A. Okines
Colombia: S. Franco, G. Adolfo Rojas Uribe
Costa Rica: L. Corrales
Brazil: L. Andrade, F. Jose Cruz
Czech Republic: B. Melichar, K. Petrakova
Denmark: E. Harder Brix, J. Dupont Jensen
Finland: T. Alanko, A. Jekunen
Portugal: C. Abreu, F. Branco
Romania: M. Schenker
Austria: R. Bartsch
Belgium: C. Vulsteke
Bulgaria: T. Karanikolova
Canada: A. Kumar
France: F. Le Du
India: S. Nag
Israel: H. Goldvaser
Switzerland: C. Kurzeder
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
P. Schmid reports grants from AstraZeneca, Genentech, Roche, Oncogenex, Novartis, Astellas, and Medivation during the conduct of the study, as well as personal fees from Pfizer, AstraZeneca, Gilead, Roche, Merck, MSD, BI, Seagen, Amgen, Bayer, Eisai, Celgene, Lilly, Puma, and Boehringer Ingelheim outside the submitted work. N.C. Turner reports advisory board honoraria from AstraZeneca, Lilly, Pfizer, Roche/Genentech, Novartis, GlaxoSmithKline, Repare Therapeutics, Relay Therapeutics, Zentalis, Gilead, Inivata, Guardant, and Exact Sciences, as well as research funding from AstraZeneca, Pfizer, Roche/Genentech, Merck Sharpe and Dohme, Guardant Health, Invitae, Inivata, Personalis, and Natera. C.H. Barrios reports grants, personal fees, and nonfinancial support from AstraZeneca, Daiichi Sankyo, Gilead, Lilly, MSD, Novartis, Pfizer, Roche, and Janssen; grants from Amgen, Aveo, Biomarin, BMS, Celgene, Checkpoint, DOCS, Exelixis, GSK, Iqivia, Labcorp, Medivation, Medplace, Merk, Myovant, Nektar, Novocure, Nuvisant, OBI Pharma, Parexel, Pharmamar, Polyphor, PPD, PSI, Regeneron, Sanofi, Seagen, Syneos Health, Takeda, Trio, and Shanghai Henlius; and personal fees from Adium Pharma during the conduct of the study. S. Saji reports grants and personal fees from Chugai during the conduct of the study. S. Saji also reports grants and personal fees from Chugai, MSD, Eisai, Takeda, Daiichi Sankyo, AstraZeneca, and Taiho, as well as personal fees from Kyowa Kirin, Novartis, Eli Lilly, Pfizer, Ono, and Nippon Kayaku outside the submitted work. S. Saji also reports a relationship with Breast International Group (Member of Board of Directors), Japan Breast Cancer Research Group (Member of Board of Directors), Japanese Breast Cancer Society (Member of Board of Directors), and Japanese Society of Medical Oncology (Member of Board of Directors). P. Savas reports grants from Roche-Genentech outside the submitted work, as well as serving as an uncompensated consultant to Roche-Genentech. G.A. Vidal reports personal fees and other support from Genentech/Roche, Eli Lilly, Pfizer, AstraZeneca, Daiichi Sankyo, and Merck; personal fees from Novartis, Biotheranostics, Stemline, Concert AI, and Myriad Genetics; grants, personal fees, and other support from Gilead and BMS; other support from Puma, Celcuity, and NAPO; and grants and other support from GSK during the conduct of the study. G.A. Vidal also reports other support from Oncodisc outside the submitted work. M. Oliveira reports grants, personal fees, and nonfinancial support from Roche during the conduct of the study. M. Oliveira also reports grants, personal fees, and nonfinancial support from AstraZeneca and Gilead; grants from Ayala Pharmaceuticals, Boehringer Ingelheim, Genentech, GSK, Novartis, and Zenith Epigenetics; grants and personal fees from Seagen; personal fees from Daiichi-Sankyo/AstraZeneca, iTEOS, Lilly, MSD, Relay Therapeutics, Libbs, Novartis, and Pfizer; and personal fees and nonfinancial support from Pierre-Fabre and Eisai outside the submitted work. J. O'Shaughnessy reports personal fees from Agendia, Aptitude Health, AstraZeneca, Daiichi Sankyo, Eisai, G1 Therapeutics, Lilly, LOXO Oncology, Merck, Novartis, Ontada, Pfizer, Pierre Fabre, Puma Biotechnology, Roche, Samsung Bioepis, Sanofi, Seagen, and Stemline Therapeutics outside the submitted work. A. Italiano reports grants and personal fees from Roche, Bayer, MSD, Merck, AstraZeneca, and BMS outside the submitted work. V. Boni reports other support from Genentech during the conduct of the study. V. Boni also reports personal fees from Merck and Nanobiotix, as well as other support from AbbVie, ACEO, Adaptimmune, Amcure, AMGEN, Amunix, AstraZeneca, Bicycle, BMS Cytomx, GSK, Genentech/Roche, Genmab, Incyte, Ipsen, Janssen, Kura, Lilly, Loxo, Nektar, Macrogenics, Menarini, Merck, Merus, Nanobiotix, Novartis, Pfizer, PharmaMar, Principia, PUMA, Ryvu, Ribbon, Sanofi, Taiho, Tesaro, BeiGene, Transgene, Takeda, Incyte, Innovio, MSD, PsiOxus, Seattle Genetics, Mersana, Daiichi, Nektar, Astellas, ORCA, Boston Therapeutics, Dynavax, DebioPharm, Boehringer Ingelheim, Regeneron, Rigontec, Millennium, Seagen, Synthon, Spectrum, Urogen, and Zenith outside the submitted work. C. Torres Mattos reports other support from Roche Farma outside the submitted work. L.H. Lam reports nonfinancial support from Roche during the conduct of the study, as well as personal fees and other support from Roche outside the submitted work. S.-J. Reilly reports employment with Roche Products Ltd. and holds shares in Roche. X. Huang reports personal fees from Roche outside the submitted work. K. Shah reports other support from Genentech/Roche outside the submitted work. R. Dent reports other support from Roche outside the submitted work. No disclosures were reported by the other authors.
Authors' Contributions
P. Schmid: Conceptualization, resources, methodology, writing–review and editing. N.C. Turner: Conceptualization, resources, methodology, writing–review and editing. C.H. Barrios: Conceptualization, resources, methodology, writing–review and editing. S.J. Isakoff: Conceptualization, resources, methodology, writing–review and editing. S.-B. Kim: Conceptualization, resources, methodology, writing–review and editing. M.-P. Sablin: Conceptualization, resources, methodology, writing–review and editing. S. Saji: Conceptualization, resources, methodology, writing–review and editing. P. Savas: Conceptualization, resources, methodology, writing–review and editing. G.A. Vidal: Conceptualization, resources, methodology, writing–review and editing. M. Oliveira: Conceptualization, resources, methodology, writing–review and editing. J. O'Shaughnessy: Conceptualization, resources, methodology, writing–review and editing. A. Italiano: Resources, writing–review and editing. E. Espinosa: Resources, writing–review and editing. V. Boni: Resources, writing–review and editing. S. White: Resources, writing–review and editing. B. Rojas: Resources, writing–review and editing. R. Freitas-Junior: Resources, writing–review and editing. Y. Chae: Resources, writing–review and editing. I. Bondarenko: Resources, writing–review and editing. J. Lee: Resources, writing–review and editing. C. Torres Mattos: Resources, writing–review and editing. J.L. Martinez Rodriguez: Resources, writing–review and editing. L.H. Lam: Conceptualization, data curation, supervision, visualization, methodology, writing–original draft, project administration, writing–review and editing. S. Jones: Software, formal analysis, validation, visualization, writing–review and editing. S.-J. Reilly: Conceptualization, data curation, supervision, methodology, writing–review and editing. X. Huang: Software, formal analysis, investigation, visualization, methodology, writing–original draft, writing–review and editing. K. Shah: Conceptualization, software, formal analysis, investigation, visualization, methodology, writing–original draft, writing–review and editing. R. Dent: conceptualization, resources, methodology, writing–review and editing.
References
- 1. Asleh K, Riaz N, Nielsen TO. Heterogeneity of triple negative breast cancer: current advances in subtyping and treatment implications. J Exp Clin Cancer Res 2022;41:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burstein MD, Tsimelzon A, Poage GM, Covington KR, Contreras A, Fuqua SA, et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res 2015;21:1688–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020;396:1817–28. [DOI] [PubMed] [Google Scholar]
- 4. Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 2018;379:2108–21. [DOI] [PubMed] [Google Scholar]
- 5. Emens LA, Adams S, Barrios CH, Diéras V, Iwata H, Loi S, et al. First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: IMpassion130 final overall survival analysis. Ann Oncol 2021;32:983–93. [DOI] [PubMed] [Google Scholar]
- 6. Cortes J, Rugo HS, Cescon DW, Im SA, Yusof MM, Gallardo C, et al. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N Engl J Med 2022;387:217–26. [DOI] [PubMed] [Google Scholar]
- 7. Miles D, Gligorov J, Andre F, Cameron D, Schneeweiss A, Barrios C, et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann Oncol 2021;32:994–1004. [DOI] [PubMed] [Google Scholar]
- 8. LoRusso PM. Inhibition of the PI3K/AKT/mTOR pathway in solid tumors. J Clin Oncol 2016;34:3803–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Savage H, Lin W, Oliveira M, Barrios C, O'Shaughnessy J, Turner N, et al. Prospective testing for PIK3CA/AKT1/PTEN alterations in tumor tissue from 1440 patients with advanced hormone receptor-positive HER2-negative breast cancer (HR+/HER2- BC) or triple-negative breast cancer (TNBC) screened for the IPATunity130 randomized phase 3 trial. Cancer Res 2021;81(4_Suppl):PS5-06-PS5. [Google Scholar]
- 10. Davies BR, Greenwood H, Dudley P, Crafter C, Yu DH, Zhang J, et al. Preclinical pharmacology of AZD5363, an inhibitor of AKT: pharmacodynamics, antitumor activity, and correlation of monotherapy activity with genetic background. Mol Cancer Ther 2012;11:873–87. [DOI] [PubMed] [Google Scholar]
- 11. Janku F, Wheler JJ, Naing A, Falchook GS, Hong DS, Stepanek VM, et al. PIK3CA mutation H1047R is associated with response to PI3K/AKT/mTOR signaling pathway inhibitors in early-phase clinical trials. Cancer Res 2013;73:276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim SB, Dent R, Im SA, Espie M, Blau S, Tan AR, et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 2017;18:1360–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schmid P, Abraham J, Chan S, Wheatley D, Brunt AM, Nemsadze G, et al. Capivasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer: the PAKT trial. J Clin Oncol 2020;38:423–33. [DOI] [PubMed] [Google Scholar]
- 14. Dent R, Oliveira M, Isakoff SJ, Im SA, Espié M, Blau S, et al. Final results of the double-blind placebo-controlled randomized phase 2 LOTUS trial of first-line ipatasertib plus paclitaxel for inoperable locally advanced/metastatic triple-negative breast cancer. Breast Cancer Res Treat 2021;189:377–86. [DOI] [PubMed] [Google Scholar]
- 15. Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, et al. Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discov 2016;6:202–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crompton JG, Sukumar M, Roychoudhuri R, Clever D, Gros A, Eil RL, et al. Akt inhibition enhances expansion of potent tumor-specific lymphocytes with memory cell characteristics. Cancer Res 2015;75:296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rogel A, Willoughby JE, Buchan SL, Leonard HJ, Thirdborough SM, Al-Shamkhani A. Akt signaling is critical for memory CD8(+) T-cell development and tumor immune surveillance. Proc Natl Acad Sci U S A 2017;114:E1178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gubser PM, Bantug GR, Razik L, Fischer M, Dimeloe S, Hoenger G, et al. Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch. Nat Immunol 2013;14:1064–72. [DOI] [PubMed] [Google Scholar]
- 19. Xue G, Zippelius A, Wicki A, Mandalà M, Tang F, Massi D, et al. Integrated Akt/PKB signaling in immunomodulation and its potential role in cancer immunotherapy. J Natl Cancer Inst 2015;107:djv171. [DOI] [PubMed] [Google Scholar]
- 20. Zhang Y, Chen H, Mo H, Hu X, Gao R, Zhao Y, et al. Single-cell analyses reveal key immune cell subsets associated with response to PD-L1 blockade in triple-negative breast cancer. Cancer Cell 2021;39:1578–93. [DOI] [PubMed] [Google Scholar]
- 21. Sai J, Owens P, Novitskiy SV, Hawkins OE, Vilgelm AE, Yang J, et al. PI3K inhibition reduces mammary tumor growth and facilitates antitumor immunity and anti-PD1 responses. Clin Cancer Res 2017;23:3371–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schmid P, Loirat D, Savas P, Espinosa E, Boni V, Italiano A, et al. Phase Ib study evaluating a triplet combination of ipatasertib (IPAT), atezolizumab (atezo), and paclitaxel (PAC) or nab-PAC as first-line (1L) therapy for locally advanced/metastatic triple-negative breast cancer (TNBC). Clin Cancer Res 2019;79(13_Suppl):CT049. [Google Scholar]
- 23. Dent R, Kim S-B, Oliveira M, Barrios C, O'Shaughnessy J, Isakoff S, et al. Double-blind placebo (PBO)-controlled randomized phase III trial evaluating first-line ipatasertib (IPAT) combined with paclitaxel (PAC) for PIK3CA/AKT1/PTEN-altered locally advanced unresectable or metastatic triple-negative breast cancer (aTNBC): primary results from IPATunity130 Cohort A. Cancer Res 2021;81(4_Suppl):GS3-04. [Google Scholar]
- 24. Turner N, Dent RA, O'Shaughnessy J, Kim SB, Isakoff SJ, Barrios C, et al. Ipatasertib plus paclitaxel for PIK3CA/AKT1/PTEN-altered hormone receptor-positive HER2-negative advanced breast cancer: primary results from cohort B of the IPATunity130 randomized phase 3 trial. Breast Cancer Res Treat 2022;191:565–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhu AX, Abbas AR, de Galarreta MR, Guan Y, Lu S, Koeppen H, et al. Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat Med 2022;28:1599–611. [DOI] [PubMed] [Google Scholar]
- 26. Chen DS, Mellman I. Elements of cancer immunity and the cancer–immune set point. Nature 2017;541:321–30. [DOI] [PubMed] [Google Scholar]
- 27. Oliveira M, Saura C, Nuciforo P, Calvo I, Andersen J, Passos-Coelho JL, et al. FAIRLANE, a double-blind placebo-controlled randomized phase II trial of neoadjuvant ipatasertib plus paclitaxel for early triple-negative breast cancer. Ann Oncol 2019;30:1289–97. [DOI] [PubMed] [Google Scholar]
- 28. Shi Z, Wulfkuhle J, Nowicka M, Gallagher RI, Saura C, Nuciforo PG, et al. Functional mapping of AKT signaling and biomarkers of response from the FAIRLANE trial of neoadjuvant ipatasertib plus paclitaxel for triple-negative breast cancer. Clin Cancer Res 2022;28:993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Emens L, Molinero L, Adams S, Rugo HS, Schneeweiss A, Diéras V, et al. Genomic profiling and clinical outcomes with first-line atezolizumab and nab-paclitaxel in triple-negative breast cancer: an exploratory analysis from the phase 3 IMpassion130 trial. Cancer Res 2021;81(4_Suppl):PD14-05. [Google Scholar]
- 30. Wagle M-C, Kirouac D, Klijn C, Liu B, Mahajan S, Junttila M, et al. A transcriptional MAPK Pathway Activity Score (MPAS) is a clinically relevant biomarker in multiple cancer types. NPJ Precis Oncol 2018;2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Molinero L, Guan X, Deurloo R, Gozlan Y, Sharim H, Emens LA, et al. Impact of steroid premedication on atezolizumab (atezo)-induced immune cell activation: a comparative analysis of IMpassion130 and IMpassion131 peripheral blood mononuclear cells (PBMCs). J Clin Oncol 2022;40(16_Suppl):1083. [Google Scholar]
- 32. Robson ME, Tung N, Conte P, Im SA, Senkus E, Xu B, et al. OlympiAD final overall survival and tolerability results: olaparib versus chemotherapy treatment of physician's choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol 2019;30:558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med 2017;377:523–33. [DOI] [PubMed] [Google Scholar]
- 34. Litton JK, Rugo HS, Ettl J, Hurvitz SA, Gonçalves A, Lee KH, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med 2018;379:753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Litton JK, Hurvitz SA, Mina LA, Rugo HS, Lee KH, Gonçalves A, et al. Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: final overall survival results from the EMBRACA trial. Ann Oncol 2020;31:1526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bardia A, Hurvitz SA, Tolaney SM, Loirat D, Punie K, Oliveira M, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med 2021;384:1529–41. [DOI] [PubMed] [Google Scholar]
- 37. Rong G, Yi Z, Ma F, Guan Y, Xu Y, Li L, et al. DNA damage response as a prognostic indicator in metastatic breast cancer via mutational analysis. Ann Transl Med 2021;9:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barroso-Sousa R, Keenan TE, Pernas S, Exman P, Jain E, Garrido-Castro AC, et al. Tumor mutational burden and PTEN alterations as molecular correlates of response to PD-1/L1 blockade in metastatic triple-negative breast cancer. Clin Cancer Res 2020;26:2565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pascual J, Turner NC. Targeting the PI3-kinase pathway in triple-negative breast cancer. Ann Oncol 2019;30:1051–60. [DOI] [PubMed] [Google Scholar]
- 40. Rocca A, Braga L, Volpe MC, Maiocchi S, Generali D. The predictive and prognostic role of RAS-RAF-MEK-ERK pathway alterations in breast cancer: revision of the literature and comparison with the analysis of cancer genomic datasets. Cancers (Basel) 2022;14:5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Representativeness of study participants Table S2: Baseline characteristics Table S3: Treatment exposure Table S4: Grade ≥3 AEs Table S5: GSEA analysis Fig S1: Trial designs Fig S2: pAKT model Figs S3–S5: Efficacy by trial Fig S6: PD-L1, pAKT and response
Data Availability Statement
For up-to-date details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see: https://go.roche.com/data_sharing. Anonymized records for individual patients across more than one data source external to Roche cannot, and should not, be linked due to a potential increase in risk of patient re-identification.