Abstract
The capacity to form attaching and effacing (A/E) lesions on the surfaces of enterocytes is an important virulence trait of several enteric pathogens, including enteropathogenic Escherichia coli (EPEC) and Shiga-toxigenic E. coli (STEC). Formation of such lesions depends upon an interaction between a bacterial outer membrane protein (intimin) and a bacterially encoded receptor protein (Tir) which is exported from the bacterium and translocated into the host cell membrane. Intimin, Tir, and several other proteins necessary for generation of A/E lesions are encoded on a chromosomal pathogenicity island termed the locus for enterocyte effacement (LEE). Reports of sequence heterogeneity and antigenic variation in the region of intimin believed to be responsible for receptor binding raise the possibility that the receptor itself is also heterogeneous. We have examined this by cloning and sequencing tir genes from three different STEC strains belonging to serogroups O26, O111, and O157. The deduced amino acid sequences for the Tir homologues from these strains varied markedly, exhibiting only 65.4, 80.2, and 56.7% identity, respectively, to that recently reported for EPEC Tir. STEC Tir is also highly immunogenic in humans. Western blots of E. coli DH5α expressing the various STEC tir genes cloned in pBluescript [but not E. coli DH5α(pBluescript)] reacted strongly with convalescent sera from patients with hemolytic-uremic syndrome (HUS) caused by known LEE-positive STEC. Moreover, no reaction was seen when the various clone lysates were probed with serum from a patient with HUS caused by a LEE-negative STEC or with serum from a healthy individual. Covariation of exposed epitopes on both intimin and Tir may be a means whereby STEC avoid host immune responses without compromising adhesin-receptor interaction.
Since their initial recognition over 20 years ago (20), Shiga-toxigenic strains of Escherichia coli (STEC) have emerged as an important cause of serious human gastrointestinal disease, which may result in life-threatening complications such as hemolytic-uremic syndrome (HUS) (16). Food-borne outbreaks of STEC disease appear to be increasing and, when mass-produced and mass-distributed foods are involved, can affect large numbers of people. Development of therapeutic and preventative strategies to combat STEC disease requires a thorough understanding of the mechanisms by which STEC colonize the human intestinal tract and cause local and systemic pathology. While our knowledge remains incomplete, recent studies have improved our understanding of these processes (for recent reviews, see references 24 and 28).
It has been recognized for a number of years that STEC strains causing human disease may belong to a very broad range of O serogroups (16). However, many of the STEC strains found in the gastrointestinal tracts of domestic animals (the principal source of human infections) may be of low virulence for humans. Within the human disease-associated strains, those belonging to a limited range of serogroups (notably O157, O111, and O26) are responsible for the majority of serious infections (16, 24, 28). Previous studies have compared properties of STEC from human and animal sources in order to identify the traits which distinguish human-pathogenic strains from those of lesser clinical significance. The capacity to produce attaching and effacing (A/E) lesions on intestinal mucosa, production of a plasmid-encoded enterohemolysin, and production of Shiga toxin type 2 (Stx2) rather than Shiga toxin type 1 (Stx1) have all been associated preferentially with human STEC isolates or with more-severe cases (4, 6, 19, 25, 32). Compared with STEC isolates from cases of uncomplicated diarrhea or isolates from nonhuman sources (27), STEC associated with HUS cases also have an enhanced capacity to adhere to intestinal epithelial cells in vitro.
The capacity to produce A/E lesions was initially recognized in enteropathogenic E. coli (EPEC) strains, and recent studies have elucidated the molecular events involved in their generation, as reviewed by Donnenberg et al. (9). The mechanism whereby STEC strains generate A/E lesions is less well characterized but is essentially analogous to that for EPEC (24). All of the genes necessary for generation of A/E lesions in EPEC are located on a 35.5-kb pathogenicity island termed the locus for enterocyte effacement (LEE). LEE encodes proteins with a range of functions, including a type III secretion system, various secreted effector proteins and their chaperons, and the outer membrane protein intimin, which mediates intimate attachment to the enterocyte cell surface (9). Interestingly, Kenny et al. (17) have recently reported that the receptor for intimin is also encoded by LEE. This protein was previously referred to as Hp90 but has now been renamed Tir (for translocated intimin receptor). Tir is secreted from EPEC as a 78-kDa species, and efficient delivery into the host cell is dependent upon the type III secretion system and other LEE-encoded secreted proteins. Tyrosine phosphorylation of Tir after insertion into the epithelial cell membrane increases its apparent mass to 90 kDa, but this is not essential for intimin binding, at least in vitro (17). Moreover, it does not appear to occur in STEC-infected cultures (at least those belonging to serogroup O157) (14).
Interestingly, the sequence of the C-terminal portion of intimin is known to vary markedly between EPEC and STEC and between different STEC strains (5, 15, 22, 31, 33). This region includes the putative Tir-binding domain (11), and it is possible that such heterogeneity influences the interaction between the bacterium and the enterocyte. It may also be antigenically significant, as we have recently shown that sera from several HUS patients infected with a O111:H− STEC reacted with intimin from an enteropathogenic E. coli O111 strain, as well as several other eaeA-positive STEC isolates, but not with an eaeA-positive STEC belonging to serotype O157:H−. The latter strain did, however, react with serum from a patient infected with both O111:H− and O157:H− STEC (31). Variation of exposed intimin epitopes may be a means whereby STEC avoid host immune responses, but significant changes could be potentially deleterious unless compatible variation also occurred in Tir. In the present study, we have cloned and sequenced the tir genes from STEC belonging to diverse serogroups to determine the degree of heterogeneity of this protein. We have also examined the reactivity of Tir-producing E. coli clones with convalescent sera from patients with HUS.
Bacterial strains and cloning vectors.
STEC strains 95NR1 (O111:H−), 95SF2 (O157:H−), and 95ZG1 (O26:H−) were isolated at the Women’s and Children’s Hospital (WCH), North Adelaide, South Australia, Australia, as previously described (26). The O111 EPEC strain 87A was also isolated at WCH and has been described previously (27). E. coli K-12 strain DH5α was obtained from Gibco-BRL, Gaithersburg, Md., and the phagemid pBluescript SK was obtained from Stratagene, La Jolla, Calif. All E. coli strains were routinely grown in Luria-Bertani (LB) medium (23) with or without 1.5% Bacto Agar (Difco Laboratories, Detroit, Mich.). Where appropriate, ampicillin was added to growth medium at a concentration of 50 μg/ml.
Cloning and sequence analysis of tir genes.
We have previously described the sequence of a portion of the LEE locus from STEC strain 95NR1, which contained orfU and eaeA (31). Comparison of this sequence with that of EPEC tir (17) indicated that it also included the 3′ portion of the STEC tir homologue. In order to isolate the remainder of the tir gene, we performed inverse PCR amplification on 95NR1 DNA which had been digested with BglII, recircularized, and ligated. The primers were 5′-CGTTAAGAATTCAGAGAACAACGTTGCAGC-3′ and 5′-CTGGGAATTCCCCATTAACCTTCCGGTAAC-3′, and amplification was performed with the Expand High Fidelity PCR System (Boehringer GmbH, Mannheim, Germany). This generated a 3.0-kb fragment which overlapped our previous sequence and yielded an additional 1,645 bp of 5′-flanking DNA. The purified PCR product was digested with EcoRI (the PCR primers contained EcoRI sites), cloned into pBluescript SK, and transformed into E. coli DH5α. The sequences of both strands of the STEC DNA insert, as well as nested deletions thereof (constructed by the method of Henikoff [12]), were then determined by using dye terminator chemistry on an Applied Biosystems model 373A automated DNA sequencer. The sequence was analyzed using DNASIS and PROSIS version 7.0 software (Hitachi Software Engineering, San Bruno, Calif.). Comparison of this sequence with sequence databases with the program BLASTX (2) confirmed that the inverse PCR product contained a region of STEC 95NR1 LEE encoding a homologue of an EPEC LEE open reading frame (orf19) with as yet unknown function (10) and the first 503 amino acids of the Tir homologue.
Two additional primers (5′-AATTGTGAATTCATATTGTAGTCCTGTCATTC-3′ and 5′-TTACAGGAATTCAAGAGTTACCCATGCTGC-3′) were then used for direct PCR amplification of approximately 3.1-kb fragments containing the complete orf19 and tir homologues from 95NR1, as well as from the STEC strains 95ZG1 (O26) and 95SF2 (O157:H−) and from the O111 EPEC strain 87A. PCR products from these four strains were cloned into pBluescript (recombinant plasmids were designated pJCP580, pJCP581, pJCP582, and pJCP583, respectively) and sequenced. Each of the cloned DNA inserts contained orf19 genes encoding 203-amino-acid polypeptides, immediately preceded by ribosome binding sites, and the degree of deduced amino acid sequence identity between these and the recently published sequence for Orf19 from EPEC strain E2348/69 (10) is shown in Table 1. The Orf19 homologue from 95NR1 was the most closely related to the published EPEC sequence (89.2% identity), while the least related was that from 95SF2 (74.4% identity).
TABLE 1.
Deduced amino acid sequence identity among Orf19 homologues
| Orf19 source | % Amino acid identitya
|
|||
|---|---|---|---|---|
| 95SF2 | 95ZG1 | EPEC 87A | EPEC E2348/69 | |
| 95NR1 | 75.9 | 81.3 | 80.3 | 89.2 |
| 95SF2 | 80.8 | 79.8 | 74.4 | |
| 95ZG1 | 98.0 | 79.3 | ||
| EPEC 87A | 78.3 | |||
Percentage of identical amino acids between the indicated pairs of 203-amino-acid Orf19 homologues from E. coli strains. Identity was determined with FASTA as implemented in PROSIS.
Interestingly, a much greater degree of deduced amino acid sequence divergence was observed among the Tir homologues, which also varied in length from 538 amino acids for Tir from strain 95ZG1 and EPEC 87A to 558 amino acids for 95SF2. The percent amino acid identity between the various Tir-related proteins is shown in Table 2. The greatest divergence was observed between two recently published (but slightly different) sequences for Tir from EPEC strain E2348/69 (10, 17) and the homologue from 95SF2 (56.7 to 58.1% identity). The difference in the two published EPEC Tir sequences is largely attributable to a frameshift affecting 19 residues in the C-terminal portion. Interestingly, the EPEC Tir sequence published by Elliott et al. (10) is identical to that for Tir from strain 95NR1 at 17 of 19 residues in this region. Tir from 95NR1 was the most closely related to the published EPEC sequences (80.2 to 83.3% identity). Tir from 95NR1 exhibited only 59.0 to 64.1% identity to Tir from the other two STEC strains. Remarkably, however, there was 97.0% identity between Tir from STEC 95ZG1 and our EPEC strain 87A. The alignment of various Tir-related proteins (constructed with the program CLUSTAL [13]) is shown in Fig. 1. Extensive heterogeneity is observed in both the N-terminal portion, which is believed to be exposed on the external surface of the epithelial cell and to interact with intimin, and in the C-terminal portion, which is believed to penetrate into the host cell cytoplasm (17). For example, Tir from strains 95NR1 and 95SF2 exhibit 61.6% identity for the 230-amino-acid N-terminal portion and 42.9% identity for the 160-amino-acid C-terminal portion (residues 391 to 551). The central portion of Tir (amino acids 231 to 390), which contains two putative membrane-spanning domains (17), is more conserved and exhibits 73.1% identity between the two STEC strains. Interestingly, in spite of the low homology within the C-terminal portion, six Tyr residues, at least one of which is presumed to be a substrate for phosphorylation by a host tyrosine kinase in EPEC (17), are conserved in all the Tir homologues except for the residue at position 478 in Tir from 95SF2 (Fig. 1).
TABLE 2.
Deduced amino acid sequence identity among Tir homologues
| Tir source | % Amino acid identitya
|
||||
|---|---|---|---|---|---|
| 95SF2 (558 aa) | 95ZG1 (538 aa) | EPEC 87A (538 aa) | E2348/69b
|
||
| AF013122 (549 aa) | AF022236 (550 aa) | ||||
| 95NR1 | 59.0 | 64.1 | 63.4 | 80.2 | 83.3 |
| 95SF2 | 65.5 | 64.8 | 56.7 | 58.1 | |
| 95ZG1 | 97.0 | 65.4 | 66.7 | ||
| EPEC 87A | 64.3 | 65.6 | |||
| E2348/69 AF013122 | 96.5 | ||||
Percentage of identical amino acids between the indicated pairs of Tir homologues from E. coli strains. Identity was determined with FASTA as implemented in PROSIS. The length of each protein (in amino acids [aa]) is also given in parentheses.
FIG. 1.
Alignment of the deduced amino acid sequences of Tir from STEC strains 95NR1 (O111:H−), 95SF2 (O157:H−), and 95ZG1 (O26) and from EPEC 87A (O111) with two published sequences for Tir from EPEC strain E2348/69 (GenBank accession nos. AF013122 and AF022236, respectively [10, 17]). Residues at a given position which are identical to that for 95NR1 Tir are boxed, while dashes indicate the absence of an amino acid. Conserved tyrosine residues in the C-terminal half of Tir are shaded, and the location of the Tyr residue predicted by the PROSITE algorithm (3) to be the site for tyrosine kinase phosphorylation is indicated with an asterisk.
Western blot analysis using HUS patient sera.
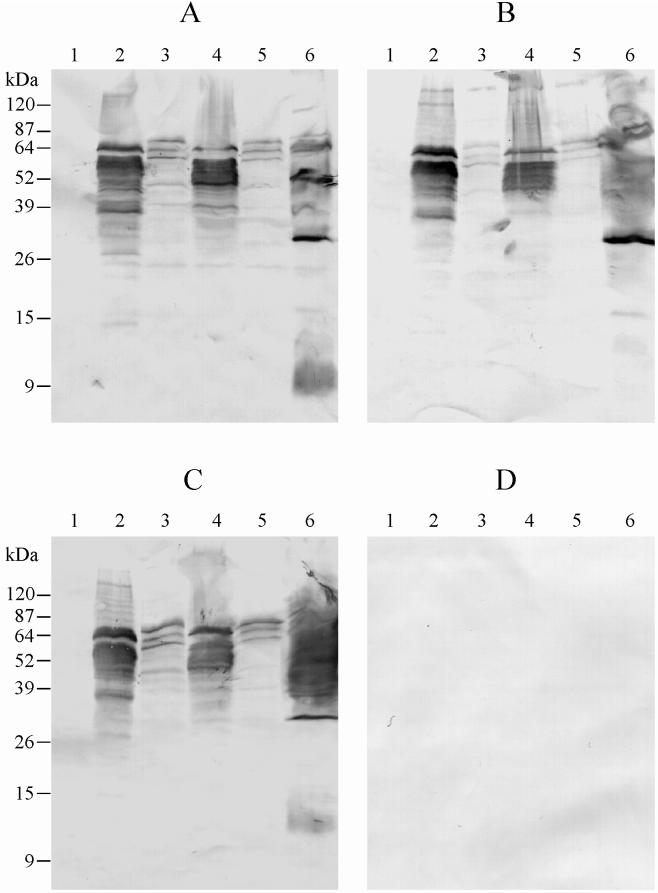
To examine the expression of cloned tir, lysates of E. coli DH5α carrying pBluescript, pJCP580, pJCP581, pJCP582, or pJCP583, or E. coli 95NR1 were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (21), and antigens were electrophoretically transferred to nitrocellulose filters (30). Filters were then probed with convalescent sera from various HUS patients (kindly provided by K. F. Jureidini, Renal Unit, WCH) or from a healthy donor (all sera were used at a dilution of 1:3,000), followed by goat anti-human immunoglobulin G conjugated to alkaline phosphatase. Immunoreactive bands were visualized using chromogenic substrate (4-nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate). The serum used in Fig. 2A was from a patient with HUS associated with an outbreak caused by dry-fermented sausage contaminated with a range of STEC including 95NR1 (26); this patient had an O111:H− STEC similar to 95NR1 isolated from feces. This serum labelled a number of protein species in the lysate of E. coli DH5α(pJCP580) (Fig. 2A, lane 2), the largest and one of the most prominent having an apparent mass of 64 kDa, which is slightly higher than that predicted for 95NR1 Tir by sequence analysis (57 kDa). It also appeared to comigrate with a weakly immunoreactive species in the lysate of the wild-type STEC 95NR1 (lane 6). Other prominently labelled protein species in the E. coli DH5α(pJCP580) lysate were in the range 38 to 60 kDa and presumably reflect degradation products of Tir, as the only other open reading frame in the portion of 95NR1 LEE cloned in pJCP580 encoded Orf19, which has a predicted mass of 22.5 kDa (a weak immunoreactive band is seen at this apparent mass). The HUS patient serum reacted with a very similar pattern of protein species in the lysate of E. coli DH5α(pJCP582) (lane 4) which produces Tir from the O157:H− STEC, in spite of the fact that there is only 59% amino acid identity between the two proteins. However, the labelling pattern in lysates of E. coli DH5α(pJCP581) and DH5α(pJCP583) (lanes 3 and 5), which produce Tir from the O26 STEC 95ZG1 and the O111 EPEC strain 87A, respectively, was much weaker; the principal immunoreactive species had apparent masses of 72, 64, and 60 kDa. The Tir from these two strains exhibit approximately 64% amino acid identity with 95NR1 Tir. Significantly, there were no detectable immunoreactive species in the lysate of E. coli DH5α(pBluescript) (lane 1). Two other convalescent sera from patients with HUS associated with the same outbreak were also tested. The serum used in Fig. 2C was from a patient who was also infected with an O111:H− STEC closely related to 95NR1, while that used in Fig. 2B yielded both O111:H− and O157:H− STEC isolates from feces. Both these sera exhibited labelling patterns with the various E. coli lysates similar to that seen in Fig. 2A. No immunoreactive species were detected in the various clone lysates when healthy donor serum was used (result not shown). Furthermore, when convalescent serum from a HUS patient who was infected with a LEE-negative STEC strain belonging to serotype OR:H9 was used to probe filters, there were also no immunoreactive species detected (Fig. 2D). When this serum was used to probe a lysate from the causative OR:H9 strain, very few protein bands were labelled (result not shown), but this was not unexpected, as we have recently demonstrated that the bulk of antibody responses of HUS patients with LEE-positive STEC disease appear to be directed at either lipopolysaccharide or LEE-encoded proteins (31). None of these antigens are present in the OR:H9 strain. There was also no evidence that the OR:H9-infected HUS patient had defective immune responses.
FIG. 2.
Western immunoblot analysis of Tir-expressing clones using convalescent sera from HUS patients. Lysates of E. coli DH5α carrying pBluescript (lane 1), pJCP580 (lane 2), pJCP581 (lane 3), pJCP582 (lane 4), or pJCP583 (lane 5) or STEC 95NR1 (lane 6) were separated by SDS-PAGE, electroblotted, and probed with convalescent sera from HUS patients infected with O111:H− STEC (A and C), both O111:H− and O157:H− STEC (B), or a LEE-negative OR:H9 STEC (D). The mobility of protein mass markers is indicated to the left.
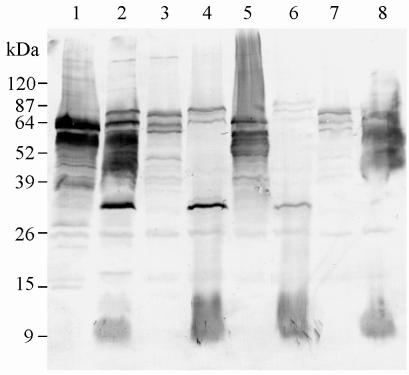
An additional Western blot was performed to examine whether the apparent size of immunoreactive tir gene products in the lysates of E. coli DH5α carrying pJCP580, pJCP581, pJCP582, or pJCP583 coincided with an immunoreactive species in the respective wild-type E. coli strain (Fig. 3). The lysates of E. coli DH5α(pJCP580) and strain 95NR1 (lanes 1 and 2) both contained major immunoreactive species with an apparent mass of 64 kDa; 95NR1 also contained a larger immunoreactive species (electrophoretic mobility suggested a mass of approximately 90 kDa), which could be intimin. The lysates of both 95ZG1 and EPEC 87A (lanes 4 and 8) also contained 64-kDa immunoreactive species comigrating with one of the major labelled proteins in E. coli DH5α(pJCP581) and DH5α(pJCP583), respectively (lanes 3 and 7). However, the lysate of 95SF2 (lane 6) reacted only weakly with the HUS patient serum.
FIG. 3.
Reactivity of HUS patient serum with STEC and EPEC strains and Tir-producing E. coli DH5α clones. The same serum used in Fig. 2A was used to probe Western blots of the indicated E. coli strains. Lanes: 1, E. coli DH5α(pJCP580); 2, STEC 95NR1; 3, E. coli DH5α(pJCP581); 4, STEC 95ZG1; 5, E. coli DH5α(pJCP582); 6, STEC 95SF2; 7, E. coli DH5α(pJCP583); 8, EPEC 87A. The mobility of protein mass markers is indicated to the left.
Conclusions.
The capacity to produce A/E lesions on enterocytes, mediated by the outer membrane protein intimin, is clearly a significant virulence trait of both EPEC and STEC (24). However, intimin is highly immunogenic and is exposed on the bacterial cell surface, and systemic or local immune responses to this protein may be capable of blocking the interaction between the bacterium and the intestinal epithelium. Antigenic variation, particularly in the exposed intimin domains, may therefore confer a significant selective advantage upon the bacterium, assuming such variation does not compromise receptor interactions. Amino acid sequence heterogeneity, particularly in the C-terminal portion of intimin which is responsible for receptor binding (11), is now well documented (5, 15, 22, 31, 33). Furthermore, Adu-Bobie et al. (1) have recently demonstrated the existence of at least five distinct classes of intimin in EPEC and STEC strains on the basis of reactivity with polyclonal antisera raised against the C-terminal portions of intimin from various strains. We have also provided direct evidence for antigenic diversity of intimin in STEC strains, as judged by reactivity of convalescent sera from HUS patients with various STEC strains (31). The fact that the intimin receptor Tir is encoded by the bacterium rather than the host (17) provides a mechanism whereby substantial intimin sequence (and hence antigenic) variation can be tolerated without compromising ligand-receptor interactions, assuming that compensatory changes also occur in the amino acid sequence of Tir.
In the present study, we have demonstrated that there is indeed marked heterogeneity in the amino acid sequence of Tir from diverse STEC and EPEC strains. The lowest overall amino acid identity between any given pair was only 56.7% between Tir from O157:H− STEC 95SF2 and EPEC 2348/69. Marked diversity occurs both in the N-terminal portion, which is thought to be responsible for intimin binding, as well as in the C-terminal portion, which is believed to penetrate the host cell cytoplasm (17). This latter region contains six Tyr residues, all of which are conserved in all the STEC and EPEC Tir proteins examined to date, with the exception of Tir from 95SF2, which has only five Tyr residues.
While this manuscript was being revised, Deibel et al. (8) reported the sequence of a secreted protein which they designated EspE, from an O26 STEC strain 413/89-1. The amino acid sequence of EspE is identical to that we report here for Tir from the O26 STEC strain 95ZG1. Interestingly, Deibel et al. demonstrated that unlike the situation for O157 STEC, EspE (Tir) from 413/89-1 is tyrosine phosphorylated after insertion into the membrane of infected cells. Thus, the absence of Tyr at position 478 of Tir from the O157 STEC strain 95SF2 observed in the present study provides an explanation for the lack of tyrosine phosphorylation of Tir by cells infected with this STEC serogroup. Indeed, of the six Tyr-containing domains in the other Tir sequences, the one in the vicinity of position 478 is the only one predicted by the PROSITE algorithm as a potential tyrosine kinase phosphorylation site (3, 8). The C-terminal portion of Tir is also presumably involved in other functions, such as localization of actin in the host cell cytoplasm immediately beneath the adherent bacteria (29) and transmission of additional signals to host cells once the Tir-intimin interaction occurs (17, 18). To date, the impact of Tir heterogeneity on these functions is unknown.
In this study, we have also demonstrated that convalescent sera from patients with HUS caused by STEC which are LEE positive contain significant levels of antibody to Tir. The convalescent sera labelled a large number of protein species in lysates of E. coli DH5α carrying cloned tir genes. The apparent mass of the largest immunoreactive species was slightly greater than that predicted by the sequence of the respective tir gene (64 kDa versus 57 kDa for 95NR1). However, an even greater discrepancy has been reported between the sizes predicted by SDS-PAGE and by sequence analysis for Tir from EPEC 2348/69 (78 kDa versus 57 kDa) (17). It seems likely that either the amino acid composition or conformational features of Tir result in anomalous behaviour on SDS-polyacrylamide gels. The large number of smaller immunoreactive species seen in tir clone lysates cannot all be accounted for by the presence of additional ATG codons within the coding sequence, suggesting that extensive proteolytic breakdown occurs in the recombinant host. Expression of tir in various E. coli DH5α clones is presumably under the direction of the endogenous promoter, as in each of the clones, the tir gene lies in the orientation opposite that of the vector lac promoter. The high copy number of the cloning vector used (pBluescript) may also have contributed to the level of expression of the tir gene in E. coli DH5α, which was significantly greater than that in the respective wild-type STEC strains. There also appeared to be very little Western blot evidence for proteolytic degradation of Tir in the wild-type strains. It is possible that the E. coli DH5α clones used in the present study are unable to export Tir efficiently due to lack of other LEE-encoded functions and that Tir accumulating in the cytoplasm as a consequence is more prone to proteolysis. The assumption that the various immunoreactive species with masses of <64 kDa observed in the E. coli DH5α clones are indeed Tir degradation products is supported by the fact that the only other open reading frame in the cloned portion of LEE from the various strains is orf19, which encodes a 22.5-kDa product of unknown function, and only weak immune responses to a protein of this size were observed. Moreover, the convalescent sera from HUS patients infected with LEE-positive STEC did not react with any protein species in lysates of E. coli DH5α(pBluescript). Similarly, serum from a HUS patient whose illness was caused by a LEE-negative STEC strain or serum from a healthy individual did not react with any protein in E. coli DH5α clones expressing tir.
We conclude from this study that patients with HUS caused by LEE-positive STEC mount significant serum antibody responses to STEC Tir. Immune responses to STEC proteins such as Tir and intimin may play an important role in resolution of infection and may also provide a degree of protection against subsequent colonization by STEC. Indeed, intimin is currently being considered as a candidate vaccine antigen for prevention of LEE-positive STEC disease (7). Tir may also be a protective immunogen against homologous STEC strains. However, given the marked amino acid sequence heterogeneity reported in this study, it may be of limited use as a vaccine antigen unless the intimin-binding domain is located in one of the more-conserved regions. Further research to delineate the intimin-binding domain of Tir is clearly warranted.
Nucleotide sequence accession number.
The nucleotide sequences described in this study have been deposited with GenBank under accession numbers AF025311, AF070067, AF070068, and AF070069.
Acknowledgments
We thank K. F. Jureidini for providing the convalescent sera from HUS patients used in this study.
This work was supported in part by a grant from the National Health and Medical Research Council of Australia. A.W.P. holds an NHMRC Australian Postdoctoral Fellowship.
REFERENCES
- 1.Adu-Bobie J, Frankel G, Bain C, Goncalves A G, Trabulsi L R, Douce G, Knutton S, Dougan G. Detection of intimins α, β, γ, and δ, four intimin derivatives expressed by attaching and effacing microbial pathogens. J Clin Microbiol. 1998;36:662–668. doi: 10.1128/jcm.36.3.662-668.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Bairoch A. PROSITE: a dictionary of sites and patterns in proteins. Nucleic Acids Res. 1992;20:2013–2018. doi: 10.1093/nar/20.suppl.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrett T J, Kaper J B, Jerse A E, Wachsmuth I K. Virulence factors in Shiga-like toxin-producing Escherichia coli isolated from humans and cattle. J Infect Dis. 1992;165:979–980. doi: 10.1093/infdis/165.5.979. [DOI] [PubMed] [Google Scholar]
- 5.Beebakhee G, Louie M, De Azavedo J, Brunton J. Cloning and nucleotide sequence of the eae gene homologue from enterohemorrhagic Escherichia coli serotype O157:H7. FEMS Microbiol Lett. 1992;91:63–68. doi: 10.1016/0378-1097(92)90563-4. [DOI] [PubMed] [Google Scholar]
- 6.Beutin L, Geier D, Zimmermann S, Karch H. Virulence markers of Shiga-like toxin-producing Escherichia coli strains originating from healthy domestic animals of different species. J Clin Microbiol. 1995;33:631–635. doi: 10.1128/jcm.33.3.631-635.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butterton J R, Ryan E T, Acheson D W K, Calderwood S B. Coexpression of the B subunit of Shiga toxin 1 and EaeA from enterohemorrhagic Escherichia coli in Vibrio cholerae vaccine strains. Infect Immun. 1997;65:2127–2135. doi: 10.1128/iai.65.6.2127-2135.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deibel C, Krämer S, Chakraborty T, Ebel F. EspE, a novel secreted protein of attaching and effacing bacteria, is directly translocated into infected host cells, where it appears as a tyrosine-phosphorylated 90 kDa protein. Mol Microbiol. 1998;28:463–474. doi: 10.1046/j.1365-2958.1998.00798.x. [DOI] [PubMed] [Google Scholar]
- 9.Donnenberg M S, Kaper J B, Finlay B B. Interactions between enteropathogenic Escherichia coli and host epithelial cells. Trends Microbiol. 1997;5:109–114. doi: 10.1016/S0966-842X(97)01000-7. [DOI] [PubMed] [Google Scholar]
- 10.Elliott S J, Wainwright L A, McDaniel T K, Jarvis K G, Deng Y K, Lai L-C, McNamara B P, Donnenberg M S, Kaper J B. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Mol Microbiol. 1998;28:1–4. doi: 10.1046/j.1365-2958.1998.00783.x. [DOI] [PubMed] [Google Scholar]
- 11.Frankel G, Candy D C A, Fabiani E, Adu-Bobie J, Gil S, Novakova M, Phillips A D, Dougan G. Molecular characterization of a carboxy-terminal cell-binding domain of intimin from enteropathogenic Escherichia coli. Infect Immun. 1995;63:4323–4328. doi: 10.1128/iai.63.11.4323-4328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984;28:351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- 13.Higgins D G, Sharp P M. CLUSTAL: a package for performing multiple sequence alignments on a microcomputer. Gene. 1988;73:237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- 14.Ismaili A, Philpott D J, Dytoc M T, Sherman P M. Signal transduction responses following adhesion of verocytotoxin-producing Escherichia coli. Infect Immun. 1995;63:3316–3326. doi: 10.1128/iai.63.9.3316-3326.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jerse A E, Kaper J B. The eae gene of enteropathogenic Escherichia coli encodes a 94-kilodalton protein, the expression of which is influenced by the EAF plasmid. Infect Immun. 1991;59:4302–4309. doi: 10.1128/iai.59.12.4302-4309.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karmali M A. Infection by verotoxin-producing Escherichia coli. Clin Microbiol Rev. 1989;2:15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kenny B, DeVinney R, Stein M, Reinscheid D J, Frey E A, Finlay B B. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 18.Kenny B, Finlay B B. Intimin-dependent binding of enteropathogenic Escherichia coli to host cells triggers novel signalling events, including tyrosine phosphorylation of phospholipase C-γ1. Infect Immun. 1997;65:2528–2536. doi: 10.1128/iai.65.7.2528-2536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleanthous H, Smith H R, Scotland S M, Gross R J, Rowe B, Taylor C M, Milford D V. Haemolytic uraemic syndromes in the British Isles, 1985-8: association with verocytotoxin producing Escherichia coli. Part 2: microbiological aspects. Arch Dis Child. 1990;65:722–727. doi: 10.1136/adc.65.7.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konowalchuk J, Speirs J I, Stavric S. Vero response to a cytotoxin of Escherichia coli. Infect Immun. 1977;18:775–779. doi: 10.1128/iai.18.3.775-779.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Louie M, De-Azavedo J, Clarke R, Borczyk A, Lior H, Richter M, Brunton J. Sequence heterogeneity of the eae gene and detection of verotoxin-producing Escherichia coli using serotype-specific primers. Epidemiol Infect. 1994;112:449–461. doi: 10.1017/s0950268800051153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 24.Nataro J P, Kaper J B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ostroff S M, Tarr P I, Neill M A, Lewis J H, Hargrett-Bean N, Kobayashi J M. Toxin genotypes and plasmid profiles as determinants of systemic sequelae in Escherichia coli O157:H7 infections. J Infect Dis. 1989;160:994–999. doi: 10.1093/infdis/160.6.994. [DOI] [PubMed] [Google Scholar]
- 26.Paton A W, Ratcliff R, Doyle R M, Seymour-Murray J, Davos D, Lanser J A, Paton J C. Molecular microbiological investigation of an outbreak of hemolytic-uremic syndrome caused by dry fermented sausage contaminated with Shiga-like toxin-producing Escherichia coli. J Clin Microbiol. 1996;34:1622–1627. doi: 10.1128/jcm.34.7.1622-1627.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paton A W, Voss E, Manning P A, Paton J C. Shiga toxin-producing Escherichia coli isolates from cases of human disease show enhanced adherence to intestinal epithelial (Henle 407) cells. Infect Immun. 1997;65:3799–3805. doi: 10.1128/iai.65.9.3799-3805.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paton J C, Paton A W. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin Microbiol Rev. 1998;11:450–479. doi: 10.1128/cmr.11.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenshine I, Donnenberg M S, Kaper J B, Finlay B B. Signal transduction between enteropathogenic Escherichia coli (EPEC) and epithelial cells: EPEC induces tyrosine phosphorylation of host cell proteins to initiate cytoskeletal rearrangement and bacterial uptake. EMBO J. 1992;11:3551–3560. doi: 10.1002/j.1460-2075.1992.tb05438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voss E, Paton A W, Manning P A, Paton J C. Molecular analysis of Shiga toxigenic Escherichia coli O111:H− proteins which react with sera from patients with hemolytic-uremic syndrome. Infect Immun. 1998;66:1467–1472. doi: 10.1128/iai.66.4.1467-1472.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willshaw G A, Scotland S M, Smith H R, Cheasty T, Thomas A, Rowe B. Hybridization of strains of Escherichia coli O157 with probes derived from the eaeA gene of enteropathogenic E. coli and the eaeA homolog from a Vero cytotoxin-producing strain of E. coli O157. J Clin Microbiol. 1994;32:897–902. doi: 10.1128/jcm.32.4.897-902.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu J, Kaper J B. Cloning and characterization of the eae gene of enterohaemorrhagic Escherichia coli O157:H7. Mol Microbiol. 1992;6:411–417. doi: 10.1111/j.1365-2958.1992.tb01484.x. [DOI] [PubMed] [Google Scholar]