Abstract
Purpose:
The MONALEESA-2, -3, -7 trials demonstrated statistically significant and clinically meaningful progression-free survival and overall survival (OS) benefits with ribociclib plus endocrine therapy (ET) versus ET alone in hormone receptor–positive, HER2-negative (HR+/HER2−) advanced breast cancer (ABC). Understanding the association of intrinsic subtypes with survival outcomes could potentially guide treatment decisions. Here, we evaluated the association of intrinsic subtypes with OS in MONALEESA-2, -3, -7.
Experimental Design:
Tumor samples from MONALEESA-2, -3, -7 underwent PAM50-based subtyping. The relationship between subtypes and OS was assessed using univariable and multivariable Cox proportional hazards models. Multivariable models were adjusted for clinical prognostic factors.
Results:
Overall, 990 tumors (among 2,066 patients) from ribociclib (n = 580) and placebo (n = 410) arms were profiled. Subtype distribution was luminal A, 54.5%; luminal B, 28.0%; HER2-enriched (HER2E) 14.6%; and basal-like, 2.8%; and was consistent across treatment arms. The luminal A subtype had the best OS outcomes in both arms, while basal-like had the worst. Patients with HER2E (HR, 0.60; P = 0.018), luminal B (HR, 0.69; P = 0.023), and luminal A (HR, 0.75; P = 0.021) subtypes derived OS benefit with ribociclib. Patients with basal-like subtype did not derive benefit from ribociclib (HR, 1.92; P = 0.137); however, patient numbers were small (n = 28).
Conclusions:
The prognostic value of intrinsic subtypes for OS was confirmed in this pooled analysis of the MONALEESA trials (largest dataset in HR+/HER2− ABC). While basal-like subtype did not benefit, a consistent OS benefit was observed with ribociclib added to ET across luminal and HER2E subtypes.
Translational Relevance.
Hormone receptor–positive (HR+)/HER2-negative (HER2−) advanced breast cancer (ABC) is a heterogeneous disease that can be classified into intrinsic subtypes (IS) with differing prognoses and responses to treatment. Non-luminal subtypes [HER2-enriched (HER2E)] are associated with poorer outcomes than luminal subtypes (luminal A, luminal B). Here, we report the findings of pooled analysis of the MONALEESA-2, -3, -7 trials evaluating association of IS and overall survival (OS). The data show that significant OS benefit with ribociclib plus endocrine therapy was observed across luminal A, luminal B, and HER2E subtypes. In addition, the findings, which are from the largest dataset correlating OS and IS in HR+/HER2− ABC, confirmed the prognostic value of PAM50-based IS. OS benefit with ribociclib was not observed in patients with the basal-like subtype; however, the sample size of this subgroup was small. The consistent OS benefit with ribociclib in patients with the HER2E subtype, which is characterized by relative endocrine resistance, warrants further investigation.
Introduction
Hormone receptor–positive (HR+), HER2-negative (HER2−) advanced breast cancer (ABC) is a clinically and biologically heterogeneous disease; one way that this heterogeneity can be categorized is through known intrinsic subtypes, which differ in prognosis, incidence, and response to treatment (1–3). The four intrinsic subtypes of HR+/HER2− breast cancer are the luminal A, luminal B, HER2-enriched (HER2E), and basal-like subtypes. These subtypes are defined by their gene expression profiles and are each associated with different prognoses (1–3). Luminal A and B subtypes are associated with more favorable outcomes compared with the non-luminal (HER2E and basal-like) subtypes (4–7). The HER2E subtype is associated with poor clinical outcomes and endocrine therapy (ET) resistance (5, 6, 8, 9). Basal-like tumors, which more closely resemble triple-negative breast cancer tumors, typically have poorest outcomes (6). Luminal A, luminal B, HER2E, and basal-like subtypes are also observed in HER2-positive (HER2+) breast cancer and triple-negative breast cancer, although in different proportions than observed in HR+/HER2− breast cancer (3, 10, 11). Notably, intrinsic subtypes may change over time, and subtype switching has been demonstrated to occur between primary and metastatic tumors and with disease progression. In a prospective, longitudinal study of patients with HR+/HER2− metastatic breast cancer, switching to more aggressive subtypes was frequent among tumor samples from patients who progressed after treatment with palbociclib (12, 13).
The cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors ribociclib, abemaciclib, and palbociclib have demonstrated statistically significant and clinically meaningful improvements in progression-free survival (PFS) in patients with HR+/HER2− ABC (14–21). Ribociclib has also demonstrated statistically significant and clinically meaningful improvements in overall survival (OS) in patients with HR+/HER2− ABC across three phase III clinical trials, regardless of ET partner, line of therapy, and menopausal status (MONALEESA-2, -3, and -7), and abemaciclib has shown a significant OS benefit in its second-line phase III trial (MONARCH 2) to date (22–25). The first-line MONARCH 3 study has yet to report final OS, although a trend was seen in interim analyses (26). An improvement in median OS was reported in patients with pretreated HR+/HER2− ABC treated with palbociclib in the phase III PALOMA-3 trial, although the difference was not statistically significant, while no clinically meaningful or statistically significant improvement in OS was observed with first-line palbociclib plus letrozole in the phase III PALOMA-2 trial (12, 27).
A prior pooled analysis of the MONALEESA trials evaluating the correlation of PAM50-based intrinsic subtype with PFS confirmed the independent prognostic value of intrinsic subtype in patients treated with ribociclib plus ET and ET alone (1). In this pooled intrinsic subtype dataset, luminal A (47%) and luminal B (24%) were the most prevalent subtypes, and HER2E (13%) and basal-like (3%) were the least frequent (1). Differences in subtype distribution were observed across the MONALEESA trials (P < 0.001) and across the type of tumor tissue (metastatic and primary; P = 0.005), which was expected and consistent with prior published analyses (e.g., differences in subtype distribution by menopausal status; primary vs. metastatic tumor; refs. 13, 28). This pooled analysis demonstrated a significant PFS benefit with ribociclib plus ET treatment in the luminal A (n = 540; HR, 0.63; P = 0.0007), luminal B (n = 277; HR, 0.52; P < 0.0001), and HER2E (n = 145; HR, 0.40; P < 0.0001) subtypes. Consistent with previous findings, patients with the HER2E subtype had poor PFS when treated with ET alone. Patients with basal-like (HR, 1.14; P = 0.78) subtype did not derive a PFS benefit with ribociclib plus ET treatment, although the sample size in this subgroup was small (n = 28) and these results should be interpreted with caution.
At the time of the PFS analysis, OS data for the key first-line MONALEESA-2 trial were not yet mature, and a pooled analysis of intrinsic subtype and OS could not be performed. Recently, the MONALEESA-2 trial demonstrated a statistically significant OS benefit with first-line ribociclib plus letrozole compared with letrozole alone in postmenopausal patients with HR+/HER2− ABC, and an analysis of intrinsic subtype and OS in the MONALEESA trials could now be completed (25). In this retrospective exploratory analysis, we evaluated the prognostic and predictive value of baseline intrinsic subtypes for OS using tumor samples pooled from the MONALEESA-2, -3, and -7 trials. This OS analysis parallels the previously reported PFS analysis except for the exclusion of the normal-like intrinsic subtype; given the frequent contamination of the normal-like intrinsic subtype with normal breast tissue, this subset was not analyzed for OS.
Materials and Methods
Study designs and patients
The phase III MONALEESA studies were randomized, double-blind, placebo-controlled, multicenter studies. The MONALEESA-2 trial (NCT01958021) enrolled postmenopausal women with locally determined HR+/HER2− ABC who had not received previous systemic therapy for ABC; patients could have received prior (neo)adjuvant ET, but a treatment-free interval of >12 months from completion of (neo)adjuvant treatment to randomization was required for prior nonsteroidal aromatase inhibitors (14). A total of 668 postmenopausal women with HR+/HER2− recurrent/metastatic breast cancer underwent 1:1 randomization to receive either ribociclib plus letrozole or placebo plus letrozole (14).
The MONALEESA-3 trial (NCT02422615) assessed ribociclib plus fulvestrant in postmenopausal patients with locally determined HR+/HER2− ABC who were treatment naïve or had received up to one line of prior ET in the advanced setting (15). Overall, 726 patients underwent 2:1 randomization to receive ribociclib plus fulvestrant or placebo plus fulvestrant.
The MONALEESA-7 trial (NCT02278120) enrolled premenopausal or perimenopausal patients with locally determined HR+/HER2− ABC who were ET naïve in the advanced setting but could have received up to one prior line of chemotherapy for ABC (16). In total, 672 patients underwent 1:1 randomization to receive ribociclib or matching placebo with either tamoxifen or a nonsteroidal aromatase inhibitor (letrozole or anastrozole), all with goserelin. Patients in the trial could have prior (neo)adjuvant ET if the treatment-free interval was ≥12 months from completion of (neo)adjuvant treatment to randomization; if the treatment-free interval was <12 months, the choice of ET therapy partner depended on the patient's previous (neo)adjuvant therapy and investigator or patient preference.
In each of the MONALEESA trials, the primary endpoint was locally assessed PFS. OS was a key secondary endpoint in each trial, and the trials were designed to have adequate power to detect a difference in OS between the study arms. Written informed consent was provided by all patients. The MONALEESA trials were conducted in accordance with the World Medical Association Declaration of Helsinki and the Good Clinical Practice guidelines. Study protocols and any modifications were approved by an independent ethics committee or an Institutional Review Board at each site. Trial conduct was supervised by a steering committee including participating international investigators and Novartis representatives. An independent data monitoring committee evaluated safety data.
Procedures
As described previously (1), for each MONALEESA trial, collection of formalin-fixed paraffin-embedded (FFPE) tumor samples (i.e., a tumor block or slides) was mandatory; metastatic samples were preferred. Tumor samples were reviewed by a central laboratory to confirm a minimum of 10% tumor content. Gene expression was evaluated using a custom CodeSet gene panel (list available on request) and the nCounter platform (both from NanoString Technologies), including 36 of the 50 research-based PAM50 genes (29). Gene expression analyses were performed using input of 100 ng of total RNA extracted from primary or metastatic tumors. Positive control and housekeeping gene normalization was performed on the NanoString raw counts; log2 transformation was performed on the normalized counts. Samples with fewer than 20 counts in >80% of genes were excluded from this analysis.
Gene sets with fewer than 50 PAM50 genes have poor accuracies in subtype calling, particularly for luminal B and HER2E subtype tumors (30). To mitigate this, and to robustly identify PAM50 subtypes in the MONALEESA tumor samples, 48 independent FFPE breast tumor samples with a known PAM50/Prosigna (NanoString Technologies) subtype [luminal A (HR+/HER2−), 10; luminal B (HR+/HER2−), 10; HER2E (HER2+), 10; basal-like (triple-negative), 9; true normal, 9] were characterized using the same protocol utilized for the samples from the MONALEESA program. In total, 152 genes were selected on the basis of their ability to identify the PAM50 subtypes in this 48-sample set and the original PAM50 microarray training dataset (29). PAM50 subtyping of the MONALEESA tumors was performed as described previously (31) using the 152 PAM50-based genes. Genomic analyses were conducted blinded from clinical data. Samples with less than 50% of housekeeping genes above the background noise (defined at 26 counts) were excluded from the analysis. Samples with normal-like subtype were excluded from the analysis because the subtype has a high proportion of normal tissue.
Statistical analysis
For the analysis of OS, the data cut-off dates were June 10, 2021, for MONALEESA-2, October 30, 2020, for MONALEESA-3, and June 29, 2020, for MONALEESA-7. The relationship between PAM50-based subtypes and OS was evaluated using univariable and multivariable Cox proportional hazards models. Multivariable Cox proportional hazards regression analyses were used to evaluate the association of the intrinsic subtypes with OS. Multivariable models were adjusted for known clinical prognostic factors, including age, prior chemotherapy, prior ET, Eastern Cooperative Oncology Group performance status, visceral disease (presence of liver/lung metastases), bone-only metastases, histologic grade, number of metastatic sites, tumor type, and de novo metastatic disease. Kaplan–Meier curves were generated, and median OS [95% confidence interval (CI)] was estimated by subtype and treatment arm. Statistical analyses were performed using the R project software (32). The P values generated are descriptive and were not adjusted for multiplicity or false discovery.
Data availability
Novartis is committed to sharing with qualified external researchers, access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trials in line with applicable laws and regulations. All requests should be sent to the corresponding author.
Results
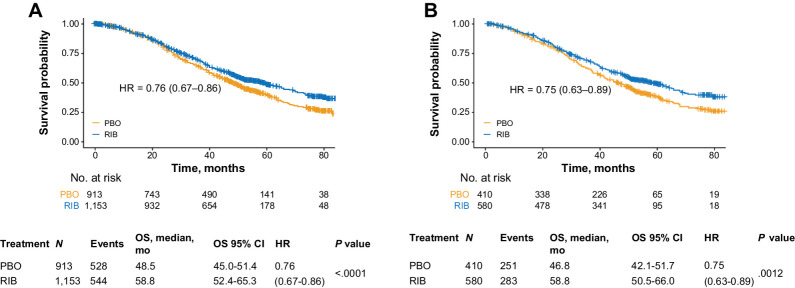
From the MONALEESA-2, -3, and -7 pooled patient population (N = 2,066), the pooled dataset with available subtype data was n = 1,153. (33). After removing 163 (14.1%) normal-like tumor samples, a total of 990 tumor samples from the ribociclib plus ET (n = 580) and placebo plus ET (n = 410) arms were included in this analysis, consisting of 318 samples from MONALEESA-2, 409 samples from MONALEESA-3, and 263 samples from MONALEESA-7 (Supplementary Fig. S1A–S1C). Of the samples that were profiled, 71% were from primary tumors and 28% were from metastatic samples (1% source unknown), similarly distributed across trials. Subtype distribution was luminal A, 54.5% (n = 540); luminal B, 28.0% (n = 277); HER2E, 14.6% (n = 145); and basal-like, 2.8% (n = 28). The distribution of subtypes was consistent across treatment arms (Table 1). Representativeness of the study is listed in Supplementary Table S1. Generally, no significant differences in baseline characteristics were observed across intrinsic subtypes, except for prior chemotherapy and histologic grade (Supplementary Table S2). However, it is unknown whether chemotherapy exposure was before or after tumor biopsy collection. Median follow-up time for the pooled cohort was 58.2 months. A consistent significant OS benefit with ribociclib plus ET versus placebo plus ET was observed in the intention-to-treat (N = 2,066; HR, 0.76; 95% CI, 0.67–0.86; P < 0.0001) and biomarker (n = 990; HR, 0.75; 95% CI, 0.63–0.89; P = 0.0012) populations with a 12-month improvement in median OS observed by adding ribociclib to ET in the biomarker population (Fig. 1A and B).
Table 1.
Predictive value of intrinsic subtype on OS by treatment arm.
| Subtype | Treatment arm | Distribution, n (%) | Events, n (%)a | Median OS estimate | Median OS 95% CI | HR estimate | HR 95% CI | P value |
|---|---|---|---|---|---|---|---|---|
| Luminal A | PBO | 221 (54) | 122 (55) | 54.6 | 48.3–66.2 | 0.75 | 0.58–0.96 | 0.021 |
| RIB | 319 (55) | 135 (42) | 68.0 | 61.5-NA | ||||
| Luminal B | PBO | 124 (30) | 79 (64) | 44.9 | 35.5–52.6 | 0.69 | 0.50–0.95 | 0.023 |
| RIB | 153 (26) | 75 (49) | 58.8 | 48.3–79.2 | ||||
| HER2E | PBO | 51 (12) | 39 (76) | 29.4 | 23.9–42.0 | 0.60 | 0.40–0.92 | 0.018 |
| RIB | 94 (16) | 59 (63) | 40.3 | 33.4–49.0 | ||||
| Basal-like | PBO | 14 (3) | 11 (79) | 21.2 | 12.8-NA | 1.92 | 0.81–4.53 | 0.137 |
| RIB | 14 (2) | 14 (100) | 19.0 | 10.7–33.2 |
Abbreviations: HER2E, human epidermal growth factor receptor 2–enriched; HR, hazard ratio; NA, not achieved; PBO, placebo; RIB, ribociclib.
aPercentage of patients within each row experiencing an event.
Figure 1.
OS in the intention-to-treat and biomarker populations. A, All patients pooled from the MONALEESA-2, -3, and -7 trials. B, Patients with PAM50 analysis of tumor samples. HR, hazard ratio; PBO, placebo; RIB, ribociclib.
Prognosis by intrinsic subtype
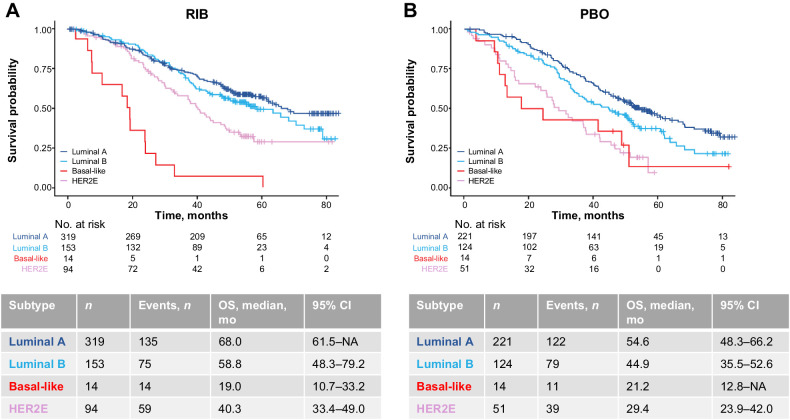
Intrinsic subtype was prognostic for OS in both the ribociclib plus ET (P < 0.0001) and placebo plus ET (P < 0.0001) arms (Fig. 2A and B). In both treatment arms, the median OS differed across intrinsic subtypes, with patients with luminal A subtype exhibiting the longest median OS and patients with basal-like subtype having the shortest median OS: median OS with ribociclib plus ET versus placebo plus ET was 68.0 versus 54.6 months for luminal A, 58.8 versus 44.9 months for luminal B, 40.3 versus 29.4 months for HER2E, and 19.0 versus 21.2 months for basal-like, respectively (Fig. 2A and B; Table 1). With luminal A as the reference group in the OS model, the luminal B (difference not statistically significant), HER2E, and basal-like subtypes showed increased risk of death in both treatment arms (Table 2). Intrinsic subtype remained prognostic for OS in the ribociclib plus ET (P < 0.0001) and placebo plus ET (P < 0.0001) arms, after adjusting for clinicopathological variables (Tables 2 and 3). In both the placebo and ribociclib arms, a higher risk of death was observed for the luminal B, HER2E, and basal-like subtypes versus the luminal A subtype [1.2 (P = 0.32), 1.8 (P = 0.00022), and 7.2 (P < 0.0001) times higher risks of death in the ribociclib arm, respectively] after adjustment for other clinicopathological variables (Table 2). This higher risk versus luminal A was significant for all subtypes except for luminal B in the ribociclib arm.
Figure 2.
OS based on intrinsic subtype in the combined MONALEESA dataset: in the RIB arm (A); in the PBO arm (B). HER2E, human epidermal growth factor receptor 2–enriched; NA, not achieved; PBO, placebo; RIB, ribociclib.
Table 2.
OS HR by subtype relative to luminal A in the combined MONALEESA dataset.
| Ribociclib Arm | Placebo Arm | |||
|---|---|---|---|---|
| HRa (95% CI) | P value | HRa (95% CI) | P value | |
| Univariate analysis | ||||
| Luminal Ab | 1.00 (–) | – | 1.00 (–) | – |
| Luminal B | 1.22 (0.92–1.62) | 0.17 | 1.41 (1.06–1.87) | 0.018 |
| HER2E | 1.94 (1.42–2.65) | <0.0001 | 2.62 (1.81–3.79) | <0.0001 |
| Basal-like | 7.84 (4.43–13.88) | <0.0001 | 2.56 (1.37–4.76) | 0.0031 |
| Multivariable Cox analyses of prognostic variables c | ||||
| Luminal Ab | 1.00 (–) | – | 1.00 (–) | – |
| Luminal B | 1.16 (0.86–1.57) | 0.32 | 1.47 (1.08–2.00) | 0.014 |
| HER2E | 1.83 (1.33–2.52) | 0.00022 | 2.87 (1.93–4.26) | <0.0001 |
| Basal-like | 7.22 (3.81–13.70) | <0.0001 | 2.35 (1.20–4.57) | 0.012 |
Abbreviation: HER2E, human epidermal growth factor receptor 2–enriched.
aAdjusted HR for multivariate analyses.
bLuminal A is used as the reference group in this analysis; thus, 95% CIs and P values could not be calculated for the luminal A subgroup here.
cAdjusted HRs obtained from multivariable Cox model including age, prior chemotherapy, prior endocrine therapy, Eastern Cooperative Oncology Group performance status, visceral disease (presence of liver/lung metastases), bone-only metastases, histologic grade, number of metastatic sites, tumor type, and de novo metastatic disease.
Table 3.
Multivariable Cox analysis in the combined MONALEESA dataset.
| Cohort | Variable | Adjusted HRa (95% CI) | P value |
|---|---|---|---|
| Ribociclib | Age | 1.01 (1.00–1.02) | 0.24 |
| Race | |||
| Asian | 1.00 (–) | – | |
| White | 0.98 (0.66–1.45) | 0.93 | |
| Other | 1.35 (0.71–2.57) | 0.35 | |
| Unknown | 1.07 (0.56–2.07) | 0.84 | |
| CT yes vs. no | 1.15 (0.84–1.59) | 0.39 | |
| Subtype | |||
| Luminal A | 1.00 (–) | – | |
| Luminal B | 1.16 (0.86–1.57) | 0.32 | |
| HER2E | 1.83 (1.33–2.52) | 0.00022 | |
| Basal-like | 7.22 (3.81–13.70) | <0.0001 | |
| ECOG PS 1 vs. 0 | 1.73 (1.33–2.24) | <0.0001 | |
| De novo disease vs. not | 0.55 (0.34–0.87) | 0.011 | |
| Visceral disease vs. not | 1.27 (0.93–1.74) | 0.14 | |
| Bone only vs. not | 1.03 (0.69–1.54) | 0.88 | |
| Histologic grade | |||
| Poorly differentiated/undifferentiated histologic grade | 1.41 (1.05–1.89) | 0.022 | |
| Unknown histologic grade | 1.35 (0.97–1.89) | 0.073 | |
| Well-differentiated histologic grade | 0.87 (0.52–1.45) | 0.59 | |
| No. of metastatic sites ≥3 vs. fewer | 1.11 (0.84–1.47) | 0.44 | |
| Tumor type (metastatic vs. primary) | 1.11 (0.84–1.48) | 0.46 | |
| ETS1b | 0.76 (0.48–1.20) | 0.24 | |
| ETS2c | 1.28 (0.85–1.91) | 0.24 | |
| ETS3d | 1.24 (0.72–2.12) | 0.44 | |
| Placebo | Age | 1.01 (1.00–1.02) | 0.21 |
| Race | |||
| Asian | 1.00 (–) | – | |
| White | 0.98 (0.67–1.42) | 0.90 | |
| Other | 1.18 (0.56–2.51) | 0.66 | |
| Unknown | 0.55 (0.27–1.14) | 0.11 | |
| CT yes vs. no | 1.55 (1.09–2.21) | 0.016 | |
| Subtype | |||
| Luminal A | 1.00 (–) | – | |
| Luminal B | 1.47 (1.08–2.00) | 0.014 | |
| HER2E | 2.87 (1.93–4.26) | <0.0001 | |
| Basal-like | 2.35 (1.20–4.57) | 0.012 | |
| ECOG 1 vs. 0 | 1.32 (1.00–1.74) | 0.052 | |
| De novo disease vs. not | 1.09 (0.64–1.83) | 0.75 | |
| Visceral disease vs. not | 1.26 (0.89–1.79) | 0.20 | |
| Bone only vs. not | 1.25 (0.82–1.90) | 0.29 | |
| Histologic grade | |||
| Poorly differentiated/undifferentiated histologic grade | 1.11 (0.81–1.52) | 0.53 | |
| Unknown histologic grade | 1.16 (0.80–1.68) | 0.43 | |
| Well-differentiated histologic grade | 0.84 (0.52–1.36) | 0.49 | |
| No. of metastatic sites ≥3 vs. fewer | 1.16 (0.85–1.58) | 0.35 | |
| Tumor type (metastatic vs. primary) | 1.16 (0.84–1.61) | 0.36 | |
| ETS1b | 0.82 (0.51–1.33) | 0.42 | |
| ETS2c | 1.65 (1.04–2.63) | 0.034 | |
| ETS3d | 1.43 (0.70–2.89) | 0.33 | |
| All patients | Age | 1.01 (0.998–1.02) | 0.10 |
| Race | |||
| Asian | 1.00 (–) | – | |
| White | 0.97 (0.74–1.26) | 0.79 | |
| Other | 1.27 (0.79–2.05) | 0.33 | |
| Unknown | 0.79 (0.49–1.28) | 0.34 | |
| CT yes vs. no | 1.32 (1.04–1.67) | 0.021 | |
| Subtype | |||
| Luminal A | 1.00 (–) | – | |
| Luminal B | 1.42 (1.06–1.91) | 0.019 | |
| HER2E | 2.66 (1.82–3.88) | <0.0001 | |
| Basal-like | 2.26 (1.19–4.28) | 0.013 | |
| ECOG PS 1 vs. 0 | 1.52 (1.26–1.83) | <0.0001 | |
| De novo disease vs. not | 0.72 (0.51–1.01) | 0.060 | |
| Visceral disease vs. not | 1.26 (1.01–1.59) | 0.045 | |
| Bone only vs. not | 1.11 (0.83–1.47) | 0.48 | |
| Histologic grade | |||
| Poorly differentiated/undifferentiated histologic grade | 1.28 (1.04–1.59) | 0.021 | |
| Unknown histologic grade | 1.31 (1.03–1.68) | 0.028 | |
| Well-differentiated histologic grade | 0.89 (0.63–1.25) | 0.49 | |
| No. of metastatic sites ≥3 vs. fewer | 1.14 (0.93–1.39) | 0.22 | |
| Tumor type (metastatic vs. primary) | 1.15 (0.94–1.42) | 0.17 | |
| ETS1b | 0.76 (0.55–1.05) | 0.093 | |
| ETS2c | 1.40 (1.04–1.89) | 0.026 | |
| ETS3d | 1.33 (0.87–2.01) | 0.19 | |
| Treatment (ribociclib vs. placebo) | 0.77 (0.60–0.99) | 0.039 | |
| Treatment*luminal B | 0.83 (0.55–1.25) | 0.36 | |
| Treatment*HER2E | 0.69 (0.42–1.12) | 0.13 | |
| Treatment*basal-like | 3.62 (1.51–8.69) | 0.004 | |
Abbreviations: CT, chemotherapy; ECOG PS, Eastern Cooperative Oncology Group performance status; ET, endocrine therapy; ETS, ET sensitive; HER2E, human epidermal growth factor receptor 2–enriched.
aObtained from multivariable Cox model including age, race, prior chemotherapy, ECOG PS, presence of visceral disease (liver/lung metastases), presence of bone-only metastases, histological grade, number of metastatic sites, prior ET, presence of de novo metastatic disease, and tumor type (primary or metastatic) as covariates.
bETS1 includes patients considered ET sensitive who exhibited progression >12 months after end of ET.
cETS2 includes patients considered ET sensitive who exhibited progression at or within 12 months of end of ET.
dETS3 includes patients who received second-line ET.
Treatment benefit based on intrinsic subtype
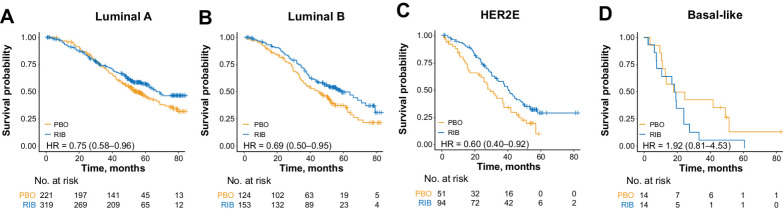
All subtypes except basal-like exhibited a significant OS benefit with ribociclib (Fig. 3A–D; Table 1). Patients with HER2E (HR, 0.60; 95% CI, 0.40–0.92; P = 0.018), luminal B (HR, 0.69; 95% CI, 0.50–0.95; P = 0.023), and luminal A (HR, 0.75; 95% CI, 0.58–0.96; P = 0.021) subtypes all derived substantial benefit from ribociclib (Fig. 3A–D; Table 1). The absolute median OS benefit from ribociclib was 10.9 months in the HER2E, 13.9 months in the luminal B, and 13.4 months in the luminal A subtypes. After adjusting for known clinical prognostic factors, a consistent OS benefit was observed with ribociclib in patients with luminal A (adjusted HR, 0.77; 95% CI, 0.60–0.99), luminal B (adjusted HR, 0.63; 95% CI, 0.46–0.88), and HER2E (adjusted HR, 0.53; 95% CI, 0.35–0.80) subtypes (Supplementary Table S3). Patients with the basal-like subtype (n = 28) did not derive benefit from ribociclib (unadjusted HR, 1.92; 95% CI, 0.81–4.53; P = 0.137), although these results should be interpreted with caution due to the small sample size in this subgroup (2%–3% in each arm). The interaction test between PAM50 subtype and treatment arm was statistically significant (P = 0.014). PAM50 subtype remained predictive of OS after adjusting for clinical covariates in a multivariable model (Pinteraction = 0.0057; Supplementary Table S3). Upon removal of the basal-like subtype from the analysis, the interaction test was no longer statistically significant [P = 0.48 (unadjusted); P = 0.33 (adjusted for clinical covariates)].
Figure 3.
OS based on treatment within each intrinsic subtype in the combined MONALEESA dataset: within luminal A (A); within luminal B (B); within HER2E (C); within basal-like (D). HER2E, human epidermal growth factor receptor 2–enriched; HR, hazard ratio; PBO, placebo; RIB, ribociclib.
Discussion
To our knowledge, this analysis of the MONALEESA-2, -3, and -7 trials is the largest evaluating the correlation of intrinsic subtype with OS in patients with HR+/HER2− ABC treated with ET alone or in combination with a CDK4/6 inhibitor. The results demonstrate the prognostic value of PAM50-based intrinsic subtype for OS in patients treated with ribociclib plus ET and those treated with ET alone. These results are also remarkably consistent with a prior analysis of PFS using the pooled MONALEESA dataset, which showed the value of intrinsic subtype for predicting outcomes on ET for HR+/HER2− ABC (1).
A retrospective exploratory analysis of the EGF30008 trial, which evaluated letrozole with or without lapatinib in patients with HR+ invasive breast cancer, also showed that intrinsic subtypes were associated with differences in PFS and OS (5). Among patients in that study who were HR+/HER2−, patients with PAM50-luminal A disease had the longest median OS, whereas the PAM50-HER2E and PAM50-basal-like subtypes had the shortest median OS (5). In addition, in the BOLERO-2 study that tested the mTOR inhibitor everolimus plus ET in ABC, non-luminal subtypes were independently associated with worse PFS and OS outcomes than luminal subtypes (6). Furthermore, an exploratory real-world analysis of 141 patients with HR+/HER2− ABC showed that the median OS was shorter in patients with PAM50-HER2E disease [30.9 months; 95% CI, 13.2–not reached (NR)] compared with patients with non–PAM50-HER2E disease (NR; 95% CI, 47.2–NR; ref. 34). Taken together, these studies along with the current analysis support the prognostic value of intrinsic subtype for PFS and OS in patients with HR+/HER2− metastatic breast cancer.
The consistent PFS benefit with ribociclib plus ET by PAM50 subtype is replicated consistently in the pattern of OS benefit in luminal A (PFS HR, 0.63; OS HR, 0.75), luminal B (PFS HR, 0.52; OS HR, 0.69), and HER2E (PFS HR, 0.40; OS HR, 0.60); thus, the impact of ribociclib on PFS was carried through to OS as well (1).
The limited activity of ribociclib in the basal-like subgroup with respect to PFS and OS may be explained by the observation that the basal-like subtype is clinically and biologically similar to triple-negative breast cancer; thus, this subtype is not responsive to ET (1, 11). However, it is important to note that the number of patients in this subgroup is small (n = 28); therefore, these results should be interpreted with caution. The interaction test between PAM50 subtype and treatment arm was statistically significant when all subtypes were included, but when the basal-like subtype was excluded, the test was not significant; this observation suggests that differences in OS benefit among subtypes were driven by the basal-like subtype. Similarly, the lack of significance in the interaction test when only the PAM50-luminal A, PAM50-luminal B, and PAM50-HER2E subtypes were included supports the consistent OS benefit of ribociclib across these subtypes.
The consistent PFS and OS benefit seen with ribociclib plus ET in the HER2E population is of particular interest given that the HER2E subtype is associated with not only poor prognosis compared with luminal subtypes but also poor response to ET alone (5, 6, 8, 9). HER2E HR+/HER2− tumors have been shown to express higher levels of Ki-67 and lower levels of estrogen receptor/progesterone receptor compared with luminal subtypes (35). Several retrospective analyses of large clinical trials that included patients with HR+/HER2− treated with ET support the poor outcomes in patients with HER2E compared with luminal subtypes (5, 8). It has been shown that the HER2E subtype of HR+/HER2− breast cancer has high proliferation rates and dampened dependency on the HR pathway, but despite these observations, ribociclib plus ET demonstrated a remarkable PFS and OS benefit in this particular subtype.
Data with other CDK4/6 inhibitors are lacking (in the case of abemaciclib) or less compelling (in the case of palbociclib). Exploratory analyses of the PALOMA-2 and PALOMA-3 trials evaluating the association of intrinsic subtype (as determined by AIMS methodology) with PFS in patients with HR+/HER2− ABC treated with palbociclib plus ET have shown a PFS benefit in the AIMS-luminal A and AIMS-luminal B subtypes only, with little to no PFS benefit observed in the non-luminal subtypes (AIMS-HER2E and AIMS-basal-like; refs. 4, 36). A reanalysis of PALOMA-2 using the research use Prosigna-PAM50 methodology showed increased PFS benefit for palbociclib in the HER2E subtype; however, both arms of the analysis had low patient numbers (palbociclib arm, n = 12; placebo arm, n = 8; ref. 37). In the PATRICIA trial, which evaluated patients with HR+/HER2+ ABC treated with palbociclib and an anti-HER2 regimen, a trend of low PFS benefit with palbociclib in patients with non-luminal subtypes (defined by PAM50) compared with luminal subtypes was also observed (38). These findings from PATRICIA are relevant given that the HER2E subtype is biologically similar regardless of the context of HR+/HER2+ or HR+/HER2– disease as defined by IHC or ISH (39).
In contrast to the findings reported with palbociclib, ribociclib has now demonstrated a consistent PFS and OS benefit in luminal (PAM50-luminal A and B) and PAM50-HER2E subtypes in HR+/ HER2− ABC clinical trials. However, cross-trial comparisons cannot be made in the absence of well-controlled, head-to-head studies. The different intrinsic subtype methodologies mostly used in the ribociclib (PAM50) and palbociclib (AIMS) studies (reported concordance rate, ≈77%), as well as the different proportion of primary or metastatic samples, should also be noted (1, 40). Interestingly, despite small numbers, an exploratory real-world analysis of 141 patients with HR+/HER2− ABC (from two centers in Spain) also showed a numerically greater PFS benefit in the PAM50-HER2E subgroup with ribociclib (n = 7) compared with patients treated with palbociclib or abemaciclib (n = 12; HR, 0.44; ref. 34). However, it should be noted that given the OS benefit observed with ribociclib in ABC (MONALEESA-2,-3, -7) and the significant invasive disease-free survival (iDFS) benefit observed with ribociclib in early breast cancer (NATALEE) contrasted with the lack of OS benefit with palbociclib in the advanced setting (PALOMA-2, -3) and lack of iDFS benefit in the early setting (PALLAS, PENELOPE-B), the differences in efficacy observed between ribociclib and palbociclib may not be limited to just the HER2E subtype alone (12, 22, 23, 25, 27, 41–43). These differences between ribociclib and palbociclib likely extend to the other subtypes as well. Indeed, while ribociclib has demonstrated OS benefit in the luminal A, luminal B, and HER2E subtypes, it remains unknown which subtypes, if any, may be associated with palbociclib OS benefit, given the lack of OS benefit in the PALOMA-2 and PALOMA-3 overall populations. To date, OS analysis by intrinsic subtype has not yet been published for any palbociclib trial.
Subtype switching is also an important concept to consider. Results from the AURORA program, which evaluated the genomic and transcriptomic profiles of matched primary and metastatic samples from 381 patients with breast cancer, demonstrated that tumors can switch to more aggressive intrinsic subtypes at disease progression (44). In AURORA, 14.3% of PAM50-luminal A or B tumors converted to PAM50-HER2E in the metastatic setting (44). Conversion was also observed in a prospective, longitudinal multiomics study of palbociclib plus ET, in which frequent switching from luminal A to luminal B (45%) and HER2E (36%) was observed at disease progression (45).
Observations from the neoadjuvant setting can provide valuable insights into differential treatment effects by intrinsic subtypes and the impact of treatment on tumor biology because tumor tissue can be obtained longitudinally during the course of treatment and at surgery as applicable (46). In an analysis of samples from the neoadjuvant phase II PAMELA trial as well as from breast cancer cell lines, dual HER2 blockade in PAM50-HER2E tumors induced switching to a PAM50-luminal A subtype with a lower proliferative phenotype (47). In the CORALLEEN study, after 6 months of neoadjuvant treatment with ribociclib, 88% of patients with PAM50-luminal B subtype switched to PAM50-luminal A at surgery, suggesting that ribociclib can change tumor biology by inducing reversion to a less aggressive subtype (48). Similarly, preclinical analyses evaluating gene expression in patient-derived HER2E xenografts showed that ribociclib treatment induced switching to a luminal phenotype (49). However, it should be noted that the stability of these subtype changes is unknown.
Pooling the MONALEESA-2, -3, and -7 trials allowed for the inclusion of patients treated in the first- or second-line setting for ABC, patients of premenopausal, perimenopausal, and postmenopausal status, and patients receiving different ET partners. While the large sample size in this analysis is a major strength, this analysis has several limitations. In the pooled MONALEESA dataset, data from only 36 of 50 PAM50 genes were available. Because use of fewer genes reduces accuracy in subtype prediction, a 152-gene set was derived from an independent set of tumor samples with known intrinsic subtype, and this custom CodeSet was used to identify the PAM50 intrinsic subtypes in this analysis (1, 30). Furthermore, a large proportion of the samples included in this analysis was from primary tumors; thus, it is possible that subtype switching could have occurred and could not be accounted for. Finally, results were not adjusted for false discovery or multiplicity.
Evaluating the treatment effect of different CDK4/6 inhibitors on tumor biology in the metastatic setting and how subtype switching occurs during treatment and at progression could be valuable in understanding differences in long-term efficacy outcomes, including OS, with these agents. Ribociclib exhibits higher CDK4 versus CDK6 inhibition and has higher free drug concentrations compared with palbociclib at clinically relevant doses; these features might be more or less important to treat tumors that are often ET resistant, such as the HER2E subtype (50–53). In support of the potential importance of these features, a recent preclinical report demonstrated that a significant decrease of the HER2E signature in breast cancer cell lines was only observed with higher doses of ribociclib or palbociclib (54). In addition, analysis of data from patients in the CORALLEEN and NEOPALANA trials suggested that in the neoadjuvant setting, the reduction of the HER2E signature was better maintained with ribociclib than with palbociclib after both drugs were stopped for > 8 days (54).
The phase III HARMONIA trial (NCT05207709) will evaluate the activity of ribociclib plus ET compared with palbociclib plus ET in the HER2E population. Whether ribociclib may be unique in sensitizing HER2E tumors to endocrine-based therapy, leading to improved long-term efficacy outcomes, will be determined. It is important to note that while HARMONIA focuses on the HER2E subtype for comparison of ribociclib versus palbociclib, it is unlikely that the differences in OS benefit observed with ribociclib and palbociclib over multiple clinical trials in ABC are driven solely by this single subtype. In addition, it is becoming increasingly important to further understand the value of intrinsic subtype in early breast cancer, where CDK4/6 inhibitors are beginning to have a role in the treatment paradigm.
Future trials in breast cancer should take into consideration patient selection based on tumor biology to develop more personalized and biomarker-guided treatment strategies that aim to improve patient survival outcomes.
Supplementary Material
Supplemental Table 1. Representativeness of study participants
Supplemental Table 2. Patient characteristics by subtype
Supplemental Table 3. Multivariable analysis of treatment benefit by subtype
Supplemental Figure 1: CONSORT diagrams of the MONALEESA (ML) trials.
Acknowledgments
This study was funded by Novartis, Instituto de Salud Carlos III (to A. Prat) and RESCUER (to A. Prat), funded by European Union's Horizon 2020 Research and Innovation Programme under Grant Agreement No. 847912. Medical writing assistance was provided by Casey Nielsen, PhD, of MediTech Media and was funded by Novartis Pharmaceuticals.
We thank the patients who participated in these trials, their families and caregivers, the study investigators (a list of investigators was published with the primary results of each respective MONALEESA trial), the data-monitoring committee members, the study steering committee members, and the staff who assisted with the trial at each site. Ribociclib was discovered by Novartis Institutes for BioMedical Research in collaboration with Astex Pharmaceuticals.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
A. Prat reports grants and personal fees from NanoString Technologies, Veracyte, and Novartis during the conduct of the study; grants and personal fees from AstraZeneca, DaiichiSankyo, and Roche outside the submitted work; in addition, A. Prat has a patent for DNADX pending. N. Solovieff reports other support from Novartis outside the submitted work. F. André reports grants from Novartis during the conduct of the study; grants from AstraZeneca, Guardant Health, Owkin, Pfizer, Lilly, Roche, DaiichiSankyo, and Lilly outside the submitted work. J. O'Shaughnessy reports personal fees from Agendia, Aptitude Health, DaiichiSankyo, Eisai, G1 Therapeutics, Genentech, Gilead Sciences, Lilly, Merck, Novartis, Ontada, Pfizer, Pierre Fabre Pharmaceuticals, Puma Biotechnology, Roche, Samsung Bioepis, Sanofi, Seagen, Stemline Therapeutics, Synthon, and AstraZeneca outside the submitted work. D.A. Cameron reports non-financial support and other support from Novartis and Lilly, grants from Novartis, and other support from Pfizer outside the submitted work. W. Janni reports grants and personal fees from Novartis during the conduct of the study; as well as grants and personal fees from Novartis outside the submitted work. G.S. Sonke reports grants from Novartis during the conduct of the study; as well as grants from Agendia, AstraZeneca, Merck, Roche, and Seagen outside the submitted work. Y.-S. Yap reports personal fees from Novartis, Pfizer, Eisai, Roche, Specialised Therapeutics, and Inivata; personal fees and non-financial support from Lilly/DKSH, AstraZeneca; and grants and personal fees from MSD outside the submitted work. D.A. Yardley reports grants from Novartis during the conduct of the study; grants from AbbVie, Ambrx, Amgen, BIOMARIN, Biothera Pharmaceuticals, Clovis Pharma, Dana-Farber Cancer Institute, Eisai, Lilly, Roche/Genentech, G1 Therapeutics, Incyte, Innocrin Pharmaceuticals, MacroGenics, MedImmune, Medivation, Merck, Merrimack Pharmaceuticals, Nektar Therapeutics, NSABP, Odonate Therapeutics, Pfizer, Polyphor, US Oncology, and UT Southwestern; grants and other support from AstraZeneca, Gilead Sciences, Novartis, Stemline Therapeutics; other support from Immunomedics, Integra Connect, and Sanofi-Aventis outside the submitted work. A. Thuerigen reports personal fees and other support from Novartis Pharma AG outside the submitted work. J.P. Zarate reports personal fees and other support from Novartis Pharmaceuticals during the conduct of the study; personal fees and other support from Novartis Pharmaceuticals outside the submitted work. A. Lteif reports other support from Novartis outside the submitted work; and A. Lteif is an employee of Novartis Pharmaceuticals Corporation. F. Su reports other support from Novartis Pharmaceuticals during the conduct of the study. No disclosures were reported by the other authors.
Authors' Contributions
A. Prat: Writing–review and editing. N. Solovieff: Data curation, formal analysis, validation, writing–review and editing. F. André: Writing–review and editing. J. O'Shaughnessy: Writing–review and editing. D.A. Cameron: Writing–review and editing. W. Janni: Writing–review and editing. G.S. Sonke: Writing–review and editing. Y.-S. Yap: Writing–review and editing. D.A. Yardley: Writing–review and editing. A.H. Partridge: Writing–review and editing. A. Thuerigen: Writing–review and editing. J.P. Zarate: Writing–review and editing. A. Lteif: Writing–review and editing. F. Su: Data curation, validation, writing–review and editing. L.A. Carey: Writing–review and editing.
References
- 1. Prat A, Chaudhury A, Solovieff N, Pare L, Martinez D, Chic N, et al. Correlative biomarker analysis of intrinsic subtypes and efficacy across the MONALEESA phase III studies. J Clin Oncol 2021;39:1458–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eroles P, Bosch A, Perez-Fidalgo JA, Lluch A. Molecular biology in breast cancer: intrinsic subtypes and signaling pathways. Cancer Treat Rev 2012;38:698–707. [DOI] [PubMed] [Google Scholar]
- 3. Cheang MC, Martin M, Nielsen TO, Prat A, Voduc D, Rodriguez-Lescure A, et al. Defining breast cancer intrinsic subtypes by quantitative receptor expression. Oncologist 2015;20:474–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Turner NC, Liu Y, Zhu Z, Loi S, Colleoni M, Loibl S, et al. Cyclin E1 expression and palbociclib efficacy in previously treated hormone receptor-positive metastatic breast cancer. J Clin Oncol 2019;37:1169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prat A, Cheang MC, Galvan P, Nuciforo P, Pare L, Adamo B, et al. Prognostic value of intrinsic subtypes in hormone receptor-positive metastatic breast cancer treated with letrozole with or without lapatinib. JAMA Oncol 2016;2:1287–94. [DOI] [PubMed] [Google Scholar]
- 6. Prat A, Brase JC, Cheng Y, Nuciforo P, Pare L, Pascual T, et al. Everolimus plus exemestane for hormone receptor-positive advanced breast cancer: a PAM50 intrinsic subtype analysis of BOLERO-2. Oncologist 2019;24:893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cejalvo JM, Martinez de Duenas E, Galvan P, Garcia-Recio S, Burgues Gasion O, Pare L, et al. Intrinsic subtypes and gene expression profiles in primary and metastatic breast cancer. Cancer Res 2017;77:2213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Prat A, Parker JS, Fan C, Cheang MCU, Miller LD, Bergh J, et al. Concordance among gene expression-based predictors for ER-positive breast cancer treated with adjuvant tamoxifen. Ann Oncol 2012;23:2866–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ellis MJ, Suman VJ, Hoog J, Lin L, Snider J, Prat A, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype–ACOSOG Z1031. J Clin Oncol 2011;29:2342–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yin L, Duan JJ, Bian XW, Yu SC. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res 2020;22:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prat A, Adamo B, Cheang MC, Anders CK, Carey LA, Perou CM. Molecular characterization of basal-like and non-basal-like triple-negative breast cancer. Oncologist 2013;18:123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med 2018;379:1926–36. [DOI] [PubMed] [Google Scholar]
- 13. Jorgensen CLT, Larsson AM, Forsare C, Aaltonen K, Jansson S, Bradshaw R, et al. PAM50 intrinsic subtype profiles in primary and metastatic breast cancer show a significant shift toward more aggressive subtypes with prognostic implications. Cancers 2021;13:1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 2016;375:1738–48. [DOI] [PubMed] [Google Scholar]
- 15. Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol 2018;36:2465–72. [DOI] [PubMed] [Google Scholar]
- 16. Tripathy D, Im SA, Colleoni M, Franke F, Bardia A, Harbeck N, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol 2018;19:904–15. [DOI] [PubMed] [Google Scholar]
- 17. Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med 2016;375:1925–36. [DOI] [PubMed] [Google Scholar]
- 18. Rugo HS, Finn RS, Dieras V, Ettl J, Lipatov O, Joy AA, et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat 2019;174:719–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 2016;17:425–39. [DOI] [PubMed] [Google Scholar]
- 20. Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 2017;35:3638–46. [DOI] [PubMed] [Google Scholar]
- 21. Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 2017;35:2875–84. [DOI] [PubMed] [Google Scholar]
- 22. Im SA, Lu YS, Bardia A, Harbeck N, Colleoni M, Franke F, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med 2019;381:307–16. [DOI] [PubMed] [Google Scholar]
- 23. Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med 2020;382:514–24. [DOI] [PubMed] [Google Scholar]
- 24. Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol 2020;6:116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Hart L, et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N Engl J Med 2022;386:942–50. [DOI] [PubMed] [Google Scholar]
- 26. Goetz MP, Toi M, Huober J, Sohn J, Tredan O, Park IH, et al. LBA15 MONARCH 3: interim overall survival (OS) results of abemaciclib plus a nonsteroidal aromatase inhibitor (NSAI) in patients (pts) with HR+, HER2- advanced breast cancer (ABC). Ann Oncol 2022;33:S1384. [DOI] [PubMed] [Google Scholar]
- 27. Finn RS, Rugo HS, Dieras VC, Harbeck N, Im S-A, Gelmon KA, et al. Overall survival (OS) with first-line palbociclib plus letrozole (PAL+LET) versus placebo plus letrozole (PBO+LET) in women with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer (ER+/HER2− ABC): analyses from PALOMA-2. J Clin Oncol 40:17s, 2022. (suppl; abstr LBA1003). [Google Scholar]
- 28. Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006;295:2492–502. [DOI] [PubMed] [Google Scholar]
- 29. Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol 2009;27:1160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prat A, Parker JS, Fan C, Perou CM. PAM50 assay and the three-gene model for identifying the major and clinically relevant molecular subtypes of breast cancer. Breast Cancer Res Treat 2012;135:301–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Llombart-Cussac A, Cortes J, Pare L, Galvan P, Bermejo B, Martinez N, et al. HER2-enriched subtype as a predictor of pathological complete response following trastuzumab and lapatinib without chemotherapy in early-stage HER2-positive breast cancer (PAMELA): an open-label, single-group, multicentre, phase 2 trial. Lancet Oncol 2017;18:545–54. [DOI] [PubMed] [Google Scholar]
- 32. R Core Team. R: A language and environment for statistical computing. Vienna, Austria, R Foundation for Statistical Computing; 2017. Available from: https://www.r-project.org/. [Google Scholar]
- 33. Yersal O, Barutca S. Biological subtypes of breast cancer: prognostic and therapeutic implications. World J Clin Oncol 2014;5:412–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Martinez-Sáez O, Tolosa P, Sánchez De Torre A, Pascual T, Brasó-Maristany F, Rodriguez Hernandez A, et al. 23P CDK4/6 inhibition and endocrine therapy in the HER2-enriched subtype in hormone receptor-positive/HER2-negative advanced breast cancer: a retrospective analysis of real-world data. Ann Oncol 2021:32;S30. [Google Scholar]
- 35. Fernandez-Martinez A, Pascual T, Perrone G, Morales S, de la Haba J, Gonzalez-Rivera M, et al. Limitations in predicting PAM50 intrinsic subtype and risk of relapse score with Ki67 in estrogen receptor-positive HER2-negative breast cancer. Oncotarget 2017;8:21930–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Finn RS, Liu Y, Zhu Z, Martin M, Rugo HS, Dieras V, et al. Biomarker analyses of response to cyclin-dependent kinase 4/6 inhibition and endocrine therapy in women with treatment-naive metastatic breast cancer. Clin Cancer Res 2020;26:110–21. [DOI] [PubMed] [Google Scholar]
- 37. Cheang M, Dowsett M, Rimawi M, Johnston S, Jacobs S, Bliss J, et al. Impact of using cross-platform gene expression profiling technologies and computational methods for intrinsic breast cancer subtyping in PALOMA-2 and PALLET [abstract]. In:Proceedings of the 2021 San Antonio Breast Cancer Symposium; 2021Dec 7–10;San Antonio, TX. Philadelphia (PA): AACR; Cancer Res 2022;82(4 Suppl):Abstract nr PD2-07. [Google Scholar]
- 38. Ciruelos E, Villagrasa P, Pascual T, Oliveira M, Pernas S, Pare L, et al. Palbociclib and trastuzumab in HER2-positive advanced breast cancer: results from the phase II SOLTI-1303 PATRICIA trial. Clin Cancer Res 2020;26:5820–9. [DOI] [PubMed] [Google Scholar]
- 39. Prat A, Carey LA, Adamo B, Vidal M, Tabernero J, Cortes J, et al. Molecular features and survival outcomes of the intrinsic subtypes within HER2-positive breast cancer. J Natl Cancer Inst 2014;106:dju152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Paquet ER, Hallett MT. Absolute assignment of breast cancer intrinsic molecular subtype. J Natl Cancer Inst 2015;107:357. [DOI] [PubMed] [Google Scholar]
- 41. Mayer EL, Dueck AC, Martin M, Rubovszky G, Burstein HJ, Bellet-Ezquerra M, et al. Palbociclib with adjuvant endocrine therapy in early breast cancer (PALLAS): interim analysis of a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2021;22:212–22. [DOI] [PubMed] [Google Scholar]
- 42. Loibl S, Marme F, Martin M, Untch M, Bonnefoi H, Kim SB, et al. Palbociclib for residual high-risk invasive HR-positive and HER2-negative early breast cancer-the Penelope-B trial. J Clin Oncol 2021;39:1518–30. [DOI] [PubMed] [Google Scholar]
- 43. Slamon D, Stroyakovskiy D, Yardley D, Huang C-S, Fasching PA, Crown J, et al. Ribociclib and endocrine therapy as adjuvant treatment in patients with HR+/HER2− early breast cancer: primary results from the phase III NATALEE trial. J Clin Oncol 41:17s, 2023. (suppl; abstr LBA500). [Google Scholar]
- 44. Aftimos P, Oliveira M, Irrthum A, Fumagalli D, Sotiriou C, Gal-Yam EN, et al. Genomic and transcriptomic analyses of breast cancer primaries and matched metastases in AURORA, the Breast International Group (BIG) molecular screening initiative. Cancer Discov 2021;11:2796–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Park YH, Im S-A, Park KH, Wen J, Min A, Bonato V, et al. Prospective longitudinal multi-omics study of palbociclib resistance in hormone receptor+/HER2– metastatic breast cancer. J Clin Oncol 39:15s, 2021. (suppl; abstr 1013). [Google Scholar]
- 46. Selli C, Sims AH. Neoadjuvant therapy for breast cancer as a model for translational research. Breast Cancer 2019;13:1178223419829072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brasó-Maristany F, Griguolo G, Pascual T, Paré L, Nuciforo P, Llombart-Cussac A, et al. Phenotypic changes of HER2-positive breast cancer during and after dual HER2 blockade. Nat Commun 2020;11:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Prat A, Saura C, Pascual T, Hernando C, Munoz M, Pare L, et al. Ribociclib plus letrozole versus chemotherapy for postmenopausal women with hormone receptor-positive, HER2-negative, luminal B breast cancer (CORALLEEN): an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol 2020;21:33–43. [DOI] [PubMed] [Google Scholar]
- 49. Brasó-Maristany F, Palafox M, Monserrat L, Bellet M, Oliveira M, Capelán M, et al. 16P Understanding the biologic determinants of ribociclib efficacy in breast cancer. Ann Oncol 2021;32:S27. [Google Scholar]
- 50. Infante JR, Cassier PA, Gerecitano JF, Witteveen PO, Chugh R, Ribrag V, et al. A phase I study of the cyclin-dependent kinase 4/6 inhibitor ribociclib (LEE011) in patients with advanced solid tumors and lymphomas. Clin Cancer Res 2016;22:5696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Patnaik A, Rosen LS, Tolaney SM, Tolcher AW, Goldman JW, Gandhi L, et al. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, and other solid tumors. Cancer Discov 2016;6:740–53. [DOI] [PubMed] [Google Scholar]
- 52. Flaherty KT, Lorusso PM, Demichele A, Abramson VG, Courtney R, Randolph SS, et al. Phase I, dose-escalation trial of the oral cyclin-dependent kinase 4/6 inhibitor PD 0332991, administered using a 21-day schedule in patients with advanced cancer. Clin Cancer Res 2012;18:568–76. [DOI] [PubMed] [Google Scholar]
- 53. Gelbert LM, Cai S, Lin X, Sanchez-Martinez C, Del Prado M, Lallena MJ, et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest New Drugs 2014;32:825–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lorman-Carbó N, Martínez-Sáez O, Fernandez-Martinez A, Galván P, Chic N, Adamo B, et al. Dissecting the biological activity of different CDK4/6 inhibitors (CDK4/6i) in hormone receptor-positive/HER2-negative (HR+/HER2-) breast cancer (BC) [abstract]. In:Proceedings of the 2022 San Antonio Breast Cancer Symposium; 2022Dec 6–10; San Antonio, TX. Philadelphia (PA): AACR; Cancer Res 2023;83(5 Suppl):Abstract nr P1-13-16. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Representativeness of study participants
Supplemental Table 2. Patient characteristics by subtype
Supplemental Table 3. Multivariable analysis of treatment benefit by subtype
Supplemental Figure 1: CONSORT diagrams of the MONALEESA (ML) trials.
Data Availability Statement
Novartis is committed to sharing with qualified external researchers, access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trials in line with applicable laws and regulations. All requests should be sent to the corresponding author.