Abstract
Purpose
Colorectal liver metastases (CLMs) represent a radioresistant histology. We aimed to investigate CLM radiation therapy (RT) outcomes and explore the association with treatment parameters.
Methods and Materials
This retrospective analysis of CLM treated with RT at Memorial Sloan Kettering Cancer Center used Kaplan-Meier analysis to estimate freedom from local progression (FFLP), hepatic progression-free, progression-free, and overall survival (OS). Cox proportional hazards regression was used to evaluate association with clinical factors. Dose-response relationship was further evaluated using a mechanistic tumor control probability (TCP) model.
Results
Ninety patients with 122 evaluable CLMs treated 2006 to 2019 with a variety of RT fractionation schemes with a median biologically effective dose (α/β = 10; BED10) of 97.9 Gy (range, 43.2-187.5 Gy) were included. Median lesion size was 3.5 cm (0.7-11.8 cm). Eighty-seven patients (97%) received prior systemic therapy, and 73 patients (81%) received prior liver-directed therapy. At a median follow-up of 26.4 months, rates of FFLP and OS were 62% (95% CI, 53%-72%) and 75% (66%-84%) at 1 year and 42% (95% CI, 32%-55%) and 44% (95% CI, 34%-57%) at 2 years, respectively. BED10 below 96 Gy and receipt of ≥3 lines of chemotherapy were associated with worse FFLP (hazard ratio [HR], 2.69; 95% CI, 1.54-4.68; P < .001 and HR, 2.67; 95% CI, 1.50-4.74; P < .001, respectively) and OS (HR, 2.35; 95% CI, 1.35-4.09; P = .002 and HR, 4.70; 95% CI, 2.37-9.31; P < .001) on univariate analyses, which remained significant or marginally significant on multivariate analyses. A mechanistic Tumor Control Probability (TCP) model showed a higher 2-Gy equivalent dose needed for local control in patients who had been exposed to ≥ 3 lines of chemotherapy versus 0 to 2 (250 ± 29 vs 185 ± 77 Gy for 70% TCP).
Conclusions
In a large single-institution series of heavily pretreated patients with CLM undergoing liver RT, low BED10 and multiple prior lines of systemic therapy were associated with lower local control and OS. These results support continued dose escalation efforts for patients with CLM.
Introduction
Colorectal cancer (CRC) accounts for 9% of cancer-related deaths in the United States.1 Up to 27% of patients with CRC develop CRC liver metastases (CLM) within 5 years of the initial diagnosis.2 Surgery is the treatment of choice for resectable disease,3 with reported 5-year survival ranging from 28% to 58%.4,5 However, only 10% to 26% of the patients with CLM are candidates for resection.6,7 For patients with inoperable or unresectable liver metastases, liver-directed therapies include hepatic artery infusion (HAI), radiofrequency ablation, microwave ablation, cryoablation, transarterial chemoembolization, radioembolization with β-emitters such as yttrium-90, and external beam radiation therapy (EBRT).3
Each modality has inherent benefits and limitations.8,9 EBRT, and specifically stereotactic body RT (SBRT), is noninvasive and may be more effective than ablation for larger lesions.10 Several phase 1 SBRT trials have established doses up to 60 Gy in 3 to 5 fractions (biologically effective dose [α/β = 10] [BED10] = 132-180 Gy) as safe with 2-year local control (LC) between 56% and 100%.11, 12, 13 More recently, dose escalation efforts above 200 Gy BED showed more promising LC.14
Numerous factors can influence LC of CLM treated with RT including RT dose, exposure to chemotherapy, and lesion size.15, 16, 17, 18 Tumor control probability (TCP) models using CLM data sets have estimated BED10 to achieve 90% LC to be between 142 and 257 Gy.16,17 The goal of this work was to analyze outcomes of patients with CLM managed with EBRT at our institution and explore the association with clinical factors including BED10, prior exposure to systemic therapy, and lesion size. Additionally, we aimed to use a validated mechanistic TCP model to predict RT dose (in 2-Gy dose equivalent [EQD2]) required to achieve acceptable LC.19,20
Methods and Materials
Patients
We conducted a retrospective study of patients who underwent RT for CLM at Memorial Sloan Kettering Cancer Center (MSKCC) between February 2006 and February 2019. The study was approved by the institutional review board (IRB #16-370). Clinical factors including lesion size, KRAS mutation status, number of liver lesions, extrahepatic disease status, prior systemic- and liver-directed therapies, and dose/fractionation used to treat the patients were collated in a database.
RT
All patients underwent simulation using a dedicated computed tomography scanner, with or without intravenous contrast. In some cases, target delineation was assisted with the use of positron emission tomography scans. Patients were treated on a linear accelerator with 6 to 15 mV energy. A variety of RT dose prescriptions and fractionation schemes were used over the study period and reflected available data at the time and physician preference (Table E1). All plans aimed to achieve planning target volume 95% above 90%, but lower coverage was accepted to respect departmental normal tissue constraints. One hundred twenty-one lesions (99.2%) were treated with daily image guidance, aligning to surgical clips or fiducial markers, or liver shape where fiducial placement was precluded, and 1 patient was treated with 37.5 Gy in 15 fractions with weekly setup to bone.
Follow-up
Standard follow-up included physical examinations, routine laboratory evaluations, and imaging every 3 to 6 months. Freedom from local progression (FFLP), hepatic progression-free survival (hPFS), and progression-free survival (PFS) were scored by Response Evaluation Criteria on Solid Tumors 1.1 based on imaging alone (biopsy confirmation was not required). Treatment-related toxicity was tabulated using Common Terminology Criteria for Adverse Events v5.0.
Statistics
Kaplan-Meier method was used to estimate rates of FFLP, hPFS, PFS, and overall survival (OS). Patients were censored at last follow-up or death. Reverse Kaplan-Meier was used to estimate median follow-up. Cox regression models were fit to evaluate the association between prognostic factors and FFLP and OS. R version 4.2.2 was used to perform all statistical computations.
TCP model
A previously validated mechanistic TCP model based on classical radiobiological mechanisms and a local energy budget was used in this analysis.19,20 Treatment efficacy of various fractionation schedules was normalized through model simulation in a conventional 2-Gy weekday fractionation schedule. Then the outcome data were fitted on the logistic TCP equation:
where TD50 is the tumor dose at which 50% of TCP is expected, γ50 is the slope of the curve at TD50, and D is the total dose of the treatment using the EQD2 model.
Results
There were 97 patients with 129 CLM lesions treated with radiation at MSKCC from February 2006 to February 2019. Of these, 90 patients and 122 metastatic lesions were evaluable. Seven patients with 7 lesions were excluded because of insufficient follow-up (5 patients with no follow-up and 2 patients with limited radiographic evaluation; Table 1). Most patients (n = 59, 66%) presented with metastatic disease at initial diagnosis, had a single liver lesion at the time of RT (n = 54, 60%) with a median lesion size of 3.5 cm (range, 0.7-11.8 cm), and had extrahepatic metastasis at the time of RT (62%). Ninety-seven percent of patients received systemic therapy before liver RT, 54% receiving 3 or more lines of chemotherapy. Eighty-one percent of the patients received at least 1 form of liver-directed therapy before liver RT, with 62% receiving 2 or more forms of liver-directed therapies.
Table 1.
Patient characteristics
| Characteristic | Overall* | BED < 96 Gy* | BED ≥ 96 Gy* | P value† |
|---|---|---|---|---|
| No. of patients/lesions | 90/122 | 30/36 | 60/86 | |
| Median age at diagnosis of primary in years (range) | 53.2 (22.6-85.0) | 54.2 (37.5-85.0) | 52.9 (22.6-78.7) | .5 |
| Median time from diagnosis of primary to liver metastasis in months (range)‡ | 0.0 (0-168.0) | 11.0 (0-83.0) | 0.0 (0-168.0) | <.001 |
| Median time from diagnosis of liver metastasis to RT in months (range) | 35.1 (0-127.2) | 35.1 (3.8-127.2) | 33.4 (0-109.2) | .2 |
| Median age at RT in years (range) | 57.1 (26.1-88.8) | 58.6 (42.1-88.8) | 57.1 (26.1-80.3) | .3 |
| Gender | .5 | |||
| Male | 58 (64.4) | 18 (60.0) | 40 (66.7) | |
| Female | 32 (35.6) | 12 (40.0) | 20 (33.3) | |
| Stage at initial diagnosis | <.001 | |||
| M0 | 30 (33.3) | 18 (60.0) | 12 (20.3) | |
| M1 | 59 (65.6) | 12 (40.0) | 47 (78.3) | |
| KRAS mutation status | .6 | |||
| Wild type | 42 (46.7) | 10 (33.3) | 32 (53.3) | |
| Mutated | 27 (30.0) | 5 (16.7) | 22 (36.7) | |
| Unknown | 21 (23.3) | 15 (50.0) | 6 (10.0) | |
| Median CEA at RT (range)§ | 10.6 (0.9-7724.7) | 17.1 (1.2-7724.7) | 9.6 (0.9-1754.0) | .11 |
| Number of liver lesions at time of RT | .2 | |||
| 1 lesion | 54 (60.0) | 21 (70.0) | 33 (55.0) | |
| ≥2 lesions | 36 (40.0) | 9 (30.0) | 27 (45.0) | |
| Extrahepatic metastatic sites at RT | .3 | |||
| No | 34 (37.8) | 9 (30.0) | 25 (41.7) | |
| Yes | 56 (62.2) | 21 (70.0) | 35 (58.3) | |
| Median lesion size in cm (range) | 3.47 (0.73-11.8) | 4.14 (1.26-11.8) | 2.94 (0.73-9.41) | .006 |
| Median BED in Gy (range) | 97.9 (43.2-187.5) | 66.0 (43.2-85.5) | 100.0 (96.0-187.5) | |
| Prior therapies, N (%) | ||||
| Systemic therapy | 87 (96.7) | 30 (100.0) | 57 (95.0) | .5 |
| Lines of chemotherapy | .2 | |||
| 0 | 1 (1.1%) | 0 (0%) | 1 (1.7%) | |
| 1 | 14 (15.6) | 3 (10.0) | 11 (18.3) | |
| 2 | 26 (28.9) | 8 (26.7) | 18 (30.0) | |
| 3 | 21 (23.3) | 7 (23.3) | 14 (23.3) | |
| 4 | 18 (20.0) | 5 (16.7) | 13 (21.7) | |
| ≥5 | 10 (11.1) | 7 (23.3) | 3 (5.0) | |
| Any liver-directed therapy | 73 (81%) | 24 (80%) | 49 (82%) | .8 |
| Hepatectomy | 63 (70.0) | 20 (66.7) | 43 (71.7) | .6 |
| HAI pump | 56 (62.2) | 18 (60.0) | 38 (63.3) | .8 |
| RFA | 31 (34.4) | 7 (23.3) | 24 (40.0) | .12 |
| Y90 | 5 (5.6) | 0 (0) | 5 (8.3) | .2 |
| Embolization | 8 (8.9) | 2 (6.7) | 6 (10.0) | .7 |
Abbreviations: BED = biologically effective dose; CEA = carcinoembryonic antigen; HAI = hepatic artery infusion; RFA = radiofrequency ablation; RT = radiation therapy.
Median (range); n (%).
Wilcoxon rank sum test; Pearson's χ2 test; Fisher's exact test.
Time from diagnosis of primary to liver metastasis was reported as simultaneous if occurred within 4 weeks of each other.
CEA at RT was log transformed to stabilize regression results.
Patients received a range of dose and fractionation schemes, reflecting physician preference and evolution of institutional standards over time (Table E1; prescription doses are listed). Sixty-one lesions (50%) were treated with fractionation schemes consisting of 1 to 5 fractions, whereas another 61 (50%) received >5 fractions. The median BED10 was 97.9 Gy (range, 43.2-187.5 Gy). D95% was above 95% in 84.4% of plans. All treatment courses delivered in 1 to 5 fractions (n = 61) or those in >5 fractions exceeding BED10 of 60 Gy (n = 60) were treated with daily image guidance.
Based on the nonnormal bimodal distribution of BED10 values, for the purposes of this analysis these were dichotomized around a clinically meaningful cutoff of 96 Gy into a low BED group (n = 30; median BED10 = 66.0 Gy; range, 43.2-85.5 Gy) and a high BED10 group (n = 60; median BED10 = 100.0 Gy; range, 96.0-187.5 Gy). Patients treated to a high BED were more likely to have smaller tumors (P = .006), metastatic disease at initial diagnosis (P < .001), and shorter time to diagnosis of CLM (P < .001; Table 1). There were no differences in performance status, age, the number of liver lesions, or extrahepatic metastases between the groups. Likewise, similar proportions of patients had prior liver-directed therapies and systemic therapy.
LC and OS
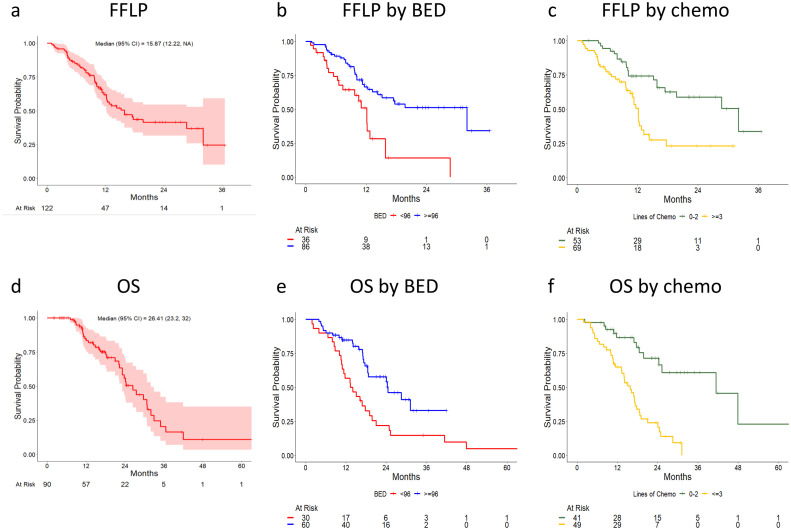
Median follow-up was 26.4 months (95% CI, 23.2-32.0 months). One- and 2-year rates of FFLP were 62% (95% CI, 53%-72%) and 42% (95% CI, 32%-55%), respectively (Fig. 1a). One- and 2-year rates of OS were 75% (95% CI, 66%-84%) and 44% (95% CI, 34%-57%), respectively (Fig. 1d). One-year rates of hPFS and PFS were 24% (95% CI, 16%-35%) and 11% (95% CI, 6%-20%), respectively (Supplementary Materials Figs. S1 and S2).
Figure 1.
Association of freedom from local progression and overall survival with biologically effective dose for α/β of 10 and number of lines of chemotherapy. Kaplan-Meier estimates of freedom from local progression (a-c) and overall survival (d-f) are shown, stratified by biologically effective dose for α/β of 10 (b,e) and by the number of lines of chemotherapy (c,f).
Factors associated with LC and OS
BED10 below 96 Gy (hazard ratio [HR], 2.69; 95% CI, 1.54-4.68; P < .001), 3 or more lines of chemotherapy (HR, 2.67; 95% CI, 1.50-4.74; P < .001), lesion size above the median of 2.9 cm (HR, 2.30; 95% CI, 1.33-4.00; P = .003), and KRAS mutation (HR, 0.47; 95% CI, 0.22-0.97; P = .042) were associated with inferior FFLP on univariate analysis (UVA). One- and 2-year FFLP rates for lesions treated with BED10 ≥ 96 versus < 96 Gy were 66% (95% CI, 56%-78%) versus 51% (95% CI, 35%-74%) and 51% (95% CI, 40%-66%) versus 14% (95% CI, 4.3%-47%), respectively (Fig. 1b). One- and 2-year FFLP rates after 0 to 2 versus ≥3 lines of chemotherapy were 74% (95% CI, 63%-87%) versus 50% (95% CI, 38%-67%) and 59% (95% CI, 45%-76%) versus 23% (95% CI, 12%-44%), respectively. Of note, gender, age, synchronous versus metachronous nature of metastatic disease, and number of liver lesions at RT were not associated with FFLP. On multivariate analysis (MVA), the association remained statistically significant for lines of chemotherapy (HR, 2.58; 95% CI, 1.42-4.66; P = .002) and lesion size (HR, 1.94; 95% CI, 1.04-3.60; P = .037), whereas borderline significance was observed for BED10 (HR, 1.83; 95% CI, 0.98-3.42; P = .057; Table 2).
Table 2.
Univariate and multivariate analyses of factors associated with FFLP and OS
| FFLP |
OS |
|||||||
|---|---|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
Univariate |
Multivariate |
|||||
| Variable | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| Female sex (vs male) | 1.44 (0.84-2.46) | .188 | - | - | 0.82 (0.45-1.47) | .498 | - | - |
| Age at RT | 1.01 (0.99-1.03) | .197 | - | - | 1.01 (0.99-1.03) | .552 | - | - |
| M1 stage at diagnosis (vs M0) | 0.68 (0.39-1.19) | .181 | - | - | 0.87 (0.49-1.54) | .631 | - | - |
| ≥2 hepatic lesions at RT (vs 1) | 1.54 (0.89-2.67) | .122 | - | - | 1.88 (1.07-3.28) | .027⁎ | 1.79 (0.99-3.23) | .052⁎ |
| Extrahepatic disease at RT (vs none) | 1.19 (0.69-2.04) | .535 | - | - | 4.10 (1.96-8.55) | <.001⁎ | 1.89 (0.79-4.51) | .2 |
| BED10 < 96 Gy (vs ≥96 Gy) | 2.69 (1.54-4.68) | <.001⁎ | 1.83 (0.98-3.42) | .057⁎ | 2.35 (1.35-4.09) | .002⁎ | 2.10 (1.17-3.76) | .013⁎ |
| Size above median 2.9 cm (vs <2.9 cm) | 2.30 (1.33-4.0) | .003⁎ | 1.94 (1.04-3.60) | .037⁎ | 2.81 (1.56-5.06) | <.001⁎ | 1.89 (0.98-3.63) | .058⁎ |
| CEA at RT | 1.10 (0.97-1.26) | .148 | - | - | 1.53 (1.32-1.77) | <.001⁎ | 1.31 (1.10-1.57) | .002⁎ |
| ≥3 lines of chemo (vs <3) | 2.67 (1.50-4.74) | <.001⁎ | 2.58 (1.42-4.66) | .002⁎ | 4.70 (2.37-9.31) | <.001⁎ | 2.78 (1.31-5.91) | .008⁎ |
| Liver-directed therapy (vs none) | 0.84 (0.40-1.80) | .658 | - | - | 0.56 (0.29- 1.11) | .098 | - | - |
| KRAS (vs wild type) | 0.47 (0.22-0.97) | .042⁎ | - | - | 1.71 (0.89-3.31) | .108 | - | - |
Abbreviations: BED = biologically effective dose; CEA = carcinoembryonic antigen; FFLP = freedom from local progression; HR = hazard ratio; OS = overall survival; RT = radiation therapy.
P values that are statistically significant or suggestive of a trend.
Likewise, BED10 below 96 Gy (HR, 2.35; 95% CI, 1.35-4.09; P = .002) and 3 or more lines of chemotherapy (HR, 4.70; 95% CI, 2.37-9.31; P < .001) were associated with inferior OS on UVA (Table 2). One- and 2-year OS for lesions treated with BED10 ≥ 96 versus < 96 Gy were 85% (76%-94%) versus 57% (41%-77%) and 58% (45%-74%) versus 22% (11%-44%), respectively (Fig. 1e). One- and 2-year OS after 0 to 2 versus ≥3 lines of chemotherapy were 87% (76%-98%) versus 65% (53%-80%) and 71% (57%-90%) versus 24% (14%-41%), respectively (Fig. 1f). Other significant factors in UVA included presence of extrahepatic metastasis (HR, 4.10; 95% CI, 1.96-8.55; P < .001), lesion size above the median (HR, 2.81; 95% CI, 1.56-5.06; P < .001), 2 or more liver metastases (HR, 1.88; 95% CI, 1.07-3.28; P = .027), and carcinoembryonic antigent (CEA) at the time of RT (HR, 1.53; 95% CI, 1.32-1.77; P < .001). Gender, age, and synchronous versus metachronous nature of metastatic disease were not associated with OS. After adjusting for confounders, BED10 and 3 or more lines of chemotherapy remained significantly associated with OS on MVA.
Dose effect using TCP
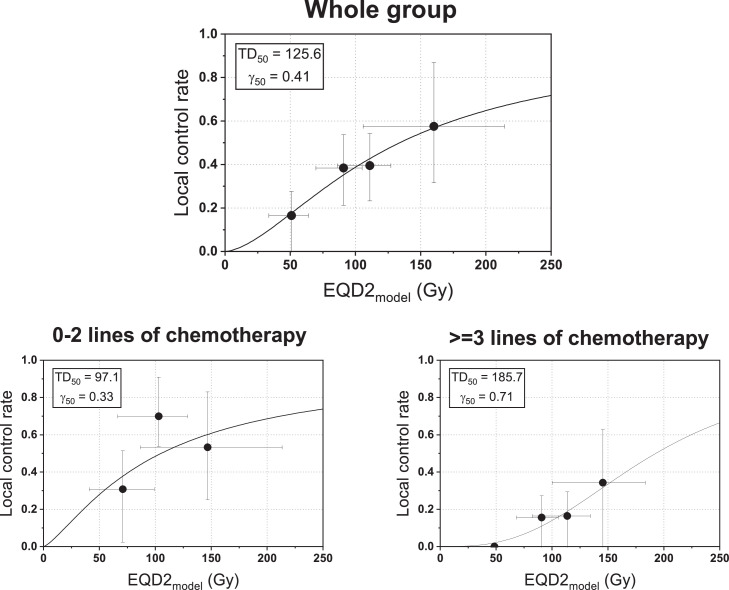
Dose-response curves were derived for the whole cohort and dichotomized by chemotherapy (0-2 lines vs 3 or more; Fig. 2). The TD50 of the whole group was 125.6 Gy (in EQD2), and 97.1 Gy versus 185.7 Gy for 0 to 2 versus 3 or more lines of chemotherapy. Similarly, the EQD2 needed to achieve 70% tumor control rate was 185 ± 77 Gy versus 250 ± 29 Gy for 0 to 2 versus 3 or more lines of chemotherapy.
Figure 2.
Dose-response curve using tumor control probability model for the whole group (top) and for patients exposed to prior 0 to 2 lines of chemotherapy (bottom left) and ≥3 lines of chemotherapy (bottom right). The asymmetrical error bars on local control rate (y-axis) indicate 95% confidence interval based on the Clopper-Pearson method. The x-error bars were estimated from the conversion of the standard error of the total local control rate into the uncertainty of the equivalent dose. Abbreviations: γ50 = the slope of the curve at TD50; EQD2 = 2 Gy dose equivalent; TD50 = tumor dose at which 50% of tumor control probability is expected.
Toxicity
All grade 3 and higher toxic events regardless of attribution are shown in Table 3. There were 2 grade 3 toxic events at least possibly related to RT (occurring within RT field), including a hepatic abscess and biliary stricture. Most hepatic/biliary events (24 out of 26, 92%) were recorded in patients who had received hepatic arterial infusion pump chemotherapy, and only 2 were at least possibly related to RT.
Table 3.
Toxicity results
| Grade |
||||||
|---|---|---|---|---|---|---|
| Toxicity | Number N = 90 | Attributed to RT N = 90 | HAI pump N = 56 | 3 | 4 | 5 |
| Hepatic abscess | 5 | 1 | 5 | 5 | - | - |
| Cholangitis | 6 | 0 | 6 | 4 | 2 | - |
| Biloma | 5 | 0 | 5 | 3 | - | - |
| Biliary stricture | 10 | 1 | 8 | 10 | - | - |
| Ascites requiring drainage | 9 | - | 8 | - | - | - |
| GI bleed (requiring transfusion) | 5 | 0 | 4 | 5 | - | - |
Abbreviations: GI = gastrointestinal; HAI = hepatic artery infusion; RT = radiation therapy.
Discussion
In this work, we present outcomes for CLM treated with RT for a large single-institution cohort of heavily pretreated patients. Metastases from CRC are considered to be radioresistant,21,22 and CLM, in particular, has been shown to have inferior LC after stereotactic body RT compared with other histologies.23, 24, 25 Our LC was lower than some of the previously published series of CLM (Table 4).14,15,26, 27, 28, 29 This likely reflects the frequent use of lower BED regimens, especially at the beginning of the study period, inclusion of very large lesions, and significant exposure to chemotherapy. Eighty-three percent of participants had received 2 or more lines.
Table 4.
Outcomes of CLM treated with RT
| Study first author | #patients | #lesions | Median size in cm (range) | 2 y LC | 2 y OS |
|---|---|---|---|---|---|
| Hoyer26 (BED 112.5 Gy) |
64 | 141 | 3.5 (1-8.8) | 86% | 38% |
| Van der Pool27 (BED 84.38 Gy) |
20 | 31 | 2.3 (0.7-6.2) | 74% | 83% |
| Chang16 | 65 | 102 | 30.1 mL (0.6-3088) | 55% | 38% |
| BED >= 75 Gy | - | 51 | - | 71% | - |
| BED < 75 Gy | - | 51 | - | 31% | - |
| Scorsetti28 (BED 262.5 Gy) |
42 | 52 | 3.5 (1.1-5.4) | 91% | 65% |
| Joo15 | 70 | 103 | 2.9 (-) | 73% | 75% |
| BED >= 112 Gy | 26 | 30 | - | 92% | - |
| BED < 112 Gy | 44 | 73 | - | 61% | - |
| Present study | 90 | 122 | 3.47 (0.73-11.8) | 42% | 44% |
| BED < 96 Gy | 30 | 36 | 2.71 (0.33-9.41) | 14% | 22% |
| BED >= 96 Gy | 60 | 86 | 3.86 (1.26-11.8) | 51% | 58% |
Abbreviations: BED = biologically effective dose; CLM = colorectal liver metastases; LC = local control; OS = overall survival; RT = radiation therapy.
Similar to prior studies, we observed an association of LC and survival outcomes with clinical factors, including BED10 and exposure to chemotherapy.4,13,15, 16, 17, 18,29 A dose-response relationship has been demonstrated for CLM over a range of fractionation schemes used, with most studies suggesting that 2 year LC above 80% requires doses above 100 Gy BED10.15, 16, 17,28 TCP modeling aims to provide a quantitative method to predict the likelihood of LC for different fractionation schemes. Modeling can be mostly based on clinical data (empiric) or can incorporate a more mechanistic radiobiological understanding of tumor response to RT. Using an empiric TCP model on data from 623 mixed histology metastases, it has been shown that exposure to chemotherapy significantly decreases control rate for a given RT dose for CLM compared with breast cancer metastasis, an effect that is most pronounced over the lower RT dose spectrum.17 We used a mechanistic as opposed to an empirical TCP model for this analysis, which specifically models the tumor cellular response to RT as the interplay of hypoxia and proliferation.20 Importantly, our model has been shown to robustly reproduce tumor dose-response across the complete range of clinical fractionation regimens for lung adenocarcinoma in multiple clinical data sets. Thus, it is particularly useful in examining the dose response relationship in this data set where a wide variety of fractionation schemes was used. Our model confirms the previously described requirement for a higher BED to achieve a similar level of tumor control for CLM after exposure to chemotherapy. Moreover, our data set had more detailed clinical annotation with regard to prior chemotherapy exposure. Here, we show the extent to which additional lines of chemotherapy further exacerbate this phenomenon—the dose of 250 Gy EQD2 or 300 Gy BED10 would only yield a 70% control rate in a patient with 3 or more prior lines of chemotherapy. Previously suggested hypotheses for the association of chemotherapy exposure and TCP include possible effect on RT coverage (chemotherapy causes some gross tumor shrinkage, thereby leading to undercontouring of residual microscopic disease along the margins) and biologic selection of a more aggressive phenotype with enhanced DNA damage repair abilities.17 Based on the practice pattern within our institution, most of the patients with CLM referred for liver-directed RT are referred at the time of CLM progression as opposed to consolidation after prior systemic therapy. As such, the second hypothesis seems more likely, especially given that a greater effect on TCP is seen after developing chemoresistance to several lines of therapy.
Of note, in addition to systemic chemotherapy, 62% of the participants also received prior HAI pump (HAIP) chemotherapy. The use of HAIP is very heterogenous across institutions, and the high use of HAIP in this cohort sets it apart from other series. Although HAIP chemotherapy (or receipt of any liver-directed therapy) was not a significant predictor of LC in our analysis, all patients who received HAIP chemotherapy also received systemic chemotherapy, and as such we cannot rule out the possibility that HAIP use contributed to the relative radioresistance observed in our study.
In addition to the close association with LC, both BED10 and the number of lines of chemotherapy also showed a statistically significant association with OS. The association of these factors with both LC and OS suggests that their effect on LC may be contributing to OS. Alternatively, 1 or both factors may also influence OS directly, especially the number of lines of chemotherapy. In this regard, it should be noted that patients in the higher BED group were more likely to have metastatic disease at initial diagnosis and shorter median time to diagnosis of liver metastasis; both characteristics are suggestive of a more aggressive disease biology and are expected to confer inferior survival.30 Thus, the positive association of BED with OS despite the imbalance in prognostic factors that influence survival is particularly meaningful. Others have also shown that sustained LC of CLM correlates with improved OS, supporting the clinical value of controlling hepatic disease.16 In view of recent data that presence of liver metastasis may induce immune tolerance elsewhere in the body and blunt response to immune therapy,31 a particularly intriguing speculation is that more effective control of CLM also contributes to enhanced natural antitumor immune response.
Similar to prior analyses, CLM size was a factor associated with LC and OS in our database.18 Large lesions can limit the dose that can be safely delivered, especially when short fractionation schemes are used. Although CLMs in the lower BED10 group were significantly larger than in the higher BED10 group in our study, this association persisted after adjusting for other factors including BED10 on MVA. A larger number of clonogens, and factors like hypoxia, subclonal heterogeneity, and intercellular communications may all contribute to the greater radioresistance of larger lesions.32
Our results support further dose escalation for CLM, when it can be safely achieved, especially for heavily pretreated patients or larger lesions. They also suggest that offering RT early in the treatment course before exposure to multiple lines of chemotherapy may be associated with a better chance of tumor control. This is particularly relevant when considering the growing evidence that local therapy is associated with improved survival compared with systemic therapy alone in some oligometastatic and oligoprogressive states.33
Finally, our toxicity results demonstrate radiation to be a relatively safe, noninvasive alternative for patients for whom resection or other liver-directed therapies may not be a viable option. Although toxicities can be underreported in retrospective studies, we focused on grade 3 and higher adverse events that can be reliably ascertained from the review of hospital admission, imaging, interventions, and operative reports. We attribute our low rate of toxicity to the strict adherence to our institutional constraints. This is particularly notable given the high number of patients with prior HAIP chemotherapy in our cohort, who are known to be at risk of biliary sclerosis and other associated biliary toxicity.34 Not unexpectedly, patients with history of HAIP chemotherapy had biliary events noted over the course of the study, but only 2 of 26 events were within the radiation field, suggesting that RT did not significantly increase the risk of biliary toxicity. These data support consideration of RT as a safe option for patients with CLM and prior HAIP chemotherapy where there is concern for additive hepatic and biliary toxicity from salvage liver-directed interventions.
Limitations of this study include retrospective design and heterogeneous patient population. With >12 years of study duration, there were likely multiple shifts in the institutional approach to patients with CLM, not including the change in fractionation schemes used for treatment of CLM over time. Furthermore, the TCP model parameters are derived from lung cancer primaries, and the results should be interpreted with caution. On the whole, this study adds value to the current literature because of the unique aspects of the patient cohort and the in-depth analysis of the dose-response relationship.
Conclusion
In a large single-institution series of heavily pretreated patients with CLM undergoing liver RT, low BED10 and multiple prior lines of systemic therapy were associated with lower LC and OS. A mechanistic TCP model confirmed the effect of prior chemotherapy and RT dose on LC. These results provide a rationale for continued dose-escalation efforts for patients with CLM.
Disclosures
This was partly funded by the National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748.
Paul B. Romesser received research funding (2019) and serves as a consultant for EMD Serono (2018-present), receives research funding from XRAD Therapeutics (2022-present), is a consultant for Faeth Therapeutics (2022-present), is a consultant for Natera (2022-present), and is a volunteer on the advisory board for the HPV Alliance and Anal Cancer Foundation nonprofit organizations. Paul B. Romesser is also supported by an NIH/NCI grant (K08CA255574). Abraham J. Wu receives unrelated grants funded by CivaTech Oncology and participates on the scientific advisory board for Simphotek. Marsha Reyngold has Elekta and Varian research grants and speaker fees from Elekta outside of the submitted work.
Footnotes
Sources of support: This work was supported in part by National Institutes of Health/National Cancer Institute (NIH/NCI) Memorial Sloan Kettering Cancer Center (MSK) support grant (P30 CA008748).
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.adro.2023.101382.
Appendix. Supplementary materials
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Engstrand J, Nilsson H, Stromberg C, Jonas E, Freedman J. Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer. 2018;18:78. doi: 10.1186/s12885-017-3925-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark ME, Smith RR. Liver-directed therapies in metastatic colorectal cancer. J Gastrointest Oncol. 2014;5:374–387. doi: 10.3978/j.issn.2078-6891.2014.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 5.Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–722. doi: 10.1097/01.sla.0000160703.75808.7d. discussion 722-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hackl C, Neumann P, Gerken M, et al. Treatment of colorectal liver metastases in Germany: A ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer. 2014;14:810. doi: 10.1186/1471-2407-14-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdalla EK, Adam R, Bilchik AJ, Jaeck D, Vauthey J-N, Mahvi D. Improving resectability of hepatic colorectal metastases: Expert consensus statement. Ann Surg Oncol. 2006;13:1271–1280. doi: 10.1245/s10434-006-9045-5. [DOI] [PubMed] [Google Scholar]
- 8.Hunt TM, Flowerdew AD, Birch SJ, Williams JD, Mullee MA, Taylor I. Prospective randomized controlled trial of hepatic arterial embolization or infusion chemotherapy with 5-fluorouracil and degradable starch microspheres for colorectal liver metastases. Br J Surg. 1990;77:779–782. doi: 10.1002/bjs.1800770720. [DOI] [PubMed] [Google Scholar]
- 9.Mahnken AH, Pereira PL, Baère TD. Interventional oncologic approaches to liver metastases. Radiology. 2013;266:407–430. doi: 10.1148/radiol.12112544. [DOI] [PubMed] [Google Scholar]
- 10.Jackson WC, Tao Y, Mendiratta-Lala M, et al. Comparison of stereotactic body radiation therapy and radiofrequency ablation in the treatment of intrahepatic metastases. Int J Radiat Oncol Biol Phys. 2018;100:950–958. doi: 10.1016/j.ijrobp.2017.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee MT, Kim JJ, Dinniwell R, et al. Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol. 2009;27:1585–1591. doi: 10.1200/JCO.2008.20.0600. [DOI] [PubMed] [Google Scholar]
- 12.Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27:1572–1578. doi: 10.1200/JCO.2008.19.6329. [DOI] [PubMed] [Google Scholar]
- 13.Rule W, Timmerman R, Tong L, et al. Phase I dose-escalation study of stereotactic body radiotherapy in patients with hepatic metastases. Ann Surg Oncol. 2011;18:1081–1087. doi: 10.1245/s10434-010-1405-5. [DOI] [PubMed] [Google Scholar]
- 14.Folkert MR, Meyer JJ, Aguilera TA, et al. Long-term results of a phase 1 dose-escalation trial and subsequent institutional experience of single-fraction stereotactic ablative radiation therapy for liver metastases. Int J Radiat Oncol Biol Phys. 2021;109:1387–1395. doi: 10.1016/j.ijrobp.2020.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Joo JH, Park J-H, Kim JC, et al. Local control outcomes using stereotactic body radiation therapy for liver metastases from colorectal cancer. Int J Radiat Oncol Biol Phys. 2017;99:876–883. doi: 10.1016/j.ijrobp.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 16.Chang DT, Swaminath A, Kozak M, et al. Stereotactic body radiotherapy for colorectal liver metastases: A pooled analysis. Cancer. 2011;117:4060–4069. doi: 10.1002/cncr.25997. [DOI] [PubMed] [Google Scholar]
- 17.Klement RJ, Guckenberger M, Alheid H, et al. Stereotactic body radiotherapy for oligo-metastatic liver disease - Influence of pre-treatment chemotherapy and histology on local tumor control. Radiother Oncol. 2017;123:227–233. doi: 10.1016/j.radonc.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Mahadevan A, Blanck O, Lanciano R, et al. Stereotactic body radiotherapy (SBRT) for liver metastasis - clinical outcomes from the international multi-institutional RSSearch(R) Patient Registry. Radiat Oncol. 2018;13:26. doi: 10.1186/s13014-018-0969-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeong J, Shoghi KI, Deasy JO. Modelling the interplay between hypoxia and proliferation in radiotherapy tumour response. Phys Med Biol. 2013;58:4897–4919. doi: 10.1088/0031-9155/58/14/4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeong J, Oh JH, Sonke J-J, et al. Modeling the cellular response of lung cancer to radiation therapy for a broad range of fractionation schedules. Clin Cancer Res. 2017;23:5469–5479. doi: 10.1158/1078-0432.CCR-16-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh D, Chen Y, Hare MZ, et al. Local control rates with five-fraction stereotactic body radiotherapy for oligometastatic cancer to the lung. J Thorac Dis. 2014;6:369. doi: 10.3978/j.issn.2072-1439.2013.12.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baschnagel A, Mangona VS, Robertson JM, Welsh RJ, Kestin LL, Grills IS. Lung metastases treated with image-guided stereotactic body radiation therapy. Clin Oncol (R Coll Radiol) 2013;25:236–241. doi: 10.1016/j.clon.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed KA, Caudell JJ, El-Haddad G, et al. Radiosensitivity differences between liver metastases based on primary histology suggest implications for clinical outcomes after stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2016;95:1399–1404. doi: 10.1016/j.ijrobp.2016.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herfarth KK, Debus J, Lohr F, et al. Stereotactic single-dose radiation therapy of liver tumors: Results of a phase I/II trial. J Clin Oncol. 2001;19:164–170. doi: 10.1200/JCO.2001.19.1.164. [DOI] [PubMed] [Google Scholar]
- 25.Katsoulakis E, Riaz N, Cannon DM, et al. Image-guided radiation therapy for liver tumors: Gastrointestinal histology matters. Am J Clin Oncol. 2014;37:561–567. doi: 10.1097/COC.0b013e318282a86b. [DOI] [PubMed] [Google Scholar]
- 26.Hoyer M, Roed H, Traberg Hansen A, et al. Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol. 2006;45:823–830. doi: 10.1080/02841860600904854. [DOI] [PubMed] [Google Scholar]
- 27.van der Pool AE, Mendez Romero A, Wunderink W, et al. Stereotactic body radiation therapy for colorectal liver metastases. Br J Surg. 2010;97:377–382. doi: 10.1002/bjs.6895. [DOI] [PubMed] [Google Scholar]
- 28.Scorsetti M, Comito T, Tozzi A, et al. Final results of a phase II trial for stereotactic body radiation therapy for patients with inoperable liver metastases from colorectal cancer. J Cancer Res Clin Oncol. 2015;141:543–553. doi: 10.1007/s00432-014-1833-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrelli F, Comito T, Barni S, et al. Stereotactic body radiotherapy for colorectal cancer liver metastases: A systematic review. Radiother Oncol. 2018;129:427–434. doi: 10.1016/j.radonc.2018.06.035. [DOI] [PubMed] [Google Scholar]
- 30.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: Analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. discussion 318-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu J, Green MD, Li S, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med. 2021;27:152–164. doi: 10.1038/s41591-020-1131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brenner DJ. Dose, volume, and tumor-control predictions in radiotherapy. Int J Radiat Oncol Biol Phys. 1993;26:171–179. doi: 10.1016/0360-3016(93)90189-3. [DOI] [PubMed] [Google Scholar]
- 33.Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: Long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol. 2020;38:2830–2838. doi: 10.1200/JCO.20.00818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito K, Ito H, Kemeny NE, et al. Biliary sclerosis after hepatic arterial infusion pump chemotherapy for patients with colorectal cancer liver metastasis: Incidence, clinical features, and risk factors. Ann Surg Oncol. 2012;19:1609–1617. doi: 10.1245/s10434-011-2102-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.