Abstract
Clinical paracoccidioidomycosis is 13 times more common in men than in women. Estrogen inhibits the transition of mycelia or conidia (the saprophytic form of Paracoccidoides brasiliensis) to yeasts (the parasitic form) in vitro. Here, we show that, in male mice that were infected intranasally (mimicking natural infection) the transition of conidia in bronchoalveolar lavage fluids to intermediate forms and yeasts occurred over 24 to 96 h; CFU and yeasts (shown by histopathology) increased subsequently. In females, transition did not occur and infection cleared. These events in vivo are consistent with epidemiological and in vitro observations, suggesting that female hormones block transition and are responsible for resistance.
Paracoccidioidomycosis is one of the most important systemic mycoses affecting residents of Latin America. Its etiological agent is the dimorphic fungus Paracoccidioides brasiliensis (2). This organism probably occurs in the mycelial form (M) in nature, and the disease is thought to be acquired by inhalation of the propagules produced by this phase. Clinical manifestations are reflected in diverse forms, ranging from asymptomatic pulmonary lesions to systemic generalized infections. The tissue form of P. brasiliensis is a multiply budding yeast (Y) (26, 33).
Clinical disease is more common in adult males, with a male/female ratio of 13:1 in some areas where it is endemic (2, 20, 33). In Colombia this ratio is 78:1 (20). The greater incidence of clinical disease in adult males led to the hypothesis that hormonal factors might play a role in the pathogenesis of paracoccidioidomycosis (15, 23, 30). In contrast to overt disease, subclinical infection, which is detected by delayed-type hypersensitivity to paracoccidioidin in healthy individuals from areas where the disease is endemic, does not reveal this sex-based difference. Thus, it appears that both sexes acquire subclinical infections at the same rate (19) but that progression to overt disease is much more frequent in males (33).
A number of hormonal systems in various fungi have been described (10, 30). Several fungi have cytosolic proteins that bind mammalian hormones with high affinity and stereospecificity (8, 15, 30, 31).
Data from in vitro studies also support the hypothesis that hormones might influence the pathogenesis of paracoccidioidomycosis in humans. In vitro, the inhibition of transition of conidia (C) or M to Y by estrogens has been described (23, 25). However, the in vivo effect of the female hormonal milieu on the transition of C to Y has not been described previously.
The primary objective of this study was to examine the possible in vivo effect of the different hormonal environments in the different sexes on the pathogenesis of the experimental infection induced by C in BALB/c mice.
Fungus and inoculum.
P. brasiliensis ATCC 60855 (21) in M was used to produce C (22). The C viability was determined by ethidium bromide fluorescence (3), and the inoculum was adjusted so that 3 × 106 to 4 × 106 viable propagules were contained in 0.06 ml. The inoculum was administered by intranasal instillation under methoxyflurane anesthesia (24).
Animals.
Specific-pathogen-free male and female BALB/c mice from Simonsen Laboratories (Gilroy, Calif.) were used. The animals were 4 to 6 weeks old and were supplied with sterilized food and acidified water ad libitum.
Three mice of each sex (of a total of 33 males and 33 females) were euthanized per interval postinfection (1, 24, 48, 72, and 96 h) for bronchoalveolar lavage (BAL) and for CFU determination and histopathology at 2, 4, and 6 weeks. The results of BAL studies were confirmed in a second identical experiment of the same proportions.
BAL and lung examination for fungal morphology and quantitation.
Samples were collected after the mice were euthanized with CO2. The lungs were inflated with RPMI 1640 medium; 0.5-ml volumes were injected and withdrawn several times to obtain samples of BAL fluid. This suspension was centrifuged at 4°C, and the pellet was suspended in 2 ml of physiologic saline. After harvesting of the cells, cytospin slides were prepared and stained with Grocott methenamine-silver nitrate stain (GMS). Fungal cells were counted, and their morphology was assessed (C, intermediate [INT], or Y). The number of fungal cells was expressed as the log10.
Because of the rarity of fungal cells in BAL fluids ≥96 hours postinfection in preliminary experiments, we monitored the course of the infection at later times by quantitating CFU and examining the pulmonary histopathology. Lungs were removed, weighed, and homogenized in tissue grinders. The homogenates were serially diluted in saline and plated in duplicate on modified Sabouraud glucose agar plates with 0.01% thiamine (Mycosel; BBL, Cockeysville, Md.). The plates were incubated at room temperature for 2 months. Mean colony counts were obtained and expressed as log10 CFU per entire organ. For histopathology, lungs were removed and fixed in 10% buffered formalin and were paraffin embedded. Serial sections were stained with GMS. The fungi were counted, and their morphology was assessed. Hematoxylin-and-eosin-stained sections were used to observe the inflammatory response and the tissue cellularity.
Statistical analyses.
Statistical analyses were done on the pooled data from two experiments for the total fungal cells recovered from BAL fluids and for the proportions of C, INT, or Y from the sexes at various times. Analyses of the total fungal cells recovered at each time were done with a Mann-Whitney U test on log10 transformed numbers (GB-STAT, version 6.0; Dynamic Microsystems, Silver Spring, Md.). Changes with time were analyzed with a Kruskal-Wallis one-way analysis of variance (ANOVA). Comparative analyses of the proportions were done by parametric tests with a one- or a two-way ANOVA. Proportions were first transformed with an arcsin transformation to normalize the data (29). An analysis of the changes over time for each sex was done with a one-way ANOVA. Comparisons between sexes with time were done with a two-way ANOVA, and comparisons at each time between sexes were done with a one-way ANOVA, with the C data considered alone and the INT and Y data grouped together. Analyses of the CFU and fungal cell counts in the histologic preparations were done with Student’s t test. A P value of <0.05 was considered significant.
Fungi in BAL fluid.
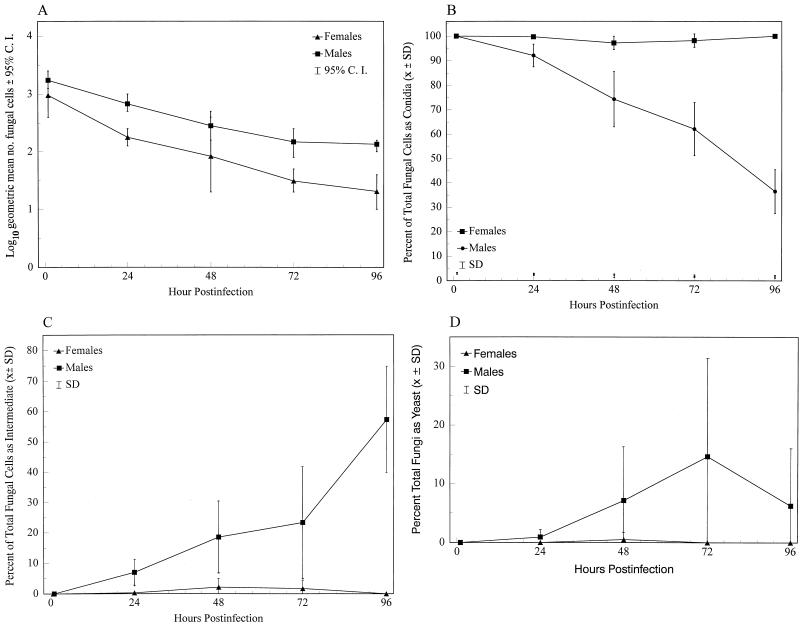
At 1 h postinfection there was no difference between sexes in the morphology of the fungus, because only C were observed (Fig. 1). In the males, INT and Y were observed as early as 24 h postinfection. In contrast, few INT and Y were observed in BAL fluids from females up to 96 h.
FIG. 1.
Appearance of P. brasiliensis cells at 1, 24, 48, 72, and 96 h, in the BAL fluids of mice infected intranasally with C. Data are pooled results from two experiments (n = 6, except for females at 96 h, n = 5) and show total fungal cells (A) and percentages of total cells as C (B), INT (C), and Y (D). C. I., confidence interval; SD, standard deviation.
The total fungal cells recovered in BAL fluids significantly diminished with time in males and females (P values of <0.0001 and <0.0009, respectively) (Fig. 1A). A significantly greater number of fungal cells was recovered from males at 24, 72, and 96 h (P values of <0.002, <0.002, and <0.004, respectively).
Two major factors affected the morphological transition of the organisms: gender and the length of time after infection. Analyses of the proportions of the C, INT, and Y forms showed that both factors were significant in their effects on morphological transition (P < 0.0001) (Fig. 1B to D). In males the proportion of fungal cells remaining as C significantly decreased with time (P < 0.0001), while numbers of INT and Y increased (P < 0.0001).
Comparisons between genders of the proportions of fungal cells appearing as C, INT, and Y at different times showed significant differences at 24 to 96 h. The proportion of C at 24 to 96 h was significantly higher for females (P < 0.004, at all times), and the proportion of cells as INT or Y was significantly greater at 24 to 96 h in males (P < 0.004, at all times).
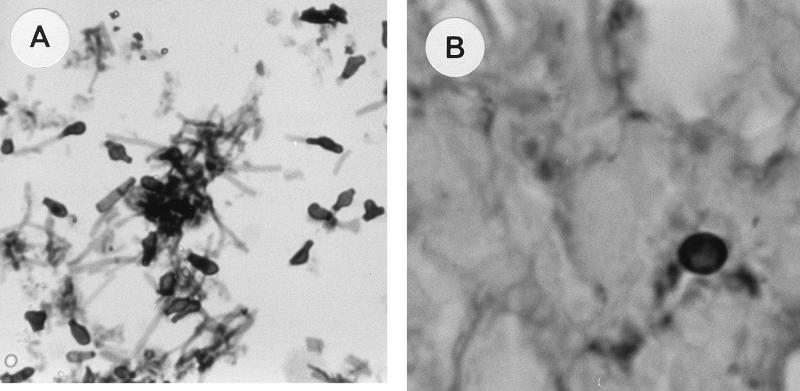
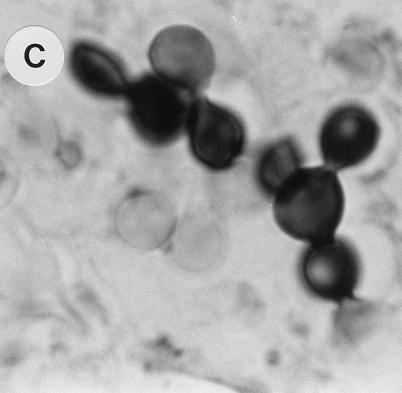
The various stages in the C-to-Y transformation are illustrated in Fig. 2. The inoculated propagules (Fig. 2A) changed over the observation time in males. Initially, C converted into INT (Fig. 2B) and later to Y. Multiply budding Y (Fig. 2C) were observed only toward the end of the 96-h period.
FIG. 2.
Stages in the C-to-Y transition in lungs of mice. (A) Inoculum of C obtained from M phase in BAL fluid 1 h postchallenge, stained with GMS. Magnification, ×40. (B) INT cells developing in males 48 h postchallenge, stained with GMS. Magnification, ×100. (C) Y cells in males 48 to 96 h postchallenge, stained with GMS. Magnification, ×100. Note that the diameter of Y (cells in the plane of focus) is twice that of cells in panel B, and note the reproduction by budding in Y.
CFU in lung cultures.
Infected males were unable to control the infection, as shown by CFU recovered from the lungs at 2 to 6 weeks (Table 1).
TABLE 1.
Fungal burden in the lungs of male and female mice infected with P. brasiliensis C
| Wk postinfection | CFU in the lungs ofa:
|
||
|---|---|---|---|
| Males
|
Females | ||
| Mean | 95% CI | ||
| 2 | 2.42 | 2.4–2.5 | 0 |
| 4 | 2.09 | 2.0–2.1 | 0 |
| 6 | 2.55 | 2.5–2.6 | 0 |
a Data are log10 geometric means of CFU. P < 0.05 for all values. CI, confidence interval.
Histopathology of the lungs.
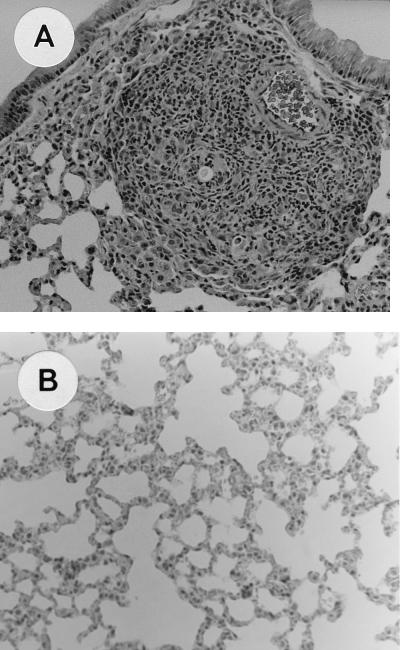
In the experiments enumerating fungal cells in lung sections at 2, 4, and 6 weeks postchallenge, significant differences were observed between male and female mice. In females, fungal cells were not found at any time. In contrast, in males, 10 to 20 fully transformed Y were counted per section (P < 0.05).
Histological observation showed a chronic granulomatous inflammatory response in males. Lesions were localized in the peribronchiolar and perivascular spaces (Table 2) with many histiocytes and lymphocytes at all times; as the lesions evolved, some plasmacytes and giant cells appeared. In contrast, no granulomas, plasmacytes, or giant cells were seen in females, and histiocytes were less numerous and later disappeared (Fig. 3).
TABLE 2.
Histological assessment of cell types present in the lungs of mice infected with P. brasiliensis C
| Group and wk postinfection | Cell type or inflammatory responsea
|
||||
|---|---|---|---|---|---|
| L | H | PC | GC | GF | |
| Males | |||||
| 2 | +++ | +++ | − | A | P |
| 4 | +++ | +++ | ++ | A | P |
| 6 | ++ | +++ | + | P | P |
| Females | |||||
| 2 | ++ | + | − | A | A |
| 4 | ++ | + | − | A | A |
| 6 | ++ | − | − | A | A |
a Average dimensions of the total area of lung sections examined was 15 by 10 mm. L, lymphocytes; H, histiocytes; PC, plasma cells; GC, giant cells; GF, granuloma formation. −, 0 cells; +, 1 to 9 cells; ++, 10 to 24 cells; +++, 25 to 50 cells. A, absent; P, present.
FIG. 3.
Histological observation of the lungs of mice 2 to 6 weeks after intranasal infection with P. brasiliensis C. (A) Chronic granulomatous reaction in a male mouse (hematoxylin and eosin stain; magnification, ×40). (B) Lung section with no inflammatory reaction in a female mouse (hematoxylin and eosin stain; magnification, ×40).
Various observations suggest sex-based differences in the immune response (6, 9, 11, 18, 32). Gender-associated differences in patterns of resistance to bacterial, viral, or parasitic infection, tumor development, and autoimmune responses are well documented (6, 13, 17, 32). Women appear to be less susceptible to some fungal, bacterial, and viral infections, presumably due to hormonal factors (7, 32). It has been reported that natural estrogens, such as estradiol, strongly stimulate macrophage activity, whereas testosterone has only a marginal effect (9, 11, 32).
Active and progressive paracoccidioidomycosis is seen markedly more frequently in men (33). This suggests that female sex hormones are important in the progression of subclinical paracoccidioidal infection towards overt disease and that small differences in hormonal influence on immune responses (as cited above) are greatly augmented by a hormone-specific effect on this pathogen. In vitro the direct inhibitory effect of estradiol on the M- or C-to-Y transformation has been shown (23, 25). These observations and the finding of estrogen-binding proteins in the cytosol of P. brasiliensis suggest that endogenous host estrogens may inhibit the adaptation of the fungus to tissue conditions (15, 31).
Animal experimentation has contributed little to our knowledge concerning the influence of sex hormones in paracoccidioidomycosis or the hormonal influence on P. brasiliensis adaptation to host conditions (30). When infected experimentally with Y inocula by the intratracheal route (4, 5) or by the intraperitoneal route (1), female mice were more resistant than males. In contrast, in intraperitoneal infection (12), intact female rats were more susceptible to infection with Y. McEwen et al. (16), using a C inoculum given intranasally, demonstrated that severe pulmonary paracoccidioidomycosis occurred at the same rate in female and male mice. The susceptibility of female mice during the various stages of the estrous cycle to intravenous inoculation with Y of P. brasiliensis has been compared with that of males (27, 28). Female mice, especially those at metestrus II, developed invasive disease more often (27, 28). Generally, at proestrus and estrus, the blood estrogen is at its highest level, while at metestrus II, it is at its lowest (14, 28). These experiments showed that female hormones affect the clearance of P. brasiliensis Y from tissues.
However, five of the six experiments mentioned (1, 4, 5, 12, 27, 28) used Y inocula, i.e., the tissue form of the fungus. The use of Y circumvents natural events because the infectious particles (M fragments or C) are presumably M derived. C were used in only one study (16). There were differences with our study: the use of mice that were 12 weeks old (16); concentration of the inoculum (fourfold lower) (16); the anesthetic used, diethyl ether (16), known to destroy alveolar lining, versus methoxyflurane in our study, the source of BALB/c mice (Corporacion para Investigaciones Biologicas) (16); and the experiment duration (up to 6 months) (16). On the other hand, the P. brasiliensis strain used was the same, identified as Gr (16); later, the strain was registered in the American Type Culture Collection as ATCC 60855. In additional experiments (32a), it was also found that female BALB/c mice, 6 to 8 weeks old, were resistant to intravenous challenge with C. In those experiments, the number of CFU in lungs and spleen were significantly lower in females 3 weeks after challenge. The infection continued to progress in males 4 to 8 weeks after challenge, whereas in females the infection had cleared by 4 weeks. In contrast, intravenous infection with Y was cleared from lungs and spleens in both sexes at the same rate.
To prove that sex hormones did indeed have an inhibitory effect in the transition of P. brasiliensis from C (infectious form) to Y (parasitic form), we studied sequential stages of the transition in mice. In males, even during the earliest stages of the infectious process (24 to 48 h), C had already begun to transform, with many INT cells and even some Y. This indicates that, at least in males, the transition of C to Y cells does not take long. Other studies have shown that after 96 h, a large proportion of C had already transformed into Y (16). Remarkably, in the present study, during the same interval, C but no INT or Y cells were found in females.
We noted during examination of the BAL preparations that although macrophages comprised ≥95% of host cells in both sexes prior to infection, polymorphonuclear neutrophils comprised the majority of cells in males after infection, whereas in females, cells other than macrophages were rarely seen. This suggests that the chemotactic response might be hormonally modulated. Thus, a hormonally mediated influence on the immune system could be an additional or adjunct explanation for the observed sex-based differences in resistance. The different profile could also relate to different chemotaxins and chemotaxinogens produced by different morphological forms of the fungus, i.e., INT and Y eliciting polymorphonuclear neutrophils in males and C eliciting macrophages in females. In addition, the macrophage response may be the more protective one (11, 13).
These findings suggest that the inhibition of the fungal transition process by female hormones results in protection. In addition, it is possible that males suffer a suppression of their cell-mediated immune responses to P. brasiliensis due to androgens, as androgens have been shown to possess both immunoinhibitory and immunostimulatory capacities (13, 17).
The histopathology samples obtained at later time points showed the presence of abundant budding Y, accompanied by an intense inflammatory response with epithelioid granuloma formation. Also, numbers of CFU from lungs were higher in males. This suggests that males develop chronic and progressive paracoccidioidomycosis more frequently, because females are able to halt the transformation process before 96 h. The female immune response was strong enough to kill the fungus, as indicated by negative cultures ≥2 weeks postchallenge.
The initial events that we describe demonstrate, for the first time in vivo, that female resistance to paracoccidioidomycosis is related to early events after infection and that these events might be hormonally modulated. These findings support our previous hypothesis, derived from in vitro observations (23, 25), that female hormones inhibit the transition of P. brasiliensis from M or C to Y, thus making females less susceptible to paracoccidioidomycosis.
Acknowledgments
This work was supported in part by the Instituto Colombiano para el Desarrollo de la Ciencia y la Tecnologia Francisco Jose de Caldas (COLCIENIAS), Bogota, Colombia, and the Corporacion para Investigaciones Biologicas, Medellin, Colombia.
We thank B. L. Gomez for technical assistance, R. Azzi for assistance with histopathology, E. Brummer for contributions to BAL data, and L. Treat-Clemons and F. Montoya for the statistical analyses.
REFERENCES
- 1.Borelli D, Marcano C. Mus musculus como modelo para la paracoccidioidomicosis experimental. Dermatol Venez. 1971;10:1271–1273. [Google Scholar]
- 2.Brummer E, Castaneda E, Restrepo A. Paracoccidioidomycosis: an update. Clin Microbiol Rev. 1993;6:89–117. doi: 10.1128/cmr.6.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calich V L G, Purchio A, Paula C R. A new fluorescent viability test for fungi cells. Mycopathologia. 1985;66:175–177. doi: 10.1007/BF00683967. [DOI] [PubMed] [Google Scholar]
- 4.Calich V L G, Singer-Vermes L M, Siqueira A M, Burger E. Susceptibility and resistance of inbred mice to Paracoccidioides brasiliensis. Br Soc Exp Pathol. 1985;66:585–594. [PMC free article] [PubMed] [Google Scholar]
- 5.Calich V L G, Burger E, Kashino S S, Fazioli R A, Singer-Vermes L M. Resistance to Paracoccidioides brasiliensis in mice is controlled by a single dominant autosomal gene. Infect Immun. 1987;55:1919–1923. doi: 10.1128/iai.55.8.1919-1923.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chao T-C, VanAlten P J, Greager J A, Walter R J. Steroid sex hormones regulate the release of tumor necrosis factor by macrophages. Cell Immunol. 1995;160:43–49. doi: 10.1016/0008-8749(95)80007-6. [DOI] [PubMed] [Google Scholar]
- 7.Clemons K V, Stover E P, Schar G, Stathis P A, Chan K, Tokes L, Stevens D A, Feldman D. Steroid metabolism as a mechanism of escape from progesterone-mediated growth inhibition in Trichophyton mentagrophytes. J Biol Chem. 1989;264:11186–11192. [PubMed] [Google Scholar]
- 8.Clemons K V, Schär G, Stover E P, Feldman D, Stevens D A. Dermatophyte-hormone relationships: characterization of progesterone-binding specificity and growth inhibition in the genera Trichophyton and Microsporum. J Clin Microbiol. 1988;26:2110–2115. doi: 10.1128/jcm.26.10.2110-2115.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Da Silva P J A. Sex hormones, glucocorticoids and autoimmunity: facts and hypotheses. Ann Rheum Dis. 1995;54:6–16. doi: 10.1136/ard.54.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gooday G W, Adams D J. Sex hormones and fungi. Adv Microb Physiol. 1993;34:69–145. doi: 10.1016/s0065-2911(08)60028-4. [DOI] [PubMed] [Google Scholar]
- 11.Kenny J F, Pangburn P C, Trail G. Effect of estradiol on immune competence: in vivo and in vitro studies. Infect Immun. 1976;13:448–456. doi: 10.1128/iai.13.2.448-456.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerr I B, Schaeffer G V, Miranda D S. Sex hormones and susceptibility of the rat to paracoccidioidomycosis. Mycopathologia. 1984;88:149–154. doi: 10.1007/BF00436446. [DOI] [PubMed] [Google Scholar]
- 13.Kita E, Takahashi S, Yasui K, Kashiba S. Effect of estrogen (17β-estradiol) on the susceptibility of mice to disseminated gonococcal infection. Infect Immun. 1985;49:238–243. doi: 10.1128/iai.49.1.238-243.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knobil E, Neill J D. The ovarian cycle of the rat. In: Knobil E, Neill J, et al., editors. The physiology of reproduction. Vol. 2. New York, N.Y: Raven Press; 1990. pp. 1898–1899. [Google Scholar]
- 15.Loose D S, Stover E P, Restrepo A, Stevens D A, Feldman D. Estradiol binds to a receptor-like cytosol protein and initiates a biological response in Paracoccidioides brasiliensis. Proc Natl Acad Sci USA. 1983;80:7659–7663. doi: 10.1073/pnas.80.24.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McEwen J G, Bedoya V, Patino M M, Salazar M E, Restrepo A. Experimental murine paracoccidioidomycosis induced by the inhalation of conidia. J Med Vet Mycol. 1987;25:165–175. doi: 10.1080/02681218780000231. [DOI] [PubMed] [Google Scholar]
- 17.Mock B A, Nacy C A. Hormonal modulation of sex differences in resistance to Leishmania major systemic infections. Infect Immun. 1988;56:3316–3319. doi: 10.1128/iai.56.12.3316-3319.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montoya F, Garcia-Moreno L F. Effect of sex on delayed hypersensitivity responses in experimental mouse paracoccidioidomycosis. J Reticuloendothel Soc. 1979;26:467–478. [PubMed] [Google Scholar]
- 19.Pereira A J C S. Inquerito intradermico para paracoccidioidomicose em Goiania. Rev Patol Trop. 1988;17:157–159. [Google Scholar]
- 20.Restrepo A. Ecology of P. brasiliensis. In: Franco M, Da Silva-Lacaz C, Restrepo-M. A, Del Negro G, editors. Paracoccidioidomycosis. Boca Raton, Fla: CRC Press; 1994. pp. 121–128. [Google Scholar]
- 21.Restrepo A, Jiménez B E. Growth of Paracoccidioides brasiliensis yeast phase in a chemically defined culture medium. J Clin Microbiol. 1980;12:279–281. doi: 10.1128/jcm.12.2.279-281.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Restrepo A, Salazar M E, Cano L E, Patino M M. A technique to collect and dislodge conidia produced by Paracoccidioides brasiliensis mycelial form. J Med Vet Mycol. 1986;24:247–250. [PubMed] [Google Scholar]
- 23.Restrepo A, Salazar M E, Cano L E, Stover E P, Feldman D, Stevens D A. Estrogens inhibit mycelium-to-yeast transformation in the fungus Paracoccidioides brasiliensis: implications for resistance of females to paracoccidioidomycosis. Infect Immun. 1984;46:346–353. doi: 10.1128/iai.46.2.346-353.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Restrepo S, Tobon A, Trujillo J, Restrepo A. Development of pulmonary fibrosis in mice during infection of Paracoccidioides brasiliensis conidia. J Med Vet Mycol. 1992;30:173–184. [PubMed] [Google Scholar]
- 25.Salazar M E, Restrepo A, Stevens D A. Inhibition by estrogens of conidium-to-yeast conversion in the fungus Paracoccidioides brasiliensis. Infect Immun. 1988;56:711–713. doi: 10.1128/iai.56.3.711-713.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.San-Blas, G., A. Restrepo, K. V. Clemons, D. A. Stevens, F. San-Blas, R. Puccia, L. R. Travassos, J. I. Figueroa, A. J. Hamilton, M. A. Bartholomew, T. Harada, L. Fenelon, and R. J. Hay. 1992. Paracoccidioidomycosis. J. Med. Vet. Mycol. 30(Suppl. 1):59–71. [DOI] [PubMed]
- 27.Sano A, Miyaji M, Nishimura K. Studies on the relationship between paracoccidioidomycosis in ddY mice and their estrous cycle. Mycopathologia. 1991;115:73–81. doi: 10.1007/BF00436795. [DOI] [PubMed] [Google Scholar]
- 28.Sano A, Miyaji M, Nishimura K. Studies on the relationship between the estrous cycle of BALB/c mice and their resistance to Paracoccidioides brasiliensis infection. Mycopathologia. 1992;119:141–145. doi: 10.1007/BF00448811. [DOI] [PubMed] [Google Scholar]
- 29.Sokal R R, Rohlf F J. Biometry. 2nd ed. San Francisco, Calif: W. H. Freeman & Co.; 1981. [Google Scholar]
- 30.Stevens D A. The interface of mycology and endocrinology. J Med Vet Mycol. 1989;27:133–140. doi: 10.1080/02681218980000191. [DOI] [PubMed] [Google Scholar]
- 31.Stover E P, Schär G, Clemons K V, Stevens D A, Feldman D. Estradiol-binding proteins from mycelial and yeast-form cultures of Paracoccidioides brasiliensis. Infect Immun. 1986;51:199–203. doi: 10.1128/iai.51.1.199-203.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Styrt B, Sugarman B. Estrogens and infection. Rev Infect Dis. 1991;13:1139–1150. doi: 10.1093/clinids/13.6.1139. [DOI] [PubMed] [Google Scholar]
- 32a.Villar, L. A., and A. Restrepo. Unpublished data.
- 33.Wanke B, Londero A T. Epidemiology and paracoccidioidomycosis infection. In: Franco M, Da Silva-Lacaz C, Restrepo-M. A, Del Negro G, editors. Paracoccidioidomycosis. Boca Raton, Fla: CRC Press; 1994. pp. 109–120. [Google Scholar]