Abstract
Local and regional recurrences are common following an initial course of radiotherapy, yet management of these recurrences remains a challenge. Reirradiation may be an optimal treatment approach for providing durable tumor control and even offering select patients with locoregional recurrences or new primary tumors a chance of cure, but photon reirradiation can be associated with considerable risks of high grade acute and late toxicities. The high conformality and lack of exit dose with proton therapy offer significant advantages for reirradiation. By decreasing dose to adjacent normal tissues, proton therapy can more safely deliver definitive instead of palliative doses of reirradiation, more safely dose escalate reirradiation treatment, and more safely allow for concurrent systemic therapy in the reirradiation setting. In this case-based analysis, renowned experts in the fields of proton therapy and of reirradiation present cases for which they recently employed proton reirradiation. This manuscript focuses on case studies in patients with lung cancer, head and neck malignancies, and pelvic malignancies. Considerations for when to deliver proton therapy in the reirradiation setting and the pros and cons of proton therapy are discussed, and the existing literature supporting the use of proton reirradiation for these disease sites is assessed.
Introduction
Patients who develop local or regional recurrences or new primary malignancies within the field of a previous radiotherapy treatment course represent considerable management challenges for oncologists. Local therapy, either using surgery or reirradiation, can often provide the greatest chance of long-term disease control and even have the potential to cure select recurrent patients. However, surgery after prior radiation is often not safe or feasible, and photon-based reirradiation is often associated with considerable rates of morbidities, including a real risk of life-threatening complications.1 Given these risks, patients with locoregional recurrences are more typically treated with systemic therapy alone, either with chemotherapy or increasingly immunotherapy, but these treatment approaches generally do not provide patients with an opportunity for cure.
Proton therapy can allow for optimizing disease control rates, while improving the toxicity profile compared with photon therapy. The physical properties of proton therapy allow energy to be deposited at a specific depth, termed the Bragg peak, with a rapid falloff of energy beyond this point.2 This can allow fewer normal tissues adjacent to target structures to be exposed to unnecessary irradiation, especially tissues distal to the target volume.3 When proton therapy is delivered as an initial course of radiation therapy, these physical advantages can translate, for select patients, into significant clinical toxicity reductions and better preservation of quality of life for patients4–9 or in some cases improvements in overall survival and clinical outcomes10–14 compared to photon therapy.
With a second or even third course of radiation therapy, however, the physical advantages of proton therapy can translate into the ability to deliver a safer and more effective course of reirradiation, and thus offer patients a new chance of cure. Such physical and dosimetric advantages of proton therapy are particularly important in the setting of reirradiation and can allow radiation oncologists to: (1) more safely deliver definitive instead of palliative doses of reirradiation, (2) more safely dose escalate reirradiation treatment, and (3) more safely allow for concurrent systemic therapy in the reirradiation setting.15 The high conformality and lack of exit dose with proton therapy offer significant advantages for reirradiation, particularly when normal tissues adjacent to the target volume have already received high doses close to their tolerance during the prior course of radiotherapy.15–17
In this case-based analysis, leaders in proton therapy and in reirradiation present cases for which they recently delivered proton reirradiation. With a focus on lung cancer, head and neck malignancies, and pelvic malignancies, these experts discuss considerations for when to deliver proton therapy in the reirradiation setting, the pros and cons of the advanced modality, and existing literature supporting the use of proton therapy for reirradiation.
Lung Cancer—Expert #1—Emory University School of Medicine
Patient 1 is a 73-year-old male with a prior history of pancreatic adenocarcinoma resected 18 years ago without recurrence and treated 1 year ago with conventionally fractionated radiation therapy for a medically inoperable left lower lobe cT2aN0M0 stage IB lung adenocarcinoma. The radiation dose and volume for his prior nonsmall cell lung cancer (NSCLC) were 66 Gy in 33 daily fractions to the isolated lung cancer, without regional nodal irradiation (Fig. 1A).
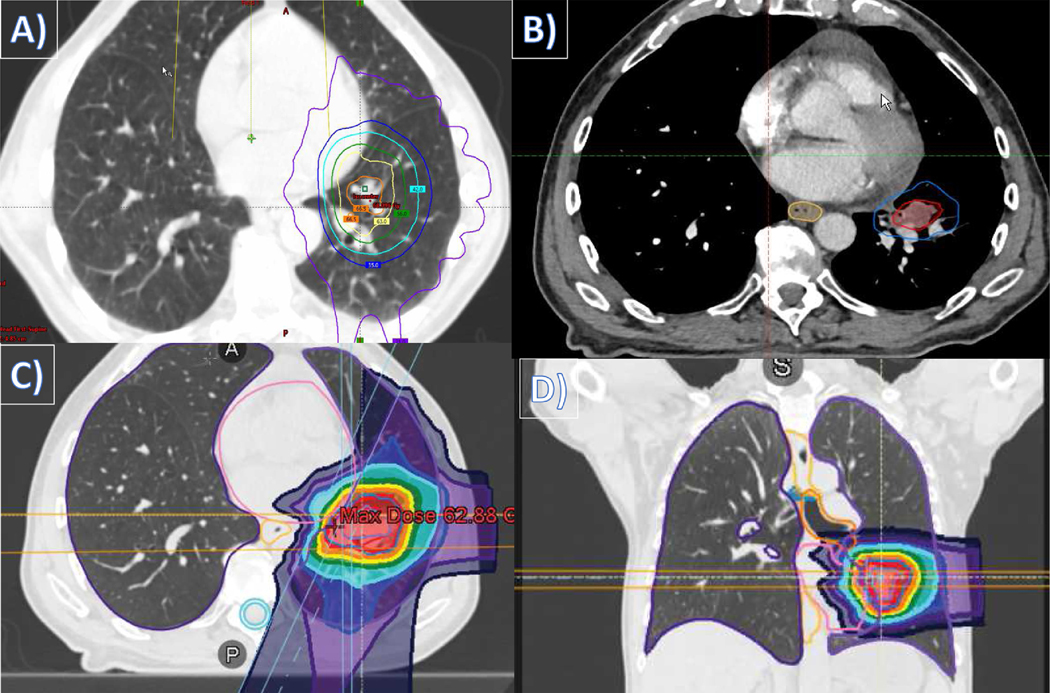
Figure 1.
Recurrent early stage nonsmall cell lung cancer. This patient had a medically inoperable left lower lobe cT2aN0M0 stage IB lung adenocarcinoma and was treated with conventionally fractionated photon therapy, and one year later he developed a left lung local recurrence adjacent to the left pulmonary vasculature treated with hypofractionated intensity-modulated proton therapy. Radiation treatment plans depicting: (A) axial image of the initial conventionally fractionated photon therapy course; (B) target volume of the recurrent left lower lung disease; (C) axial image of the proton reirradiation course; and (D) coronal image of the proton reirradiation course.
He then was found to have a left lung recurrence detected on follow-up CT imaging 12 months later. This patient was further staged with a brain MRI and a PET/CT that showed no other evidence of regional or distant disease. He was evaluated by an experienced thoracic surgeon who did not consider him operable, nor did the patient agree to consider surgery. As a result, he underwent CT simulation with contrast to plan definitive reirradiation, with the CT scan showing the lesion in the left lower lobe adjacent to the left pulmonary vasculature (Fig. 1B). The patient was treated to the left lower lobe recurrent disease to 60 CGE in 15 daily fractions using intensity-modulated proton therapy (IMPT) (Fig. 1C-D). During simulation the patient could hold his breath for >30 seconds at a time, so we treated him using a breath-hold technique to diminish the effects of tumor motion on pencil beam delivery.
This case was chosen to illustrate a few important teaching points: (a) to discuss optimal management of his initial NSCLC a year earlier, (b) to discuss options for thoracic reirradiation, and (c) to address some important considerations about using proton beam in this setting.
With regards to the initial treatment for his Stage IB NSCLC 1 year earlier, there are now randomized phase III data showing that stereotactic body radiation therapy (SBRT) more than doubles the local control rates compared to conventionally fractionated radiation therapy.18 Local failure rates in the TROG 09.02 CHISEL trial with SBRT were 14% compared to 31% (Log-rank HR 0.32; P = 0.0077). Most US clinicians stopped using conventional fractionation in this situation over a decade ago. There is no reason for any patient in the United States with inoperable stage I NSCLC to be treated in this fashion, and the Advanced Payment Model proposed by The Centers for Medicare & Medicaid Services would likely encourage clinicians to hypofractionate in situations where there are convincing data to do so.
There are a few choices available to patients with isolated recurrences following prior radiation therapy for lung cancer. We considered 3 different options for this patient: (1) fractionated proton beam therapy, (2) photon-based SBRT, and (3) fractionated photon treatment. There is no clear answer as to which option is best to use here. The pertinent organs-at-risk in this patient are the heart, left lung, and the pulmonary vasculature. Luckily, the esophagus is away from the target volume and can be well avoided with any of these options.
We chose proton beam therapy because it better avoids the adjacent heart compared with photon therapy.19 Additionally, considering the relative biological effectiveness advantage of proton therapy, protons could provide a ≥10% advantage in local control compared to photons without increasing toxicity risk. Prospective data have emerged showing an advantage for protons in the reirradiation situation, with some severe but fairly low incidence of toxicities.20,21
With respect to considerations for proton therapy, while there are advantages of better OAR sparing compared to photon therapy,19 tumor motion can be a more significant concern with proton therapy.22 This is especially true for spot scanning proton delivery, where larger tumor motion can lead to greater proton dose degradation.23 Spot scanning for moving targets has real risks for undertreating tumor or over-treating adjacent organs at risk, and of having daily variations in dose delivery via the interplay effect.23,24 Ideally, target motion would be minimal or mitigated effectively per Particle Therapy Co-operative Group Thoracic and Lymphoma Subcommittee Consensus Guidelines so that these risks are minimized.25 For this patient, on free breathing, his tumor had hysteresis based on both heart and lung motion, with a maximum of 1 cm of movement in any direction. He was able to tolerate breath-hold technique without difficulty, so we used that motion mitigation method that helped to facilitate proton-based reirradiation, which has promising early efficacy and toxicity reirradiation results.
Lung Cancer—Expert #2—The University of Texas MD Anderson Cancer Center
Patient 2 is a 74-year-old female who originally presented in 2016 with stage IV adenocarcinoma of the lung without targeted gene mutation who was initially treated with 4 cycles of carboplatin and pemetrexed chemotherapy with well controlled systemic disease but residual thoracic disease. She was then treated with consolidative radiotherapy to the residual bilateral hilar, mediastinal, and supraclavicular lymphadenopathy to 45 Gy in 15 daily fractions using intensity-modulated radiation therapy (IMRT) (Fig. 2A), followed by maintenance pemetrexed.
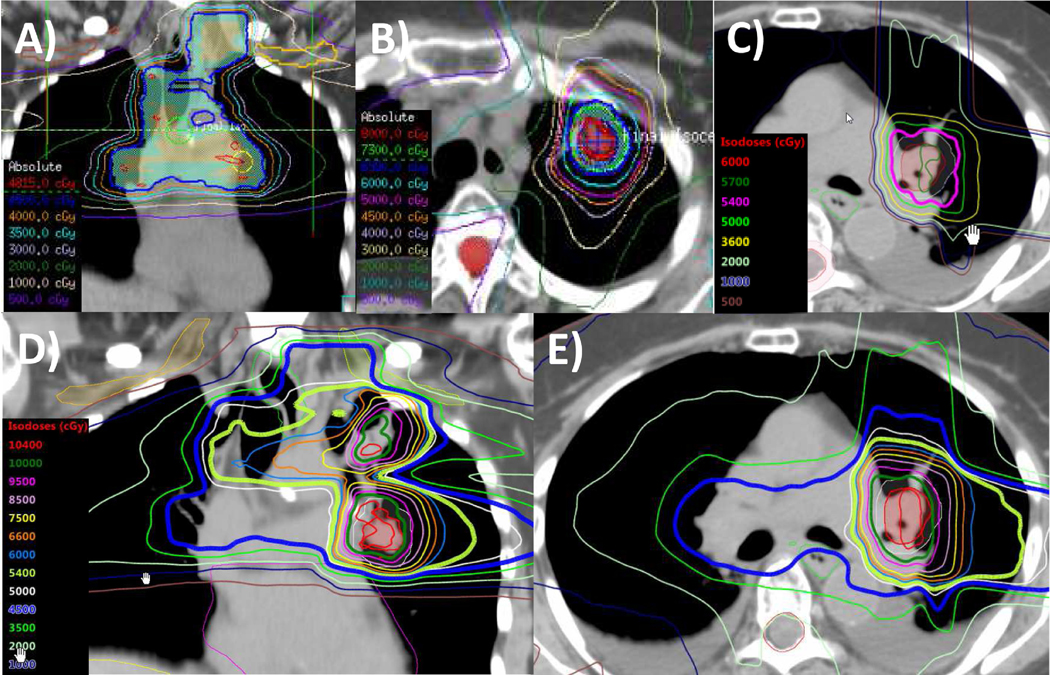
Figure 2.
Multiple thoracic irradiation courses in stage IV nonsmall cell lung cancer. This patient had a stage IV lung adenocarcinoma and underwent systemic therapy followed by consolidative thoracic intensity-modulated radiation therapy and consolidative chemotherapy. She progressed distantly the next year and was treated with palliative irradiation and immunotherapy, followed a year later by a left upper lobe lung recurrence treated with hypofractionated intensity-modulated radiation therapy. One year later, she developed a left intrabronchial recurrence and was treated with proton therapy and concurrent immunotherapy. Radiation treatment plans depicting: (A) a coronal image of the IMRT plan to 45 Gy delivered in 2016; (B) axial image of the IMRT plan to 70 Gy delivered in 2018; (C) axial image of the proton plan to 54 CGE delivered in 2019; (D) coronal image of the composite plan for all 3 courses of radiation therapy; and (E) axial image of the composite plan for all 3 courses of radiation therapy.
The next year, she developed symptomatic progression in the left humerus. Biopsy revealed recurrent adenocarcinoma with PD-L1 expression of 75%. She was treated with palliative humeral photon radiotherapy and then started pembrolizumab. Her disease remained controlled until 2018, when she developed a parenchymal recurrence in the left upper lobe. She continued receiving immunotherapy and was treated with IMRT to 70 Gy in 10 daily fractions (Fig. 2B). Then, in 2019, she developed an isolated left intrabronchial recurrence. She was treated with proton therapy to 54 CGE in 30 daily fractions with concurrent immunotherapy (Fig. 2C-E). She achieved complete clinical response with no high grade acute or, to date, late toxicities.
There is a strong rationale for26,27 and dosimetric data supporting the use of28 proton therapy for thoracic reirradiation to reduce doses relative to photon therapy to critical thoracic organs at risk that may have already received high doses with the first course of radiation therapy. Our group has shown that proton therapy29 and even IMPT30 is a safe and feasible approach for reirradiation of locoregionally recurrent NSCLC. We showed that concurrent chemotherapy given with reirradiation could increase local control, reduce distant metastasis free survival, and improve overall survival at the expensive of increased esophagitis.31
As NSCLC patients are achieving better systemic control with improvements in chemotherapy, the increasing use and multiple lines of targeted therapy, and the proliferation and success of immunotherapy, more patients can benefit from local radiotherapy, sometimes even multiple local courses, and even in patients with stage IV disease. The efficacy associated with reirradiation can play a crucial role in survival outcomes for patients, and protons can be an optimal treatment approach for minimizing side effects and preserving long-term quality of life.32
Head and Neck Cancer—Expert #1—New York Proton Center and Memorial Sloan Kettering Cancer Center
Patient 3 is a 72-year-old former smoker (20 pack-years) who initially presented with a locally advanced squamous cell carcinoma of the right retromolar trigone and underwent marginal resection of the right posterior mandible, right modified neck dissection of levels 2, 3, and 4, and reconstruction with bone plate placement and right buccal mucosa advancement flap. Pathology revealed pT4N0M0 stage IVA disease. She then underwent postoperative treatment with IMRT to 60 Gy in 2 Gy daily fractions concurrently with cetuximab completed in May 2006.
She had no evidence of disease until late 2017 (11 years later), when she presented with progressive dysphagia. Workup revealed unresectable cT4bN0M0 stage IVB squamous cell carcinoma of the right base of tongue that was p16 negative, encased the right external carotid artery, involved the sublingual, parapharyngeal and masticator spaces, and had probable involvement of the hemimandible and hyoid bone (Fig. 3). Given the extent of disease, the multidisciplinary consensus was for induction systemic therapy followed by re-evaluation for resectability. A percutaneous endoscopic gastrostomy tube was placed in the interim for failure to thrive secondary to dysphagia. She proceeded with the EXTREME regimen with 4 cycles of cetuximab, cisplatin and 5-fluorouracil (5-FU), with subsequent partial response of the tumor and improvement in her pain. Upon re-evaluation by the head and neck surgeon, it was determined that the disease was now borderline resectable, as there was still concern for carotid artery encasement.
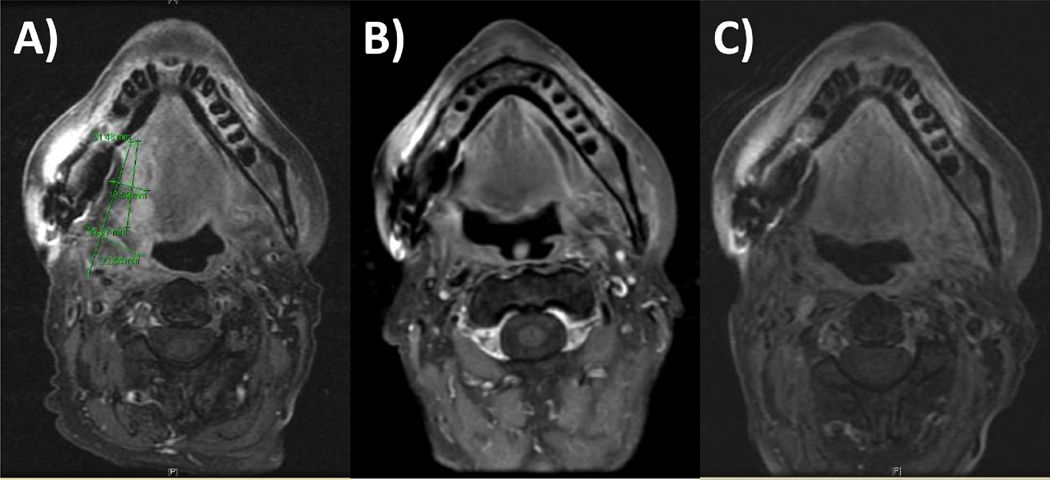
Figure 3.
Metachronous head and neck cancer. This patient had a prior pT4N0Mo stage IVA squamous cell carcinoma of the right retromolar trigone and underwent marginal resection of the right posterior mandible and adjuvant intensity-modulated radiation therapy with concurrent cetuximab, and then they presented with a new cT4bN0M0 stage IVB squamous cell carcinoma of the right base of tongue 11 years later treated with induction chemotherapy followed by concurrent chemoradiation delivering with intensity-modulated proton therapy. MRI axial images showing: (A) preinduction disease extent; (B) postinduction disease extent; and (C) surveillance imaging 4 months following the completion of proton reirradiation.
Given the potential significant morbidity of a radical surgical intervention, the patient decided to proceed with reirradiation and was enrolled in a single-institutional phase II proton reirradiation trial looking at locoregional recurrence free rates, toxicities, and patient-reported outcomes following definitive conventionally fractionated or short-course hypofractionated reirradiation. She was treated to the gross disease at the right base of tongue and right oral tongue with IMPT to 70 CGE in 2 CGE daily fractions concurrently with weekly cisplatin, with completion of treatment in July 2018. She is now 18 months from her last treatment and remains disease-free per imaging and serial flexible fiberoptic nasopharyngoscopy exams. She presently reports chronic grade 2 xerostomia and grade 2 trismus, and she continues use of the percutaneous endoscopic gastrostomy tube.
When patients present with recurrent or metachronous head and neck cancers in previously irradiated fields, we strongly consider resection when possible. Given this patient’s presentation with unresectable disease, after multiple discussions in our multidisciplinary tumor board, we felt that induction systemic therapy was the best approach to see if this tumor could be downstaged for reconsideration of resectability. In this case, however, consideration for surgery even after induction systemic therapy was fraught with likely serious morbidity; therefore, reirradiation was determined to be the best local treatment option for this patient.
In the reirradiation setting, proton therapy is almost always considered at our institution because of the physical advantage of the rapid dose fall-off beyond the Bragg peak with protons, and especially with IMPT, allowing for more conformal treatment and better-targeted dose coverage of the tumor compared with IMRT.33 Furthermore, meeting normal tissue constraints can be particularly difficult in the reirradiation setting and may necessitate reducing the target volume as was done in this situation (the neck was not treated electively), which allowed us to more safely dose escalate and treat gross disease to a definitive dose (70 CGE in this case).
Our overall head and neck reirradiation treatment paradigm is: (1) smaller volumes to minimize toxicities and allow dose escalation to known recurrent or new disease, (2) the addition of systemic therapy (if the patient is a candidate) in order to maximize the chance of cure, and (3) the use of proton therapy to decrease the risks of late sequelae from reirradiation. We also place any eligible patients on our phase II proton reirradiation protocol for recurrent head and neck cancer.
Head and Neck Cancer—Expert #2—The University of Texas MD Anderson Cancer Center
Patient 4 is a 49-year-old male who initially presented in January 2017 with cT4N0M0 stage IV nasopharyngeal carcinoma, with pathology demonstrating nonkeratinizing squamous carcinoma, EBV positive (WHO-3 disease). He was treated on protocol with concurrent chemoradiation therapy alone with IMRT and cisplatin to a dose of 70 Gy in 33 daily fractions with completion of treatment in April 2017.
In 2019, he developed recurrent tumor in the left-sided skull base centered on the sphenoid and left cavernous sinus. The recurrent tumor involved the left middle cranial fossa, the nasal cavity, and the middle meatus with blockage of the sinus drainage opening for the maxillary sinus and left ethmoids. The disease also involved the clivus, nasopharynx left petrous apex, and left-sided optic strut (Fig. 4). Biopsy of the clivus was positive for undifferentiated carcinoma; however, the tumor was EBV negative. He underwent multidisciplinary evaluation and was recommended to receive concurrent chemoradiation. He was treated with cisplatin and IMPT to the local recurrence to a dose of 66 CGE in 2 CGE daily fractions.
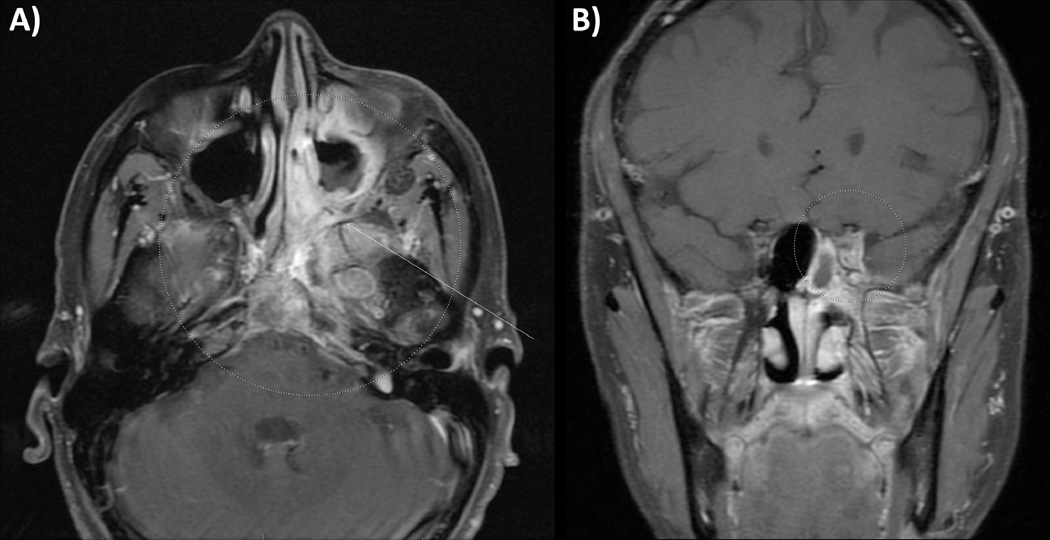
Figure 4.
Recurrent nasopharynx cancer. This patient had a cT4N0M0 stage IV nasopharyngeal carcinoma and received concurrent chemoradiation therapy alone with intensity-modulated radiation therapy, and 2 years later they developed recurrent tumor in the left-sided skull base centered on the sphenoid and left cavernous sinus treated with concurrent chemoradiation delivered with intensity-modulated proton therapy. MRI images showing: (A) axial and (B) coronal disease extent at the time of recurrence diagnosis.
Proton therapy can be an optimal choice for nasopharyngeal.34 and sinonasal cancer treatment,35 and IMPT is the appropriate choice for this unresectable nasopharynx recurrence due to the biologic enhancement (relative biological effectiveness) and physics enhancement (linear energy transfer) of proton therapy over photon therapy.36 Proton therapy also often allows for significant reductions in critical normal structures at the skull base (eg, carotid arteries, temporal lobes, brainstem, optic nerves, optic chiasm, cochlea, cranial nerves IX-XII, pituitary gland, and pterygoid muscles),37 which is particularly important in the reirradiation setting.38 These critical structures already received their maximum doses with IMRT during the first course of treatment, and IMPT was the ideal treatment option for this complex recurrence at the skull base.
Pelvic Cancer (Rectal)—Expert #1—The University of Pennsylvania
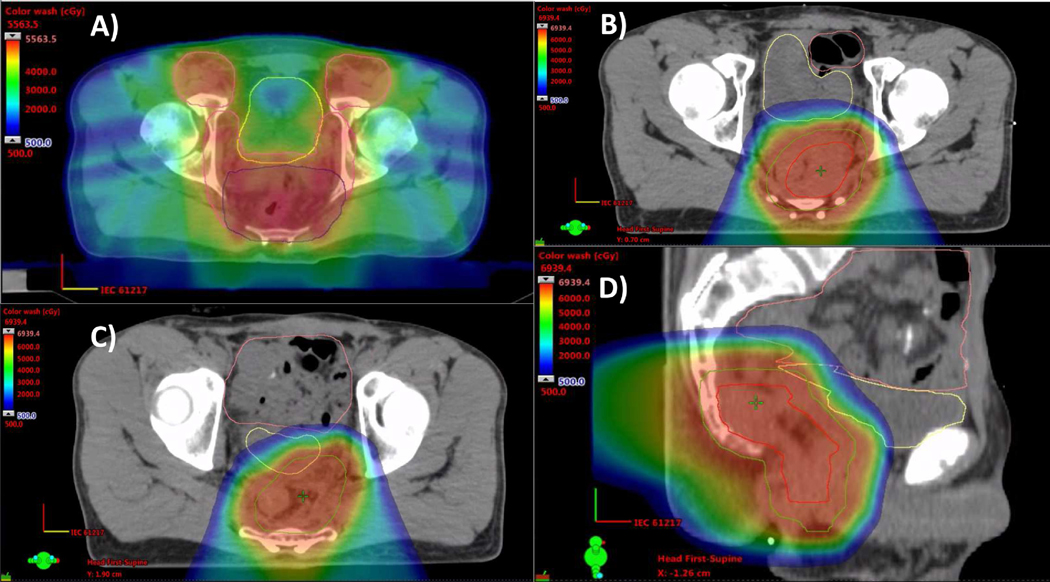
Patient 5 is a 48-year-old male who presented with a cT3N1 stage IIIB low rectal adenocarcinoma that invaded the sphincter. He received photon-based IMRT, including elective coverage of the inguinal nodes (Fig. 5A), with concurrent chemotherapy, followed by total mesorectal excision with abdominoperineal resection. Two years later, he developed an isolated local recurrence in the presacral region. He was considered inoperable due to the need for partial sacral resection. He instead was treated with systemic second-line chemotherapy, but this isolated local recurrence grew and became more symptomatic. Reirradiation to 63 CGE in 1.8 CGE daily fractions with proton therapy was chosen for this isolated, painful presacral recurrence to minimize additional irradiation dose to the bowel and bladder (Fig. 5B-D).
Figure 5.
Proton reirradiation for rectal cancer. This patient had a cT3N1M0 stage IIIB low rectal adenocarcinoma with sphincter invasion and was treated with concurrent chemoradiation with intensity-modulated radiation therapy followed by resection, then 2 years later had an isolated presacral recurrence treated with pencil beam scanning proton reirradiation. Radiation treatment plans depicting: (A) the original intensity-modulated radiation therapy plan for preoperative chemoradiation, including bilateral inguinal nodal regions; and (B-D) the reirradiation plan using pencil beam scanning proton therapy treating the isolated local recurrence using posterior oblique fields treating the FDG-PET avid gross tumor volume (red contour) with margin to create a clinical target volume (green contour), avoiding the anterior organs at risk, including the bladder (yellow contour) and bowel (orange contour).
When faced with a challenging case of local pelvic recurrence of rectal cancer, the risk of uncontrolled local progression and the potential for associated pain syndrome merits consideration of aggressive local treatment. The use of reirradiation for rectal cancer has been well-established historically with photon therapy. A series using twice-daily pelvic reirradiation found that outcomes were better if surgery was combined with reirradiation and if the interval between radiation courses was more prolonged (over 2 years).39 Overall survival at 3 years exceeded 50% in select patients treated with photon reirradiation; however, the toxicity in this series was notable, with 13 of 50 (26%) patients experiencing grade 3 or 4 late toxicities.
Proton therapy can help to reduce acute and late high-grade toxicities in the reirradiation setting for gastrointestinal malignancies.40 Our group has previously reported our prospective series of proton reirradiation for esophageal41 and pancreatic42 cancers. Proton therapy reirradiation has also been reported to be feasible and beneficial for anal and rectal recurrences.43
Specifically for rectal reirradiation, proton therapy is often better than photon therapy for typical recurrences that occur in the presacral or pelvic sidewall region. The lack of exit dose generally allows for better sparing of bladder and bowel without having to sacrifice the hip joints and pelvic bone marrow. One concern with proton reirradiation in the posterior pelvis is a potentially high skin dose that can result from using narrowly separated posterior oblique proton beams, particularly when proton therapy is delivered with passive scattering techniques. The overlap on skin can result in high surface doses and significant radiation dermatitis that can be more profound than with IMRT reirradiation. However, these high skin doses can be mitigated with the use of pencil beam scanning proton therapy and IMPT, although very posterior targets may still result in a high surface dose. In this patient’s case, he was able to take an extended flight shortly after completing reirradiation, as his dermatitis never exceeded Grade 2.
When considering reirradiation for rectal cancer when surgical resection is not possible, the use of high “tumoricidal” doses (such as 60–65 Gy) can be considered if other organs at risk can be spared with proton therapy. In general, there are often fixed loops of bowel that fall into the high-dose region. It is advisable to avoid allowing the high dose region to encompass the entire circumferential thickness of reirradiated bowel in order to minimize the risk of stricture. Other organs that can develop strictures from high cumulative radiation doses include the urethra, large bowel, vagina, and ureter. Close collaboration with surgical and interventional specialties is important to manage these patients, especially the judicious use of metal stents in reirradiated viscous organs that can develop erosions and perforations in poorly vascularized reirradiated tissues.42
One key concept to consider when reirradiating hollow viscous organs is whether the organ will be “used” by the patient. If a patient with rectal cancer has had a diversion or abdomino-perineal resection, then organ preservation is not an issue and more aggressive reirradiation doses may be considered. This is in contrast to esophageal cancer reirradiation,41 where there is usually an expectation that the organ will be used for swallowing, and stricture and/or dysmotility limiting quality of life is a more significant risk with high cumulative doses. If surgery is possible, then lower reirradiation doses may also be appropriate. In summary, proton therapy may be a useful tool when dealing with a recurrent rectal or anal cancer, but multidisciplinary care is required.
Pelvic Cancer (Endometrial)—Expert #2—Rutgers Cancer Institute of New Jersey
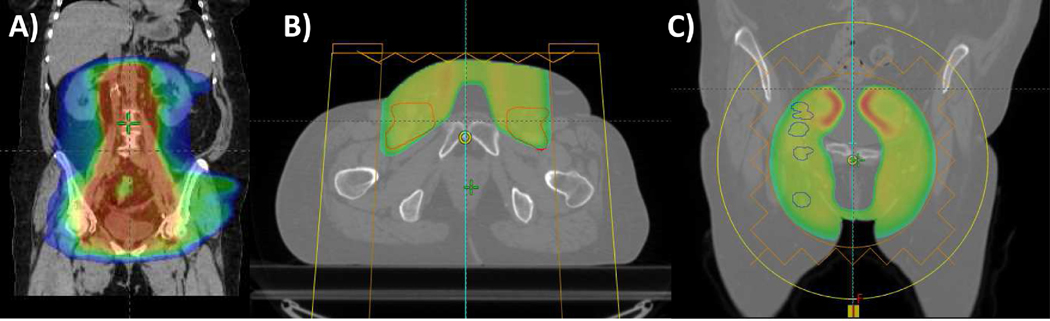
Patient 6 is a 60-year-old female who presented in 2014 with locally advanced endometrial cancer. She was treated with total abdominal hysterectomy and bilateral salpingo-oophorectomy, with pathology revealing 9 of 14 pelvic and paraaortic lymph nodes involved with disease. She then received 3 cycles of carboplatin and paclitaxel, followed by adjuvant extended-field radiation therapy encompassing the pelvis and para-aortic lymph nodes to 45 Gy in 25 daily fractions delivered with an IMRT plan (Fig. 6A), followed by 16.5 Gy via high dose-rate vaginal cuff brachytherapy, followed by 3 additional cycles of carboplatin and paclitaxel.
Figure 6.
Proton reirradiation for endometrial cancer. This patient had a locally advanced endometrial cancer and underwent total abdominal hysterectomy and bilateral salpingo-oophorectomy, followed by adjuvant chemotherapy and extended-field radiation therapy encompassing the pelvis and para-aortic lymph nodes delivered with intensity-modulated radiation therapy and high dose-rate vaginal cuff brachytherapy. Eighteen months later, she developed a right inguinal lymph node recurrence treated with stereotactic body radiation therapy, followed one year later by a right inguinal recurrence treated with immunotherapy and surgical resection, followed approximately 8 months later by diffuse pelvic lymph node recurrences treated with proton reirradiation. Radiation treatment plans depicting: (A) coronal image of the initial extended field IMRT plan; (B) axial image of inguinal lymph node proton irradiation plan; and (C) coronal image of the recurrent pelvic lymph node tumor volumes.
Restaging FDG-PET scan 18 months after the completion of therapy detected a right inguinal lymph node, and biopsy demonstrated recurrent endometrial cancer. She was treated with SBRT to 40 Gy in 5 fractions. One year later, by late 2017, she was found on surveillance imaging to have a recurrence in the right inguinal region, and she received phase I clinical trial therapy with an anti-PD-1 monoclonal antibody, followed by subsequent resection in early 2018 for poor response to checkpoint inhibition. By late 2018, she developed more diffuse pelvic lymph node recurrences, and definitive surgical resection was deemed not possible.
She was treated with passive scatter proton reirradiation, which allowed us to spare her femoral heads, bladder, rectum, and bowel, while treating her inguinal lymph node basins as well as the gross recurrences in the bilateral internal and external iliac nodes (Fig. 6B-C). The use of anterior beams to encompass these nodal volumes was well tolerated and resulted only in grade 1 skin and gastrointestinal toxicities, and it permitted the tolerance of concurrent radiosensitizing carboplatin to maximize the local effect of proton therapy. Given her intact bowel in her pelvis, we limited the dose to the pelvis to 36 CGE in 1.8 CGE per fraction.
While dosimetric studies have consistently shown significantly decreased doses to the bladder, bowel, rectum, kidneys, femoral heads, and bone marrow with proton therapy compared with photon therapy, the clinical data using proton therapy in treating gynecologic cancers are limited to date, with most prior publications reporting on the use of proton therapy for the treatment of para-aortic lymph nodes, as an alternative to brachytherapy, or for reirradiation.44
Among gynecologic malignancies, proton therapy might be most advantageous for recurrent disease in patients who have received prior pelvic radiotherapy. The concern for a long-term bowel complication including perforation, fistula, stricture, or adhesions is not trivial with reirradiation near luminal structures given the low recovery of bowel after multiple courses of radiation therapy. In the reirradiation setting, our goal is to allow limited volumes of small bowel to receive a maximum of 60 Gy, and limited volumes of large bowel to receive a maximum of 65–70 Gy. Proton therapy can minimize these maximum doses and also even more significantly limit the overall volumes of small and large bowel receiving reirradiation. Proton therapy can also help to minimize the risk of bladder damage, lymphedema, and marrow toxicity in the reirradiation setting.
Conclusions
The decision to reirradiate patients with locoregional recurrences or new primary malignancies within a previously irradiated field is nuanced and must be individualized. In select patients, proton therapy is often the best modality for delivering reirradiation. Proton therapy can allow for safer delivering of definitive reirradiation doses. Pencil beam scanning proton therapy and IMPT offer increased dose conformality and even further advantages over photon therapy in the setting of reirradiation. Furthermore, with the increasing availability of IMPT and of volumetric image-guidance with proton therapy,45 reirradiation dose escalation is now more feasible. Proton therapy has an increasing role in reirradiation for thoracic malignancies, head and neck cancers, and rectal malignancies, among other cancers, and increasing data support the use of proton reirradiation for these malignancies.
Footnotes
Conflict of Interest: None.
References
- 1.De Crevoisier R, Bourhis J, Domenge C, et al. : Full-dose reirradiation for unresectable head and neck carcinoma: Experience at the Gustave-Roussy Institute in a series of 169 patients. J Clin Oncol 16:3556–3562, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Suit H, Goldberg S, Niemierko A, et al. : Proton beams to replace photon beams in radical dose treatments. Acta Oncol 42:800–808, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Simone CB 2nd, Kramer K, O’Meara WP, et al. : Predicted rates of secondary malignancies from proton versus photon radiation therapy for stage I seminoma. Int J Radiat Oncol Biol Phys 82:242–249, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumann BC, Mitra N, Harton JG, et al. : Comparative effectiveness of proton vs photon therapy as part of concurrent chemoradiotherapy for locally advanced cancer. JAMA Oncol 2019. Dec 26. Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanchard P, Garden AS, Gunn GB, et al. : Intensity-modulated proton beam therapy (IMPT) versus intensity-modulated photon therapy (IMRT) for patients with oropharynx cancer—A case matched analysis. Radiother Oncol 120:48–55, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin SH, Hobbs B, Thall P, et al. : Results of a phase II randomized trial of proton beam therapy vs intensity modulated radiation therapy in esophageal cancer. Int J Radiat Oncol Biol Phys 105:S680–S681, 2019 [Google Scholar]
- 7.Sanford NN, Pursley J, Noe B, et al. : Protons versus photons for unresectable hepatocellular carcinoma: Liver decompensation and overall survival. Int J Radiat Oncol Biol Phys 105:64–72, 2019 [DOI] [PubMed] [Google Scholar]
- 8.Hoppe BS, Michalski JM, Mendenhall NP, et al. : Comparative effectiveness study of patient-reported outcomes after proton therapy or intensity-modulated radiotherapy for prostate cancer. Cancer 120:1076–1082, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahalley LS, Peterson R, Ris MD, et al. : Superior intellectual outcomes after proton radiotherapy compared with photon radiotherapy for pediatric medulloblastoma. J Clin Oncol 38:454–461, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel SH, Wang Z, Wong WW, et al. : Charged particle therapy versus photon therapy for paranasal sinus and nasal cavity malignant diseases: A systematic review and meta-analysis. Lancet Oncol 15:1027–1038, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Zhou J, Yang B, Wang X, et al. : Comparison of the effectiveness of radiotherapy with photons and particles for chordoma after surgery: A meta-analysis. World Neurosurg 117:46–53, 2018 [DOI] [PubMed] [Google Scholar]
- 12.Higgins KA, O’Connell K, Liu Y, et al. : National cancer database analysis of proton versus photon radiation therapy in non-small cell lung cancer. Int J Radiat Oncol Biol Phys 97:128–137, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Chi A, Chen H, Wen S, et al. : Comparison of particle beam therapy and stereotactic body radiotherapy (SBRT) for early stage non-small cell lung cancer: A systematic review and hypothesis-generating meta-analysis. Radiother Oncol 123:346–354, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasan S, Abel S, Verma V, et al. : Proton beam therapy versus stereotactic body radiotherapy for hepatocellular carcinoma: Practice patterns, outcomes, and the effect of biologically effective dose escalation. J Gastrointest Oncol 10:999–1009, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verma V, Rwigema JM, Malyapa RS, et al. : Systematic assessment of clinical outcomes and toxicities of proton radiotherapy for reirradiation. Radiother Oncol 125:21–30, 2017 [DOI] [PubMed] [Google Scholar]
- 16.Combs SE, Debus J, Schulz-Ertner D: Radiotherapeutic alternatives for previously irradiated recurrent gliomas. BMC Cancer 7:167, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guttmann DM, Frick MA, Carmona R, et al. : A prospective study of proton reirradiation for recurrent and secondary soft tissue sarcoma. Radiother Oncol 124:271–276, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Ball D, Mai GT, Vinod S, et al. : TROG 09.02 CHISEL investigators. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): A phase 3, open-label, randomised controlled trial. Lancet Oncol 20:494–503, 2019 [DOI] [PubMed] [Google Scholar]
- 19.Giaddui T, Chen W, Yu J, et al. : Establishing the feasibility of the dosimetric compliance criteria of RTOG 1308: Phase III randomized trial comparing overall survival after photon versus proton radiochemotherapy for inoperable stage II-IIIB NSCLC. Radiat Oncol 11:66, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Badiyan SN, Rutenberg MS, Hoppe BS, et al. : Clinical outcomes of patients with recurrent lung cancer reirradiated with proton therapy on the proton collaborative group and University of Florida Proton Therapy Institute prospective registry studies. Pract Radiat Oncol 9:280–288, 2019 [DOI] [PubMed] [Google Scholar]
- 21.Chao HH, Berman AT, Simone CB 2nd, et al. : Multi-Institutional prospective study of reirradiation with proton beam radiotherapy for locoregionally recurrent non-small cell lung cancer. J Thorac Oncol 12:281–292, 2017 [DOI] [PubMed] [Google Scholar]
- 22.Chang JY, Jabbour SK, De Ruysscher D, et al. : International particle therapy co-operative group thoracic subcommittee. Consensus statement on proton therapy in early-stage and locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys 95:505–516, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang M, Huang S, Solberg TD, et al. : A study of the beam-specific inter-play effect in proton pencil beam scanning delivery in lung cancer. Acta Oncol 56:531–540, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Lin L, Kang M, Huang S, et al. : Beam-specific planning target volumes incorporating 4D CT for pencil beam scanning proton therapy of thoracic tumors. J Appl Clin Med Phys 16:5678, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang JY, Zhang X, Knopf A, et al. : Consensus guidelines for implementing pencil-beam scanning proton therapy for thoracic malignancies on behalf of the PTCOG thoracic and lymphoma subcommittee. Int J Radiat Oncol Biol Phys 99:41–50, 2017 [DOI] [PubMed] [Google Scholar]
- 26.Simone CB 2nd, Rengan R: The use of proton therapy in the treatment of lung cancers. Cancer J 20:427–432, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Vyfhuis MAL, Rice S, Remick J: Reirradiation for locoregionally recurrent non-small cell lung cancer. J Thorac Dis 10(Suppl 21):S2522–S2536, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Troost EGC, Wink KCJ, Roelofs E, et al. : Photons or protons for reirradiation in (non-)small cell lung cancer: Results of the multicentric ROCOCO in silico study. Br J Radiol 2019. Epub [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McAvoy SA, Ciura KT, Rineer JM, et al. : Feasibility of proton beam therapy for reirradiation of locoregionally recurrent non-small cell lung cancer. Radiother Oncol 109:38–44, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Ho JC, Nguyen QN, Li H, et al. : Reirradiation of thoracic cancers with intensity modulated proton therapy. Pract Radiat Oncol 8:58–65, 2018 [DOI] [PubMed] [Google Scholar]
- 31.McAvoy S, Ciura K, Wei C, et al. : Definitive reirradiation for locoregionally recurrent non-small cell lung cancer with proton beam therapy or intensity modulated radiation therapy: Predictors of high-grade toxicity and survival outcomes. Int J Radiat Oncol Biol Phys 90:819–827, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Verma V, Simone CB 2nd, Mishra MV: Quality of life and patient-reported outcomes following proton radiation therapy: A systematic review. J Natl Cancer Inst 110, 2018 [DOI] [PubMed] [Google Scholar]
- 33.Simone CB 2nd, Ly D, Dan TD, et al. : Comparison of intensity-modulated radiotherapy, adaptive radiotherapy, proton radiotherapy, and adaptive proton radiotherapy for treatment of locally advanced head and neck cancer. Radiother Oncol 101:376–382, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewis GD, Holliday EB, Kocak-Uzel E, et al. : Intensity-modulated proton therapy for nasopharyngeal carcinoma: Decreased radiation dose to normal structures and encouraging clinical outcomes. Head Neck 38(Suppl 1):E1886–E1895, 2016 [DOI] [PubMed] [Google Scholar]
- 35.Yu NY, Gamez ME, Hartsell WF, et al. : A multi-institutional experience of proton beam therapy for sinonasal tumors. Adv Radiat Oncol 4:689–698, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L, Wang X, Li Y, et al. : Human papillomavirus status and the relative biological effectiveness of proton radiotherapy in head and neck cancer cells. Head Neck 39:708–715, 2017 [DOI] [PubMed] [Google Scholar]
- 37.Blanchard P, Gunn GB, Lin A, et al. : Proton therapy for head and neck cancers. Semin Radiat Oncol 28:53–63, 2018 [DOI] [PubMed] [Google Scholar]
- 38.Phan J, Sio TT, Nguyen TP, et al. : Reirradiation of head and neck cancers with proton therapy: Outcomes and analyses. Int J Radiat Oncol Biol Phys 96:30–41, 2016 [DOI] [PubMed] [Google Scholar]
- 39.Das P, Delclos ME, Skibber JM, et al. : Hyperfractionated accelerated radiotherapy for rectal cancer in patients with prior pelvic irradiation. Int J Radiat Oncol Biol Phys 77:60–65, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Verma V, Lin SH, Simone CB 2nd, et al. : Clinical outcomes and toxicities of proton radiotherapy for gastrointestinal neoplasms: A systematic review. J Gastrointest Oncol 7:644–664, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernandes A, Berman AT, Mick R, et al. : A prospective study of proton beam reirradiation for esophageal cancer. Int J Radiat Oncol Biol Phys 95:483–487, 2016 [DOI] [PubMed] [Google Scholar]
- 42.Boimel PJ, Berman AT, Li J, et al. : Proton beam reirradiation for locally recurrent pancreatic adenocarcinoma. J Gastrointest Oncol 8:665–674, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moningi S, Ludmir EB, Polamraju P, et al. : Definitive hyperfractionated, accelerated proton reirradiation for patients with pelvic malignancies. Clin Transl Radiat Oncol 19:59–65, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verma V, Simone CB 2nd, Wahl AO, et al. : Proton radiotherapy for gynecologic neoplasms. Acta Oncol 55:1257–1265, 2016 [DOI] [PubMed] [Google Scholar]
- 45.Veiga C, Janssens G, Teng CL, et al. : First clinical investigation of cone beam computed tomography and deformable registration for adaptive proton therapy for lung cancer. Int J Radiat Oncol Biol Phys 95:549–559, 2016 [DOI] [PubMed] [Google Scholar]