Abstract
Objective
We evaluated the impact of reimbursement for NFFCCM on healthcare utilization among Medicare beneficiaries with type 2 diabetes in Louisiana.
Methods
We implemented group-based trajectory balancing and propensity score matching to obtain comparable treatment (with NFFCCM) and control (without NFFCCM) groups at baseline. Diabetes patients with Medicare as their primary payer at baseline were extracted using electronic health records of 3 health systems from REACHnet, a Clinical Research Network. The study period is from 2013 to early 2020. Our outcomes include general healthcare utilization (outpatient, Emergency Department (ED), and inpatient encounters) and health utilization related to diabetic complications. We tested each of these outcomes according to multiple treatment definitions and different subgroups.
Results
Receiving any NFFCCM was associated with an increase in outpatient visits of 657 (95% CI: 626 to 687; p < 0.001) per 1,000 patients per month, a decrease in inpatient admissions of 5 (95% CI: 2 to 7; p < 0.001) per 1,000 patients per month, and a decrease in ED visits of 4 (95% CI: 1 to 7; p = 0.005) per 1,000 patients per month after 24-month follow-up from initial NFFCCM encounter. Both complex and non-complex NFFCCM significantly increased visits to outpatient services and inpatient admissions per month. Receiving NFFCCM has a dose-response association with increasing outpatient visits per month.
Conclusions
Diabetes patients in Louisiana who received NFFCCM had more low-cost primary health care and less high-cost healthcare utilization in general. The cost savings of NFFCCM in diabetes management could be further explored in the future.
Keywords: non-face-to-face, remote care, diabetes care, chronic care management
Introduction
Chronic diseases such as diabetes are increasingly common in the world, which has brought increased healthcare costs to individuals and the economy.1 According to the National Diabetes Statistics Report 2020, 34.2 million people have diabetes in the United States, which accounts for 10.5% of the US population.2 Approximately 90-95% of them have type 2 diabetes (T2D). The common risk factors coexisting with T2D, such as older age, hypertension, dyslipidemia, and smoking, are risk factors for cardiovascular disease (CVD), and T2D itself also confers an independent risk for CVD.3 As a result, elderly patients with diabetes covered by Medicare usually have multiple chronic conditions.4 Any policymakers who are considering public policies (e.g., Medicare’s payment systems) for Medicare must recognize the need to target patients with multiple chronic conditions.5 As a result, CMS has defined that “chronic care management (CCM) is a specific care management service that provides coverage for patients with two or more chronic conditions for a continuous relationship with their care team.” There is a critical need for care coordination and chronic care management in diabetes management because CCM and care coordination in clinical practice may be highly correlated or spilled over. Various approaches have been implemented to improve chronic disease management.6 Research studies have demonstrated that CCM can reduce total costs of care for chronic disease patients while improving their overall health.7,8
However, patients frequently face barriers to seeking chronic care, such as lack of appointment times, long waiting times, or distance from provider locations.9 Supplementing in-person care with remote care has been promoted in recent years to improve the access to chronic care especially given pandemics, hurricanes, and other disruptions that can impact continuity of care. In 2015, the Centers for Medicare and Medicaid Services (CMS) began reimbursing chronic care management (CCM) services for Medicare beneficiaries who have multiple (two or more) chronic conditions expected to last at least 12 months, or until the death of the patient.10 The first Current Procedural Terminology (CPT) billing code for non-complex CCM (99490) was issued in 2015, and the CMS released two more supplementary billing codes (99487 and 99489) for complex CCM in 2017.10 The CMS has adopted CPT 99490 for Medicare NFFCCM services, which is defined in the CPT Professional Codebook as follows: “Chronic care management services, at least 20 minutes of clinical staff time directed by a physician or other qualified health care professional, per calendar month, with the following required elements: multiple (two or more) chronic conditions expected to last at least 12 months, or until the death of the patient; chronic conditions place the patient at significant risk of death, acute exacerbation/decompensation, or functional decline; comprehensive care plan established, implemented, revised, or monitored.”11 As of 2017, CPT 99487 has been used for reimbursement by Medicare to account for extended care coordination time spent with especially complex patients. This code reimburses for the first 60 minutes of non-face-to-face care coordination by clinical staff. This contrasts with CPT 99490 which was introduced on January 1, 2015, and reimburses for only 20 minutes per month. The two key differentiators between 99487 and 99490 are the additional time (60 minutes for CPT 99487 versus 20 minutes for CPT 99490) and the requirement for medical decision-making. In addition, a code reimbursing for additional time (CPT 99489) is available for complex CCM patients being billed under CPT 99487. These non-face-to-face chronic care management (NFFCCM) services aim to be a critical component of primary care that promotes better health and reduces overall health care costs of chronic diseases. These services are likely to benefit patients with type 2 diabetes because patients with diabetes often experience long-term microvascular and macrovascular complications. Multiple chronic conditions covered by this reimbursement are common diabetes complications or can lead to complications, such as cardiovascular disease, hypertension, and hyperlipidemia.
Previous evidence has shown that CCM has a significant effect in reducing health care costs among Medicare beneficiaries, likely through decreased use of inpatient hospital and post-acute care services12, while achieving substantial additional revenue for practices13. These studies only evaluated the impact of the 2015 CCM national payment policy. It is still unclear whether the policy revisions for CCM payments in 2017 could further encourage better chronic care management. Several qualitative studies explored the barriers and facilitators in implementing NFFCCM for diabetes in Louisiana from different perspectives.14-16 Few quantitative studies have been published on the effect of NFFCCM on healthcare utilization for patients with type 2 diabetes. Therefore, we conducted this quasi-experimental study to examine the impact of multiple NFFCCM services, complex and non-complex, on utilization among Medicare beneficiaries with type 2 diabetes in Louisiana.
Methods
Study design and data sources
This is a quasi-experimental study using electronic health records (EHR) between 2013 and March 2020 from Research Action for Health Network (REACHnet), a clinical research network in PCORnet, the National Patient-Centered Clinical Research Network. Records stored in REACHnet are from several health systems in Louisiana and Texas and standardized to the PCORnet Common Data Model. We obtained data on all type 2 diabetes patients from 3 health systems in Louisiana using the Surveillance Prevention, and Management of Diabetes Mellitus (SUPREME-DM) definition.17,18 The data were collected from three health systems in the Greater New Orleans Area, including Ochsner Health System, Tulane Medical Center, and University Medical Center New Orleans. The study and analysis plan followed the STROBE reporting guidelines and were approved by Tulane University Institutional Review Board (IRB# 906810).
Interventions and comparators or controls
The intervention group is those who received any NFFCCM (complex or non-complex) under three billing codes (99490, 99487, and/or 99489). We then used propensity score weighting to balance the selected comparison group who did not receive any NFFCCM services.
Outcome measures
We assessed several outcomes in the present study including healthcare utilization of different encounter types (non-NFFCCM outpatient visits, all-cause hospitalizations, all-cause emergency department (ED) visits), and any hospitalizations or ED visits related to diabetes complications in the follow-up period. The outpatient visits were identified using the encounter type in the EHR, including ambulatory visits and other ambulatory visits. Healthcare utilization was measured by month. We included three main diabetes complications: stroke, coronary heart disease (CHD), and MACE (major adverse cardiovascular events), which were defined as stroke or CHD. We examined the proportion of patients who had any inpatient or ED encounters with a diagnosis code of stroke, CHD, or MACE separately. Diagnosis codes of these chronic conditions are available at the CMS Chronic Conditions Data Warehouse.19
Covariates
We collected a set of observable characteristics including age at the initiation of NFFCCM, race, ethnicity, chronic conditions, health care utilization, and several diabetic biomarkers from the REACHnet EHR database. A full list is shown in Table 1.
Table 1.
Baseline characteristics before and after propensity score weighting for HbA1c measurements.
| Non-weighted | Weighted | |||||
|---|---|---|---|---|---|---|
| Treatment | Control | SMD | Treatment | Control | SMD | |
| Age at first NFFCCM (years) | 72.560 | 72.322 | −2.5% | 72.560 | 72.597 | 0.4% |
| Female (%) | 59.7 | 54.4 | −10.6% | 59.7 | 59.4 | −0.5% |
| Black (%) | 46.0 | 38.7 | −14.8% | 46.0 | 46.0 | −0.1% |
| Hispanic (%) | 1.7 | 2.5 | 5.6% | 1.7 | 1.7 | −0.1% |
| Stroke (%) | 15.6 | 15.7 | 0.2% | 15.6 | 15.5 | −0.4% |
| Hypertension (%) | 95.9 | 95.4 | −2.3% | 95.9 | 96.0 | 0.5% |
| Alzheimer's (%) | 2.0 | 1.6 | −3.1% | 2.0 | 2.0 | −0.1% |
| Arthritis (%) | 52.3 | 46.0 | −12.7% | 52.3 | 52.4 | 0.1% |
| Asthma (%) | 14.3 | 11.3 | −8.8% | 14.3 | 14.3 | 0.0% |
| Atrial Fibrillation (%) | 14.7 | 14.5 | −0.6% | 14.7 | 14.8 | 0.2% |
| Cancer (%) | 12.6 | 12.2 | −1.2% | 12.6 | 12.7 | 0.2% |
| Chronic Obstructive Pulmonary Disease (%) | 25.8 | 22.8 | −7.2% | 25.8 | 25.8 | −0.1% |
| Chronic Kidney Disease (%) | 45.0 | 45.5 | 1.1% | 45.0 | 44.9 | 0.0% |
| Depression (%) | 26.4 | 23.6 | −6.5% | 26.4 | 26.3 | −0.3% |
| Heart Failure (%) | 22.7 | 21.6 | −2.6% | 22.7 | 22.5 | −0.4% |
| Hyperlipidemia (%) | 89.3 | 88.6 | −2.2% | 89.3 | 89.3 | 0.0% |
| Coronary Heart Disease (%) | 35.5 | 35.5 | 0.0% | 35.5 | 35.2 | −0.6% |
| Osteoporosis (%) | 13.2 | 11.5 | −5.0% | 13.2 | 13.2 | 0.1% |
| Outpatient visits/month | 0.617 | 0.545 | −13.0% | 0.617 | 0.617 | 0.1% |
| Emergency department visits/month | 0.018 | 0.016 | −3.5% | 0.018 | 0.017 | −0.5% |
| Have any inpatient admissions (%) | 25.5 | 25.1 | −0.8% | 25.5 | 25.4 | −0.3% |
| Number of HbA1c tests | 4.119 | 3.66 | −24.5% | 4.119 | 4.13 | 0.5% |
| HbA1c>8% (%) | 14.7 | 15.5 | 2.1% | 14.7 | 14.6 | −0.5% |
| BMI>=35 (kg/m2) (%) | 31.2 | 27.2 | −8.9% | 31.2 | 31.4 | 0.6% |
| LDL>=110 (mg/dl) (%) | 19.7 | 23.0 | 8.1% | 19.7 | 19.6 | −0.2% |
| HDL<40 (mg/dl) (%) | 53.4 | 53.4 | −0.1% | 53.4 | 53.3 | −0.3% |
| eGFR>=30 (mL/min/1.73 m2) (%) | 95.7 | 94.5 | −5.8% | 95.7 | 95.8 | 0.2% |
| Trajectory group 1 (%) | 30.9 | 37.2 | 13.3% | 30.9 | 30.8 | −0.2% |
| Trajectory group 2 (%) | 54.7 | 52.4 | −4.5% | 54.7 | 54.8 | 0.3% |
| Trajectory group 3 (%) | 14.4 | 10.4 | −12.2% | 14.4 | 14.4 | −0.2% |
| N | 1,668 | 20,574 | 1,668 | 20,574 | ||
Note: NFFCCM: non-face-to-face chronic care management; SMD: standardized mean difference.
Time frame for the study
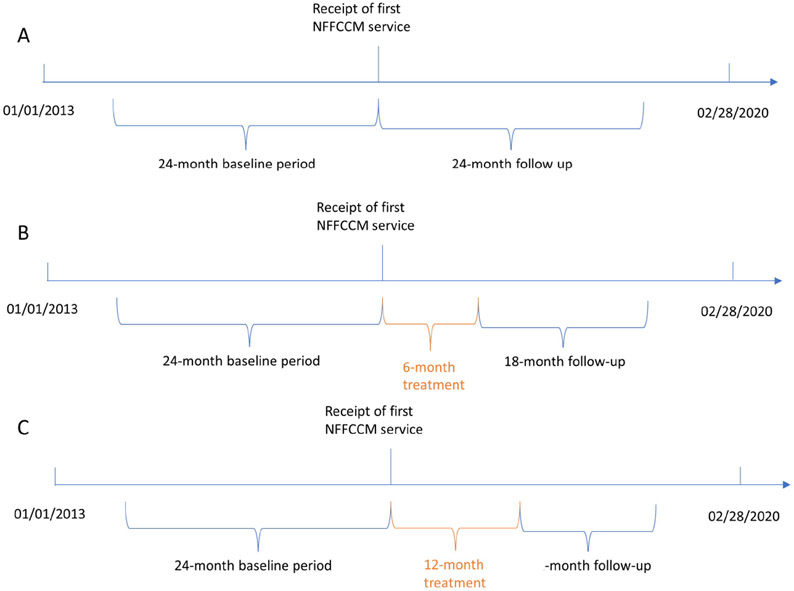
We used electronic health records (EHRs) from 01/01/2013 to 02/28/2020 stored in REACHnet. We defined the treatment group as patients with at least one record of NFFCCM (at least one CPT code: 99490, 99487, 99489), and the dates of the first NFFCCM coded were the initiation dates. We then assigned initiation dates randomly for untreated patients based on the distribution of initiation dates in the treated population. The baseline period was 24 months before the initiation dates. The evaluation or follow-up period was 24 months following the initiation dates (Figure 1. Panel A).
Figure 1.
Time frame of the study. NFFCCM indicates non-face-to-face chronic care management.
Statistical analysis
To have success in balancing, we first used group-based trajectory modeling to categorize individuals into latent groups with similar patterns of outpatient visits over 24 months before the initiation dates. The 24 monthly indicators of outpatient visits before treatment were modeled using the zero-inflated Poisson model for the group-specific models with time defined by months. Once the best group-based trajectory model has been chosen, we can incorporate measures of group membership as control variables in regression models or propensity score weighting protocols.20 We selected the optimal number of trajectory groups based on the Bayesian information criterion (BIC). Additionally, each trajectory group should have more than 5 percent of the population contributing to it.21 Detailed explanations of using group-based trajectory models can be found in prior work of other studies.21-24 We then estimated the propensity scores of getting the NFFCCM using a probit regression model, controlling for the covariates of baseline characteristics and binary indicators of each trajectory group (listed in Table 1). The propensity score is estimated from a probit regression model fitted on our analytic sample that includes both NFFCCM beneficiaries and non-NFFCCM beneficiaries. NFFCCM beneficiaries were assigned a weight of 1 and non-NFFCCM beneficiaries were assigned weights based on propensity scores (weight= pscore / (1-pscore)). These weights were used in our subsequent outcome modeling. We used this matched sample in a weighted linear regression to implement the doubly robust estimator by controlling the same set of variables used in the weighting step.
In addition to assessing the marginal association between ever having received NFFCCM and outcomes during the 24-month follow-up, we also conducted multiple subgroup analyses based on the complexity of NFFCCM and different baseline levels of HbA1c (⩾ 7.5% vs. < 7.5%), respectively. As the sensitivity analysis, we tested different treatment definitions based on different treatment periods (6-month or 12-month) and frequency of non-complex NFFCCM (CPT: 99490) use because of limited patients with complex NFFCCM. The outcomes were collected in the following 18 months after 6-month treatment (Figure 1. Panel B) and outcomes were collected in the following 12 months after 12-month treatment (Figure 1. Panel C). We then divided the treated patients into groups based on the frequency of non-complex NFFCCM use during the treatment period. The treatment sample may change with different treatment definitions; therefore, we repeated our matching process and regenerated propensity scores for non-NFFCCM beneficiaries to approximate the corresponding counterfactuals. For each matching, we checked the standardized mean difference between treatment and control before and after matching to ensure successful matching defined as differences within 10% for all baseline characteristics. All analyses were performed using SAS 9.4 and Stata 15.1.
Results
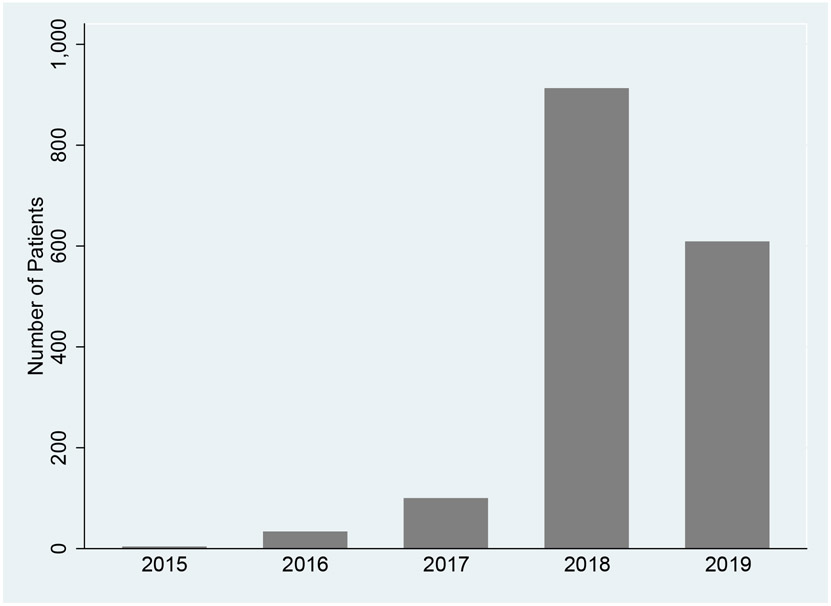
We identified 22,242 T2D patients with non-missing baseline characteristics and at least one outpatient visit during the 24 months before the first treatment. We checked the distribution of patients who used NFFCCM services per year (Figure 2). There was a very small number of patients who used any NFFCCM services in the first three years after implementing the reimbursement policy. In 2018, more than 800 patients used any NFFCCM services, either complex or non-complex. However, the number of patients decreased to about 600 in 2019. We obtained 3 groups by using group-based trajectory modeling and patients in each group shared a similar trend of outpatient visits during 24 months at baseline (Appendix. Figure A1). A total of 1,668 patients were NFFCCM beneficiaries and 20,574 were non-NFFCCM beneficiaries. The baseline characteristics of this sample are shown in Table 1. All baseline characteristics were successfully balanced within 10% of a standardized mean difference after being weighted by propensity scores. In the matched sample, the mean age was about 72.6 years old and 60% were female. Nearly half were black. Hypertension was the most common chronic disease among this diabetes population, about 96%. About 16% were diagnosed with stroke and 35% were diagnosed with CHD at baseline. Outpatient visits and ED visits were about 0.617 and 0.02 per patient per month, respectively. 25% had any inpatient admissions 24 months before the first treatment. We then repeated matching procedures and successfully matched all characteristics for each sample listed in the following outcome tables.
Figure 2.
The distribution of NFFCCM services utilization, 2015-2019.
Our main outcomes, general healthcare utilization and complication-specific healthcare utilization, are presented in Table 2. First, we compared patients with any NFFCCM (complex or non-complex) services with patients without any NFFCCM services. Receiving any NFFCCM was significantly associated with an increase in outpatient visits of 657 per 1,000 patients per month (95% CI: 626 to 687; p<0.001), a decrease in inpatient admissions of 5 (95% CI: 2 to 7; p<0.001), and a decrease in ED visits of 4 per 1,000 patients per month (95% CI: 1 to 7; p=0.005). We then made two comparisons: patients with any complex NFFCCM services versus those without any NFFCCM services and patients with only non-complex NFFCCM services versus those without any NFFCCM services. Similar effects on outpatient visits and inpatient admissions were detected when we considered the complexity of NFFCCM. However, receiving any complex NFFCCM failed to decrease ED utilization. We then examined the effect of receiving any NFFCCM on these outcomes by considering the baseline HbA1c (Table 3). Receiving at least one NFFCCM service among patients with higher baseline HbA1c (>= 7.5%) was associated with an increase in outpatient visits of 747 (95% CI: 685 to 810; p<0.001) and a decrease in ED visits of 11 (p < 0.01) per 1,000 patients per month. For patients with lower baseline HbA1c (< 7.5%), receiving at least one NFFCCM encounter was associated with an increase in outpatient visits of 638 (95% CI: 603 to 673; p < 0.001) and a decrease in inpatient admissions of 8 (95% CI: 5 to 10; p < 0.001) per 1,000 patients per month. We found no significant associations between receiving any NFFCCM and improvements in controlling diabetes complications related hospitalizations or ED visits.
Table 2.
The effect of NFFCCM on healthcare utilization by the complexity of NFFCCM.
| Any NFFCCM vs non- NFFCCM |
Any complex NFFCCM vs non-NFFCCM |
Non-complex NFFCCM only vs non-NFFCCM |
|
|---|---|---|---|
| All-cause utilization (visits per month) | |||
| Outpatient | 0.657*** | 0.845*** | 0.636*** |
| [0.626,0.687] | [0.811,0.879] | [0.605,0.666] | |
| <0.001 | <0.001 | <0.001 | |
| Inpatient | −0.005*** | −0.006*** | −0.005*** |
| [−0.007,−0.002] | [−0.009,−0.003] | [−0.007,−0.002] | |
| <0.001 | <0.001 | <0.001 | |
| ED | −0.004** | 0.013*** | −0.005*** |
| [−0.007,−0.001] | [0.009,0.017] | [−0.008,−0.003] | |
| 0.005 | <0.001 | <0.001 | |
| Any inpatient admissions or ED visits related to | |||
| CHD | 0.002 | 0.048*** | −0.003 |
| [−0.007,0.010] | [0.038,0.059] | [−0.011,0.006] | |
| 0.729 | <0.001 | 0.514 | |
| Stroke | 0.005* | −0.001 | 0.006* |
| [0.001,0.010] | [−0.006,0.004] | [0.001,0.010] | |
| 0.021 | 0.709 | 0.013 | |
| MACE | 0.002 | 0.047*** | −0.003 |
| [−0.007,0.010] | [0.037,0.058] | [−0.012,0.006] | |
| 0.740 | <0.001 | 0.523 | |
| N treatment | 1,668 | 166 | 1,502 |
| N control | 20,574 | 19,372 | 20,618 |
| N total | 22,242 | 19,538 | 22,120 |
Notes: NFFCCM: non-face-to-face chronic care management. ED: emergency department. CHD: coronary heart disease. MACE: major adversed cardiovascular disease. Non-complex NFFCCM: identified using the CPT code of 99490. Complex NFFCCM: identified using the CPT codes of 99487 and 99489. For each outcome, the coefficient is listed and followed with its 95% confidence interval and p value. * p< 0.05, ** p< 0.01, *** p< 0.001.
Table 3.
The effect of NFFCCM on healthcare utilization: HbA1c >= 7.5% vs HbA1c < 7.5%.
| Any NFFCCM vs non-NFFCCM | ||
|---|---|---|
| Baseline HbA1c >= 7.5% | Baseline HbA1c < 7.5% | |
| All-cause utilization (visits per month) | ||
| Outpatient | 0.747*** | 0.638*** |
| [0.685,0.810] | [0.603,0.673] | |
| <0.001 | <0.001 | |
| Inpatient | 0.004 | −0.008*** |
| [−0.001,0.009] | [−0.010,−0.005] | |
| 0.137 | <0.001 | |
| ED | −0.011*** | −0.002 |
| [−0.017,−0.006] | [−0.005,0.001] | |
| <0.001 | 0.263 | |
| Any inpatient admissions or ED visits related to | ||
| CHD | −0.016 | 0.007 |
| [−0.033,0.002] | [−0.002,0.017] | |
| 0.083 | 0.141 | |
| Stroke | 0.027*** | −0.002 |
| [0.015,0.039] | [−0.006,0.003] | |
| <0.001 | 0.409 | |
| MACE | −0.001 | 0.002 |
| [−0.019,0.018] | [−0.008,0.012] | |
| 0.952 | 0.638 | |
| N treatment | 386 | 1,282 |
| N control | 5,050 | 15,540 |
| N total | 5,436 | 16,822 |
Notes: NFFCCM: non-face-to-face chronic care management. ED: emergency department. CHD: coronary heart disease. MACE: major adversed cardiovascular disease. For each outcome, the coefficient is listed and followed with its 95% confidence interval and p value. * p< 0.05, ** p< 0.01, *** p< 0.001.
Last, we examined the effect of non-complex NFFCCM intensity on healthcare utilization and complications event rate (Table 4). During a 6-month treatment period, about 51% of non-complex NFFCCM beneficiaries received non-complex NFFCCM services more than 5 times. We only found higher frequency (over 5 times) of non-complex NFFCCM was associated with a significant increase in outpatient visits of 134 (95% CI: 6 to 263; p = 0.040) per 1,000 patients per month after 18-month follow-up from the end of the treatment period. Among patients receiving any non-complex NFFCCM in 12 months since the initial NFFCCM, about 52% of them received it more than 10 times. Higher frequency (over 10 times) was associated with decreased outpatient visits by 186 per 1,000 patients per month (95% CI: 35 to 337; p=0.016) after 12 months from the end of the treatment period.
Table 4.
The effect of non-complex NFFCCM intensity on healthcare utilization
| 6-month treatment | 12-month treatment | |||||
|---|---|---|---|---|---|---|
| 1-5 visits | 6+ visits | 6+ vs 1-5 | 1-10 visits | 11+ visits | 11+ vs 1-10 | |
| All-cause utilization (visits per month) | ||||||
| Outpatient | 0.458*** | 0.640*** | 0.134* | 0.312*** | 0.578*** | 0.186* |
| [0.423,0.494] | [0.603,0.676] | [0.006,0.263] | [0.269,0.354] | [0.536,0.619] | [0.035,0.337] | |
| <0.001 | <0.001 | 0.040 | <0.001 | <0.001 | 0.016 | |
| Inpatient | 0.003 | 0.002 | −0.004 | 0.003 | −0.001 | −0.002 |
| [−0.000,0.006] | [−0.001,0.005] | [−0.015,0.007] | [−0.001,0.006] | [−0.004,0.003] | [−0.015,0.010] | |
| 0.096 | 0.312 | 0.519 | 0.166 | 0.647 | 0.710 | |
| ED | −0.004* | −0.010*** | −0.005 | 0.000 | −0.001 | 0.000 |
| [−0.008,−0.000] | [−0.014,−0.007] | [−0.016,0.006] | [−0.006,0.005] | [−0.005,0.003] | [−0.015,0.015] | |
| 0.033 | <0.001 | 0.365 | 0.881 | 0.689 | 0.966 | |
| Any inpatient admissions or ED visits related to | ||||||
| CHD | −0.004 | 0.010* | 0.019 | 0.010* | 0.005 | −0.002 |
| [−0.013,0.005] | [0.000,0.019] | [−0.014,0.052] | [0.001,0.020] | [−0.005,0.014] | [−0.036,0.031] | |
| 0.406 | 0.044 | 0.249 | 0.037 | 0.333 | 0.899 | |
| Stroke | −0.001 | 0.004 | 0.007 | −0.006** | 0.001 | 0.007 |
| [−0.005,0.003] | [−0.000,0.009] | [−0.009,0.023] | [−0.009,−0.002] | [−0.003,0.005] | [−0.006,0.020] | |
| 0.740 | 0.059 | 0.371 | 0.002 | 0.587 | 0.295 | |
| MACE | −0.003 | 0.002 | 0.013 | 0.007 | 0.001 | −0.002 |
| [−0.013,0.006] | [−0.008,0.011] | [−0.021,0.047] | [−0.003,0.017] | [−0.009,0.010] | [−0.035,0.032] | |
| 0.480 | 0.706 | 0.443 | 0.201 | 0.916 | 0.923 | |
| N treatment | 660 | 687 | 679 | 510 | 553 | 551 |
| N control | 17,636 | 17,505 | 660 | 13,958 | 13,906 | 506 |
| N total | 18,296 | 18,192 | 1,339 | 14,468 | 14,459 | 1,057 |
Notes: NFFCCM: non-face-to-face chronic care management. ED: emergency department. CHD: coronary heart disease. MACE: major adversed cardiovascular disease. For each outcome, the coefficient is listed and followed with its 95% confidence interval and p value. * p< 0.05, ** p< 0.01, *** p< 0.001.
Discussion
Our analysis of the impact of reimbursement of NFFCCM found fewer inpatient admissions and ED visits among patients with type 2 diabetes in Louisiana, which provides strong evidence to encourage the use of NFFCCM in diabetes care. Less utilization of more expensive healthcare services, inpatient stays, and ED visits is likely driven by increased access to outpatient services. While we found no significant improvements in controlling diabetes complications related hospitalizations or ED visits, findings in the present study are still consistent with the expected benefits of NFFCCM to reduce the general utilization of more costly healthcare services.
A CMS-sponsored Mathematica evaluation using Medicare claims showed a similar impact of NFFCCM on health care utilization among Medicare beneficiaries.12 The NFFCCM could significantly reduce health care costs among Medicare beneficiaries, likely through decreased use of inpatient hospital and post-acute care services. Compared with the CMS evaluation, our study significantly enhanced the policy evaluation. Aligning with our results above, a higher rate of outpatient visits was observed after the initiation of NFFCCM services. But previous findings were limited to only non-complex NFFCCM in Medicare beneficiaries with any two types of chronic conditions. Our findings further demonstrated the positive effects of NFFCCM, including complex NFFCCM, for diabetes patients with any other chronic diseases. In addition, these effects were even stronger among patients with diabetes who had higher HbA1c of at least 7.5% at baseline. In general, the CCM evaluations in a systematic review of 12 randomized clinical trials only showed evidence of the effectiveness of the CCM for T2D management in primary care as well as significant improvements in clinical outcomes in 6 studies.8 Several clinical trials were performed to examine the effectiveness of other related approaches (e.g., telemedicine) to prompt diabetes care management.25,26 Findings from these trials indicated remote care management has the potential to serve as a supplement to usual in-person care for diabetes. We also found that NFFCCM could be a supplement to usual in-person primary care for diabetes because of increases in non-NFFCCM outpatient visits. More outpatient visits or primary care would provide more opportunities for patients to get tested, monitored, and early detection and treatment of disease, which would finally result in better health.27-29 Communities that are predominantly Black and Latinx/Hispanic tend to have fewer primary care providers and lower-quality health care facilities than communities that are mostly White.30-32 Receiving NFFCCM services may help to reduce such disparities in primary care through remote chronic care management and more access to outpatient services. According to our results, receiving NFFCCM was also associated with a decrease in ED visits if we used a longer follow-up period, such as 18 months and 24 months. Therefore, decreasing ED visits is highly possible driven by increased access to outpatient services in the long term.
The findings may raise concerns that estimates overstate the true impacts of the intervention. We used alternative treatment definitions and also conducted multiple subgroup analyses that support the findings. Importantly we identified that both patients with non-complex conditions, as well as patients with complex chronic conditions, could benefit from NFFCCM. In addition, the magnitude of the impact of NFFCCM is larger among patients with relatively worse glycemic control at baseline. We performed intensity analyses for only non-complex NFFCCM because of the small sample of patients using complex NFFCCM. The magnitude of this effect on outpatient visits was associated with different frequency levels of non-complex NFFCCM use.
Our sample was balanced well on observable characteristics between the NFFCCM and comparison groups by using trajectory balancing and propensity score matching. Without randomly assigning the treatment, however, our findings might be biased if the two groups are not balanced on unobservable characteristics, such as provider characteristics, or family or community support. Socio-economic background may be also closely associated with health care utilization, such as income and education. However, such information was not included in the dataset we used in this study, which could bias our findings. We excluded patients with any missing baseline characteristics. Our findings may also be biased if the characteristics of excluded patients were highly different from the patients included in this study. While reimbursement of NFFCCM has been shown to reduce expensive healthcare utilization in general, the current study indicates that at least the existing NFFCCM in such a short duration has not been as successful as expected in reducing the proportion of patients with any ED visits and inpatient admissions related to diabetes complications. Management of these diabetes complications is complex and is highly related to diabetes duration, disease severity, diet and nutrition, and other factors not captured by the current data. Although five years have passed since the initiation of NFFCCM, the uptake of these services is still quite a small proportion (about 7% of T2D patients) in our sample. More NFFCCM services are likely provided in primary care clinics that are not covered in this study. A study conducted by our team has examined the barriers to implementing the NFFCCM services among the elderly population with diabetes.14 Possible reasons included burden on staff and time commitment, financial sustainability, selection, and retention of patients. Therefore, it is not yet clear if this reimbursement policy would subsequently lower the event rate of diabetes complications in the larger population. Therefore, these factors limit the generalizability of our results and stronger evidence of the impact of NFFCCM may be found in a larger database with more NFFCCM recipients, such as a statewide administrative database. The present study only evaluated the impact of NFFCCM reimbursement on healthcare utilization. Further study needs to evaluate its impact on clinical outcomes and health care spending, such as glycemic control, the mortality rate from T2D complications, and out-of-pocket spending, to better understand the true value of reimbursing the NFFCCM for diabetes care.
Despite the limitations noted above, the findings in the present study are still notable because this is the largest study using real-world evidence/natural experiment to examine the impact of reimbursement of NFFCCM on healthcare utilization in diabetes patients in Louisiana. Remote management in diabetes is evolving with the incorporation of sophisticated technologies utilizing patient wearables such as continuous glucose monitoring, activity trackers, and smart weighing scales. Effective translation of the data from these devices may be enhanced with NFFCCM. The natural experiment of this CMS reimbursement policy incorporates elements of the structure of the chronic care model. The chronic care model emphasizes the importance of chronic disease management in the context of the primary care setting and incorporates the community, health system, patient, and practice team in productive interactions which result in improved functional and clinical outcomes. Important elements of the chronic care model that are relevant to CCM services include patient self-management support, delivery system design, decision support, and clinical information systems. Better coordinated care, consisting primarily of cross-cutting patient self-management support, delivery system design, decision support, and clinical information systems, will facilitate overcoming health care system and provider barriers as well as patients’ barriers.
Conclusions
Our study is the first real-world study showing evidence that the reimbursement of NFFCCM, especially non-complex NFFCCM, has benefited diabetes patients in Louisiana by shifting high-cost health utilization to low-cost primary health care settings in general. While barriers and challenges still exist to implementing this policy, our findings provide evidence to support NFFCCM as an effective tool for diabetes care. Policymakers could consider covering more NFFCCM services or other remote services of CCM in the future. Incorporating modern technology for more effective disease monitoring could be coupled with NFFCCM to provide significant clinical benefits.
Supplementary Material
Acknowledgments
The research described in this study was supported by a Patient-Centered Outcomes Research Institute® (PCORI) cooperative agreement (NEN-1508-32257) as part of Natural Experiments for Translation in Diabetes 2.0 (NEXT-D2) and was conducted in partnership with Research Action for Health Network (REACHnet, funded by PCORI RI-CRN-2020-008). Dr. Gang Hu was partly supported by grant from the National Institute of General Medical Sciences (U54GM104940) of the National Institutes of Health. REACHnet is a partner in PCORnet®, the National Patient-Centered Clinical Research Network, which was developed with funding from PCORI®. The content of this report is solely the responsibility of the author(s) and does not necessarily represent the views of other organizations participating in, collaborating with, providing data or funding REACHnet or PCORnet®, or of PCORI®. The LEAD Study would like to acknowledge the contributions of our partners. The success of this study depended on their ongoing support and expertise. These partners include Ochsner Health and the Ochsner Patient Research Advisory Board; Tulane Medical Center; University Medical Center New Orleans; Louisiana Public Health Institute, the REACHnet Coordinating Center, and their multi-stakeholder Diabetes Advisory Groups; Pennington Biomedical Research Center; Access Health Louisiana; EXCELth, Inc.; Blue Cross and Blue Shield of Louisiana; and our patient partners Patricia Dominick and Cathy Glover.
Funding source:
The research described in this study was supported by a Patient-Centered Outcomes Research Institute® (PCORI) cooperative agreement (NEN-1508-32257) as part of Natural Experiments for Translation in Diabetes 2.0 (NEXT-D2) and was conducted in partnership with Research Action for Health Network (REACHnet, funded by PCORI RI-CRN-2020-008).
Role of the Funder/Sponsor:
The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest disclosure:
Dr Fonseca reports receiving Research Support (to Tulane): Grants from Fractyl, Jaguar Gene Therapy, honoraria for Consulting and Lectures: Takeda, Novo Nordisk, Sanofi-Aventis, Bayer Abbott, Astra-Zeneca, Intarcia, Asahi; stock options: Mellitus Health, BRAVO4Health; Stock: Amgen. Dr Nauman reports grants from Patient Centered Outcomes Research Institute, during the conduct of the study; grants from Patient Centered Outcomes Research Institute, outside the submitted work. Dr Shi is an editor for Value in Health and had no role in the peer-review process of this article. No other disclosures were reported.
References
- 1.Forouhi NG, Wareham NJ. Epidemiology of diabetes. Medicine (Abingdon). 2014;42(12):698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC. National diabetes statistics report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services. 2020. [Google Scholar]
- 3.American Diabetes A. Standards of medical care in diabetes--2014. Diabetes Care. 2014;37 Suppl 1:S14–80. [DOI] [PubMed] [Google Scholar]
- 4.Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Archives of internal medicine. 2002;162(20):2269–2276. [DOI] [PubMed] [Google Scholar]
- 5.Anderson GF. Medicare and chronic conditions. New England Journal of Medicine. 2005;353(3):305. [DOI] [PubMed] [Google Scholar]
- 6.Del Valle KL, McDonnell ME. Chronic Care Management Services for Complex Diabetes Management: a Practical Overview. Curr Diab Rep. 2018;18(12):135. [DOI] [PubMed] [Google Scholar]
- 7.Lorig KR, Sobel DS, Stewart AL, et al. Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: a randomized trial. Med Care. 1999;37(1):5–14. [DOI] [PubMed] [Google Scholar]
- 8.Peikes D, Chen A, Schore J, Brown R. Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries: 15 randomized trials. Jama. 2009;301(6):603–618. [DOI] [PubMed] [Google Scholar]
- 9.Strunk BC, Cunningham PJ. Treading water: Americans' access to needed medical care, 1997-2001. Track Rep. 2002(1):1–6. [PubMed] [Google Scholar]
- 10.CMS. Chronic Care Management Services. The Centers for Medicare & Medicaid Services (CMS). The Centers for Medicare & Medicaid Services (CMS) Web site. https://www.cms.gov/outreach-and-education/medicare-learning-network-mln/mlnproducts/downloads/chroniccaremanagement.pdf. Published 2019. Accessed December 20th, 2020. [Google Scholar]
- 11.CMS. CY 2015 PFS Final Rule. . Centers for Medicare&Medicaid Services. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices-Items/CMS-1612-FC.html?DLPage=1&DLSort=2&DLSortDir=descending. . Published 2015. Accessed December 15, 2021. [Google Scholar]
- 12.Schurrer J, O’Malley A, Wilson C, McCall N, Jain N. Evaluation of the diffusion and impact of the Chronic Care Management (CCM) services. Mathematica Policy Research;2017. [Google Scholar]
- 13.Basu S, Phillips RS, Bitton A, Song Z, Landon BE. Medicare Chronic Care Management Payments and Financial Returns to Primary Care Practices: A Modeling Study. Ann Intern Med. 2015;163(8):580–588. [DOI] [PubMed] [Google Scholar]
- 14.Bazzano AN, Wharton MK, Monnette A, et al. Barriers and Facilitators in Implementing Non-Face-to-Face Chronic Care Management in an Elderly Population with Diabetes: A Qualitative Study of Physician and Health System Perspectives. J Clin Med. 2018;7(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wharton MK, Shi L, Eragoda S, et al. Qualitative Analysis of Health Systems Utilizing Non-Face-to-Face Chronic Care Management for Medicare-Insured Patients With Diabetes. J Ambul Care Manage. 2020;43(4):326–334. [DOI] [PubMed] [Google Scholar]
- 16.Yeager VA, Wharton MK, Monnette A, et al. Non-Face-to-Face Chronic Care Management: A Qualitative Study Assessing the Implementation of a New CMS Reimbursement Strategy. Popul Health Manag. 2018;21(6):454–461. [DOI] [PubMed] [Google Scholar]
- 17.Nichols GA, Desai J, Elston Lafata J, et al. Construction of a multisite DataLink using electronic health records for the identification, surveillance, prevention, and management of diabetes mellitus: the SUPREME-DM project. Prev Chronic Dis. 2012;9:E110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen Y, Shi L, Nauman E, et al. Inverse Association Between HDL (High-Density Lipoprotein) Cholesterol and Stroke Risk Among Patients With Type 2 Diabetes Mellitus. Stroke. 2019;50(2):291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CMS. Chronic Conditions Data Warehouse. Centers for Medicare & Medicaid Services (CMS). https://www2.ccwdata.org/web/guest. Accessed Feburary 17th, 2021. [Google Scholar]
- 20.Sweeten G. Group-Based Trajectory Models. In: Bruinsma G, Weisburd D, eds. Encyclopedia of Criminology and Criminal Justice. New York, NY: Springer New York; 2014:1991–2003. [Google Scholar]
- 21.Nagin DS, Jones BL, Passos VL, Tremblay RE. Group-based multi-trajectory modeling. Stat Methods Med Res. 2018;27(7):2015–2023. [DOI] [PubMed] [Google Scholar]
- 22.Haviland A, Nagin DS, Rosenbaum PR, Tremblay RE. Combining group-based trajectory modeling and propensity score matching for causal inferences in nonexperimental longitudinal data. Dev Psychol. 2008;44(2):422–436. [DOI] [PubMed] [Google Scholar]
- 23.Nagin DS. Group-based trajectory modeling: an overview. Ann Nutr Metab. 2014;65(2-3):205–210. [DOI] [PubMed] [Google Scholar]
- 24.Shi Q, Mendoza TR, Gunn GB, Wang XS, Rosenthal DI, Cleeland CS. Using group-based trajectory modeling to examine heterogeneity of symptom burden in patients with head and neck cancer undergoing aggressive non-surgical therapy. Qual Life Res. 2013;22(9):2331–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonnell ME. Telemedicine in Complex Diabetes Management. Curr Diab Rep. 2018;18(7):42. [DOI] [PubMed] [Google Scholar]
- 26.Piette JD, Weinberger M, McPhee SJ. The effect of automated calls with telephone nurse follow-up on patient-centered outcomes of diabetes care: a randomized, controlled trial. Med Care. 2000;38(2):218–230. [DOI] [PubMed] [Google Scholar]
- 27.Hostetter J, Schwarz N, Klug M, Wynne J, Basson MD. Primary care visits increase utilization of evidence-based preventative health measures. BMC Family Practice. 2020;21(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedberg MW, Hussey PS, Schneider EC. Primary care: a critical review of the evidence on quality and costs of health care. Health Aff (Millwood). 2010;29(5):766–772. [DOI] [PubMed] [Google Scholar]
- 29.Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83(3):457–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howell EA, Egorova N, Balbierz A, Zeitlin J, Hebert PL. Black-white differences in severe maternal morbidity and site of care. American Journal of Obstetrics & Gynecology. 2016;214(1):122.e121–122.e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown EJ, Polsky D, Barbu CM, Seymour JW, Grande D. Racial Disparities In Geographic Access To Primary Care In Philadelphia. Health Aff (Millwood). 2016;35(8):1374–1381. [DOI] [PubMed] [Google Scholar]
- 32.Gaskin DJ, Dinwiddie GY, Chan KS, McCleary RR. Residential Segregation and the Availability of Primary Care Physicians. Health Services Research. 2012;47(6):2353–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.