Abstract
The stereoselective reduction of the steroidal 4-ene-3-ketone moiety (enone) affords the 5β-steroid backbone that is a key structural element of biologically important neuroactive steroids. Neurosteroids have been currently studied as novel and potent central nervous system drug-like compounds for the treatment of, e.g., postpartum depression. As a green methodology, we studied the palladium-catalyzed hydrogenation of steroidal 4-ene-3-ketones in the presence of ionic liquids derived from natural carboxylic acids. The hydrogenation proceeds with improved 5β-selectivity in the presence of tetrabutylammonium carboxylates as additives compared to the exclusive use of an organic solvent. Under optimal conditions, using tetrabutylammonium d-mandelate, the reduction of testosterone led to 5β-dihydrotestosterone in high yield and stereoselectivity and no byproduct formation was observed. Moreover, the catalyst could be recycled. The presence of additional substituents on the steroid backbone showed a significant effect on the 5β-selectivity.
Introduction
Ionic liquids are extensively studied in a wide range of synthetic procedures and are applied in a variety of industrial processes.1−3 Ionic liquids are often referred to as attractive alternatives to conventional organic solvents due to their good chemical stability, excellent solvation ability, and negligible vapor pressure. However, some ionic liquids or their degradation products are found to be toxic.4,5 One possibility to improve their green character is their synthesis from easily available, renewable resources.6 Amino acids7 available from proteins or natural carboxylic acids from biomass, such as l-lactic acid,8l-malic acid,9,10 or l-mandelic acid,11 can serve as building blocks of greener ionic liquids. By using them as chiral solvents or additives, they present an opportunity to utilize chirality in synthetic procedures.8,12 Hydrogenation reactions in ionic liquids are widely studied13,14 as the immobilization of catalysts in ionic liquids facilitates the separation of the products and the recycling of the catalytic species. Using the advantageous properties of ionic liquids, such a methodology has great applicability in the transformation of steroidal enones to biologically important 5β-steroid structures under mild reaction conditions.
Neurosteroids are endogenous compounds synthesized in the nervous tissue from cholesterol or steroidal precursors from peripheral sources.15,16 As shown in Scheme 1, cholesterol and its metabolite pregnenolone bear a double bond in positions C-5 to C-6 (Δ5-double bond). The following isomerization to progesterone affords the 4-ene-3-ketone moiety.
Scheme 1. Schematic Biosynthesis of Progesterone from Cholesterol by Enzymes Cholesterol Desmolase (CYP11A) and 3β-Hydroxysteroid Dehydrogenase (HSD3B) with Ring Lettering.
The numbering of the carbons is simplified.
The moiety of 4-ene-3-ketone of progesterone is then reduced by 5α-reductase or 5β-reductase affording 5α- or 5β-pregnane-3,20-dione (Scheme 2). The 5α-steroidal skeleton has a planar A/B-trans configuration, while the 5β-steroid has a bent A/B-cis configuration. In vivo, the 5α-reduction is the major metabolic pathway in the brain.17
Scheme 2. Schematic Reduction of Progesterone.
In contrast, the chemical modifications under the conditions of catalytic hydrogenation of 4-enes are markedly dependent on the presence of the functional group(s) at C-17 and also on the character of the solvent. Moreover, the stereochemistry of the catalytic hydrogenation of the Δ4-double bond in the 4-ene-3-ketones shows sensitivity to reaction conditions and remote structural features. The metal used as a catalyst and the pH of the solution also have a considerable influence on the reaction. Table 1 demonstrates the various results that have been reported.
Table 1. Stereochemistry of Reduction of Steroidal 4-Ene-3-ketones.
| compound | catalyst | solvent | C5-configuration Isolated yield | C5-configuration GC yield | reference |
|---|---|---|---|---|---|
| progesterone | Pd/CaCO3 | EtOH/KOH | 5β (60%) | (18) | |
| testosterone | Pd(OH)2 | EtOH/NaOH | 5β (86%), 5α (14%) | (19) | |
| 11-ketoprogesterone | Pd/BaSO4 | Ethyl acetate | 5α (68%) | (20) | |
| corticosterone acetate | Pd/BaSO4 | Ethyl acetate | 5α (70%) | (20) | |
| testosterone | Pd(OH)2 | EtOH | 5β (34%), 5α (66%) | (19) | |
| testosterone | Pd(OH)2 | iPrOH | 5β (43%), 5α (57%) | (19) | |
| testosterone | Pd/C | MeOH | 5β (42%), 5α (54%) | (21) | |
| testosterone | PdO | HOAc/HCl | 5β (49%), 5α (51%) | (19) | |
| cortisol | PtO2 | HOAc | 5β (66%), 5α (10%) | (22) | |
| cortisol | Rh on Al2O3 | HOAc | 5β (50%), 5α (40%) | (22) |
As mentioned previously, the 5α reduction of progesterone is the major metabolic pathway in the brain. The 5β-metabolites are minor, yet recent research on neurosteroids as novel drug-like compounds has demonstrated great potential for 5β-reduced steroids. Figure 1 shows the structure of recently approved neurosteroids by the Food and Drug Administration (FDA). Zuranolone was approved as the first-in-class oral treatment for women with postpartum depression.23 Zuranolone acts as a positive allosteric modulator of the γ-aminobutyric acid A receptor (GABAAR), the major inhibitory signaling pathway of the brain and central nervous system. Zuranolone structure was designed and developed based on the structures of brexanolone and ganaxolone. Brexanolone was approved by the FDA in 2019 as the first-in-class treatment of postpartum depression (i.v. administration only).24,25 Ganaxolone was approved in 2022 as the first-in-class medication for the treatment of seizures in CDKL5 deficiency.26
Figure 1.
Structures of the FDA-approved neurosteroids.
Current research, therefore, shows the profound potential of neurosteroids in drug development targeting brain health disorders. The spectrum of effects of various diastereomers then raises the possibility that neurosteroids, yet considered only as tools for basic research or preclinical studies, may have therapeutic potential that complements and, in some cases, may exceed their natural counterparts.
The selective reduction of the Δ4-double bond in the steroid skeleton is critical and usually one of the first steps in the synthesis of novel neurosteroids. Therefore, the development of novel and efficient methods for the synthesis of 5β-reduced steroids is of great interest to medicinal chemists.
In this work, we aimed to study the applicability of a variety of ionic liquids to the stereoselective reduction of steroidal enones. Tetraalkylammonium-based ionic liquids containing prolinate anions were found to be good chiral modifiers of the stereoselective hydrogenation of α,β-unsaturated ketones.27 To the best of our knowledge, so far, only tetrabutylammonium l-prolinate ionic liquid as an additive was tested in the diastereoselective hydrogenation of progesterone and 4-cholest-3-one with different selectivities, de: 25 and 67, respectively. Testosterone was chosen as a model compound as its 5β-dihydro derivative, 5β-dihydrotestosterone, is a key intermediate in the synthesis of neuroactive steroids.28
Results and Discussion
We have studied the reduction of testosterone using the reaction conditions for progesterone and 4-cholest-3-one published by Ferlin and co-workers.27 The results are summarized in Table 2.
Table 2. Palladium-Catalyzed Hydrogenation of Testosterone in the Presence of Tetrabutylammonium-Based Ionic Liquids and Saltsa.
| entry | additive | isolated yield (%)b | drc |
|---|---|---|---|
| 1 | 82 | 61:39 | |
| 2 | [TBA][l-prolinate] | 27 | 80:20 |
| 3d | [TBA][l-prolinate] | 41 | 86:14 |
| 4e | [TBA][l-prolinate] | 70 | 75:25 |
| 5d | [TBA][l-alanate] | 32 | 84:16 |
| 6 | [TBA][l-mandelate] | 94 | 82:18 |
| 7 | [TBA][d-mandelate] | 99 | 84:16 |
| 8 | [TBA][l-lactate] | 92 | 85:15 |
| 9 | [TBA]2[l-malate] | 75 | 84:16 |
| 10 | [TBA][l-hydrogenmalate] | n.d. (72) | 85:15 |
| 11 | TBA acetate | 99 | 78:22 |
| 12 | TBA benzoate | 98 | 84:16 |
| 13 | [emim][l-lactate] | 99 | 80:20 |
| 14 | [Ch][l-lactate] | n.d. (95) | 75:25 |
| 15f | [TBA][d-mandelate] | 94 | 84:16 |
| 16g | [TBA][d-mandelate] | 99 | 84:16 |
| 17h | [TBA][d-mandelate] | 83 | 84:16 |
| 18i | [TBA][d-mandelate] | n.d. (92) | 85:15 |
Reaction conditions: 200 mg of IL, 1 mmol of steroid, 0.01 mmol (1 mol %) of PdCl2, mass ratio of iPrOH/IL = 5, 18 h, rt, 1 bar H2.
Isolated yield of the 2a/3a mixture after chromatography. The number in parentheses shows the conversion of testosterone, determined by quantitative 1H NMR measurement.
The diastereomeric ratio (dr) was determined by quantitative 1H NMR measurement.
Solvent: iPrOH/water (10% v/v) mixture.
Solvent: iPrOH/water (30% v/v) mixture.
0.02 mmol (2 mol %) of PdCl2.
Reaction time: 2h.
Mass ratio of iPrOH/IL = 2.
Mass ratio of iPrOH/IL = 1.
In order to evaluate the effect of the ionic liquids on the outcome of the hydrogenation selectivity, initially, testosterone (Scheme 3, 1a) was hydrogenated in iPrOH for 18 h in the presence of PdCl2 without a chiral additive.27 The reaction proceeded with a good isolated yield of 82% but with low 5β-selectivity, 2a/3a = 61/39 (Table 2, entry 1).
Scheme 3. Palladium-Catalyzed Hydrogenation of Testosterone Leading to 5β-Product 2a and 5α-Product 3a.
The 5β and 5α product ratio was determined by quantitative 1H NMR measurement (for details, see Materials and Methods and Supporting Information). Similarly, low selectivities were achieved in the hydrogenation of testosterone using another palladium salt, Pd(OH)2, as a catalyst precursor in EtOH (2a/3a = 34/66) and slightly improved selectivity was obtained in iPrOH (2a/3a = 43/57).19 Also, iPrOH was found to be the best solvent for the stereoselective reduction of isophorone in the presence of tetrabutylammonium l-prolinate, while no enantioselectivity was observed in the presence of aprotic solvents. Therefore, iPrOH was used in our further screening.
The reaction was repeated with tetrabutylammonium l-prolinate (Figure 2). After 18 h, iPrOH was removed from the crude material, and the product was isolated by extraction from the ionic liquid. The chromatographic purification afforded the 5α- and 5β-dihydrotestosterone mixture in poor yield of 27% (Table 2, entry 2); however, the diastereomeric ratio was improved to 2a/3a = 80/20. In order to explain the low yield of the reaction, the ionic liquid residue was purified by column chromatography as some byproducts could have had low solubility in the solvent used for the extraction. Only a few milligrams of a steroid compound were isolated. According to mass spectrometry and NMR spectroscopy, the isolated material was identified as an isomeric mixture of N-alkylated proline derivatives (4a, Supporting Information). A side reaction with l-proline as a chiral additive in the enantioselective hydrogenation of isophorone was observed.29 It was proposed that in the presence of l-proline, the hydrogenation proceeds via an iminium intermediate that is further hydrogenated to an N-alkylated proline derivative. In our case, the side reaction could occur with the proline component of the ionic liquid, and the presence of the tetrabutylammonium counterion may explain the high polarity of the compound and the unsuccessful chromatographic isolation. In order to prevent this side reaction, water was added to the reaction mixture: the hydrogenation of testosterone was performed in the presence of iPrOH/water (10% v/v and 30% v/v) mixture (Table 2, entries 3 and 4). The addition of water resulted in a higher isolated yield of the product mixture, 41 and 70%. However, the higher ratio of water led to decreased selectivity; the measured diastereomeric ratios were 2a/3a = 86/14 (iPrOH/10% water) and 75/15 (iPrOH/30% water), respectively.
Figure 2.
Structures of additives used in this study.
In order to describe the applicability of other amino acid-based ionic liquids under these hydrogenation conditions, we tested tetrabutylammonium l-alanate (Figure 2) using identical reaction parameters. We observed a similar outcome in the presence of iPrOH/water (10% v/v), and the product mixture was isolated in the yield of 32%, with a diastereomeric ratio of 2a/3a = 84/16 (Table 2, entry 5).
Effect of Different Tetrabutylammonium α-Hydroxy-Carboxylates on the Selectivity
As our methodology for amino acid-based ILs was not optimal due to the low isolated yields, we tested other chiral tetrabutylammonium carboxylates as additives (Figure 2). First, mandelic acid-based ionic liquids were evaluated. In the presence of tetrabutylammonium l-mandelate, the hydrogenation led to the products with a high isolated yield of 94% and a good diastereoselectivity of 2a/3a = 82/18 (Table 2, entry 6). The reaction was repeated with tetrabutylammonium d-mandelate, which led to an excellent yield of 99% and high selectivity, 2a/3a = 84/16 (Table 2, entry 7). These results show that the different configurations of the chiral centers of the mandelate counterions do not have a significant influence on the selectivities. Moreover, the reduction proceeded with high chemoselectivity, and no byproduct formation was observed under these conditions.
Next, tetrabutylammonium l-lactate and l-malate were tested as these ionic liquids were successfully used in the selective catalytic hydrogenation of 1,5-cyclooctadiene under mild conditions.9 In the case of tetrabutylammonium l-lactate, the hydrogenation resulted in a high isolated yield of 92% and high selectivity, 2a/3a = 85/15 (Table 2, entry 8). Bis(tetrabutylammonium) l-malate showed very similar selectivity; however, the products were isolated only in 75% yield (Table 2, entry 9). The residue of the ionic liquid was dissolved in dichloromethane, and the thin-layer chromatography (TLC) analysis proved the presence of 5β and 5α products in the ionic liquid. Presumably, because of the different viscosity of the ionic liquid, the extraction with diethyl ether was incomplete. Next, tetrabutylammonium l-hydrogenmalate failed to improve the yield and selectivity. After 18 h, testosterone was still present in the product mixture (Table 2, entry 10). Finally, we tested achiral tetrabutylammonium salts. The selectivity of the reaction in the presence of tetrabutylammonium acetate was 2a/3a = 78/22 with an isolated yield of 99% (Table 2, entry 11). A similar trend was observed for tetrabutylammonium benzoate; the selectivity of the reaction in the presence of 2a/3a = 84/16 with an isolated yield of 98% (Table 2, entry 12). These results show that there is no marked difference in the selectivities using various carboxylate counterions. Moreover, in the presence of an achiral aromatic counterion, such as benzoate, high selectivity can also be achieved.
Effect of Imidazolium and Choline Counterions on the 5β-Selectivity
Next, we studied the effect of different cations on hydrogenation selectivity. Imidazolium-based ionic liquids are widely used for the immobilization of transition metal catalyst precursors in hydrogenation reactions.13 Therefore, 1-ethyl-3-methylimidazolium lactate ([emim][l-lactate]) was selected, and the reaction led to excellent yield (99%) but lower selectivity than with the tetrabutylammonium cation, 2a/3a = 80/20 (Table 2, entry 13). As choline-based ionic liquids are referred to as biocompatible ILs,30 the effect of the choline cation was also tested. Using [Ch][l-lactate] as an additive, the hydrogenation resulted in low selectivity 2a/3a = 75/25 (Table 2, entry 14).
Taken together, we conclude that [TBA][d-Man] and [TBA][l-Lac] were found to be the best additives for the diastereoselective hydrogenation of testosterone.
Effect of Various Reaction Conditions on the 5β-Selectivity
Using [TBA][d-Man], additional reaction conditions were studied (Table 2, entry 7). First, the increased amount of PdCl2 from 1 to 2 mol %, as a precursor, did not influence the selectivity of the reaction (Table 2, entry 15 versus entry 7). Second, a reduction of reaction time to only 2 h was tested. The conversion of the reaction was complete, and no difference in the selectivities was found (Table 2, entry 16 versus entry 7). Therefore, further experiments were carried out with a reaction time of 2 h. Using a higher mass ratio of ionic liquid (iPrOH/IL = 2 and iPrOH/IL = 1), the same selectivities were observed (2a/3a = 84/16) (Table 1, entries 17 and 18). However, the higher ratio of ionic liquid (mass ratio of iPrOH/IL = 1) slowed down the reaction, and testosterone was not fully converted after 2 h. It can be explained by the higher viscosity of the reaction medium.
We conclude that the optimal reaction conditions were identified as follows: 0.01 equiv of PdCl2, mass ratio of iPrOH/[TBA][d-Man] = 5, and 2 h reaction time at 1 bar H2 atmosphere and room temperature.
Influence of C-17 Substituents on the 5β-Selectivity
Synthesis of novel neurosteroids offers a great variety as many steroidal skeletons are commercially available in large quantities for synthesis. As the nature of the C-17 substituent defines the type of skeleton and steroid, a series of steroids with various C-17 substituents was selected (Scheme 4), and the influence of different steroid skeletons on the 5β-selectivity was tested. Since the reactivity of different C-17 substituted steroids can differ, all reactions were carried out using 18 h of reaction time.
Scheme 4. Palladium-Catalyzed Hydrogenation of Steroidal Enones with Different C-17 Substituents.

First, the androst-4-ene 3-ketone skeletons (1b and 1c) were tested. For testosterone acetate and androstenedione (Table 3, entries 1 and 2), the isolated yields were high (97 and 94%, respectively), but the selectivities decreased as compared to testosterone (2b/3b = 76/24 and 2c/3c = 70/30). Second, a steroid with a pregnane skeleton (1d) was studied. The hydrogenation of progesterone 1d resulted in a good isolated yield of 82% but a moderate selectivity of 2d/3d ratio = 60/40 (Table 3, entry 3). Finally, the skeleton bearing an additional 21-hydroxy moiety was tested. Hydrogenation of 11-deoxycorticosterone 1e afforded the product mixture with an isolated yield of 74%, without any selectivity for the 5β product (2e/3e = 55/45) (Table 3, entry 4).
Table 3. Effect of C-17 Substituents in the Palladium-Catalyzed Hydrogenation of Steroidal Enonesa.
Reaction conditions: 200 mg of [TBA][d-Man], 1 mmol of steroid, 0.01 mmol (1 mol %) of PdCl2, mass ratio of iPrOH/IL = 5, 18 h, rt, 1 bar H2.
Isolated yield of the 5β/5α mixture after chromatography.
The diastereomeric ratio (dr) was determined by quantitative 1H NMR measurement.
It can be concluded that different C-17 substituents have a significant influence on the 5β-selectivity. The long-range effect of substituents in the 17-position on the hydrogenation of the double bond of the steroidal enones has been described for hydrogenations using Pd(OH)2 as a catalyst precursor in iPrOH or acidic conditions,31 a Pd/C catalyst in pyridines,32 and also a platinum catalyst in acetic acid.33 Besides the presence of different C-17 substituents, the C-17 configuration and length of the side chain also affected the selectivities.
Recycling of the Catalyst
As the possible recycling of the catalytic species is an advantage of the use of ionic liquids, we tested it in the presence of [TBA][d-Man] using a 2 h reaction time. After the first use of the ionic liquid, iPrOH was removed in vacuo, and the product was extracted by diethyl ether from the ionic liquid. Then, the ionic liquid was dried in a vacuum to remove the solvent residues. Afterward, iPrOH and testosterone were added, and the mixture was hydrogenated for 2 h. In the second run, the conversion of testosterone was decreased to 69%.
In the first run, the reaction mixture turned to a black solution upon exposure to hydrogen, but at the end of the reaction, aggregation of the catalyst was observed. We hypothesized that the ionic liquid does not provide effective protection against aggregation of the catalyst using the ratio of iPrOH/IL = 5, leading to reduced catalytic activity. Therefore, the reusability was tested using a higher ratio of the ionic liquid to iPrOH. In the case of the ratio of iPrOH/IL = 2, the conversion was significantly improved in the second run to 83%, without any change in the selectivity (Figure 3, column 2). Interestingly, using the initial iPrOH/IL = 5 ratio and implementing increased reaction time (18 h), high conversion was achieved while preserving the selectivity (Figure 3, column 4). These results show that the catalyst can be recycled.
Figure 3.
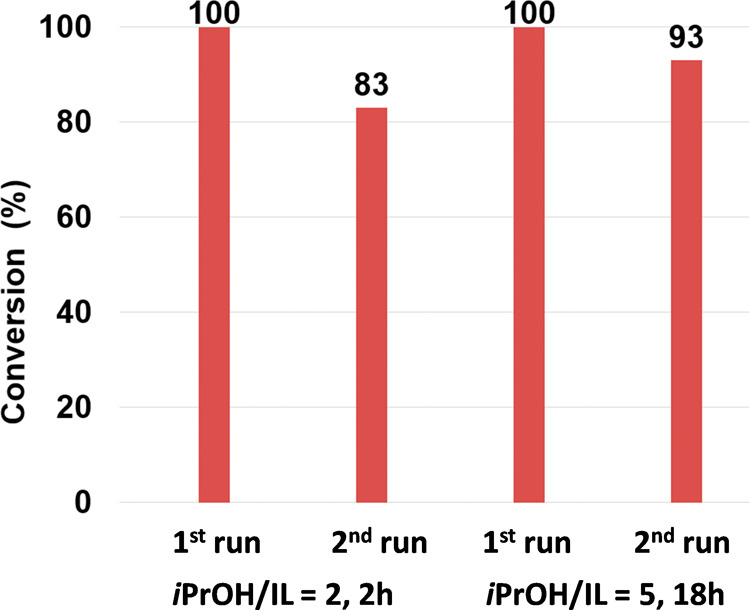
Reuse of the ionic liquid at different iPrOH/ionic liquid ratios.
Characterization of the Isolated Catalyst by IR Spectroscopy
It has been shown that primary Pd salts, such as PdCl2 reduced by H2 in alcoholic solvents, generate Pd(0) and HCl as a byproduct.34 It was proposed that the generated Pd(0) species is possibly coordinated with the solvent and/or the reactants. Tetrabutylammonium salts35,36 and ionic liquids14,37 were shown as ideal immobilizing agents of Pd(0) species in hydrogenation reactions. In these materials, the ionic liquid forms a liquid barrier surrounding the catalyst and may control the access of the substrates dependent on their solubility in the ionic layer.38 According to these findings, we assume the stabilizing effect of tetrabutylammonium-based additives of the formed palladium catalyst under our reaction conditions. In order to analyze the catalytic species, two independent hydrogenation experiments were performed with [TBA][l-Man] and [TBA][l-Lac], respectively, in the presence of PdCl2 and iPrOH. After 10 min of hydrogenation, a black solution was formed, and the formed particles were isolated by centrifugation (for details, see Materials and Methods). The isolated particles and supernatant were analyzed by IR spectroscopy. Both obtained IR spectra have a similar pattern reflecting the composition of the ionic liquid (Figure 4, more details in the Supporting Information). However, some spectral differences such as small frequency shifts were observed, which could be due to the interaction of the ionic liquid with the palladium particles. The latter results suggest that the ionic liquid may serve as a protecting layer for the formed catalyst. According to these findings, we hypothesize that the selectivity of the hydrogenation may depend on the nature of the formed catalyst, such as particle size, and also the presence of tetrabutylammonium carboxylates. The proximity of a bulkier anion, such as mandelate, may lead to a favorable orientation of the steroid molecule and attachment of the steroid with the β-side to the catalyst surface. Besides the steric effect, the basic character of the ionic liquid may also contribute to the 5β-selectivity. It has been previously described that in the catalytic hydrogenation of steroidal enones under basic conditions, the α-attack of the double bond is suppressed. It was suggested that in the presence of a base, Δ2,4-dienolate anion may form, and the absence of the axial 2β proton should favor the approach to the catalyst from the β-side. In contrast, the axial hydrogens at C-7 and C-9 hinder the α-face of the Δ4 bond. Therefore, the presence of the basic carboxylate anion of the ionic liquid may contribute to the 5β-selectivity.
Figure 4.

Details of infrared spectra of the isolated catalyst formed in the presence of [TBA][l-Man] (black line) and the supernatant (red line).
Analysis of Pd Content in Isolated Products by ICP-OES
In order to verify the purity of the obtained steroidal samples, the palladium content of the crude steroid material was measured by inductively coupled plasma optical emission spectrometry (ICP-OES) measurements after hydrogenation in the presence of [TBA][d-Man]. The results show low levels of palladium impurity of 6.05 ppm in the crude material after ethereal extraction, while after chromatographic purification, the palladium content was 1.53 ppm.
Comparison of the Isolated Yield of 5β-Dihydrotestosterone Obtained by Different Methods
Finally, using the best reaction conditions, we isolated pure 5β-dihydrotestosterone (2a) by chromatography, which yielded 78%. We compared our method to previously reported procedures for the stereoselective hydrogenation of testosterone (Table 4). The use of traditional hydrogenation conditions such as the Pd/C catalyst in organic solvents led to low selectivity (Table 4, entry 1). Tsuji and co-workers32 improved the latter method by replacing traditional organic solvents with pyridine derivatives, which led to high yield (Table 4, entry 2); however, the toxicity of pyridine derivatives is disadvantageous, especially in a larger reaction scale. Next, the diastereoselective synthesis of a series of 5β-steroids has been reported via organocatalytic transfer hydrogenation (Table 4, entry 3).39 The drawback of organocatalytic transfer hydrogenation is that pyridine derivatives, as byproducts, are generated in the reaction. Moreover, the catalyst cannot be reused. Recently, the stereoselective reduction of testosterone in the presence of cobalt nanoparticles was published, which leads to good yield but requires more harsh reaction conditions (Table 4, entry 4).40
Table 4. Comparison of Different Methods for the Reduction of Testosterone to 5β-Dihydrotestosterone.
| entry | reaction conditions | isolated yield (%) | reference |
|---|---|---|---|
| 1 | Pd/C, THF, rt, 6 h, 1 bar H2 | 39 | (41) |
| 2 | Pd/C, 4-MeO-pyridine, rt, 42 h, 1 bar H2 | 70 | (32) |
| 3 | (S)-(+)-1-(2-pyrrolidinylmethyl)pyrrolidine, d-camphor sulfonic acid, Hantzsch ester, acetonitrile, reflux, 72 h | 82a | (39) |
| 4 | CoOx@NC-800, water, 110 °C, 24 h, 20 bar H2 | 77 | (40) |
| 5 | PdCl2, [TBA][d-Man], iPrOH, rt, 2 h, 1 bar H2 | 78 | this work |
de: 96.
Conclusions
In conclusion, the palladium-catalyzed hydrogenation of steroidal 4-ene-3-ketones in the presence of tetrabutylammonium-carboxylate ionic liquids is an efficient method for the synthesis of biologically important 5β-steroid structures. Not only chiral additives but also achiral tetrabutylammonium salts favor 5β-selectivity. The latter observation suggests that the presence of the ionic liquid itself has a greater influence on the selectivity than the chiral center in the additive. Under optimal conditions, in the presence of [TBA][d-Man], 1 mol % PdCl2 in iPrOH at 1 bar H2 atmosphere and room temperature, the reaction is highly selective for 5β-dihydrotestosterone, without the formation of byproducts. In comparison with published methods, this work was found to be the most efficient to date for the preparation of 5β-dihydrotestosterone. The utilized ionic liquids can be prepared from readily available cheap resources, making possible easy product isolation with low palladium content; moreover, the catalyst can be reused for a large-scale synthesis.
Materials and Methods
General
For the optical rotation measurements, an AUTOPOL IV instrument (Rudolph Research Analytical, Hackettstown, NJ) was used. All samples were measured at 20 °C at a given concentration in a given solvent at 589 nm. 1H and 13C NMR spectra were recorded on a Bruker Avance III HD 400 instrument (400 MHz for 1H and 101 MHz for 13C). Chemical shifts are given in parts per million (δ) and are referenced to the solvent signal (CDCl3, δ 7.26 for 1H NMR and δ 77.16 for 13C NMR). The coupling constants J are given in Hz. The 1H and 13C NMR spectra of the synthesized ionic liquids were recorded in D2O by using tBuOH as an external standard. Quantitative 1H NMR was acquired on a Bruker Avance III HD 400 instrument and a Bruker Avance III HD 500 spectrometer (500.0 MHz for 1H) using 30° flip angle, inverse gated 13C decoupling, and a relaxation delay of 10 s during pulse sequence. Manual integration of relevant signals was applied after phase and baseline correction. The signal deconvolution was used for the integration of slightly overlapping C-19 methyl signals in the case of 2c–e and 3c–e. FTIR spectra were recorded on a Nicolet 6700 spectrometer (Thermo Scientific) equipped with a standard MIR source, a KBr beamsplitter, and a DTGS detector in ATR-FTIR mode using ATR-MIRacl, a single-reflection diamond horizontal ATR prism (Pike Technologies), in the 4000–600 cm–1 spectral range with the following setup: 256 scans, 4 cm–1 spectral resolution, and Happ-Genzel apodization function. The spectrum of water vapor was always subtracted. A drop of the catalyst suspended in iPrOH or the supernatant (isopropanolic solution, 10 μL) was deposited on the ATR prism. The IR spectra were obtained from the dried film on the ATR prism. The HRMS spectra were performed with an LTQ Orbitrap XL instrument (Thermo Fischer Scientific, Waltham, MA) using electrospray ionization (ESI). For elemental analysis, a PE 2400 Series II CHNS/O Analyzer (PerkinElmer, Waltham, MA) was used with a microbalance MX5 (Mettler Toledo, Switzerland). The palladium content was determined using inductively coupled plasma optical emission spectroscopy (ICP-OES) with direct sample introduction via an electrothermal vaporization unit (ETV). An Arcos I spectrometer (Spectro Analytical Instruments, Kleve, Germany) and an ETV 4000c unit (Spectral Systems Peter R. Perzl, Fürstenfeldbruck, Germany) were used. Approximately 1–2 mg of the sample was precisely weighed into a graphite boat and inserted into the ETV unit. Each sample was analyzed in three replicates. TLC was performed on silica gel 60 F254-coated aluminum sheets (VWR). The TLC plates were visualized by exposure to ultraviolet light, and then, the spots were stained by a chemical reagent: the TLC plate was immersed into a methanol/sulfuric acid (10% v/v) mixture and then heated briefly to 200 °C with a heat gun. Flash chromatography was performed on a puriFlash 5.250 instrument (Interchim, Montluçon, France) using neutral silica gel (Merck, 40–63 μm) and an evaporative light-scattering detector (ELSD) detector. All commercially available solvents and reagents were used as received. Tetrabutylammonium hydroxide 30-hydrate, tetrabutylammonium hydroxide solution (40 wt % in H2O), l-proline, l-alanine, d-(−)-mandelic acid, l-(+)-mandelic acid, l-(+)-lactic acid, l-(−)-malic acid, tetrabutylammonium acetate, 1-ethyl-3-methylimidazolium l-(+)-lactate, 2-hydroxyethyl-trimethylammonium l-(+)-lactate, androstenedione, 21-hydroxyprogesterone, 21-hydroxy-5β-pregnane-3,20-dione (used as a reference compound for the characterization of 2e), palladium acetate, and palladium(II) chloride (ReagentPlus, 99%) were purchased from Sigma-Aldrich (Prague, Czech Republic). Testosterone and progesterone were purchased from Steraloids (Newport, RI). Testosterone acetate was obtained by the acetylation of testosterone with acetic anhydride in pyridine.42 Isopropanol was purchased from Penta s.r.o.
General Procedure for the Synthesis of Ionic Liquids27,43
In a round-bottom flask, amino acid (6 mmol) or carboxylic acid (5 mmol) was dissolved in distilled water (50 mL). An aqueous solution of TBAOH·30H2O (5 mmol in 100 mL of water) or an aqueous solution of TBAOH (5 mmol, 40 wt % in water) was added, and the mixture was stirred at 65 °C for 6 h. After cooling, the water was evaporated under reduced pressure. To the crude product, acetonitrile was added, and the unreacted amino acid was filtered off. The filtrate was dried over Na2SO4, and the solvent was removed in vacuo to afford the desired ionic liquid. The ionic liquid was dried further in a vacuum (0.25 kPa) at 50 °C for 3 h upon slow stirring. The ionic liquids [TBA][l-Pro], [TBA][l-Ala], [TBA][l-Man], [TBA][d-Man], [TBA][l-Lac], [TBA]2[l-Mal], and [TBA][l-HMal] have been reported previously, and their analytical data correspond to the literature data.43,44 (1H and 13C NMR data and spectra are in the Supporting Information.)
General Procedure for the Palladium-Catalyzed Hydrogenation
Into a round-bottom flask equipped with an adapter with a two-way tap, 172 mg of ionic liquid (200 mg of IL to 1 mmol of steroid), 1.5 mg of PdCl2 (0.0086 mmol, 0.01 equiv), and 1.1 mL of iPrOH (mass ratio of ionic liquid/iPrOH = 1:5) were introduced under an argon atmosphere. After 10 min of stirring, the steroid (0.86 mmol, 1 equiv) was added under an argon atmosphere. Then, the equipment was carefully evacuated and refilled with hydrogen gas (three short evacuation and refill cycles) with the help of a gas balloon. The mixture was stirred at room temperature for 2 or 18 h. After the reaction was completed, the equipment was evacuated and refilled with argon three times. The solvent was removed in vacuo from the crude reaction mixture. Next, the product was extracted with diethyl ether (5 × 5 mL) from the ionic liquid. The organic extract was dried on Na2SO4, and the solvent was evaporated. The crude product was further purified by flash chromatography. For the quantitative NMR measurements, the 5α and 5β products were collected together, and after the removal of the solvent, the obtained material was further dried over phosphorus pentoxide at 40 °C in vacuum (0.25 kPa).
Determination of the Ratio of 5α and 5β Products by Quantitative 1H NMR Measurements
It has been previously reported that in the 1H NMR spectra of the 3-oxo-5β-steroids (without any other substituents in the A and B rings), a characteristic signal of the axial 4α hydrogen appears around δ 2.7 ppm as a “pseudo triplet” (J = 13–15 Hz).45 For 3-oxo-5α-steroids, this signal appears at higher fields (δ < 2.4 ppm). Therefore, the ratio of the 5β- and 5α-dihydrotestosterone (2a and 3a, respectively) can be calculated from the integrals of the axial 4α hydrogen at δ 2.67 ppm (5β product, 2a) and the triplet (J = 8.5 Hz) of 17α hydrogen at 3.66 ppm (overlapping signals of the 5α and 5β products). If the mixture contains unreacted testosterone (1a) as well, the signal of the C-4 olefinic hydrogen appears separately at 5.73 ppm. Then, with the integration of these three signals, the ratio of compounds can be obtained (spectra in the Supporting Information). Similarly, for testosterone acetate (1b), the axial 4α hydrogen appears at δ 2.68 ppm (5β product, 2b), while the 17α hydrogen appears at 4.61 ppm (overlapping signals of the 5α and 5β products (2b and 3b)). In the case of 2c-e and 3c-e mixtures, the 5α/5β ratio was obtained from the integration of the C-19 methyl signals: for the 2c/3c mixture, the singlets of the methyl groups appear at 1.05 ppm (2c) and 1.04 ppm (3c); for the 2d/3d mixture, those appear at 1.02 ppm (2d) and 1.01 ppm (3d); and for the 2e/3e mixture, those appear at 1.02 ppm (2e) and 1.01 ppm (3e) (for spectra, see the Supporting Information).
17β-Hydroxy-5β-androstan-3-one (2a)
Purification by chromatography using silica gel with a gradient of acetone (1–10% v/v over 20 column volumes) in dichloromethane gave pure 5β product (195 mg, 78%) as a white solid. [α]D +27.8, c 0.395, CHCl3. Selected signals of 2a in 1H NMR (400 MHz, CDCl3) δ 3.66 (t, J = 8.5 Hz, 1H, H-17), 2.67 (t, J = 14.0 Hz, 1H, H-4α), 1.03 (s, 3H, H-19), 0.76 (s, 3H, H-18). 13C{1H} NMR (101 MHz, CDCl3) δ 213.4, 82.0, 51.2, 44.5, 43.3, 42.5, 41.1, 37.3, 37.2, 37.0, 35.8, 35.1, 30.7, 26.6, 25.5, 23.5, 22.8, 20.9, 11.3. The NMR analysis is consistent with previously reported data.39 MS (ESI+): calcd for C19H31O2 [M + H]+ 291.2319, found 291.2318; anal. calcd for C19H30O2: C, 78.57; H, 10.41, found: C, 78.32; H, 10.48.
3-Oxo-5β-androstan-17β-yl Acetate (2b/3b = 76/24)
Purification by chromatography using silica gel with a gradient of acetone (0–5% v/v over 20 column volumes) in dichloromethane gave 5α and 5β product mixture (277 mg, 97%) as a white solid. Selected signals of 2b in 1H NMR (400 MHz, CDCl3): δ 4.61 (dd, J = 8.3 Hz, 1H, H-17), 2.68 (t, J = 14.2 Hz, 1H, H-4α), 2.04 (s, 3H, H-21), 1.03 (s, 3H, H-19), 0.80 (s, 3H, H-18). Selected signals of 2b in 13C NMR (101 MHz, CDCl3): δ 213.2, 171.3, 82.8, 50.9, 44.4, 42.9, 42.5, 41.0, 37.3, 37.2 (2C), 35.5, 35.1, 27.7, 26.6, 25.5, 23.6, 22.8, 21.3, 20.8, 12.3. The NMR analysis is consistent with previously reported data.46 MS (ESI+): calcd for C21H33O3 [M + H]+ 333.2424, found 333.2423.
5β-Androstane-3,17-dione (2c/3c = 70/30)
Purification by chromatography using silica gel with a gradient of acetone (0–5% v/v over 20 column volumes) in dichloromethane gave 5α and 5β product mixture (233 mg, 94%) as a white solid. Selected signals of 2c in 1H NMR (400 MHz, CDCl3): δ 2.67 (t, J = 14.3 Hz, 1H, H-4α), 1.05 (s, 3H, H-19), 0.89 (s, 3H, H-18). Selected signals of 2c in 13C NMR (101 MHz, CDCl3): δ 221.0, 212.9, 51.6, 48.0, 44.3, 42.4, 41.2, 37.3, 37.1, 36.0, 35.3, 35.2, 31.8, 26.5, 24.9, 22.7, 21.9, 20.6, 14.0. The NMR analysis is consistent with previously reported data.39 MS (ESI+): calcd for C19H29O2 [M + H]+ 289.2162, found 289.2160.
5β-Pregnan-3,20-dione (2d/3d = 60/40)
Purification by chromatography using silica gel with a gradient of acetone (0–8% v/v over 20 column volumes) in dichloromethane gave 5α and 5β product mixture (223 mg, 82%) as a white solid. Selected signals of 2d in 1H NMR (400 MHz, CDCl3): δ 2.68 (t, J = 14.3 Hz, 1H, H-4α), 2.55 (t, J = 9.0 Hz, 1H, H-17), 2.12 (s, 3H, H-21), 1.02 (s, 3H, H-19), 0.63 (s, 3H, H-18). Selected signals of 2d in 13C NMR (101 MHz, CDCl3): δ 213.2, 209.6, 63.9, 56.8, 44.3, 42.4, 40.9, 39.3, 37.3, 37.1, 35.7, 35.1, 31.7, 26.6, 25.9, 24.5, 23.1, 22.8, 21.3, 13.6. The NMR analysis is consistent with previously reported data.39 MS (ESI+): calcd for C21H33O2 [M + H]+ 317.2475, found 317.2473.
21-Hydroxy-5β-pregnane-3,20-dione (2e/3e=55/45)
Purification by chromatography using silica gel with a gradient of acetone (0–20% v/v over 20 column volumes) in dichloromethane gave 5α and 5β product mixture (212 mg, 74%) as a white solid. Selected signals of 2e in 1H NMR (400 MHz, CDCl3): δ 4.21 and 4.16 (d, J = 18.9 Hz, 2H, 2 x H-21), 2.67 (t, J = 14.3 Hz, 1H, H-4α), 1.02 (s, 3H, H-19), 0.66 (s, 3H, H-18). Selected signals of 2e in 13C NMR (101 MHz, CDCl3): δ 213.1, 210.4, 69.6, 59.4, 56.8, 45.2, 44.2, 42.4, 40.9, 38.9, 37.3, 37.1, 35.7, 35.1, 26.6, 25.9, 24.6, 23.2, 22.8, 21.2, 13.7. The 1H NMR analysis is consistent with previously reported data,47 and the 13C NMR data are consistent with the spectrum of a commercially available sample. MS (ESI+): calcd for C21H33O3 [M + H]+ 333.2424, found 333.2423.
Isolation of the Initially Formed Catalyst in the Reaction Mixture
Using identical conditions with the hydrogenation experiment, [TBA][l-Man] and [TBA][l-Lac] ionic liquid and palladium chloride in iPrOH were stirred under a hydrogen atmosphere for 10 min to afford a black solution. The formed black particles were isolated by centrifugation. The supernatant was removed, and the particles were washed twice with iPrOH. The isolated black particles were characterized by IR spectroscopy and compared with the IR spectrum of the removed supernatant after centrifugation.
Acknowledgments
Supported by the project National Institute for Research of Metabolic and Cardiovascular Diseases (Programme EXCELES, ID Project No. LX22NPO5104) – Funded by the European Union – Next Generation EU, and by the Czech Academy of Sciences institutional supports RVO:61388963. The authors would like to thank IOCB Core Facilities (Analytical Laboratories, Mass Spectrometry, NMR) for continuous support.
Glossary
Abbreviations
- IL
ionic liquid
- GC
gas chromatography
- de
diastereomeric excess
- iPrOH
2-propanol
- TBA
tetrabutylammonium
- Ch
cholinium
- dr
diastereomeric ratio
- rt
room temperature
- TLC
thin-layer chromatography
- emim
1-ethyl-3-methylimidazolium
- tBuOH
tert-butyl alcohol
- ICP-OES
inductively coupled plasma optical emission spectroscopy
- ELSD
evaporative light-scattering detector
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c08963.
1H NMR and 13C NMR spectra of compounds, quantitative 1H NMR spectra, 1H NMR and MS spectra of byproduct 4a, IR spectra of the isolated catalysts, and 1H NMR and 13C NMR spectra of the synthesized ionic liquids (PDF)
Author Contributions
The manuscript was written through contributions of all authors and all authors have approved the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Hallett J. P.; Welton T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 2011, 111 (5), 3508–3576. 10.1021/cr1003248. [DOI] [PubMed] [Google Scholar]
- Plechkova N. V.; Seddon K. R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37 (1), 123–150. 10.1039/B006677J. [DOI] [PubMed] [Google Scholar]
- Greer A. J.; Jacquemin J.; Hardacre C. Industrial Applications of Ionic Liquids. Molecules 2020, 25 (21), 5207. 10.3390/molecules25215207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty K. M.; Kulpa J. C. F. Toxicity and antimicrobial activity of imidazolium and pyridinium ionic liquids. Green Chem. 2005, 7 (4), 185–189. 10.1039/B419172B. [DOI] [Google Scholar]
- Stolte S.; Arning J.; Bottin-Weber U.; Matzke M.; Stock F.; Thiele K.; Uerdingen M.; Welz-Biermann U.; Jastorff B.; Ranke J. Anion effects on the cytotoxicity of ionic liquids. Green Chem. 2006, 8 (7), 621–629. 10.1039/b602161a. [DOI] [Google Scholar]
- Hulsbosch J.; De Vos D. E.; Binnemans K.; Ameloot R. Biobased Ionic Liquids: Solvents for a Green Processing Industry?. ACS Sustainable Chem. Eng. 2016, 4 (6), 2917–2931. 10.1021/acssuschemeng.6b00553. [DOI] [Google Scholar]
- Kirchhecker S.; Esposito D. Amino acid based ionic liquids: A green and sustainable perspective. Curr. Opin. Green Sustainable Chem. 2016, 2, 28–33. 10.1016/j.cogsc.2016.09.001. [DOI] [Google Scholar]
- Abedi E.; Hashemi S. M. B. Lactic acid production - producing microorganisms and substrates sources-state of art. Heliyon 2020, 6 (10), e04974 10.1016/j.heliyon.2020.e04974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlin N.; Courty M.; Gatard S.; Spulak M.; Quilty B.; Beadham I.; Ghavre M.; Haiß A.; Kümmerer K.; Gathergood N.; Bouquillon S. Biomass derived ionic liquids: synthesis from natural organic acids, characterization, toxicity, biodegradation and use as solvents for catalytic hydrogenation processes. Tetrahedron 2013, 69 (30), 6150–6161. 10.1016/j.tet.2013.05.054. [DOI] [Google Scholar]
- Hayouni S.; Robert A.; Ferlin N.; Amri H.; Bouquillon S. New biobased tetrabutylphosphonium ionic liquids: synthesis, characterization and use as a solvent or co-solvent for mild and greener Pd-catalyzed hydrogenation processes. RSC Adv. 2016, 6 (114), 113583–113595. 10.1039/C6RA23056C. [DOI] [Google Scholar]
- Yarolimek M. R.; Kennemur J. G. Exploration of mandelic acid-based polymethacrylates: Synthesis, properties, and stereochemical effects. J. Polym. Sci. 2020, 58 (23), 3349–3357. 10.1002/pol.20200638. [DOI] [Google Scholar]
- Prechtl M. H. G.; Scholten J. D.; Neto B. A. D.; Dupont J. Application of Chiral Ionic Liquids for Asymmetric Induction in Catalysis. Curr. Org. Chem. 2009, 13 (13), 1259–1277. 10.2174/138527209789055153. [DOI] [Google Scholar]
- Dupont J.; de Souza R. F.; Suarez P. A. Z. Ionic Liquid (Molten Salt) Phase Organometallic Catalysis. Chem. Rev. 2002, 102 (10), 3667–3692. 10.1021/cr010338r. [DOI] [PubMed] [Google Scholar]
- Migowski P.; Dupont J. Catalytic Applications of Metal Nanoparticles in Imidazolium Ionic Liquids. Chem. - Eur. J. 2007, 13 (1), 32–39. 10.1002/chem.200601438. [DOI] [PubMed] [Google Scholar]
- Baulieu E. E.Steroid Hormones in the Brain: Several Mechanisms?. In Steroid Hormone Regulation of the Brain; Fuxe K.; Gustafsson J.-Å.; Wetterberg L., Eds.; Elsevier: Pergamon, 1981; pp 3–14. [Google Scholar]
- Baulieu E. E. Neurosteroids: a novel function of the brain. Psychoneuroendocrinology 1998, 23 (8), 963–987. 10.1016/S0306-4530(98)00071-7. [DOI] [PubMed] [Google Scholar]
- Celotti F.; Negri-Cesi P.; Poletti A. Steroid Metabolism in the Mammalian Brain: 5Alpha-Reduction and Aromatization. Brain Res. Bull. 1997, 44 (4), 365–375. 10.1016/S0361-9230(97)00216-5. [DOI] [PubMed] [Google Scholar]
- MacNevin C. J.; Atif F.; Sayeed I.; Stein D. G.; Liotta D. C. Development and Screening of Water-Soluble Analogues of Progesterone and Allopregnanolone in Models of Brain Injury. J. Med. Chem. 2009, 52 (19), 6012–6023. 10.1021/jm900712n. [DOI] [PubMed] [Google Scholar]
- Nishimura S.; Shimahara M.; Shiota M. Stereochemistry of the Palladium-Catalyzed Hydrogenation of 3-Oxo-4-ene Steroids. J. Org. Chem. 1966, 31 (7), 2394–2395. 10.1021/jo01345a507. [DOI] [PubMed] [Google Scholar]
- Pataki J.; Rosenkranz G.; Djerassi C. Steroids: XXX. Partial Synthesis of Allopregnane-3β,11β,21-TRIOL-20-One and Allopregnane-3β,11β,17α,21-Tetrol-20-One. J. Biol. Chem. 1952, 195 (2), 751–754. 10.1016/S0021-9258(18)55785-9. [DOI] [PubMed] [Google Scholar]
- Parr M. K.; Zapp J.; Becker M.; Opfermann G.; Bartz U.; Schänzer W. Steroidal isomers with uniform mass spectra of their per-TMS derivatives: Synthesis of 17-hydroxyandrostan-3-ones, androst-1-, and −4-ene-3,17-diols. Steroids 2007, 72 (6), 545–551. 10.1016/j.steroids.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Caspi E. Degradation of Corticosteroids. III.1,2 Catalytic Hydrogenation of Cortisol. J. Org. Chem. 1959, 24 (5), 669–673. 10.1021/jo01087a027. [DOI] [Google Scholar]
- Meshkat S.; Teopiz K. M.; Di Vincenzo J. D.; Bailey J. B. B.; Rosenblat J. D.; Ho R. C.; Rhee T. G.; Ceban F.; Kwan A. T. H.; Cao B.; McIntyre R. S. Clinical efficacy and safety of Zuranolone (SAGE-217) in individuals with major depressive disorder. J. Affective Disord. 2023, 340, 893. 10.1016/j.jad.2023.08.027. [DOI] [PubMed] [Google Scholar]
- Pinna G.; Almeida F. B.; Davis J. M. Allopregnanolone in Postpartum Depression. Front. Glob. Womens Health 2022, 3, 823616 10.3389/fgwh.2022.823616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornett E. M.; Rando L.; Labbé A. M.; Perkins W.; Kaye A. M.; Kaye A. D.; Viswanath O.; Urits I. Brexanolone to Treat Postpartum Depression in Adult Women. Psychopharmacol Bull. 2021, 51 (2), 115–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb Y. N. Ganaxolone: First Approval. Drugs 2022, 82 (8), 933–940. 10.1007/s40265-022-01724-0. [DOI] [PubMed] [Google Scholar]
- Ferlin N.; Courty M.; Van Nhien A. N.; Gatard S.; Pour M.; Quilty B.; Ghavre M.; Haiß A.; Kümmerer K.; Gathergood N.; Bouquillon S. Tetrabutylammonium prolinate-based ionic liquids: a combined asymmetric catalysis, antimicrobial toxicity and biodegradation assessment. RSC Adv. 2013, 3 (48), 26241–26251. 10.1039/c3ra43785j. [DOI] [Google Scholar]
- Kudova E.; Chodounska H.; Slavikova B.; Budesinsky M.; Nekardova M.; Vyklicky V.; Krausova B.; Svehla P.; Vyklicky L. A New Class of Potent N-Methyl-D-Aspartate Receptor Inhibitors: Sulfated Neuroactive Steroids with Lipophilic D-Ring Modifications. J. Med. Chem. 2015, 58 (15), 5950–5966. 10.1021/acs.jmedchem.5b00570. [DOI] [PubMed] [Google Scholar]
- Tungler A.; Máthé T.; Petró J.; Tarnai T. Enantioselective hydrogenation of isophorone. J. Mol. Catal. 1990, 61 (3), 259–267. 10.1016/0304-5102(90)80001-Y. [DOI] [Google Scholar]
- Tzani A.; Karadendrou M.-A.; Kalafateli S.; Kakokefalou V.; Detsi A. Current Trends in Green Solvents: Biocompatible Ionic Liquids. Crystals 2022, 12 (12), 1776. 10.3390/cryst12121776. [DOI] [Google Scholar]
- Mori K.; Abe K.; Washida M.; Nishimura S.; Shiota M. Stereochemistry of the palladium-catalyzed hydrogenation of 3-oxo-4-ene steroids. III. Effects of the functional groups at C-11, C-17, and C-20 on the hydrogenation. J. Org. Chem. 1971, 36 (1), 231–233. 10.1021/jo00800a059. [DOI] [PubMed] [Google Scholar]
- Tsuji N.; Suzuki J.; Shiota M.; Takahashi I.; Nishimura S. Highly stereoselective hydrogenation of 3-oxo-4-ene and −1,4-diene steroids to 5β compounds with palladium catalyst. J. Org. Chem. 1980, 45 (13), 2729–2731. 10.1021/jo01301a043. [DOI] [Google Scholar]
- Sidova R.; Stransky K.; Kasal A.; Slavikova B.; Kohout L. On steroids - Part CCCXCVII - Long-range effect of 17-substituents in 3-oxo steroids on 4,5-double bond hydrogenation. Collect. Czech. Chem. Commun. 1998, 63 (10), 1528–1542. 10.1135/cccc19981528. [DOI] [Google Scholar]
- Ballesteros-Soberanas J.; Carrasco J. A.; Leyva-Pérez A. Parts-Per-Million of Soluble Pd0 Catalyze the Semi-Hydrogenation Reaction of Alkynes to Alkenes. J. Org. Chem. 2023, 88 (1), 18–26. 10.1021/acs.joc.2c00616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bras J.; Mukherjee D. K.; González S.; Tristany M.; Ganchegui B.; Moreno-Mañas M.; Pleixats R.; Hénin F.; Muzart J. Palladium nanoparticles obtained from palladium salts and tributylamine in molten tetrabutylammonium bromide: their use for hydrogenolysis-free hydrogenation of olefins. New J. Chem. 2004, 28 (12), 1550–1553. 10.1039/B409604E. [DOI] [Google Scholar]
- Mukherjee D. Potential application of palladium nanoparticles as selective recyclable hydrogenation catalysts. J. Nanopart. Res. 2008, 10 (3), 429–436. 10.1007/s11051-007-9270-2. [DOI] [Google Scholar]
- Umpierre A. P.; Machado G.; Fecher G. H.; Morais J.; Dupont J. Selective Hydrogenation of 1,3-Butadiene to 1-Butene by Pd(0) Nanoparticles Embedded in Imidazolium Ionic Liquids. Adv. Synth. Catal. 2005, 347 (10), 1404–1412. 10.1002/adsc.200404313. [DOI] [Google Scholar]
- Migowski P.; Lozano P.; Dupont J. Imidazolium based ionic liquid-phase green catalytic reactions. Green Chem. 2023, 25 (4), 1237–1260. 10.1039/D2GC04749G. [DOI] [Google Scholar]
- Ramachary D. B.; Sakthidevi R.; Reddy P. S. Direct organocatalytic stereoselective transfer hydrogenation of conjugated olefins of steroids. RSC Adv. 2013, 3 (32), 13497–13506. 10.1039/c3ra41519h. [DOI] [Google Scholar]
- Song T.; Ma Z.; Yang Y. Chemoselective Hydrogenation of α,β-Unsaturated Carbonyls Catalyzed by Biomass-Derived Cobalt Nanoparticles in Water. ChemCatChem 2019, 11 (4), 1313–1319. 10.1002/cctc.201801987. [DOI] [Google Scholar]
- Singh C.; Hassam M.; Verma V. P.; Singh A. S.; Naikade N. K.; Puri S. K.; Maulik P. R.; Kant R. Bile acid-based 1,2,4-trioxanes: synthesis and antimalarial assessment. J. Med. Chem. 2012, 55 (23), 10662–10673. 10.1021/jm301323k. [DOI] [PubMed] [Google Scholar]
- Trafalis D.; Geromichalou E.; Dalezis P.; Nikoleousakos N.; Sarli V. Synthesis and evaluation of new steroidal lactam conjugates with aniline mustards as potential antileukemic therapeutics. Steroids 2016, 115, 1–8. 10.1016/j.steroids.2016.07.009. [DOI] [PubMed] [Google Scholar]
- Allen C. R.; Richard P. L.; Ward A. J.; van de Water L. G. A.; Masters A. F.; Maschmeyer T. Facile synthesis of ionic liquids possessing chiral carboxylates. Tetrahedron Lett. 2006, 47 (41), 7367–7370. 10.1016/j.tetlet.2006.08.007. [DOI] [Google Scholar]
- Zhang S.; Huang Y.; Jing H.; Yao W.; Yan P. Chiral ionic liquids improved the asymmetric cycloaddition of CO2 to epoxides. Green Chem. 2009, 11 (7), 935–938. 10.1039/b821513h. [DOI] [Google Scholar]
- Chodounská H.; Buděšínský M.; Šídová R.; Sisa M.; Kasal A.; Kohout L. J. Simple NMR Determination of 5α/5β Configuration of 3-Oxosteroids. Collect. Czech. Chem. Commun. 2001, 66, 1529–1544. 10.1135/cccc20011529. [DOI] [Google Scholar]
- Cristofoli W. A.; Benn M. Synthesis of samanine. J. Chem. Soc., Perkin Trans. 1 1991, (8), 1825–1831. 10.1039/p19910001825. [DOI] [Google Scholar]
- Slavikova B.; Kasal A.; Budesinsky M. Autoxidation vs hydrolysis in 16 alpha-acyloxy steroids. Collect. Czech. Chem. Commun. 1999, 64 (7), 1125–1134. 10.1135/cccc19991125. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.








