Abstract
The incidence of renal cell carcinoma (RCC) is increasing worldwide, yet research within this field is lagging behind other cancers. Despite increased detection of early disease as a consequence of the widespread use of diagnostic CT scans, 25% of patients have disseminated disease at diagnosis. Similarly, around 25% progress to metastatic disease following curatively intended surgery. Surgery is the cornerstone in the treatment of RCC; however, when the disease is disseminated, immunotherapy or immunotherapy in combination with a tyrosine kinase inhibitor is the patient's best option. Immunotherapy is a potent treatment, with durable treatment responses and potential to cure the patient, but only half of the patients benefit from the administered treatment, and there are currently no methods that can identify which patients will respond to immunotherapy. Moreover, there is a need to identify the patients in greatest risk of relapsing after surgery for localized disease and direct adjuvant treatment there. Even though several molecular biomarkers have been published to date, we are still lacking routinely used biomarkers to guide optimal clinical management. The purpose of this review is to highlight some of the most promising biomarkers, discuss the efforts made within this field to date, and describe the barriers needed to be overcome to have reliable and robust predictive and prognostic biomarkers in the clinic for renal cancer.
Introduction
The incidence of renal cell carcinoma (RCC) is increasing worldwide, yet research within this field is lagging behind other cancers. Despite increased detection of early disease, as a consequence of the widespread use of diagnostic CT scans, a quarter of patients have disseminated disease at diagnosis. Similarly, around a quarter progress to metastatic disease following curatively intended surgery.
Surgery is the cornerstone in the treatment of localized RCC, however when the disease is disseminated, immunotherapy (IO) or immunotherapy in combination with a tyrosine kinase inhibitor (TKI-IO) is the patient's best option (1–5).
When counseling patients on surgical or medical treatments, urologists, and oncologists are utilizing variables such as age, performance status, blood chemistry, and histology in their decision making. These patient characteristics (biomarkers) are guiding treatment decisions to some extent, but not sufficiently for optimal risk stratifying.
In this review, we describe the current clinical needs for biomarkers in patient with renal cancer care, and discuss the challenges of biomarker development, including a highlight of the most promising biomarkers today.
Clinical Challenges in Renal Cancer Management
In this section, we will discuss some of the current challenges in patient with renal cancer management (Fig. 1).
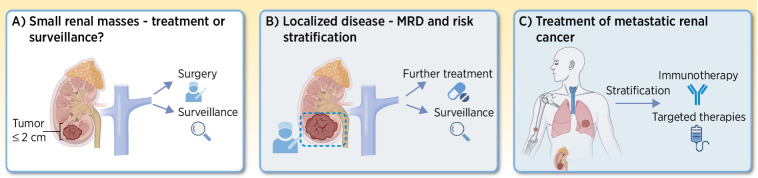
Figure 1.
Clinical challenges in renal cancer management. Listing some of the major challenges within patients with renal cancer management. A, For small renal masses, the dilemma is between operating or taking a watchful waiting strategy. B, For localized disease, risk stratification is needed. Does the patient need further treatment after curatively intended surgery? C, For metastatic disease, which treatment to offer is the main challenge. Immunotherapy should be administered only to patients most likely to respond to the treatment. (Adapted from an image created with BioRender.com.)
Localized disease
Localized RCC can be treated in various curatively intended ways, depending on the size and positioning of the tumor within the kidney. Nephron-sparing surgery is a valid treatment option for the pT1 tumors, whereas larger tumors primarily are removed by a radical nephrectomy (6). Cryoablation is also an established treatment option for pT1a tumors, given they are not placed in close vicinity of the renal pelvis or larger vessels (7). Here hydrodissection can be used to spare the neighboring organs. Finally stereotactic radiotherapy can be used in selected patients unfit for surgery or cryoablation technique (8). Patients with stage I/II RCC go on an active surveillance program following surgery, however the rate of recurrence (local or metastatic) after definitive surgery ranges widely dependent on the given risk assessment (9). Predicting RCC recurrence risk is based on prognostic systems or nomograms, with the Leibovich score system being one of the most widely used. The Leibovich score system is used to risk stratify patients with localized disease into “low,” “intermediate,” and “high risk” groups, based on histologic features and the tumor–node–metastasis (TNM) stage (9). The Leibovich score is used to determine the amount of follow-up offered; the low risk group will have CT scans at year 1, 3, and 5, “intermediate” at 6 and 12 months, and then yearly for 5 years, whereas the “high risk” group will have CT scans every 6 months for 3 years and then yearly up to 5 years.
The high recurrence rates for some patient groups may be explained by presence of micrometastatic disease prior to surgery, which is undetectable with current imaging modalities.
For patients in the Leibovich “intermediate risk” group (26% of all patients with localized disease), a clinical dilemma exists, because their risk of relapsing is 25% (9, 10). On one hand this risk is significant, but is it sufficient for offering adjuvant treatment, with the risk of adverse events this carries? Or should we rely on frequent scans? On the contrary, 75% of these patients will never relapse, and are therefore treated unnecessary with either adjuvant therapy or unnecessary and costly scans.
Minimal residual disease (MRD) is known to be closely linked to disease recurrence (11). Currently, no detection method for MRD is used in the clinic, even though MRD is undetectable with current imaging modalities. However, research of liquid biopsy-based biomarkers, including circulating tumor cells (CTC) and circulating tumor DNA (ctDNA), shows promising results for sensitive real-time cancer detection and profiling (12). Detection of MRD would provide an opportunity of intensified monitoring and early intervention, thus increasing the likelihood of successful treatment.
Small renal masses
Another clinical challenge is how to treat small renal masses (reviewed in ref. 13). With the number of abdominal imaging increasing in the diagnostic setting, the detection of small renal masses, which are often indolent, is increasing. Thus, there is potential harm derived from overdiagnosis and consequently unnecessary treatment of patients with RCC (14). This raises the question of how we determine whether a small renal mass is potentially harmful for the patient and thus needs to be removed, or whether a lesion is benign or progressing so slowly that it is not a risk to the patient.
A clinical study is currently evaluating active surveillance of patients with renal masses smaller or equal to 2 cm (NCT03804320) with patients being followed by CT scans every 3 months for the first year, and then annually for up to 5 years. This study is expected to have completed follow-up by the end of 2023, and the results from this study could potentially change the clinical management of small renal masses.
The most optimal treatment of small renal masses might in the future be determined by pretreatment characterization of biopsies. The aim will be to identify the masses with aggressive biology for treatment and spare those with indolent characteristics.
Perioperative treatment and adjuvant therapies
Historically, only the S-TRAC study evaluating the TKI Sunitinib has been approved by the FDA (15). The study showed an increase in disease-free survival (DFS) but no increase in overall survival (OS). No European Medicines Agency (EMA) approval was given. Consequently, adjuvant treatment has not been widely used in RCC.
Recent studies on adjuvant immunotherapy have shown different results in proving benefit over placebo. The IMMOTION010 (16), PROSPER RCC (17), and CheckMate 914 (part A; NCT03138512) did not succeed in showing a clinical benefit over placebo, however in the KEYNOTE-564 study (18), Pembrolizumab showed a clear DFS benefit over placebo (HR, 0.63; 95% CI, 0.50−0.80). In this study, intermediate- to high-risk patients are offered either Pembrolizumab for 1 year or placebo for 1 year. The follow-up has not yet matured sufficiently for showing a significant effect on OS, but the FDA and EMA have already approved adjuvant pembrolizumab. Interestingly, a subgroup of patients with M1 disease, but radically operated on, showed very strong results (HR, 0.28; 95% CI, 0.12–0.66), indicating that adjuvant pembrolizumab may have the highest impact in patients with the highest risk of recurrence (18). Going forward, this finding may support treating ctDNA-positive patients with adjuvant therapy.
Especially important in the adjuvant setting is the toxicity risks, because patients are curatively treated, asymptomatic, a majority of them will never relapse, and the adverse events may potentially be fatal or have a lifelong impact. Moreover, the detection of small often indolent renal masses is rising due to increased abdominal imaging, leading to potential overdiagnosis/-treatment (13, 14). A biomarker for MRD which is not detectable on conventional CT scans could potentially designate a subgroup of patients who would be much more likely to benefit from adjuvant therapy. Thereby justifying the use of adjuvant immunotherapy despite the toxicities, both clinically and financially.
Treatment of metastatic disease
Although localized disease is treated with surgery, patients with metastatic RCC (mRCC) are predominantly treated with immunotherapy. Even though immunotherapy doublet (IO-IO) delivers durable responses in one third of patients, the main clinical challenge is that 50% of patients experience disease progression within the first 12 months (19). With the introduction of TKI-IO therapy we have seen the median progression-free survival (PFS) increase [KN-426 (2), CLEAR (3), and CM9ER trials (4)]. This is a clear clinical improvement to the treatment of mRCC, however, we still lack data on the ability of TKI-IO to deliver as durable responses as the IO-IO with Ipilimumab and Nivolumab.
At present, TKI-IO is identified as the gold standard for patients in the international mRCC database consortium (IMDC) or the Memorial Sloan Kettering Cancer Center (MSK) “favorable” risk group. For patients in the “intermediate”- and “poor”-risk groups, IO-IO is the cornerstone in the treatment, alternatively the above-mentioned TKI-IO combinations (1). The COSMIC 313 trial is presently evaluating the efficacy of triplet treatment with Ipilimumab, Nivolumab, and Cabozantinib. Data are still preliminary, however PFS is particularly increased for patients in the intermediate-risk group. OS data still awaits (20). Currently, studies are evaluating the antitumor effect of Belzutifan, which seems a very promising treatment, both alone and in combination with pembrolizumab (NCT05239728) or cabozantinib (NCT03634540; ref. 21). Very recently, the chimeric antigen receptor (CAR) T-cell therapy IVS-3001 received the FDA fast-track designation, and a phase I/IIa trial has commenced (NCT05672459).
A substantial portion of patients receiving IO-IO or TKI-IO do not obtain disease control on this treatment, leading to disease progression and impaired prognosis (1). Despite attempts in finding predictive biomarkers of response to therapy (reviewed in ref. 22), it is still unclear which patients will benefit most from a given combination and what the optimal sequence of administration is. Moreover, with an evolving treatment armamentarium, where combinations of agents are being tested as compared with single-agent approaches, a mechanism by which to guide clinical decision-making is necessitated as expensive medical treatments should be given in a timely manner but only to patients who need treatment and who are likely to benefit from it.
RCC is characterized as one of the most “immune hot” tumor types, and immunotherapy has markedly improved the treatment options for mRCC and demonstrated clear benefits over standard targeted therapy (19). But, only little is known about the immunomodulatory and biological effects of the current standard treatment for the vast majority of mRCC patients, even though 50% of patients will have progressed within the first year (1). The antitumor response mounted by immunotherapy is stated to be impacted by the number of proliferating CD8 T cells recognizing tumor antigens, with results showing that exhausted T cells are enriched in mRCC (23). Moreover, recent studies elucidate that the microbiota composition may modulate the immune system and thus treatment response (24). However, the molecular mechanisms underlying the differences between responding and nonresponding patients with mRCC to immunotherapy is still unclarified (25).
Challenges in Biomarker Development
Numerous biomarkers in renal cancer management have been suggested (26, 27). Figure 2 highlights the areas where biomarker-guided treatment could have a major impact on RCC management. However, only a few biomarkers have reached the clinic to date. Currently, the Leibovich criteria is used in the clinic to risk stratify and to determine the follow-up program for patients presenting with localized disease. Here we discuss some of the challenges in biomarker development (see also Table 1 listing the biomarkers discussed below).
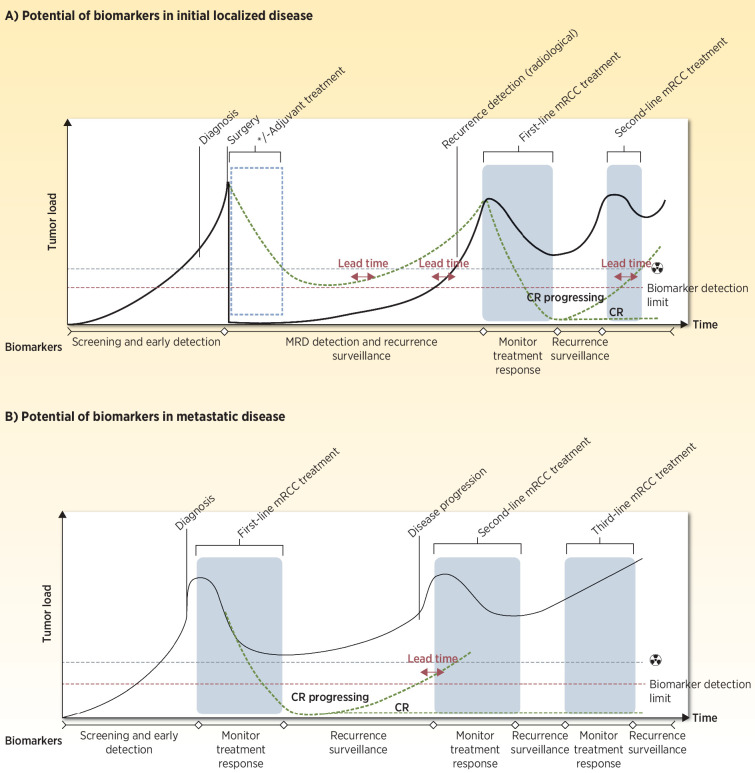
Figure 2.
Illustration of a standard disease course for (A) localized to metastatic disease and (B) for primary metastatic disease, highlighting the potential of biomarkers at the various steps. Detection limits for both radiologic assessments and potential biomarkers are indicated. Lead time indicates the time from detection of residual disease/relapse/disease progression by use of a biomarker to the time for which the residual disease/relapse/disease progression can be detected by current standard CT scans. The radiation symbol denotes imaging. (Adapted from an image created with BioRender.com.)
Table 1.
Renal cancer biomarker types, potential clinical applications, and challenges.
| Biomarker | Clinical utility | Challenges |
|---|---|---|
| Histology: subtyping based on sarcomatoid or rhabdoid features | Prognostication and treatment consideration | Applicable only to the group of patients presenting with sarcomatoid or rhabdoid features |
| Histology: nomograms | Risk stratification | The current risk stratification based on nomograms is not sufficient |
| Tumor-centric genetic biomarkers | Prognostication | Lack of treatment options based on the targets. Lack of evidence for efficacy |
| Plasma: ctDNA | Diagnosis, evaluation of treatment options, prediction of treatment outcome, monitoring, and detection of relapse | Low level of ctDNA released from renal cancers; methods need optimization |
| Plasma: CTCs | Knowledge of cancer biology and metastatic process, prediction of therapy response, and prognostication | Technical optimization of CTC enumeration |
| TCR | Immune health | Determination of neoantigen targets |
| Microbiome | Understanding the impact on tumorigenesis, immunity and immune response in relation to immunotherapies, and new treatment options | Multiple bacterial species that may have similar agonistic/antagonistic roles. Interactions with immune and other cell types are very complex |
| Single cell/spatial omics | Characterize RCC cell populations and immune composition to discover new biomarkers | Comprehensive data analysis, high levels of technical noise, lack of good reference material, and data standardization |
| Radiomics | Diagnosis of small tumors from benign cases, grading, subtyping, prediction of treatment efficacy, and prognostication | Lack of standardization of scanning protocols and analysis tools across studies |
Heterogeneity and clonal evolution
Clear cell RCC (ccRCC) represents approximately 80% of all kidney tumors, and the remaining major subtypes, defined by distinct histological and molecular features, are generally referred to as non-ccRCC (28). The morphologic subtyping constitutes an independent prognostic predictor of cancer-specific survival, and thus clinical behavior, where ccRCC tumors have a worse survival rate compared with the two most common non-ccRCC subtypes; papillary RCC (pRCC), and chromophobe RCC (chRCC; refs. 29, 30). This heterogeneity has proven to have an effect on the response to treatment with sarcomatoid differentiated tumors at a histologic level being associated with poorer outcomes in general, but shown to respond better if treated with immune checkpoint inhibitor-based therapies in the KEYNOTE-426 (2), CheckMate-214 (31), and CheckMate-025 (32) studies (33, 34). Renal tumors are in general known to be characterized with a relatively high level of both inter- and intratumoral heterogeneity, which both individually and combined can cause different patient responses to the same administered treatment, most likely due to molecular differences (35, 36). Well-established genetic mutations, including VHL, MET, and folliculin, contribute to the development of distinct RCC subtypes (ccRCC, pRCC, and chRCC, respectively) and thus different response mechanisms (36). Moreover, the intratumoral heterogeneity (ITH) presents a barrier to adequate characterization of the genome from preoperative renal tumor lesions. ITH implies multiple distinct clones co-exists within a single tumor, with branched lineages independently evolving towards increased malignancy. TCGA revealed ccRCC as having the highest degree of immune- and T-cell-infiltration based on transcriptomic analysis of 19 cancer types (37). Furthermore, the PCAWG consortium characterized the genetic ITH across 2,658 genomes from 38 cancer types and observed that the rare chRCC were among the cancers with the highest subclonal SNVs and SVs (38). However, ccRCC tumors did not exhibit a higher degree of genetic heterogeneity as compared with the other cancers. Moreover, despite being an immunogenic tumor type, renal cancers have a relatively low tumor mutational burden (TMB) as compared to other immunogenic cancers (39).
It is well-known that genetic heterogeneity fosters clonal evolution causing subclones within a tumor to change over time. TRACERx Renal showed that mRCC lesions are less heterogeneous and have fewer driver alterations than primary tumors. Consequently, driver diversity seems to accumulate in the primary tumor and metastatic-competent populations arise. The clones leading to metastatic lesions are characterized by a high proliferation rate, genomic instability, and immune evasion, with a rapid multi-tissue seeding of metastatic clones being associated with highly aggressive disease and poor OS (40, 41). Such patients may not benefit from cytoreductive nephrectomy.
Moreover, through computational modeling it has been proposed that growth on the surface of the tumor, as compared with growth in tumor volume, leads to branched evolution and consequently additional subclones with different potential arise (42). Subclone heterogeneity advances treatment resistance or may arise as a result of treatment (35). Given this diversity, the traditional approach of performing molecular and histological classification of a tumor based on a single biopsy is considerably complicated by heterogeneity both for the application of precision medicine approaches and the development of novel biomarkers of therapy resistance (43). A method to overcome heterogeneity and avoid possible sampling bias may include multiregion sequencing with phylogenetic analysis and integrated analysis of molecular data from tumor biopsies with ctDNA analysis (discussed later). Taken together, these studies highlight the importance of considering ITH both for prognostic purposes and in the development of biomarkers for surgery and therapy response.
Moreover, as immune checkpoint inhibitors execute their antitumor probabilities by activating the patient's own immune system the tumor microenvironment (TME) is known to be highly involved in the treatment response, which makes the role of the TME in renal cancer development and progression crucial to understand. ccRCC tumors are highly infiltrated with leukocytes, especially T cells of various phenotypes (44). Studies have shown that the nature and degree of the tumor infiltrate has prognostic significance, with worse outcomes being associated with the burden of accumulated T cells and the presence of specific subpopulations of tumor-associated macrophages (37, 45). The M2 macrophage phenotype has been widely associated with tumor promotion (reviewed in ref. 46), where a recent study in RCC shows implication of M2 macrophages in immune-related pathways (47). Another study of the COMPARZ (48) cohort revealed that a high tumor infiltration of macrophages in general was associated with a worse OS in patients with mRCC treated with pazopanib (HR, 2.62), where no survival difference was seen in the sunitinib-treated patient group (49). Furthermore, a study measuring CD163, a well-described marker of M2-like tumor-associated macrophages, showed that baseline soluble circulating CD163 is a novel independent biomarker of OS in mRCC (50). These findings motivate further exploration of the predictive potential for the TME in IO and TKI response. Yet, attention needs to be drawn to the risk of oversimplification when looking at only M1 and M2 phenotypes, as it has been shown that macrophages exist in a continuum of activation states (51, 52).
However, the underlying mechanisms behind the immunogenicity of ccRCC has been undefined due to the slightly atypical low mutational and neoantigen load detected in the ccRCC tumor (37, 53). It has been postulated that the epithelial compartment and an elevated antigen presentation may be involved in the attraction of immune infiltrate and the immunotherapy responsiveness (37, 54, 55). In contrast, chRCC has shown less immune filtration compared with ccRCC and is consequently defined as “immune-cold.” Both subtypes have a low TMB and despite the distinct biology, the difference in immune infiltration remains unexplained (55).
Methodological challenges
In addition to heterogeneity, technical features are also challenging the comparison between studies. More specifically, the variability in specificities, sensitivities, and assays applied, the lack of reproducibility between platforms and analyzing methods used between studies, as well as the lack of validation in and across clinical trials. Many studies performed to date are based on small retrospective cohorts and have not been validated in independent (large) cohorts, and thus the significance of these studies may be questionable. Accuracy and reproducibility both within a given study and in subsequent validation studies are utmost important.
The number of FDA-approved biomarkers used in RCC patient management are limited and to change this, collaborative strategies need to be fostered within the RCC research field. Importantly, to increase the number of patients within each study, trials across borders should be set up under prospective conditions with high accuracy, standardized data collection, and reproducibility.
Potential of Tumor-centric Biomarkers
The four most frequent mutated alterations in RCC are VHL (64%), PBRM1 (36%), SETD2 (20%), and BAP1 (13%), and their potential as biomarkers have been widely analyzed (reviewed in ref. 56). There has been debate concerning the VHL gene association with prediction and prognostics, however a meta-analysis including results from six studies showed no association between VHL mutational status and clinical parameters, such as OS, PFS, or overall response rate (57). Mutations in PBRM1 mutation is generally a favorable prognostic biomarker, but varied associations with response to VEGF or immunotherapies have been observed (58, 59). BAP1 alterations have been associated with poor prognosis with significantly shorter OS for BAP1-mutated patients (60, 61), but neither BAP1, VHL, or PBRM1 showed prognostic potential in a clinical phase III trial (JAVELIN; ref. 62). In addition, SETD2, a H3K36-trimethyltransferase, has been linked to metastatic disease, where a recent study demonstrates that SETD2 loss leads to an open chromatin structure in the renal tumors, which facilitates increased transcriptional output from oncogenic drivers (63, 64).
Recently, gene expression alterations such as metabolomics and or methylation signatures (65), as well as, distinct histological and molecular combined signatures have been suggested as promising risk stratifiers (66–70). Many biomarkers have been examined in RCC trials, especially TMB, tumor-infiltrating lymphocytes (TIL) and PD-L1 expression, but also MMR-Deficiency/MSI-High, PBRM-1, and neutrophil-to-lymphocyte ratio (NLR), however, despite major contributions within this field none of these biomarkers may successfully predict the efficacy of ICI-based therapy (71, 72).
Currently, one of the most investigated tumor-associated biomarkers in RCC is expression of the tumor cell programmed cell death 1 ligand 1 (PD-L1). PD-L1 is a transmembrane protein expressed primarily on activated T cells, and the expression of PD-L1 has shown to be a poor prognostic marker in immune checkpoint inhibitor (ICI)-based therapy naive patients and to correlate strongly with an unfavorable outcome in TKI-based therapy (73, 74). A meta-analysis of randomized PD-L1 clinical trials including in total 4,635 patients with mRCC from six clinical trials comparing ICIs with standard therapy, revealed that PD-L1 expression correlated positively with PFS but not OS (75). The latter was improved upon treatment regardless of PD-L1 levels, thus indicating that PD-L1 may not be an ideal predictive biomarker of response to ICIs (1).
However, there has been great variability in the PD-L1 positivity reported by various studies, ranging from a few percent to over 60% of cases having PD-L1 detected (76, 77). The reason for this variability can be explained by the cut-off level applied to define PD-L1 expression, the amount of tissue analyzed as tumor tissues are not uniform, heterogeneous expression, different antibodies and staining procedures as well as difficulties in distinguishing PD-L1 positive tumor cells from macrophages. These kinds of variabilities limit the progress within this field.
Potential of Liquid Biopsies as Biomarkers in Renal Cancer
The utility of liquid-based biomarkers is numerous such as: distinguishing benign from malignant renal masses, monitoring and detecting MRD beyond the resolution of imaging, predicting therapy response, prognostication, stratify patients based on risk assessments, and monitor tumor evolution (in response to treatment) using a noninvasive approach.
Circulating tumor DNA
ctDNA has proven great potential as a diagnostic, prognostic, and predictive biomarker in cancer in general, however the analysis has been challenged in RCC by the trace amounts of ctDNA shedded by RCC tumors (78); 1,237 patients have so far been examined across 19 different ctDNA-based studies.
A few studies have questioned ctDNA as a prognostic marker in RCC, and it has been shown that ctDNA levels can be used as an independent predictor of disease- and recurrence-free survival (79–82). Furthermore, some studies have reported that ctDNA changes, detected through longitudinal analysis, correlate with the clinical disease course and with changes in tumor volume as assessed by CT-imaging (79, 83–85). However, these studies have been challenged by detection of ctDNA in only a low fraction of patients. This may to some extent be explained by the use of single-biomarker strategies which may not capture the huge complexity of response. Tumor-guided methods as compared with tumor-agnostic methods, where the blood is investigated without any prior knowledge, have to some extent improved the ctDNA detection rate (78). With more sensitive markers and techniques, such as cfMeDIP-seq exploring the cfDNA methylation landscape, it has proven possible to identify ctDNA in early-stage RCC (86). Recent studies (86, 87) demonstrated that using cfMeDIP-seq it is possible to accurately classify patients across all stages of RCC, with a ctDNA detection rate of 97% at 100% specificity. Moreover, in mRCC methylation levels assessed by cfMeDIP-seq showed superior performance in ctDNA detection (100%), compared with cfDNA mutation analysis using targeted sequencing (21%), with 88% specificity (84). This work shows the potential of overcoming some of the challenges previously reported in the renal ctDNA field, so that the same sensitivity as seen in other cancers can be obtained.
As the half-life of cfDNA is <2 hours, it is intuitive that patients cured by surgery will not have ctDNA during follow-up, whereas patients receiving nonradical surgery or having occult dissemination at time of surgery are likely to have it (88). Importantly, liquid biopsies can overcome genetic heterogeneity both in space (subclones/regions with different alterations) and in time (evolution during for example selection pressure) as liquid biopsies both capture the entire tumor and can be sampled throughout disease progression. However, tumor-informed approaches to ctDNA detection may pose some challenges to capture metastatic lesions with significantly different biological backgrounds.
ctDNA analysis has potential as a tool for assessing the impact of intervention, and to provide a critical window of opportunity for intervention at an early time-point where curative modalities are still an option. Use of ctDNA analysis may be able to correctly guide when to perform CT-imaging as opposed to current surveillance programs with radiologic assessment at specific time points, with the frequency of CT-imaging correlating to a pre-assessed risk of recurrence. In addition, ctDNA analysis may be able to identify, and improve on, factors associated with suboptimal surgery, and to identify patients with subclinical metastatic disease who may benefit from adjuvant therapy, but are untreated today (stage I, II, III).
Nevertheless, more, and larger studies across centers are warranted to fully validate the potential of ctDNA as a biomarker in RCC.
Circulating tumor cells
CTCs are tumor cells shed from the primary tumor, which circulates through the peripheral blood system and are involved in the metastatic process (89). Characterizing CTCs could give valuable insight into the metastatic process in RCC, which could potentially help to diagnose metastatic lesions early as well as provide information to help hinder metastatic disease. A few studies have linked the detection of CTCs with poor response to treatment and shorter PFS in mRCC (90). At the time of radical nephrectomy, the detection of CTCs correlates with positive lymph nodes and synchronous metastasis (91). In addition, CTCs have been shown to have variable plasticity, and CTCs with stem-cell-like characteristics have been associated with shorter PFS (92). An observational clinical trial, including 246 mRCC first-line TKI patients (93) reported that patients with three or more CTCs detected at baseline had a shorter PFS and OS. Thus, detection of CTCs has the potential to be of prognostic value in RCC, however for clinical integration there exists a need for technical optimization of CTC enumeration (reviewed in ref. 94).
Other liquid biomarkers
In a recent study, T-cell receptor (TCR) clones, analyzed in the blood of patients with RCC during IO treatment, were found to be indicative of treatment response (95). Changes in TCR clones demonstrate the potential of predictive blood based markers. However, to implement this into clinical practice in the future, figuring out how to select personalized target neoantigens is of priority, as neoantigens are unique in individual cancers.
In addition, multiple types of noncoding RNAs have been tested and proposed as biomarkers in RCC (reviewed in ref. 96) and comprehensively discussed in ref. 97). However, to move this field forward consensus of methods needs to be obtained, such that results can be validated across studies.
New Avenues to Explore for Biomarker Discovery
To move the RCC biomarker field ahead new areas need to be explored.
Microbiome
The microbiome plays a role not only in the metabolism, but also in tumorigenesis, immunity, and immune response in relation to immunotherapies. Administering antibiotics prior to immunotherapy treatment is linked to a reduced PFS, which could be caused by an altered microbiome (98). The composition of the microbiome has also been shown to be altered as a consequence of TKI treatment (98). The microbiome has further been linked to ICI response; patients with a greater microbial diversity respond better (99). This knowledge has been translated into tests of fecal microbiota transplantation in mice (100), and administration of probiotic supplements in patients with RCC receiving VEGF-TKIs, regardless of the line of therapy (101). Despite increased levels of the bacteria clones in the gut, no effect was observed on the outcome for the patients treated with probiotics (101). In a recent open-label, single-center study, 30 treatment-naive patients with mRCC were randomized 2:1 to receive Nivolumab and Ipilimumab with or without daily probiotic supplement, respectively. Patients receiving probiotics had a significantly longer PFS (12.7 months vs. 2.5 months), and higher response rate (58% vs. 20%; ref. 102). Consequently, the role of the gut microbiome in modulating treatment response during treatment is intriguing and warrants further investigation.
Single-cell and spatial omics
Single-cell omics, which allow to disentangle genomic, transcriptomic, proteomic, or any other omic data for every individual cell in RCC is a next natural step to better characterize RCC cell populations to discover new biomarkers. A few small-scaled studies have started to look into this and found prognostic and predictive value for specific cell types (54, 55, 103, 104).
Moreover, to further understand the intercellular communication of cancer cells with the cells surrounding, spatial analysis of the tissue can assist single cell findings. In RCC, it has been found that tumors harboring close proximity between natural killer and T cells possess poor OS (105). Thus, single-cell and spatial omics can give novel insight into the characteristics of RCC tumor biology, immune composition, and TME, which can be linked to clinical outcome measures and subsequently be investigated as potential targets for therapy.
Radiomics
A further intriguing area advancing in the field is the use of artificial intelligence (AI) applied to RCC radiomics. Image characterization of renal masses might be able to diagnose small and indolent tumors from benign cases (106), grade tumors (107), differentiate RCC subtypes (108), and to predict treatment efficacy (109), and for prognostication (110) (reviewed in detail in ref. 111). Yet, work is still needed to standardize scanning protocols and analysis tools.
Perspectives
To optimize the treatment of patients with RCC and move towards a more personalized approach it is essential to comprehensively characterize the tumor in relation to the TME and immune landscape. Further, the utility of potential biomarkers in RCC should in the future include the development of clinically significant data in the context of prospective clinical trials, and ultimately be tested for clinical utility in biomarker-guided clinical trials. Moreover, it is important that we continue to try to understand the early steps of kidney cancer, molecular definitions, and understand the wealth of the different kidney cancer subtypes. A further understanding of why some tumors is responding to treatment whereas others do not is needed. For this, prospectively collected cohorts with excellent clinical data are needed. Furthermore, and importantly, the standardization of procedures, such as sample acquisition, storage, and analysis is needed to be tightly controlled and standardized to move this field forward.
The potential clinical implications are numerous if biomarker-guided studies could clearly demonstrate the potential of the discussed biomarkers over the current prognostic determinations and imaging modalities. First, for example, a more patient specific follow-up scheme could be accomplished using ctDNA-guided clinical management: (1) ctDNA-positive patients may benefit from immediate intervention. (2) For ctDNA-negative patients, a watchful waiting strategy, possibly involving serial ctDNA analysis, may be beneficial and could potentially spare patients from unnecessary concerns and inconvenience, as well as unnecessary use of imaging resources.
Second, prediction of therapy response could help select patients with a high probability of immunotherapy efficacy, ultimately paving the way for precision immuno-oncology.
This will benefit both patients and the healthcare system; expensive medical treatments should be given in a timely manner but only to patients who need, for example, additional oncologic treatment and who are likely to benefit from it.
In conclusion, to truly move the renal cancer biomarker field ahead collaborative forces are needed across countries to ensure sufficient enrolled patients in prospective studies, evaluating not only the tumor composition, but equally interesting the patient's immune response to the treatment, and the microbiome composition in a holistic view of the patient's cancer disease.
Acknowledgments
This work was supported through a personal grant to I. Lyskjær from the Lundbeck Foundation (R413–2022–606).
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authors' Disclosures
I. Lyskjær reports grants from Lundbeck Foundation during the conduct of the study. L. Dyrskjøt reports grants from C2i Genomics, Natera, AstraZeneca, and Photocure; grants and personal fees from Ferring; personal fees from MSD and UroGen; and other support from Pfizer and Roche outside the submitted work. N. Fristrup reports personal fees from MSD, BMS, and AstraZeneca outside the submitted work. No disclosures were reported by the other authors.
References
- 1. Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018;378:1277–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019;380:1116–27. [DOI] [PubMed] [Google Scholar]
- 3. Motzer R, Alekseev B, Rha S-Y, Porta C, Eto M, Powles T, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med 2021;384:1289–300. [DOI] [PubMed] [Google Scholar]
- 4. Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2021;384:829–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019;380:1103–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maroni P, Moss J. Nephron-sparing surgery. Semin Intervent Radiol 2014;31:104–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stacul F, Sachs C, Giudici F, Bertolotto M, Rizzo M, Pavan N, et al. Cryoablation of renal tumors: long-term follow-up from a multicenter experience. Abdom Radiol (NY) 2021;46:4476–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Siva S, Ali M, Correa RJM, Muacevic A, Ponsky L, Ellis RJ, et al. 5-year outcomes after stereotactic ablative body radiotherapy for primary renal cell carcinoma: an individual patient data meta-analysis from IROCK (the international radiosurgery consortium of the kidney). Lancet Oncol 2022;23:1508–16. [DOI] [PubMed] [Google Scholar]
- 9. Leibovich BC, Blute ML, Cheville JC, Lohse CM, Frank I, Kwon ED, et al. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer 2003;97:1663–71. [DOI] [PubMed] [Google Scholar]
- 10. Azawi NH, Tesfalem H, Mosholt KSS, Høyerup P, Jensen ES, Malchau E, et al. Recurrence rates and survival in a Danish cohort with renal cell carcinoma. Dan Med J 2016;63:A5208. [PubMed] [Google Scholar]
- 11. Larribère L, Martens UM. Advantages and challenges of using ctDNA NGS to assess the presence of minimal residual disease (MRD) in solid tumors. Cancers 2021;13:5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pantel K, Alix-Panabières C. Liquid biopsy and minimal residual disease—latest advances and implications for cure. Nat Rev Clin Oncol 2019;16:409–24. [DOI] [PubMed] [Google Scholar]
- 13. Tamara JI, Juan GR, Patricia ZJ, Roser VD, Moisés R, Dmitry E, et al. Diagnosis and treatment of small renal masses: where do we stand? Curr Urol Rep 2022;23:99–111. [DOI] [PubMed] [Google Scholar]
- 14. Sohlberg EM, Metzner TJ, Leppert JT. The harms of overdiagnosis and overtreatment in patients with small renal masses: a mini-review. Eur Urol Focus 2019;5:943–5. [DOI] [PubMed] [Google Scholar]
- 15. Ravaud A, Motzer RJ, Pandha HS, George DJ, Pantuck AJ, Patel A, et al. Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N Engl J Med 2016;375:2246–54. [DOI] [PubMed] [Google Scholar]
- 16. Pal SK, Uzzo R, Karam JA, Master VA, Donskov F, Suarez C, et al. Adjuvant atezolizumab versus placebo for patients with renal cell carcinoma at increased risk of recurrence following resection (IMmotion010): a multicentre, randomised, double-blind, phase 3 trial. Lancet 2022;400:1103–16. [DOI] [PubMed] [Google Scholar]
- 17. Patel HD, Puligandla M, Shuch BM, Leibovich BC, Kapoor A, Master VA, et al. The future of perioperative therapy in advanced renal cell carcinoma: how can we PROSPER? Future Oncol 2019;15:1683–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Powles T, Tomczak P, Park SH, Venugopal B, Ferguson T, Symeonides SN, et al. Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for clear cell renal cell carcinoma (KEYNOTE-564): 30-month follow-up analysis of a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2022;23:1133–44. [DOI] [PubMed] [Google Scholar]
- 19. Deleuze A, Saout J, Dugay F, Peyronnet B, Mathieu R, Verhoest G, et al. Immunotherapy in renal cell carcinoma: the future is now. Int J Mol Sci 2020;21:2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Choueiri TK, Powles T, Albiges L, Burotto M, Szczylik C, Zurawski B, et al. Cabozantinib plus nivolumab and ipilimumab in renal-cell carcinoma. N Engl J Med 2023;388:1767–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jonasch E, Donskov F, Iliopoulos O, Rathmell WK, Narayan VK, Maughan BL, et al. Belzutifan for renal cell carcinoma in von Hippel-Lindau disease. N Engl J Med 2021;385:2036–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Raimondi A, Sepe P, Zattarin E, Mennitto A, Stellato M, Claps M, et al. Predictive biomarkers of response to immunotherapy in metastatic renal cell cancer. Front Oncol. 2020;10:1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yao C, Zhang T, Wu T, Brugarolas J. Facts and hopes for immunotherapy in renal cell carcinoma. Clin Cancer Res 2022;28:5013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Deluce J, Maleki Vareki S, Fernandes R. The role of gut microbiome in immune modulation in metastatic renal cell carcinoma. Ther Adv Med Oncol 2022;14:17588359221122714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sobottka B, Nienhold R, Nowak M, Hench J, Haeuptle P, Frank A, et al. Integrated analysis of immunotherapy treated clear cell renal cell carcinomas: an exploratory study. J Immunother 2022;45:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li F, Aljahdali IAM, Zhang R, Nastiuk KL, Krolewski JJ, Ling X. Kidney cancer biomarkers and targets for therapeutics: survivin (BIRC5), XIAP, MCL-1, HIF1α, HIF2α, NRF2, MDM2, MDM4, p53, KRAS, and AKT in renal cell carcinoma. J Exp Clin Cancer Res 2021;40:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marchioni M, Rivas JG, Autran A, Socarras M, Albisinni S, Ferro M, et al. Biomarkers for renal cell carcinoma recurrence: state of the art. Curr Urol Rep 2021;22:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Escudier B, Porta C, Schmidinger M, Rioux-Leclercq N, Bex A, Khoo V, et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol 2019;30:706–20. [DOI] [PubMed] [Google Scholar]
- 29. Delahunt B, Cheville JC, Martignoni G, Humphrey PA, Magi-Galluzzi C, McKenney J, et al. The international society of urological pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am J Surg Pathol 2013;37:1490–504. [DOI] [PubMed] [Google Scholar]
- 30. Leibovich BC, Lohse CM, Crispen PL, Boorjian SA, Thompson RH, Blute ML, et al. Histological subtype is an independent predictor of outcome for patients with renal cell carcinoma. J Urol 2010;183:1309–15. [DOI] [PubMed] [Google Scholar]
- 31. McDermott DF, Choueiri TK, Motzer RJ, Aren OR, George S, Powles T, et al. CheckMate 214 post-hoc analyses of nivolumab plus ipilimumab or sunitinib in IMDC intermediate/poor-risk patients with previously untreated advanced renal cell carcinoma with sarcomatoid features. J Clin Oncol 2019;37:4513. [Google Scholar]
- 32. Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first-line therapy for metastatic renal cell carcinoma (mRCC): outcomes in the combined IMDC intermediate/poor risk and sarcomatoid subgroups of the phase 3 KEYNOTE-426 study. J Clin Oncol 2019;37:4500. [Google Scholar]
- 33. Motzer RJ, Rini BI, McDermott DF, Arén Frontera O, Hammers HJ, Carducci MA, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol 2019;20:1370–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Peralta-Venturina M, Moch H, Amin M, Tamboli P, Hailemariam S, Mihatsch M, et al. Sarcomatoid differentiation in renal cell carcinoma: a study of 101 cases. Am J Surg Pathol 2001;25:275–84. [DOI] [PubMed] [Google Scholar]
- 35. Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012;366:883–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Beksac AT, Paulucci DJ, Blum KA, Yadav SS, Sfakianos JP, Badani KK. Heterogeneity in renal cell carcinoma. Urol Oncol 2017;35:507–15. [DOI] [PubMed] [Google Scholar]
- 37. Şenbabaoğlu Y, Gejman RS, Winer AG, Liu M, Van Allen EM, de Velasco G, et al. Tumor immune microenvironment characterization in clear cell renal cell carcinoma identifies prognostic and immunotherapeutically relevant messenger RNA signatures. Genome Biol 2016;17:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dentro SC, Leshchiner I, Haase K, Tarabichi M, Wintersinger J, Deshwar AG, et al. Characterizing genetic intra-tumor heterogeneity across 2,658 human cancer genomes. Cell 2021;184:2239–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SAJR, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature 2013;500:415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Turajlic S, Xu H, Litchfield K, Rowan A, Horswell S, Chambers T, et al. Deterministic evolutionary trajectories influence primary tumor growth: TRACERx renal. Cell 2018;173:595–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Turajlic S, Xu H, Litchfield K, Rowan A, Chambers T, Lopez JI, et al. Tracking cancer evolution reveals constrained routes to metastases: TRACERx renal. Cell 2018;173:581–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fu X, Zhao Y, Lopez JI, Rowan A, Au L, Fendler A, et al. Spatial patterns of tumour growth impact clonal diversification in a computational model and the TRACERx renal study. Nat Ecol Evol 2022;6:88–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McGranahan N, Swanton C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell 2017;168:613–28. [DOI] [PubMed] [Google Scholar]
- 44. Yoshihara K, Shahmoradgoli M, Martínez E, Vegesna R, Kim H, Torres-Garcia W, et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun 2013;4:2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Obradovic A, Chowdhury N, Haake SM, Ager C, Wang V, Vlahos L, et al. Single-cell protein activity analysis identifies recurrence-associated renal tumor macrophages. Cell 2021;184:2988–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Komohara Y, Jinushi M, Takeya M. Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci 2014;105:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang X, Sun Y, Ma Y, Gao C, Zhang Y, Yang X, et al. Tumor-associated M2 macrophages in the immune microenvironment influence the progression of renal clear cell carcinoma by regulating M2 macrophage-associated genes. Front Oncol 2023;13:1157861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 2013;369:722–31. [DOI] [PubMed] [Google Scholar]
- 49. Hakimi AA, Voss MH, Kuo F, Sanchez A, Liu M, Nixon BG, et al. Transcriptomic profiling of the tumor microenvironment reveals distinct subgroups of clear cell renal cell cancer: data from a randomized phase III trial. Cancer Discov 2019;9:510–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lauridsen KM, Hokland M, Al-Karradi S, Møller HJ, Donskov F, Andersen MN. Soluble CD163: a novel independent prognostic biomarker in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother 2023;72:461–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008;8:958–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 2010;11:889–96. [DOI] [PubMed] [Google Scholar]
- 53. de Velasco G, Miao D, Voss MH, Hakimi AA, Hsieh JJ, Tannir NM, et al. Tumor mutational load and immune parameters across metastatic renal cell carcinoma risk groups. Cancer Immunol Res 2016;4:820–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bi K, He MX, Bakouny Z, Kanodia A, Napolitano S, Wu J, et al. Tumor and immune reprogramming during immunotherapy in advanced renal cell carcinoma. Cancer Cell 2021;39:649–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang Y, Narayanan SP, Mannan R, Raskind G, Wang X, Vats P, et al. Single-cell analyses of renal cell cancers reveal insights into tumor microenvironment, cell of origin, and therapy response. Proc Natl Acad Sci U S A 2021;118:e2103240118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bui TO, Dao VT, Nguyen VT, Feugeas J-P, Pamoukdjian F, Bousquet G. Genomics of clear-cell renal cell carcinoma: a systematic review and meta-analysis. Eur Urol 2022;81:349–61. [DOI] [PubMed] [Google Scholar]
- 57. Kim BJ, Kim JH, Kim HS, Zang DY. Prognostic and predictive value of VHL gene alteration in renal cell carcinoma: a meta-analysis and review. Oncotarget 2017;8:13979–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Braun DA, Ishii Y, Walsh AM, Van Allen EM, Wu CJ, Shukla SA, et al. Clinical validation of PBRM1 alterations as a marker of immune checkpoint inhibitor response in renal cell carcinoma. JAMA Oncol 2019;5:1631–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Miao D, Margolis CA, Gao W, Voss MH, Li W, Martini DJ, et al. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science 2018;359:801–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jin S, Wu J, Zhu Y, Gu W, Wan F, Xiao W, et al. Comprehensive analysis of somatic mutation in clear cell renal cell carcinoma to explore potential mechanisms. J Cancer 2018;9:4108–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Niersch J, Vega-Rubín-de-Celis S, Bazarna A, Mergener S, Jendrossek V, Siveke JT, et al. A synonymous mutation results in exon skipping, loss of function and worse patient prognosis. iScience 2021;24:102173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Motzer RJ, Robbins PB, Powles T, Albiges L, Haanen JB, Larkin J, et al. Avelumab plus axitinib versus sunitinib in advanced renal cell carcinoma: biomarker analysis of the phase 3 JAVELIN Renal 101 trial. Nat Med 2020;26:1733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hsieh JJ, Le VH, Oyama T, Ricketts CJ, Ho TH, Cheng EH. Chromosome 3p loss-orchestrated VHL, HIF, and epigenetic deregulation in clear cell renal cell carcinoma. J Clin Oncol 2018;36:JCO2018792549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Xie Y, Sahin M, Sinha S, Wang Y, Nargund AM, Lyu Y, et al. SETD2 loss perturbs the kidney cancer epigenetic landscape to promote metastasis and engenders actionable dependencies on histone chaperone complexes. Nat Cancer 2022;3:188–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Li Y, Lih T-SM, Dhanasekaran SM, Mannan R, Chen L, Cieslik M, et al. Histopathologic and proteogenomic heterogeneity reveals features of clear cell renal cell carcinoma aggressiveness. Cancer Cell 2023;41:139–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Büttner FA, Winter S, Stühler V, Rausch S, Hennenlotter J, Füssel S, et al. A novel molecular signature identifies mixed subtypes in renal cell carcinoma with poor prognosis and independent response to immunotherapy. Genome Med 2022;14:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Büttner F, Winter S, Rausch S, Reustle A, Kruck S, Junker K, et al. Survival prediction of clear cell renal cell carcinoma based on gene expression similarity to the proximal tubule of the nephron. Eur Urol 2015;68:1016–20. [DOI] [PubMed] [Google Scholar]
- 68. Rini B, Goddard A, Knezevic D, Maddala T, Zhou M, Aydin H, et al. A 16-gene assay to predict recurrence after surgery in localised renal cell carcinoma: development and validation studies. Lancet Oncol 2015;16:676–85. [DOI] [PubMed] [Google Scholar]
- 69. Brooks SA, Brannon AR, Parker JS, Fisher JC, Sen O, Kattan MW, et al. ClearCode34: a prognostic risk predictor for localized clear cell renal cell carcinoma. Eur Urol 2014;66:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ghatalia P, Rathmell WK. Systematic review: ClearCode 34 - a validated prognostic signature in clear cell renal cell carcinoma (ccRCC). Kidney Cancer 2018;2:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Martin SD, Bhuiyan I, Soleimani M, Wang G. Biomarkers for immune checkpoint inhibitors in renal cell carcinoma. J Clin Med Res 2023;12:4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sarkis J, Assaf J, Alkassis M. Biomarkers in renal cell carcinoma: towards a more selective immune checkpoint inhibition. Transl Oncol 2021;14:101071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Möller K, Fraune C, Blessin NC, Lennartz M, Kluth M, Hube-Magg C, et al. Tumor cell PD-L1 expression is a strong predictor of unfavorable prognosis in immune checkpoint therapy-naive clear cell renal cell cancer. Int Urol Nephrol 2021;53:2493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Choueiri TK, Figueroa DJ, Fay AP, Signoretti S, Liu Y, Gagnon R, et al. Correlation of PD-L1 tumor expression and treatment outcomes in patients with renal cell carcinoma receiving sunitinib or pazopanib: results from COMPARZ, a randomized controlled trial. Clin Cancer Res 2015;21:1071–7. [DOI] [PubMed] [Google Scholar]
- 75. Carretero-González A, Lora D, Martín Sobrino I, Sáez Sanz I, Bourlon MT, Anido Herranz U, et al. The value of PD-L1 expression as predictive biomarker in metastatic renal cell carcinoma patients: a meta-analysis of randomized clinical trials. Cancers 2020;12:1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Leite KRM, Reis ST, Junior JP, Zerati M, Gomes D de O, Camara-Lopes LH, et al. PD-L1 expression in renal cell carcinoma clear cell type is related to unfavorable prognosis. Diagn Pathol 2015;10:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Stenzel PJ, Schindeldecker M, Tagscherer KE, Foersch S, Herpel E, Hohenfellner M, et al. Prognostic and predictive value of tumor-infiltrating leukocytes and of immune checkpoint molecules PD1 and PDL1 in clear cell renal cell carcinoma. Transl Oncol 2020;13:336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Geertsen L, Koldby KM, Thomassen M, Kruse T, Lund L. Circulating tumor DNA in patients with renal cell carcinoma. A systematic review of the literature. Eur Urol Open Sci 2022;37:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yamamoto Y, Uemura M, Fujita M, Maejima K, Koh Y, Matsushita M, et al. Clinical significance of the mutational landscape and fragmentation of circulating tumor DNA in renal cell carcinoma. Cancer Sci 2019;110:617–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bacon JVW, Annala M, Soleimani M, Lavoie J-M, So A, Gleave ME, et al. Plasma circulating tumor DNA and clonal hematopoiesis in metastatic renal cell carcinoma. Clin Genitourin Cancer 2020;18:322–31. [DOI] [PubMed] [Google Scholar]
- 81. Lin Y-L, Wang Y-P, Li H-Z, Zhang X. Aberrant promoter methylation of PCDH17 (protocadherin 17) in serum and its clinical significance in renal cell carcinoma. Med Sci Monit 2017;23:3318–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jung M, Ellinger J, Gevensleben H, Syring I, Lüders C, de Vos L, et al. Cell-free DNA methylation in blood as a molecular staging parameter for risk stratification in renal cell carcinoma patients: a prospective observational cohort study. Clin Chem 2019;65:559–68. [DOI] [PubMed] [Google Scholar]
- 83. Smith CG, Moser T, Mouliere F, Field-Rayner J, Eldridge M, Riediger AL, et al. Comprehensive characterization of cell-free tumor DNA in plasma and urine of patients with renal tumors. Genome Med 2020;12:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lasseter K, Nassar AH, Hamieh L, Berchuck JE, Nuzzo PV, Korthauer K, et al. Plasma cell-free DNA variant analysis compared with methylated DNA analysis in renal cell carcinoma. Genet Med 2020;22:1366–73. [DOI] [PubMed] [Google Scholar]
- 85. Pal SK, Sonpavde G, Agarwal N, Vogelzang NJ, Srinivas S, Haas NB, et al. Evolution of circulating tumor DNA profile from first-line to subsequent therapy in metastatic renal cell carcinoma. Eur Urol 2017;72:557–64. [DOI] [PubMed] [Google Scholar]
- 86. Nuzzo PV, Berchuck JE, Korthauer K, Spisak S, Nassar AH, Abou Alaiwi S, et al. Detection of renal cell carcinoma using plasma and urine cell-free DNA methylomes. Nat Med 2020;26:1041–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Shen SY, Singhania R, Fehringer G, Chakravarthy A, Roehrl MHA, Chadwick D, et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature 2018;563:579–83. [DOI] [PubMed] [Google Scholar]
- 88. Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med 2008;14:985–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lin D, Shen L, Luo M, Zhang K, Li J, Yang Q, et al. Circulating tumor cells: biology and clinical significance. Signal Transduct Target Ther 2021;6:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bialek J, Wencker A, Kawan F, Yankulov S, Fornara P, Theil G. Potential use of CTCs as biomarkers in renal cancer patients. Life 2022;12:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bluemke K, Bilkenroth U, Meye A, Fuessel S, Lautenschlaeger C, Goebel S, et al. Detection of circulating tumor cells in peripheral blood of patients with renal cell carcinoma correlates with prognosis. Cancer Epidemiol Biomarkers Prev 2009;18:2190–4. [DOI] [PubMed] [Google Scholar]
- 92. Nel I, Gauler TC, Bublitz K, Lazaridis L, Goergens A, Giebel B, et al. Circulating tumor cell composition in renal cell carcinoma. PLoS One 2016;11:e0153018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Basso U, Facchinetti A, Rossi E, Maruzzo M, Conteduca V, Aieta M, et al. Prognostic role of circulating tumor cells in metastatic renal cell carcinoma: a large, multicenter, prospective trial. Oncologist 2021;26:740–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Couto-Cunha A, Jerónimo C, Henrique R. Circulating tumor cells as biomarkers for renal cell carcinoma: ready for prime time? Cancers 2022;15:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Carlisle JW, Jansen CS, Cardenas MA, Sobierajska E, Reyes AM, Greenwald R, et al. Clinical outcome following checkpoint therapy in renal cell carcinoma is associated with a burst of activated CD8 T cells in blood. J Immunother Cancer 2022;10:e004803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Barth DA, Drula R, Ott L, Fabris L, Slaby O, Calin GA, et al. Circulating non-coding RNAs in renal cell carcinoma-pathogenesis and potential implications as clinical biomarkers. Front Cell Dev Biol 2020;8:828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Applications of noncoding RNAs in renal cancer patients. Clinical applications of noncoding RNAs in cancer. Academic Press; 2022. pp. 211–84. [Google Scholar]
- 98. Derosa L, Hellmann MD, Spaziano M, Halpenny D, Fidelle M, Rizvi H, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol 2018;29:1437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Salgia NJ, Bergerot PG, Maia MC, Dizman N, Hsu J, Gillece JD, et al. Stool microbiome profiling of patients with metastatic renal cell carcinoma receiving anti-PD-1 immune checkpoint inhibitors. Eur Urol 2020;78:498–502. [DOI] [PubMed] [Google Scholar]
- 100. Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018;359:91–97. [DOI] [PubMed] [Google Scholar]
- 101. Dizman N, Hsu J, Bergerot PG, Gillece JD, Folkerts M, Reining L, et al. Randomized trial assessing impact of probiotic supplementation on gut microbiome and clinical outcome from targeted therapy in metastatic renal cell carcinoma. Cancer Med 2021;10:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Dizman N, Meza L, Bergerot P, Alcantara M, Dorff T, Lyou Y, et al. Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: a randomized phase 1 trial. Nat Med 2022;28:704–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Braun DA, Street K, Burke KP, Cookmeyer DL, Denize T, Pedersen CB, et al. Progressive immune dysfunction with advancing disease stage in renal cell carcinoma. Cancer Cell 2021;39:632–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Krishna C, DiNatale RG, Kuo F, Srivastava RM, Vuong L, Chowell D, et al. Single-cell sequencing links multiregional immune landscapes and tissue-resident T cells in ccRCC to tumor topology and therapy efficacy. Cancer Cell 2021;39:662–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Brück O, Lee MH, Turkki R, Uski I, Penttilä P, Paavolainen L, et al. Spatial immunoprofiling of the intratumoral and peritumoral tissue of renal cell carcinoma patients. Mod Pathol 2021;34:2229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Miskin N, Qin L, Silverman SG, Shinagare AB. Differentiating benign from malignant cystic renal masses: a feasibility study of computed tomography texture-based machine learning algorithms. J Comput Assist Tomogr 2023;47:376–81. [DOI] [PubMed] [Google Scholar]
- 107. He X-M, Zhao J-X, He D-L, Ren J-L, Zhao L-P, Huang G. Radiogenomics study to predict the nuclear grade of renal clear cell carcinoma. Eur J Radiol Open 2023;10:100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wang W, Cao K, Jin S, Zhu X, Ding J, Peng W. Differentiation of renal cell carcinoma subtypes through MRI-based radiomics analysis. Eur Radiol 2020;30:5738–47. [DOI] [PubMed] [Google Scholar]
- 109. Rossi E, Boldrini L, Maratta MG, Gatta R, Votta C, Tortora G, et al. Radiomics to predict immunotherapy efficacy in advanced renal cell carcinoma: a retrospective study. Hum Vaccin Immunother 2023;19:2172926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Deniffel D, McAlpine K, Harder FN, Jain R, Lawson KA, Healy GM, et al. Predicting the recurrence risk of renal cell carcinoma after nephrectomy: potential role of CT-radiomics for adjuvant treatment decisions. Eur Radiol 2023;33:5840–50. [DOI] [PubMed] [Google Scholar]
- 111. Ferro M, Crocetto F, Barone B, Del Giudice F, Maggi M, Lucarelli G, et al. Artificial intelligence and radiomics in evaluation of kidney lesions: a comprehensive literature review. Ther Adv Urol 2023;15:17562872231164803. [DOI] [PMC free article] [PubMed] [Google Scholar]