This randomized clinical trial assesses the effect of pegargiminase-based chemotherapy on survival in patients with nonepithelioid pleural mesothelioma.
Key Points
Question
What is the effect on survival of the arginine-depleting agent pegargiminase combined with standard platinum and pemetrexed chemotherapy in patients with nonepithelioid pleural mesothelioma?
Findings
In this pivotal randomized placebo-controlled phase 3 trial in 249 patients with nonepithelioid pleural mesothelioma, pegargiminase-chemotherapy increased significantly the median overall survival by 1.6 months and quadrupled the survival at 36 months compared to placebo-chemotherapy. Pegargiminase-based chemotherapy was well tolerated with no new safety signals.
Meaning
Arginine deprivation with pegargiminase is a novel cancer chemotherapy that improves survival in patients with nonepithelioid pleural mesothelioma and warrants additional studies in arginine-dependent cancers with poor survival outcomes.
Abstract
Importance
Arginine deprivation using ADI-PEG20 (pegargiminase) combined with chemotherapy is untested in a randomized study among patients with cancer. ATOMIC-Meso (ADI-PEG20 Targeting of Malignancies Induces Cytotoxicity-Mesothelioma) is a pivotal trial comparing standard first-line chemotherapy plus pegargiminase or placebo in patients with nonepithelioid pleural mesothelioma.
Objective
To determine the effect of pegargiminase-based chemotherapy on survival in nonepithelioid pleural mesothelioma, an arginine-auxotrophic tumor.
Design, Setting, and Participants
This was a phase 2-3, double-blind randomized clinical trial conducted at 43 centers in 5 countries that included patients with chemotherapy-naive nonepithelioid pleural mesothelioma from August 1, 2017, to August 15, 2021, with at least 12 months’ follow-up. Final follow-up was on August 15, 2022. Data analysis was performed from March 2018 to June 2023.
Intervention
Patients were randomly assigned (1:1) to receive weekly intramuscular pegargiminase (36.8 mg/m2) or placebo. All patients received intravenous pemetrexed (500 mg/m2) and platinum (75-mg/m2 cisplatin or carboplatin area under the curve 5) chemotherapy every 3 weeks up to 6 cycles. Pegargiminase or placebo was continued until progression, toxicity, or 24 months.
Main Outcomes and Measures
The primary end point was overall survival, and secondary end points were progression-free survival and safety. Response rate by blinded independent central review was assessed in the phase 2 portion only.
Results
Among 249 randomized patients (mean [SD] age, 69.5 [7.9] years; 43 female individuals [17.3%] and 206 male individuals [82.7%]), all were included in the analysis. The median overall survival was 9.3 months (95% CI, 7.9-11.8 months) with pegargiminase-chemotherapy as compared with 7.7 months (95% CI, 6.1-9.5 months) with placebo-chemotherapy (hazard ratio [HR] for death, 0.71; 95% CI, 0.55-0.93; P = .02). The median progression-free survival was 6.2 months (95% CI, 5.8-7.4 months) with pegargiminase-chemotherapy as compared with 5.6 months (95% CI, 4.1-5.9 months) with placebo-chemotherapy (HR, 0.65; 95% CI, 0.46-0.90; P = .02). Grade 3 to 4 adverse events with pegargiminase occurred in 36 patients (28.8%) and with placebo in 21 patients (16.9%); drug hypersensitivity and skin reactions occurred in the experimental arm in 3 patients (2.4%) and 2 patients (1.6%), respectively, and none in the placebo arm. Rates of poststudy treatments were comparable in both arms (57 patients [45.6%] with pegargiminase vs 58 patients [46.8%] with placebo).
Conclusions and Relevance
In this randomized clinical trial of arginine depletion with pegargiminase plus chemotherapy, survival was extended beyond standard chemotherapy with a favorable safety profile in patients with nonepithelioid pleural mesothelioma. Pegargiminase-based chemotherapy as a novel antimetabolite strategy for mesothelioma validates wider clinical testing in oncology.
Trial Registration
ClinicalTrials.gov Identifier: NCT02709512
Introduction
Cancers with loss of argininosuccinate synthetase 1 (ASS1), a tumor suppressor and urea cycle enzyme, are critically dependent on arginine for survival and intrinsically sensitive to amino acid deprivation strategies. Pegylated arginine deiminase (ADI-PEG20; pegargiminase) degrades arginine into citrulline and ammonia and triggers cytotoxicity in multiple ASS1-silenced cancers preclinically, with evidence of single-agent activity in the clinic. Malignant pleural mesothelioma has among the lowest 5-year survival rate of any solid cancer, estimated at 5% to 10%. Previously, we reported a significant 1.2-month progression-free survival (PFS) benefit for pegargiminase and best supportive care compared with best supportive care alone in the phase 2 ADAM (Arginine Deiminase And Mesothelioma) randomized clinical trial. Patients preselected for ASS1 deficiency using immunohistochemistry testing had a worse survival when compared with patients with ASS1-proficient disease, validating ASS1 as a prognostic and predictive biomarker.
To optimize arginine deprivation, we identified that pegargiminase sensitized mesothelioma and related arginine-auxotrophic tumors to the antifolate pemetrexed by suppressing the de novo synthesis and salvage of thymidine. This exploited a metabolic rewiring of ASS1-negative cancers to divert aspartate from arginine biosynthesis to the production of pyrimidines for tumor anabolism. The phase 1 TRAP trial of pegargiminase combined with pemetrexed and cisplatin in patients with ASS1-deficient thoracic cancers revealed good safety and a high disease control rate (93.5%-100%). The median overall survival (OS) was 10 to 14 months and included patients with pleural mesothelioma, especially the nonepithelioid subtype, where the median OS historically has been around 6 months with standard chemotherapy. Nonepithelioid mesothelioma exhibited a 3-fold higher rate of tumoral ASS1 deficiency compared with the epithelioid subtype (60% vs 20%). Moreover, twice as many patients enrolled with nonepithelioid than epithelioid disease, despite the former representing approximately one-third of mesothelioma cases. Mechanistically, we identified reduced 18-FLT uptake by 24 hours of pegargiminase monotherapy and robust metabolic responses on completion of chemotherapy using positron emission tomography, corroborating the thymidine salvage pathway as a downstream target of pegargiminase. Based on these findings, the ATOMIC-Meso phase 2-3 trial evaluated the primary efficacy and safety of adding pegargiminase or placebo to standard chemotherapy with pemetrexed and platinum (cisplatin or carboplatin) in patients with chemotherapy-naive nonresectable nonepithelioid pleural mesothelioma.
Methods
Trial Oversight
Polaris Pharmaceuticals, Inc, sponsored the ATOMIC-Meso trial and provided the trial drugs in collaboration with the academic institutions to develop the study, which was conducted according to Good Clinical Practice guidelines and the Declaration of Helsinki. Written informed consent was obtained from all patients. An independent data and safety monitoring board (DSMB) provided oversight of efficacy and safety. Protocol approval was secured by an independent ethics committee at each site. The study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Patients
Patients were aged 18 years or older with histologically proven nonresectable and treatment-naive nonepithelioid pleural mesothelioma. Patients had evaluable disease by modified Response Evaluation Criteria in Solid Tumors (RECIST) criteria for pleural involvement and RECIST, version 1.1, criteria for metastatic lesions. Additional inclusion criteria included Eastern Cooperative Group performance status of 0 or 1, adequate organ function, and minimum expected survival of 3 months. Key exclusion criteria included symptomatic brain or spinal cord metastases, uncontrolled intercurrent illness, recent major surgery, pregnancy, allergy to pegylated or Escherichia coli products, and prior therapy with pegargiminase. Race and ethnicity was collated to understand disease differences, trial access, pathophysiology, and response to therapy. Clinical site trial data managers entered the race and ethnicity data based on the medical records, and all database entries had principal investigator oversight.
Study Design
Eligible patients were randomized to receive weekly pegargiminase, 36 mg/m2, or placebo intramuscularly in a double-blinded fashion and up to 6 cycles of standard pemetrexed and cisplatin chemotherapy (trial protocol and statistical analysis plan in Supplement 1 and Supplement 2, respectively). All patients received standard supplementation with hydroxycobalamin (1000 mcg intramuscularly every 9 weeks), folic acid (400 mcg orally daily) commencing 7 days prior to cycle 1, and dexamethasone (4 mg twice daily starting the day before intravenous chemotherapy for 3 days) prophylaxis with each new cycle. The initial dose of pegargiminase or placebo was administered 48 hours before the first dose of cytotoxic drugs (eFigure 1 in Supplement 3). Substitution of carboplatin was permitted from cycle 1 in patients who did not or were not expected to tolerate cisplatin. Pegargiminase or placebo was continued until progression, toxicity, or 24 months.
Randomization
Patients were randomized 1:1 in this double-blind study to the pegargiminase group or the placebo group from 43 centers worldwide and stratified according to nonepithelioid histologic subtype (biphasic vs sarcomatoid). Randomization was performed centrally using SAS Programming (SAS Institute) with a randomly selected block size of 4. The randomization number was assigned based on information obtained from the interactive web response system. Unblinding was permitted only in the event of an emergency.
Outcomes
The primary efficacy end point for the phase 2 portion of the study was objective (complete and partial) response rate (ORR) via blinded independent central review. The secondary end point of the phase 2 portion was the duration of response by blinded independent central review. The ORR was tested once at the completion of the phase 2 portion by the Interim Analysis Group and DSMB for the purposes of accelerated approval only. Regardless of this decision, the study then moved to the primary efficacy end point for the phase 3 portion, which was OS. Secondary end points for phase 3 were PFS, safety, pharmacodynamics, immunogenicity, and pharmacokinetics of the pegargiminase-chemotherapy arm.
Statistical Analysis
Approximately 176 participants (88 per arm) were planned to be enrolled in the phase 2 portion of the trial, with response rate as the primary outcome measure, and 386 participants (193 per arm) in the whole phase 2-3 trial, with the phase 3 primary outcome measure of OS. The sample size calculation for the efficacy end point of response rate assumed that the ORR in the placebo-chemotherapy arm would be 15%. A total sample size of 176 participants (88 per arm) in the phase 2 portion of the study provided approximately 87% power to detect an improvement in the response rate from 15% to 35% at the first interim analysis. The treatment groups were compared using the Cochran-Mantel-Haenszel test, stratified by tumor histologic subtype (biphasic vs sarcomatoid). The significance level and coverage probability used in the response rate analysis was based on an α = .05 (2-sided).
The sample size calculation for the OS assumed that the median OS was 6 months in the placebo-chemotherapy arm. Assuming a median OS of 8.4 months in the pegargiminase-chemotherapy arm (corresponding to a 40% improvement in survival and a hazard ratio [HR] of 0.714), 338 OS events provided power of approximately 87% for the OS analysis. Based on DSMB recommendations, the original planned sample size was changed from 386 participants to all enrolled up to August 15, 2021 (249 participants), and the number of deaths required for the original planned final analysis of OS was changed from 338 to the actual number of deaths occurring by August 14, 2022. The estimated power with 249 participants was predicted to be in a range of 73% to 80% if the true HR was in a range of 0.71 to 0.68.
The primary and final analysis of OS was performed at study conclusion. The treatment effect on OS was evaluated using the stratified log-rank test (stratified by tumor histologic subtype). The significance level used in the OS calculation at the final analysis was based on an α = .05 (2-sided). There was an interim analysis of OS once 50% of the planned OS events for the phase 3 occurred, which was used to determine whether to terminate the study for futility or for possible sample size reestimation for the phase 3 portion of the trial as described in the statistical analyses plan (Supplement 2). Analyses were performed using SAS, version 9.4 (SAS Institute).
Results
Patients
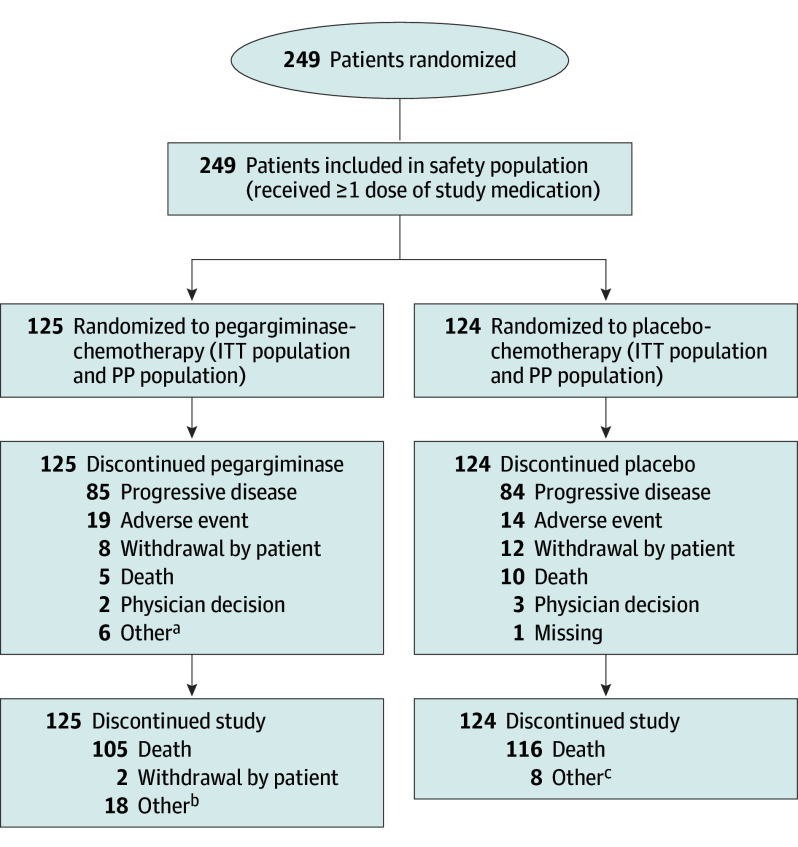
From August 2017 to August 2021, a total of 249 patients with advanced nonepithelioid pleural mesothelioma were randomly assigned to receive pegargiminase-chemotherapy (125 patients) or placebo-chemotherapy (124 patients), as depicted in Figure 1 and in eFigure 2 in Supplement 3. The trial was stopped after the second interim analysis on the recommendation of the DSMB, after consultation with the US Food and Drug Administration, due to positive results favoring the experimental arm. Thus, enrollment ceased in August 2021, and follow-up was continued until August 2022.
Figure 1. CONSORT Diagram.
ITT indicates intention-to-treat; PP, per-protocol.
aOther refers to stable disease, trial completion/stable disease, and patient continuing in the expanded phase.
bOther refers to study completion for the majority of patients with a reason of other, 1 patient with study termination, 2 patients moving away, 1 patient with an unknown reason, and 1 patient who continued treatment in the blinded expanded phase.
cOther refers to study completion.
Apart from more Australasian patients in the placebo-chemotherapy arm (6 [4.8%] in the pegargiminase-chemotherapy arm vs 16 [12.9%] in the placebo-chemotherapy arm; P = .048), the demographic and clinical characteristics of both study groups were well balanced at baseline (Table 1). There was a male predominance (206 [82.7%]), and a majority of patients were of Eastern Cooperative Oncology Group status 1, although with a nonsignificant increase in the control group (97 [78.2%] vs 87 [69.6%] in the experimental arm; P = .12). Histologic subtype and disease stage were distributed similarly across both groups.
Table 1. Baseline Patient Characteristics.
| Characteristic | Patients, No. (%) | P value | ||
|---|---|---|---|---|
| Pegargiminase-chemotherapy (n = 125) | Placebo-chemotherapy (n = 124) | Total (N = 249) | ||
| Age, y | ||||
| Mean (SD) | 69.5 (7.98) | 69.4 (7.91) | 69.4 (7.93) | .88a |
| Median (range) | 71.0 (28-84) | 70.0 (34-86) | 71.0 (28-86) | |
| Sex | ||||
| Female | 23 (18.4) | 20 (16.1) | 43 (17.3) | .64b |
| Male | 102 (81.6) | 104 (83.9) | 206 (82.7) | |
| Region | ||||
| North America | 31 (24.8) | 22 (17.7) | 53 (21.3) | .048b |
| Europe | 88 (70.4) | 86 (69.4) | 174 (69.9) | |
| Australasia | 6 (4.8) | 16 (12.9) | 22 (8.8) | |
| Race and ethnicity | ||||
| Asian | 5 (4.0) | 5 (4.0) | 10 (4.0) | .34c |
| Black or African American | 3 (2.4) | 0 | 3 (1.2) | |
| White | 116 (92.8) | 116 (93.5) | 232 (93.2) | |
| Unknown | 1 (0.8) | 3 (2.4) | 4 (1.6) | |
| ECOG performance status | ||||
| 0 | 38 (30.4) | 27 (21.8) | 65 (26.1) | .12b |
| 1 | 87 (69.6) | 97 (78.2) | 184 (73.9) | |
| Stage of pleural mesothelioma | ||||
| I | 0 | 2 (1.6) | 2 (0.8) | .39c |
| IA | 8 (6.4) | 5 (4.0) | 13 (5.2) | |
| IB | 16 (12.8) | 12 (9.7) | 28 (11.2) | |
| II | 12 (9.6) | 16 (12.9) | 28 (11.2) | |
| IIIA | 18 (14.4) | 18 (14.5) | 36 (14.5) | |
| IIIB | 13 (10.4) | 21 (16.9) | 34 (13.7) | |
| IIIC | 0 | 1 (0.8) | 1 (0.4) | |
| IV | 28 (22.4) | 29 (23.4) | 57 (22.9) | |
| Unknown | 30 (24.0) | 20 (16.1) | 50 (20.1) | |
| Histologic subtype | ||||
| Biphasic | 60 (48.0) | 60 (48.4) | 120 (48.2) | .95b |
| Sarcomatoid | 65 (52.0) | 64 (51.6) | 129 (51.8) | |
| Prior radiotherapy | ||||
| Yes | 4 (3.2) | 9 (7.3) | 13 (5.2) | .17c |
| No | 121 (96.8) | 115 (92.7) | 236 (94.8) | |
| Prior surgery | ||||
| Yes | 18 (14.4) | 19 (15.3) | 37 (14.9) | .84b |
| No | 107 (85.6) | 105 (84.7) | 212 (85.1) | |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
P value comparing pegargiminase-chemotherapy to placebo-chemotherapy is based on the t test.
P value comparing pegargiminase-chemotherapy to placebo-chemotherapy is based on the χ2 test.
P value comparing pegargiminase-chemotherapy to placebo-chemotherapy is based on Fisher exact test due to categories with fewer than 5 patients.
Most patients discontinued treatment due to disease progression in the pegargiminase group (85 patients [68.0%]) and in the placebo group (84 patients [67.7%]), with death during treatment occurring in 5 patients (4.0%) and 10 patients (8.1%), respectively. One patient was receiving study treatment at the time of analysis in September 2022; treatment continued for 2 months before stopping for disease progression. Six patients (4.8%) receiving pegargiminase and 0 patients receiving placebo completed therapy 2 years from randomization (eTable 1 in Supplement 3). Poststudy therapies were comparable in the 2 groups: 57 patients (45.6%) in the pegargiminase group and 58 patients (46.8%) in the placebo group received various systemic anticancer therapies, including immune checkpoint inhibitors (16.8% vs 8.9%, respectively, P = .77) and chemotherapy. Palliative radiotherapy was administered to 14 patients (11.2%) in the pegargiminase group and 14 patients (11.3%) in the placebo group (eTable 2 in Supplement 3).
Overall Survival
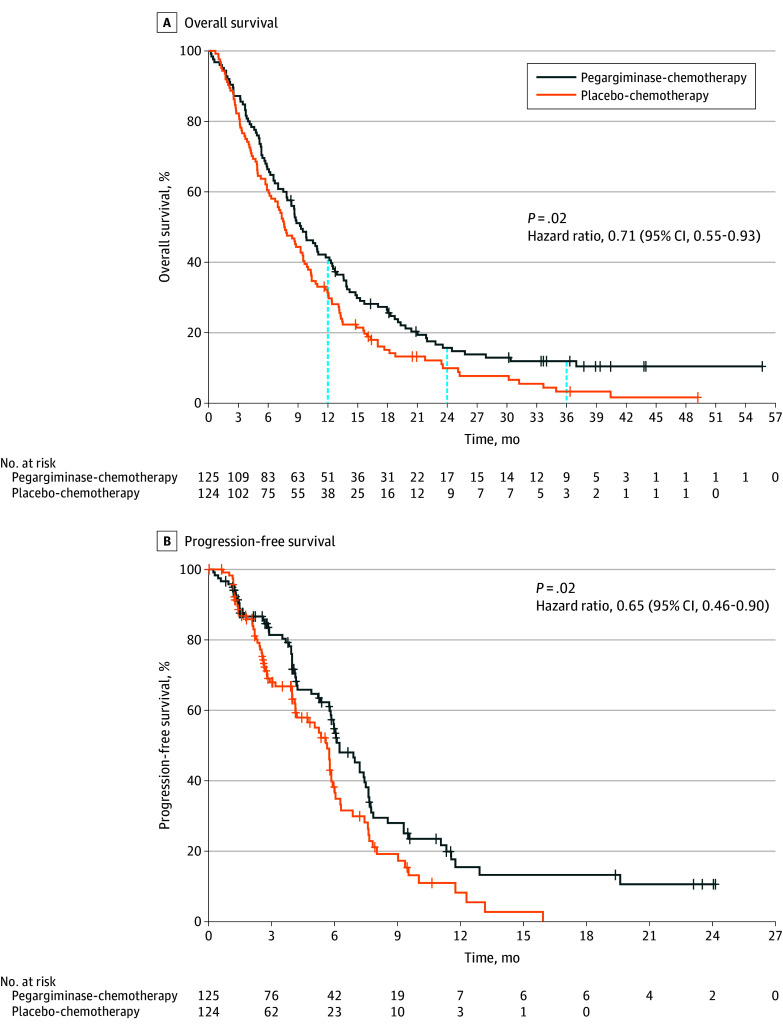
At the time of the database lock on August 19, 2022, all patients had a minimum of 12 months of follow-up for survival. A total of 105 patients (84.0%) in the pegargiminase group and 116 patients (93.5%) in the placebo group had died. OS was significantly longer in the pegargiminase group (median, 9.3 months; 95% CI, 7.9-11.8 months) than in the placebo group (median, 7.7 months; 95% CI, 6.1-9.5 months). The stratified HR for death was 0.71 (95% CI, 0.55-0.93; P = .02), and the 1-year OS rate was 41.4% (95% CI, 32.7%-49.9%) in the pegargiminase group and 31.4% (95% CI, 23.5%-39.7%) in the placebo group (Figure 2A). Notably, the survival curves diverged early and remained separate throughout the course of the study. A therapeutic plateau was reached by 36 months with 3- to 4-fold more patients alive in the pegargiminase group compared with the placebo group (11.9% vs 3.3%; eTable 3 in Supplement 3).
Figure 2. Overall and Progression-Free Survival in Phase 3 Patients (Intention-to-Treat Population).
Analysis population was the intention-to-treat population, which included all randomized patients. A, Overall survival (OS) was calculated as the time from randomization until death. In the event that no death was documented prior to study termination or analysis cutoff, OS was censored at the last known date the patient was known to be alive (using last contact day or last dose day). Total number of patients from the analysis population was 249, including 25 censored (17 from pegargiminase-chemotherapy group, 8 from placebo-chemotherapy group) and 224 with OS events (108 from pegargiminase group, 116 from placebo group). The 48-month survival percentage was not computed in the pegargiminase group due to the large number of censored patients (eTable 3 in Supplement 3). The blue vertical dashed lines indicate the 12-, 24-, and 36-month follow-up. B, Progression-free survival (PFS) was calculated as the time from randomization until date of tumor progression or death. In the event that no tumor progression or death was documented prior to end of treatment, analysis cutoff, or the start of confounding anticancer therapy, PFS was censored at the date of the last tumor assessment demonstrating no tumor progression. Total number of patients from the analysis population was 249, including 104 censored (54 from pegargiminase group, 50 from placebo group) and 145 with PFS events (71 from pegargiminase group, 74 from placebo group). Tick marks indicate censoring.
PFS and Tumor Response
The PFS was also significantly longer in the pegargiminase group (median, 6.2 months; 95% CI, 5.8-7.4 months) than in the placebo group (median, 5.6 months; 95% CI, 4.1-5.9 months). The stratified HR for disease progression or death was 0.65 (95% CI, 0.46-0.90; P = .02) (Figure 2B; eTable 4 in Supplement 3). The ORR was 13.8% in the pegargiminase group and 13.5% in the placebo group (P = .95 using the Cochran-Mantel-Haenszel test; phase 2 portion only in eTable 5 in Supplement 3). The median duration of response (the phase 2 secondary end point) was not determined in the pegargiminase group due to high censoring and was 4.6 months (95% CI, 3.7-11.8 months) in the placebo group (HR, 0.34; 95% CI, 0.10-1.14; P = .09; eTable 6 in Supplement 3). Disease control (ORR and stable disease) at the 12-week assessment was numerically greater in the pegargiminase group (85.1%; 95% CI, 75.8%-91.8%) than in the placebo group (76.4%; 95% CI, 66.2%-84.7%) (P = .15). Additional exploratory subgroup analyses are summarized in eFigure 3 in Supplement 3.
Exposure, Safety, and Pharmacodynamics
The median (range) number of doses of pegargiminase was 18 (1-104) compared with 14 (1-69) doses of placebo, with around a median (range) of 4 (1-6) doses of pemetrexed and platinum in both groups (eTable 7 in Supplement 3). A total of 46 patients (36.8%) in the pegargiminase group and 42 patients (33.9%) in the placebo group received at least 1 dose of carboplatin during chemotherapy. Treatment-emergent adverse events (TEAEs) were similar between groups, with 246 patients (98.8%) reporting at least 1 TEAE, with nausea, fatigue, and constipation being the most common with pegargiminase-chemotherapy and nausea, fatigue, and anorexia with placebo-chemotherapy (eTables 8-10 in Supplement 3). There were 7 patients (5.6%) in the pegargiminase-chemotherapy group and 12 patients (9.7%) in the placebo-chemotherapy group with fatal TEAEs, 3 of which were possibly related to pegargiminase (n = 2; sudden death, sepsis) or placebo (n = 1; sepsis). TEAEs leading to discontinuation occurred in 25 patients (20.0%)receiving pegargiminase and 17 patients (13.7%) receiving placebo (eTable 11 in Supplement 3). Grade 3 or higher TEAEs occurred in 36 patients (28.8%) receiving pegargiminase and 21 patients (16.9%) receiving placebo (P = .03), with anaphylactic hypersensitivity and skin reactions occurring in 3 patients (2.4%) and 2 patients (1.6%) in the pegargiminase group, respectively, and none in the placebo group (Table 2). Anaphylactic hypersensitivity occurred with subsequent dosing of pegargiminase, usually within cycle 1, and was managed using steroids, and depending on severity was either discontinued or administered with hydrocortisone and chlorpheniramine prophylaxis. Adding pegargiminase to chemotherapy increased neutropenia (grade 3 or higher) rates by 3-fold, but not sepsis and fever. Plasma arginine level declined with a reciprocal increase in citrulline level and antipegargiminase antibodies, which were detected in 37 of 38 patients (97.4%) by week 25 with pegargiminase (eFigure 4 in Supplement 3).
Table 2. Treatment-Emergent Adverse Events (TEAEs) Grade 3 or Higher Related to Pegargiminase or Placebo.
| System organ class/preferred terma | Patients, No. (%) | P valueb | |
|---|---|---|---|
| Pegargiminase-chemotherapy (n = 125) | Placebo-chemotherapy (n = 124) | ||
| Patients reporting ≥1 related TEAE grade ≥3 | 36 (28.8) | 21 (16.9) | .03 |
| Blood and lymphatic disorders | 12 (9.6) | 5 (4.0) | .13 |
| Anemia | 6 (4.8) | 3 (2.4) | .50 |
| Neutropenia | 6 (4.8) | 2 (1.6) | .28 |
| Investigations | 12 (9.6) | 5 (4.0) | .13 |
| Neutrophil count decreased | 7 (5.6) | 2 (1.6) | .17 |
| Platelet count decreased | 4 (3.2) | 2 (1.6) | .68 |
| White blood cell count decreased | 3 (2.4) | 0 | .25 |
| Gastrointestinal disorders | 4 (3.2) | 2 (1.6) | .68 |
| Nausea | 2 (1.6) | 1 (0.8) | >.99 |
| General/site administration | 3 (2.4) | 3 (2.4) | >.99 |
| Skin (rash, maculopapular) | 2 (1.6) | 0 | .50 |
| Fatigue | 2 (1.6) | 2 (1.6) | >.99 |
| Infections and infestations | 3 (2.4) | 2 (1.6) | >.99 |
| Neutropenic sepsis | 2 (1.6) | 1 (0.8) | >.99 |
| Metabolism and nutrition disorders | 2 (1.6) | 3 (2.4) | .68 |
| Hyponatremia | 2 (1.6) | 0 | .50 |
| Immune system disorders | 4 (3.2) | 0 | .12 |
| Anaphylactic reactionc | 3 (2.4) | 0 | .25 |
Adverse events (AEs) were coded to system organ class and preferred term using MedDRA, version 19.1. At each level of summarization (any event, system organ class, and preferred term), patients reporting more than 1 related and grade 3 or higher AE were counted only once. Related AEs were those reported as possibly, probably, or definitely related to pegargiminase or placebo.
P value comparing pegargiminase-chemotherapy to placebo-chemotherapy is based on Fisher exact test.
For 1 patient in the pegargiminase-chemotherapy group who had a grade 3 anaphylactic reaction TEAE considered related to pegargiminase, additional information was obtained that led the sponsor to disagree with the diagnosis. The most common TEAEs leading to pegargiminase discontinuation were anaphylactic reaction (3 patients [2.4%]) and fatigue, pyrexia, and cardiac arrest (2 patients [1.6%] each) (eTable 11 in Supplement 3).
Discussion
ATOMIC-Meso is a pivotal phase 3 trial conducted in patients with nonepithelioid pleural mesothelioma that evaluated standard first-line chemotherapy with the first-in-class arginine-depleting agent pegargiminase or a matched placebo. Both the primary end point of OS and the secondary end point of PFS validate pegargiminase combined with antifolate-based chemotherapy as a novel cancer treatment, in this first (to our knowledge) histologic subtype–driven study of nonepithelioid mesothelioma. The pegargiminase group had a 29% lower risk of death and a 35% lower risk of progression compared with placebo group (HR of 0.71 and 0.65, respectively, by the log-rank test).
Recently, the mesothelioma therapeutic landscape has shifted to favor immune checkpoint blockade over platinum-based chemotherapy. The CheckMate 743 study yielded a significant 4-month median OS benefit for ipilimumab and nivolumab compared with pemetrexed and platinum chemotherapy (18.1 months vs 14.1 months) that was largely driven by nonepithelioid histologic subtype (18.1 vs 8.8 months; stratified HR for death, 0.46), leading to US Food and Drug Administration approval in October 2020. While not replacing frontline immunotherapy with the more modest survival improvement in the ATOMIC-Meso study, pegargiminase nonetheless provides an incremental chemotherapeutic advance for patients with essentially chemotherapy-refractory nonepithelioid disease. Thus, deployment of pegargiminase would be envisaged second-line alongside platinum-pemetrexed. However, pegargiminase-based chemotherapy remains a practical up-front consideration for patients with active autoimmune disease. Overall, ATOMIC-Meso remains the largest study, to our knowledge, that is focused on nonepithelioid mesothelioma, and, significantly for an aggressive disease phenotype, approximately 50% of patients accessed subsequent therapies in both study treatment groups. Around 5% of patients receiving pegargiminase and none receiving placebo completed 2 years of weekly therapy, highlighting a subgroup of patients displaying exquisite sensitivity to arginine depletion that warrants further molecular characterization.
Consistent with prior studies, patients in the ATOMIC-Meso trial experienced prolonged arginine suppression with the triplet therapy over pegargiminase monotherapy, ascribed to the concurrent use of steroids and pemetrexed. Furthermore, our data justify revisiting the role of pemetrexed maintenance therapy, which has underperformed in mesothelioma. Nevertheless, how mesothelioma develops resistance to pegargiminase beyond a role for antidrug neutralizing antibodies needs to be addressed. Several studies in ASS1-silenced cancers identified reexpression of ASS1 via promoter demethylation, allowing tumors to bypass arginine restriction via the recycling of citrulline into argininosuccinate for subsequent conversion to arginine by argininosuccinate lyase. Additional pathways of pegargiminase resistance may involve metabolic rewiring via polyamines, autophagy, and macrophage recruitment.
Our study also signals further work to be done in epithelioid mesothelioma, the subtype that predominated in the ASS1-directed ADAM study. Accordingly, a recently characterized functional inverse relationship between ASS1 and the deubiquitinase BRCA1-associated protein (BAP1) provides a route for pegargiminase-based chemotherapy in epithelioid mesothelioma on biomarker selection. Several phase 3 studies of chemoimmunotherapy are also ongoing, including DREAM3R, which is assessing first-line platinum and pemetrexed plus the programmed death-ligand 1 inhibitor durvalumab, compared with standard chemotherapy or dual immune checkpoint blockade, potentially further improving survival outcomes for patients with mesothelioma, especially those with the epithelioid histologic subtype. Moreover, durvalumab-chemotherapy recorded a median OS of 9.2 months in nonepithelioid disease (compared to 24.3 months for epithelioid disease). Recently, the IND.227 phase 3 study reported the exploratory analyses for pembrolizumab-chemotherapy vs chemotherapy, noting a median OS of 12.3 months in nonepithelioid disease (compared to 19.8 months for epithelioid disease), highlighting the important role of frontline doublet immunotherapy, especially for nonepithelioid mesothelioma.
The clinical development of asparaginase for the treatment of acute lymphoblastic leukemia in the 1960s established the paradigm of amino acid deprivation to treat malignant disease. Our novel data add further impetus to the field of cancer metabolism and particularly targeting specific amino acids. Importantly, multimodality regimens of pegargiminase with chemo-immunotherapy warrant evaluation in patients with arginine-dependent cancers closely allied with a deeper appreciation of the host-tumor context, which will be fundamental to deploying pharmacological modulators of arginine metabolism effectively.
Limitations
First, the DSMB recommended early trial discontinuation due to positive results; however, additional factors, specifically the first-line approval of ipilimumab and nivolumab for mesothelioma and the COVID-19 pandemic, potentially affected the treatment effect size. Second, ASS1 biomarker validation may have offered greater study robustness but ultimately was not required for patient enrollment (Supplement 1) due to concerns over the sensitivity of the antibody between the commercial and academic laboratories. Third, there was no difference in the ORR, highlighting the need for better assessment tools in this disease. Fourth, almost twice as many patients in the pegargiminase-chemotherapy arm accessed ipilimumab and nivolumab immunotherapy (16.8% vs 8.9%) compared to the placebo-chemotherapy arm, potentially affecting long-term survival. Indeed, post hoc sensitivity analysis revealed an impressive median OS; however, this was numerically higher in the placebo group compared to the pegargiminase group (25.3 months vs 19.3 months, P = .77; eTable 12 and eFigure 5 in Supplement 3). Fifth, while quality of life was not measured in ATOMIC-Meso, in the prior ADAM study, there was no detrimental effect on quality of life in patients receiving weekly intramuscular pegargiminase compared with best supportive care alone.
Conclusions
This global randomized clinical trial in patients with treatment-naive nonepithelioid pleural mesothelioma demonstrated significantly improved OS with pegargiminase-chemotherapy compared with chemotherapy alone. The pegargiminase-pemetrexed-platinum triplet was safe and validates arginine deprivation as a novel therapeutic antimetabolite strategy for patients with nonepithelioid mesothelioma. Additional studies of pegargiminase-based regimens are warranted in patients with other urea cycle–dysregulated cancers.
Trial Protocol
Statistical Analysis Plan
eFigure 1. ATOMIC-Meso Treatment schema
eFigure 2. ATOMIC-Meso Enrollment by Site and Country
eTable 1. Subject Disposition (All Randomized Subjects)
eTable 2. Post-study therapy
eTable 3. Overall Survival – Phase 3 Subjects (ITT Population)
eTable 4. Progression-Free Survival – Phase 3 Subjects (ITT Population)
eTable 5. Objective response rate – Phase 2 Subjects (ITT population)
eTable 6. Duration of Response – Phase 2 Subjects (ITT Population)
eFigure 3. Additional exploratory subgroup analyses (forest plot; and OS and PFS by histology subtype)
eTable 7. Extent of Drug Exposure
eTable 8. Overall Summary of TEAEs (Safety Population)
eTable 9. Most Frequently Occurring (≥10% in Either Group) TEAEs
eTable 10. Treatment-emergent serious adverse events (TESAEs)
eTable 11. TEAEs Leading to ADI PEG 20/Placebo Discontinuation
eFigure 4. Pharmacodynamic and immunogenicity responses
eTable 12. Sensitivity analysis of ipilimumab and nivolumab-treated patients
eFigure 5. Overall survival plot of sensitivity analysis (ipilimumab and nivolumab)
The ATOMIC-Meso Study Group
Data Sharing Statement
References
- 1.Delage B, Fennell DA, Nicholson L, et al. Arginine deprivation and argininosuccinate synthetase expression in the treatment of cancer. Int J Cancer. 2010;126(12):2762-2772. doi: 10.1002/ijc.25202 [DOI] [PubMed] [Google Scholar]
- 2.Ensor CM, Holtsberg FW, Bomalaski JS, Clark MA. Pegylated arginine deiminase (ADI-SS PEG20,000 mw) inhibits human melanomas and hepatocellular carcinomas in vitro and in vivo. Cancer Res. 2002;62(19):5443-5450. [PubMed] [Google Scholar]
- 3.Szlosarek PW, Klabatsa A, Pallaska A, et al. In vivo loss of expression of argininosuccinate synthetase in malignant pleural mesothelioma is a biomarker for susceptibility to arginine depletion. Clin Cancer Res. 2006;12(23):7126-7131. doi: 10.1158/1078-0432.CCR-06-1101 [DOI] [PubMed] [Google Scholar]
- 4.Bowles TL, Kim R, Galante J, et al. Pancreatic cancer cell lines deficient in argininosuccinate synthetase are sensitive to arginine deprivation by arginine deiminase. Int J Cancer. 2008;123(8):1950-1955. doi: 10.1002/ijc.23723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicholson LJ, Smith PR, Hiller L, et al. Epigenetic silencing of argininosuccinate synthetase confers resistance to platinum-induced cell death but collateral sensitivity to arginine auxotrophy in ovarian cancer. Int J Cancer. 2009;125(6):1454-1463. doi: 10.1002/ijc.24546 [DOI] [PubMed] [Google Scholar]
- 6.Delage B, Luong P, Maharaj L, et al. Promoter methylation of argininosuccinate synthetase-1 sensitises lymphomas to arginine deiminase treatment, autophagy and caspase-dependent apoptosis. Cell Death Dis. 2012;3(7):e342. doi: 10.1038/cddis.2012.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly MP, Jungbluth AA, Wu BW, Bomalaski J, Old LJ, Ritter G. Arginine deiminase PEG20 inhibits growth of small cell lung cancers lacking expression of argininosuccinate synthetase. Br J Cancer. 2012;106(2):324-332. doi: 10.1038/bjc.2011.524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang HY, Wu WR, Wang YH, et al. ASS1 as a novel tumor suppressor gene in myxofibrosarcomas: aberrant loss via epigenetic DNA methylation confers aggressive phenotypes, negative prognostic impact, and therapeutic relevance. Clin Cancer Res. 2013;19(11):2861-2872. doi: 10.1158/1078-0432.CCR-12-2641 [DOI] [PubMed] [Google Scholar]
- 9.Syed N, Langer J, Janczar K, et al. Epigenetic status of argininosuccinate synthetase and argininosuccinate lyase modulates autophagy and cell death in glioblastoma. Cell Death Dis. 2013;4(1):e458. doi: 10.1038/cddis.2012.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miraki-Moud F, Ghazaly E, Ariza-McNaughton L, et al. Arginine deprivation using pegylated arginine deiminase has activity against primary acute myeloid leukemia cells in vivo. Blood. 2015;125(26):4060-4068. doi: 10.1182/blood-2014-10-608133 [DOI] [PubMed] [Google Scholar]
- 11.Izzo F, Marra P, Beneduce G, et al. Pegylated arginine deiminase treatment of patients with unresectable hepatocellular carcinoma: results from phase I/II studies. J Clin Oncol. 2004;22(10):1815-1822. doi: 10.1200/JCO.2004.11.120 [DOI] [PubMed] [Google Scholar]
- 12.Ascierto PA, Scala S, Castello G, et al. Pegylated arginine deiminase treatment of patients with metastatic melanoma: results from phase I and II studies. J Clin Oncol. 2005;23(30):7660-7668. doi: 10.1200/JCO.2005.02.0933 [DOI] [PubMed] [Google Scholar]
- 13.Tsai HJ, Jiang SS, Hung WC, et al. A phase II study of arginine deiminase (ADI-PEG20) in relapsed/refractory or poor-risk acute myeloid leukemia patients. Sci Rep. 2017;7(1):11253. doi: 10.1038/s41598-017-10542-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janes SM, Alrifai D, Fennell DA. Perspectives on the treatment of malignant pleural mesothelioma. N Engl J Med. 2021;385(13):1207-1218. doi: 10.1056/NEJMra1912719 [DOI] [PubMed] [Google Scholar]
- 15.Szlosarek PW, Steele JP, Nolan L, et al. Arginine deprivation with pegylated arginine deiminase in patients with argininosuccinate synthetase 1-deficient malignant pleural mesothelioma: a randomized clinical trial. JAMA Oncol. 2017;3(1):58-66. doi: 10.1001/jamaoncol.2016.3049 [DOI] [PubMed] [Google Scholar]
- 16.Allen MD, Luong P, Hudson C, et al. Prognostic and therapeutic impact of argininosuccinate synthetase 1 control in bladder cancer as monitored longitudinally by PET imaging. Cancer Res. 2014;74(3):896-907. doi: 10.1158/0008-5472.CAN-13-1702 [DOI] [PubMed] [Google Scholar]
- 17.Rabinovich S, Adler L, Yizhak K, et al. Diversion of aspartate in ASS1-deficient tumours fosters de novo pyrimidine synthesis. Nature. 2015;527(7578):379-383. doi: 10.1038/nature15529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keshet R, Szlosarek P, Carracedo A, Erez A. Rewiring urea cycle metabolism in cancer to support anabolism. Nat Rev Cancer. 2018;18(10):634-645. doi: 10.1038/s41568-018-0054-z [DOI] [PubMed] [Google Scholar]
- 19.Beddowes E, Spicer J, Chan PY, et al. Phase 1 dose-escalation study of pegylated arginine deiminase, cisplatin, and pemetrexed in patients with argininosuccinate synthetase 1-deficient thoracic cancers. J Clin Oncol. 2017;35(16):1778-1785. doi: 10.1200/JCO.2016.71.3230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szlosarek PW, Phillips MM, Pavlyk I, et al. Expansion phase 1 study of pegargiminase plus pemetrexed and cisplatin in patients with argininosuccinate synthetase 1-deficient mesothelioma: safety, efficacy, and resistance mechanisms. JTO Clin Res Rep. 2020;1(4):100093. doi: 10.1016/j.jtocrr.2020.100093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szyszko TA, Dunn JT, Phillips MM, et al. Role of 3′-Deoxy-3′-[18F] fluorothymidine positron emission tomography-computed tomography as a predictive biomarker in argininosuccinate synthetase 1-deficient thoracic cancers treated with pegargiminase. JTO Clin Res Rep. 2022;3(9):100382. doi: 10.1016/j.jtocrr.2022.100382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21(14):2636-2644. doi: 10.1200/JCO.2003.11.136 [DOI] [PubMed] [Google Scholar]
- 23.Ho JC, Lam SK. Combination of arginine depletion and chemotherapy in thoracic malignancies. J Clin Oncol. 2017;35(16):1758-1759. doi: 10.1200/JCO.2017.72.7305 [DOI] [PubMed] [Google Scholar]
- 24.Mansfield AS, Symanowski JT, Peikert T. Systematic review of response rates of sarcomatoid malignant pleural mesotheliomas in clinical trials. Lung Cancer. 2014;86(2):133-136. doi: 10.1016/j.lungcan.2014.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baas P, Scherpereel A, Nowak AK, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet. 2021;397(10272):375-386. doi: 10.1016/S0140-6736(20)32714-8 [DOI] [PubMed] [Google Scholar]
- 26.Dudek AZ, Wang X, Gu L, et al. Randomized study of maintenance pemetrexed versus observation for treatment of malignant pleural mesothelioma: CALGB 30901. Clin Lung Cancer. 2020;21(6):553-561.e1. doi: 10.1016/j.cllc.2020.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feun LG, Marini A, Walker G, et al. Negative argininosuccinate synthetase expression in melanoma tumours may predict clinical benefit from arginine-depleting therapy with pegylated arginine deiminase. Br J Cancer. 2012;106(9):1481-1485. doi: 10.1038/bjc.2012.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Locke M, Ghazaly E, Freitas MO, et al. Inhibition of the polyamine synthesis pathway is synthetically lethal with loss of argininosuccinate synthase 1. Cell Rep. 2016;16(6):1604-1613. doi: 10.1016/j.celrep.2016.06.097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Battisti S, Valente D, Albonici L, Bei R, Modesti A, Palumbo C. Nutritional stress and arginine auxotrophy confer high sensitivity to chloroquine toxicity in mesothelioma cells. Am J Respir Cell Mol Biol. 2012;46(4):498-506. doi: 10.1165/rcmb.2011-0195OC [DOI] [PubMed] [Google Scholar]
- 30.Phillips MM, Pavlyk I, Allen M, et al. A role for macrophages under cytokine control in mediating resistance to ADI-PEG20 (pegargiminase) in ASS1-deficient mesothelioma. Pharmacol Rep. 2023;75(3):570-584. doi: 10.1007/s43440-023-00480-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnett SE, Kenyani J, Tripari M, et al. BAP1 loss is associated with higher ASS1 expression in epithelioid mesothelioma: implications for therapeutic stratification. Mol Cancer Res. 2023;21(5):411-427. doi: 10.1158/1541-7786.MCR-22-0635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Durvalumab with chemotherapy as first line treatment in advanced pleural mesothelioma (DREAM3R). ClinicalTrials.gov identifier: NCT04334759. Updated December 1, 2023. Accessed January 9, 2024. https://clinicaltrials.gov/study/NCT04334759
- 33.Nowak AK, Lesterhuis WJ, Kok PS, et al. Durvalumab with first-line chemotherapy in previously untreated malignant pleural mesothelioma (DREAM): a multicentre, single-arm, phase 2 trial with a safety run-in. Lancet Oncol. 2020;21(9):1213-1223. doi: 10.1016/S1470-2045(20)30462-9 [DOI] [PubMed] [Google Scholar]
- 34.Forde PM, Anagnostou V, Sun Z, et al. Durvalumab with platinum-pemetrexed for unresectable pleural mesothelioma: survival, genomic and immunologic analyses from the phase 2 PrE0505 trial. Nat Med. 2021;27(11):1910-1920. doi: 10.1038/s41591-021-01541-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piccirillo MC, Chu Q, Bradbury P, et al. Brief report: Canadian Cancer Trials Group IND.227: a phase 2 randomized study of pembrolizumab in patients with advanced malignant pleural mesothelioma (NCT02784171). J Thorac Oncol. 2023;18(6):813-819. doi: 10.1016/j.jtho.2023.02.003 [DOI] [PubMed] [Google Scholar]
- 36.Chu Q, Piccirillo MC, Greillier L, et al. IND227 phase III (P3) study of cisplatin/pemetrexed (CP) with or without pembrolizumab (pembro) in patients (pts) with malignant pleural mesothelioma (PM): a CCTG, NCIN, and IFCT trial. J Clin Oncol. 2023;41(17)(supp):LBA8505. doi: 10.1200/JCO.2023.41.17_suppl.LBA8505 [DOI] [Google Scholar]
- 37.Hill JM, Roberts J, Loeb E, Khan A, MacLellan A, Hill RW. L-asparaginase therapy for leukemia and other malignant neoplasms: remission in human leukemia. JAMA. 1967;202(9):882-888. doi: 10.1001/jama.1967.03130220070012 [DOI] [PubMed] [Google Scholar]
- 38.Luengo A, Gui DY, Vander Heiden MG. Targeting metabolism for cancer therapy. Cell Chem Biol. 2017;24(9):1161-1180. doi: 10.1016/j.chembiol.2017.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faubert B, Solmonson A, DeBerardinis RJ. Metabolic reprogramming and cancer progression. Science. 2020;368(6487):eaaw5473. doi: 10.1126/science.aaw5473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomlinson BK, Thomson JA, Bomalaski JS, et al. Phase I trial of arginine deprivation therapy with ADI-PEG 20 plus docetaxel in patients with advanced malignant solid tumors. Clin Cancer Res. 2015;21(11):2480-2486. doi: 10.1158/1078-0432.CCR-14-2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lowery MA, Yu KH, Kelsen DP, et al. A phase 1/1B trial of ADI-PEG 20 plus nab-paclitaxel and gemcitabine in patients with advanced pancreatic adenocarcinoma. Cancer. 2017;123(23):4556-4565. doi: 10.1002/cncr.30897 [DOI] [PubMed] [Google Scholar]
- 42.Harding JJ, Do RK, Dika IE, et al. A phase 1 study of ADI-PEG 20 and modified FOLFOX6 in patients with advanced hepatocellular carcinoma and other gastrointestinal malignancies. Cancer Chemother Pharmacol. 2018;82(3):429-440. doi: 10.1007/s00280-018-3635-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall PE, Lewis R, Syed N, et al. A phase I study of pegylated arginine deiminase (pegargiminase), cisplatin, and pemetrexed in argininosuccinate synthetase 1-deficient recurrent high-grade glioma. Clin Cancer Res. 2019;25(9):2708-2716. doi: 10.1158/1078-0432.CCR-18-3729 [DOI] [PubMed] [Google Scholar]
- 44.Tsai HJ, Hsiao HH, Hsu YT, et al. Phase I study of ADI-PEG20 plus low-dose cytarabine for the treatment of acute myeloid leukemia. Cancer Med. 2021;10(9):2946-2955. doi: 10.1002/cam4.3871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang KY, Chiang NJ, Wu SY, et al. Phase 1b study of pegylated arginine deiminase (ADI-PEG 20) plus pembrolizumab in advanced solid cancers. Oncoimmunology. 2021;10(1):1943253. doi: 10.1080/2162402X.2021.1943253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abou-Alfa GK, Qin S, Ryoo BY, et al. Phase III randomized study of second line ADI-PEG 20 plus best supportive care versus placebo plus best supportive care in patients with advanced hepatocellular carcinoma. Ann Oncol. 2018;29(6):1402-1408. doi: 10.1093/annonc/mdy101 [DOI] [PubMed] [Google Scholar]
- 47.Hajaj E, Sciacovelli M, Frezza C, Erez A. The context-specific roles of urea cycle enzymes in tumorigenesis. Mol Cell. 2021;81(18):3749-3759. doi: 10.1016/j.molcel.2021.08.005 [DOI] [PubMed] [Google Scholar]
- 48.Field GC, Pavlyk I, Szlosarek PW. Bench-to-bedside studies of arginine deprivation in cancer. Molecules. 2023;28(5):2150. doi: 10.3390/molecules28052150 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eFigure 1. ATOMIC-Meso Treatment schema
eFigure 2. ATOMIC-Meso Enrollment by Site and Country
eTable 1. Subject Disposition (All Randomized Subjects)
eTable 2. Post-study therapy
eTable 3. Overall Survival – Phase 3 Subjects (ITT Population)
eTable 4. Progression-Free Survival – Phase 3 Subjects (ITT Population)
eTable 5. Objective response rate – Phase 2 Subjects (ITT population)
eTable 6. Duration of Response – Phase 2 Subjects (ITT Population)
eFigure 3. Additional exploratory subgroup analyses (forest plot; and OS and PFS by histology subtype)
eTable 7. Extent of Drug Exposure
eTable 8. Overall Summary of TEAEs (Safety Population)
eTable 9. Most Frequently Occurring (≥10% in Either Group) TEAEs
eTable 10. Treatment-emergent serious adverse events (TESAEs)
eTable 11. TEAEs Leading to ADI PEG 20/Placebo Discontinuation
eFigure 4. Pharmacodynamic and immunogenicity responses
eTable 12. Sensitivity analysis of ipilimumab and nivolumab-treated patients
eFigure 5. Overall survival plot of sensitivity analysis (ipilimumab and nivolumab)
The ATOMIC-Meso Study Group
Data Sharing Statement