Abstract
The cultivation of microalgae and microalgae–bacteria consortia provide a potential efficient strategy to fix CO2 from waste gas, treat wastewater and produce value-added products subsequently. This paper reviews recent developments in CO2 fixation and wastewater treatment by single microalgae, mixed microalgae and microalgae–bacteria consortia, as well as compares and summarizes the differences in utilizing different microorganisms from different aspects. Compared to monoculture of microalgae, a mixed microalgae and microalgae–bacteria consortium may mitigate environmental risk, obtain high biomass, and improve the efficiency of nutrient removal. The applied microalgae include Chlorella sp., Scenedesmus sp., Pediastrum sp., and Phormidium sp. among others, and most strains belong to Chlorophyta and Cyanophyta. The bacteria in microalgae–bacteria consortia are mainly from activated sludge and specific sewage sources. Bioengineer in CBB cycle in microalgae cells provide effective strategy to achieve improvement of CO2 fixation or a high yield of high-value products. The mechanisms of CO2 fixation and nutrient removal by different microbial systems are also explored and concluded, the importance of microalgae in the technology is proven. After cultivation, microalgae biomass can be harvested through physical, chemical, biological and magnetic separation methods and used to produce high-value by-products, such as biofuel, feed, food, biochar, fertilizer, and pharmaceutical bio-compounds. Although this technology has brought many benefits, some challenging obstacles and limitation remain for industrialization and commercializing.
Graphical Abstract
Keywords: Microalgae–bacteria, CO2 fixation, Waste gas, Wastewater, Mechanism, Products of microalgae
Introduction
A large amount of carbon dioxide (CO2) has been emitted into the atmosphere, which exacerbates global warming and the greenhouse effect [1]. CO2 concentration in the atmosphere has reached to 420.0 ppm in 2022 in Mauna Loa, Hawaii, United States (as shown in Fig. 1) [2]. In this background, global carbon reduction and neutrality have become worldwide topics [3]. Microalgae are the main microorganisms for photosynthesis on Earth, and their carbon (C) consumption accounts for nearly 50% of global CO2 fixation. The application of microalgae to fix CO2 is considered an efficient strategy to eliminate the atmospheric CO2 concentration [4], and has great potential in combating global warming due to its green economy as well as pollution-free nature [5, 6].
Fig. 1.

CO2 concentrations in in Mauna Loa, Hawaii, United States [2]
Unreasonable disposal of wastewater discharges a large amount of nitrogen (N), phosphorus (P), carbon (C), heavy metals and other pollutants into freshwater bodies, causing the disturbance of aquatic ecosystems and destruction of species diversity. Considering the high cost of traditional microalgal cultures (i.e., using culture medium and water), replacing the culture medium with wastewater showed great promise while in effective removal of pollutants from wastewater [7, 8]. Thus, to cut down the cost of microalgae cultivation, waste gas–wastewater has been used to cultivate microalgae as nutrient source. For example, with suitable culture conditions, cultured microalgae could fix 450 tons of CO2, 25 tons of N, and 2.5 tons of P per hectare per year while simultaneously producing 200 tons of microalgal biomass [9].
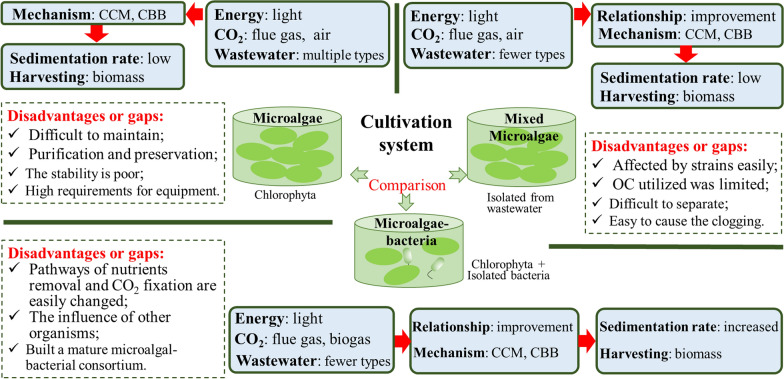
Microalgae are grown as monocultures in many studies, and applications using specific algal strains desired for harvest could result in a high yield of high-value products [10]. For instance, swine wastewater and waste CO2 were applied in cultivating Chlorella vulgaris in an integrated semi-continuous system, when wastewater renewal rate was 80%, the highest productivity of Chlorella vulgaris was obtained at 3% CO2 [11]. Then, Chlorella vulgaris biomass can be utilized for lipid extraction [12, 13]. However, due to the microalgae culture system is easily contaminated by undesired microorganisms in the industrialization or outdoor cultivation process, the culture system is difficult to maintain using a single species. Thus, consortia composed of mixed microalgae or microalgae–bacteria were proposed. Compared with a monoculture of microalgae, mixed cultures of microalgae or microalgae–bacteria are potential alternatives for tackling various pollutants due to the more robust biological system, and could improve the performance of wastewater purification [14, 15]. To strengthen the resistance of microalgae, improve the treatment effect of wastewater, and enhance the CO2 fixation ability, the novel microalgae–bacteria partnership system and its regulatory mechanism need to be explored [16].
There are huge variety of natural species of microalgae and bacteria on Earth [17]. Comparing the applications of microalgae and microalgae–bacteria grown in wastewater and waste gas is meaningful and important for the selection of suitable microorganisms, improvement of their CO2 fixation ability, and their industrialization. Moreover, it has been reported that microalgae and bacteria compete for survival in limited nutrients and space, thereby influencing their application scope and efficiency [16, 18].
In the above background, this paper reviews and compares the applications of single microalgae, mixed microalgae, and microalgae–bacteria consortia in fixing CO2 and treating wastewater, and emphasizes the gaps between the existing studies and industrialization. The influencing factors for cultivating these microorganisms along with methods to improve the performance are further discussed. It also focuses on revealing their mechanisms of CO2 fixation and nutrients removal. Moreover, the harvest method of microalgae and the high-value products from microalgae were concluded. Finally, this paper provides advice for future works to cultivate microalgae–bacteria consortium by waste gas–waste water for CO2 fixation, wastewater purification and bioproducts production.
Microalgae and microalgae–bacteria consortia
Monoculture of microalgae
Chlorophyta (green algae) are the major microalgae that have been extensively studied, including Chlorella sp., Chlorococcum sp., Pseudokirchneriella subcapitata, Scenedesmus sp., Coelastrum sp. and Nannochloropsis gaditana (Table 1). When cultivating Chlorella vulgaris in simulated municipal wastewater injected with CO2, the maximal CO2 fixation rates (56.26–85.72 mg CO2·L−1·d−1) and nutrient removal rates (96.12–99.61%) at 10% CO2 were obviously higher than those at air condition [19]. The effects of 6–16% CO2 on the CO2 fixation rate and nutrient removal rate by Coelastrum sp. were also studied, and the maximal CO2 fixation rate (302 mg·L−1·h−1), total nitrogen (TN) removal rate (84.01%) and total phosphorus (TP) removal rate (100%) were obtained at 12% CO2 [20]. TN and TP removal rates were 97.80% and 95.60% caused by Scenedesmus obliquus, respectively, and the maximal CO2 fixation rate was 26.45 mg·L−1·h−1 [21]. N and P could also be uptaken by Pseudokirchneriella subcapitata with removal rates of 100% and 51.30%, respectively, and the CO2 fixation rate reached 264 mg·L−1·d−1 [22].
Table 1.
Cultivation of microalgae by waste gas–waste water to fix CO2 and remove nutrients
| Wastewater | CO2 (% v/v) | RCO2 | RN | RP | References | |
|---|---|---|---|---|---|---|
| Chlorophyta | ||||||
|
Chlorella sp.; Chlorococcum sp. |
Industrial wastewater | 1–10 |
187.65 94.68 |
100b |
98.8 85.8c |
[7] |
| Chlorella vulgaris | Municipal wastewater | 0.04–20 | 318 | 99 a | 87.95 | [8] |
| Chlorella sp. UKM2 | Palm oil mill effluent | 10–25 | 120 | 80.9 | – | [25] |
|
Chlorella vulgaris Scenedesmus obliquus |
Municipal wastewater | 0.038–5 |
140.91 123.82 |
93.4 91.5 |
94.1 91.3 |
[26] |
| Chlorella sp. L166 | Soybean processing wastewater | 0–10 | 28.6% | 96.07 | 95.55 | [27] |
| Chlorella kessleri | Synthetic wastewater | 0–10 | 83.88 | 99 | 88 | [28] |
| Chlorella sp. GD | Aquaculture wastewater | 0.038–10 | 2333 | 90 | 99 | [29] |
| Chlorella vulgaris MBFJNU-1 | Swine slurry | 1–20 | 454 | 74 | 87 | [30] |
|
Chlorella vulgaris Chlorella pyrenoidosa Scenedesmus obliquus Scenedesmus dimorphu |
Effluent from wastewater treatment plant | 10 |
120 250 270 200 |
92.13–97.38 | > 80.43 | [31] |
| Chlorella vulgaris | Steel mill wastewater | 10.9–11.3 | 13.52 | 77a | 61c | [32] |
|
Chlorella vulgaris Pseudokirchneriella subcapitata |
Simulated domestic effluent | 0.038 |
471 264 |
99.0 100 |
67.6 51.3 |
[22] |
| Chlorella vulgaris | Simulated municipal wastewater | 10 | 170.98–220.92 | > 97.64 | > 97.64 | [12] |
| Scenedesmus obliquus | Secondary effluent | 0.03–15 | 26.45 ± 1.51 | 97.8 | 95.6 | [33] |
| Scenedesmus obliquus U169 | Tequila vinasses | 0.038–25 | 910 | 75.96 | - | [34] |
| Tetradesmus obliquus | Secondary effluent | 5–15 | 106.6–275.1 | 77.57–91.47 | > 98 | [35] |
| Cyanophyta | ||||||
| Spirulina platensis | Municipal wastewater | 2.5–15 | 378 | – | 94.0 | [18] |
|
Microcystis aeruginosa Synechocystis salina |
Simulated domestic effluent | 0.038 |
384 384 |
100 100 |
37.9 41.1 |
[22] |
RCO2 (mg CO2·L−1·d−1), CO2 fixation rate; RN (%), removal rate of nitrogen (N); RP (%), removal rate of phosphorus (P); a, ammonia nitrogen; b, nitrate and nitrite; c, orthophosphate
Spirulina platensis, Microcystis aeruginosa and Synechocystis salina, belonging to Cyanophyta (blue algae), have also been effectively cultivated in waste gas–waste water for CO2 fixation as well as wastewater purification (Table 1). Almomani et al. reported that the CO2 fixation rate of Spirulina platensis ranged from 62 to 378 mg CO2·L−1·d−1 at 2.5–15% CO2, with a maximum at 10% CO2. The NO3–N removal efficiencies from secondary effluent were 57.6–58% by Spirulina platensis [18]. In the study of Gonçalves et al., Microcystis aeruginosa and Synechocystis salina showed the potential to fix CO2 (400 mg CO2·L−1·d−1) and remove N (12.53–19.63 mg N·L−1·d−1), but the P removal rates were low (1.16–1.62 mg P·L−1·d−1, 37.9–41.1%) [22].
In addition, when Phormidium valderianum BDU 20041 was grown in ossein effluent injected with 15% v/v CO2, it fixed 56.4 mg CO2·L−1·d−1 and removed 66.35% N and 35.66% P [23]. In pharmaceutical wastewater with 0.038% CO2, the CO2 fixation rate, removal rate of NO3–N, and removal rate of PO43−–P by Tetraselmis Indica BDU 123 reached 89 mg CO2·L−1·d−1, 81.6% and 94.87%, respectively [24].
Mixed microalgae cultivation
Mixed microalgae culture is a technique in which two or more species of high-yield microalgae are cultivated in a culture system to obtain biomass. The coculture of multiple microalgae improved the biomass and CO2 fixation rate through the interaction or synergistic effects among microalgae [18, 36]. Table 2 shows that Chlorophyta are important components of mixed microalgae. For instance, Chlorella vulgaris, Botryococcus braunii and Spirulina platensis were cocultured in treated sewage and 1% v/v CO2 [37]. The maximum of CO2 fixation rate, N removal rate, P removal rate and biomass productivity of the cocultured system reached 22,400 mg CO2·m−3·d−1, 91%, 100% and 48,300 mg·d−1·m−2, respectively [37]. Microalgal consortiums, including Chlorella sp., Scenedesmus sp., Sphaerocystis sp., and Spirulina sp., isolated from a wastewater treatment plant with 50% CO2, also showed high CO2 sequestration efficiency (53–100%) and high nutrient removal efficiency [38].
Table 2.
Cultivation of mixed microalgae by waste gas–waste water to fix CO2 and remove nutrients
| Microalgae | Wastewater | CO2 (% v/v) | RCO2 | RN | RP | PB | N | References |
|---|---|---|---|---|---|---|---|---|
| Mixed algal culture from different wastewater after treatment process | Primary effluent | 2.5–15 | 492 mgC·L−1·d−1 | 58.1 | – | 0.246–0.384 gdw·L−1·d−1 | 287 mg·L−1 | [18] |
| Secondary effluent | 362 mgC L−1 d−1 | 95.7 | – | 200 mg·L−1 | ||||
| Septic tank effluent | 470 mgC L−1 d−1 | 99.6 | – | 230 mg·L−1 | ||||
| Mixed consortia of fresh water and storm water algae | Nature fresh water | Air and coal fired flue gas (0.038, 1, 3, 5.5 CO2%) | – | – | – | – | 37.73–59.75 mg·L−1·d−1 | [41] |
| Scenedesmus obliquus | Artificial wastewater | 0.038–10 | – | 80a | – | – | 30–210 105 cells·mL−1 | [42] |
|
Scenedesmus sp. LX1 and Chlorella ellipsoidea YJ1; Haematococcus and Chlorella ellipsoidea YJ1; Scenedesmus sp. LX1 and Haematococcus |
Secondary effluent | 0.038 | – | – | – | – | 183.0 mg·L−1 | [39] |
| 204.0 mg·L−1 | ||||||||
| 277.0 mg·L−1 | ||||||||
|
Chlorella vulgaris TISTR-8580 Botryococcus braunii NIES-2199 Spirulina platensis |
Simulated treated sewage | 1 | 22,400 mg·m3·day−1 | 91.0 | 100.0 | 48.3 g·(d·m2)−1 | 923.0 mg·L−1 | [37] |
|
Chlorella sp., Scenedesmus sp. Sphaerocystis sp., Spirulina sp. |
Domestic wastewater | 2–100 | 291.0 mg·g−1 | 39.0 | 59.0 | 0.114 g·(L·d)−1 | 850.0 mg·L−1 | [38] |
| Mixed microalgae in wastewater plant: cyanobacteria, diatoms, Scenedesmus sp., Chlorella sp. | Untreated urban wastewater | 20 |
24.6 mg L−1·min−1 |
100.0 | 100.0 | 28.3 g·(d·m2)−1 | – | [36] |
RCO2, CO2 fixation rate; RN (%), removal rate of nitrogen; RP (%), removal rate of phosphorus; PB, biomass productivity; N, microalgal biomass; DIC, dissolved inorganic carbon
Mixed microalgae cultivation showed better performance on biomass production, CO2 fixation, wastewater treatment than microalgal monocultivation. The growth characteristics of Scenedesmus LX1, Chlorella ellipsoidea, and Hematococcus pluvialis in monoculture and pairwise mixed culture in urban secondary effluent were investigated, it was found that the biomass and specific growth rate of mixed culture of pairwise algal species were higher than their single species [39]. Local mixed microalgae (including yellow‒green, green, blue‒green algae, etc.) from different wastewaters after the treatment process (primary effluent, secondary effluent, and septic tank effluent) were used to fix CO2 and purify wastewater [18]. The maximal biomass productivity and CO2 fixation rate of mixed microalgae in the study of Almomani et al. were 0.384 gdw·L−1·d−1 and 0.460 g C·L−1·d−1, respectively, which were obviously higher than those of Spirulina platensis [18]. In a study by Johnson et al., a polyculture of algal species (Chlorella, Scenedesmus, Chlorococcus, and Phaeodactylum tricornutum) was more stable than the cultures of single microalgal species, less susceptible to the external environment, and could reduce the risks of microalgal biomass harvesting and wastewater remediation [40]. A raceway pond (200 L), operating outdoors, was designed and used to cultivate mixed microalgae such as Scenedesmus sp. and Chlorella sp. in untreated urban wastewater injected with 20% CO2 [36]. CO2 gas was supplied continuously at different flow rates of 0.2–5.0 L·min−1 during the daytime [36]. The maximum CO2 removal rate (24.6 mg·L−1·min−1) and microalgae biomass productivity (28.3 g·d−1·m−2) were reached when the gas flow rate was 1.0 L·min−1 [36].
Microalgae–bacteria consortium cultivation
From Table 3, the bacteria in the microalgae–bacteria consortium involved in not only activated sludge but also functional microorganisms from specific sewage sources, and Chlorophyta are the most commonly used microalgae in the consortium. When Spongiochloris was cultivated with bacteria in local petroleum wastewater-injected air (0.038% CO2), the microalgal-specific growth rate, biomass productivity, COD removal rate, petroleum hydrocarbon removal rate and maximal CO2 bio-fixation rate reached 0.87 d−1, 1.5 g·L−1·d−1, 97%, 99% and 2921 mg·L−1·d−1, respectively, as the cultivation progressed [43]. A microalgae–bacteria consortium could grow under simulated flue gas from a power plant and achieved effective removal of CO2 (4.7–18.4 mg CO2·L−1·d−1), SOx (99%) and NOx (87%) [44]. Scenedesmus was inoculated into sterilized wastewater and unsterilized wastewater-injected with 10% CO2 for cultivation, and it was found that the COD removal rate in unsterilized wastewater group was 90%, much higher than that of sterilized wastewater (42%) [45]. This result implies that microalgae in the consortium are responsible for fixing CO2, while bacteria generally utilize organic carbon.
Table 3.
Cultivation of microalgae–bacteria consortium by waste gas–waste water to fix CO2 and remove nutrients
| Microorganisms | Wastewater and gas | RCO2 | RN | RP | RCOD | Biomass | Rpol | References |
|---|---|---|---|---|---|---|---|---|
| Tetradesmus obliquus PF3 and bacteria in sewage |
0.038% CO2 10% CO2 |
551 mg CO2·L−1·d−1 |
93 ± 3 81 ± 1 |
99 95 |
90 ± 3 65 ± 2 |
1.8 g·L−1 0.9 g·L−1 |
– | [45] |
| Spongiochloris sp. and Hydrocabonoclastic |
Petroleum wastewater 0.038% CO2 |
2921 mg·L−1·d−1 |
– | – | 97 | 1.5 ± 0.3 g·L−1·d−1 | – | [43] |
| Microalgae (four strains) and aerobic activated sludge |
Primary treated sewage Flue gas (12% CO2, 289 ppmv NO, 197 ppmv SO2) |
4.7–18.4 mg CO2·L−1·d−1 | – | – | – | 0.153–0.181 g·L−1·d−1 |
87% for NOx; 99% for SO2 |
[44] |
|
Chlorella vulgaris (FACHB-8) and Endophytic bacteria S395-1 and S395-2 |
Biogas slurry Biogas (62.17 ± 2.44% CH4, 34.21 ± 1.29% CO2, 0.54 ± 0.03% O2, 3.07 ± 0.21% H2O) |
68.13% ± 1.69% | 88.31 ± 4.19 | 88.21 ± 4.51 | 88.29 ± 5.03 |
50–250 μg·L−1 |
– | [46] |
| Microalgae and algae and bacteria (including Scenedesmus spp., Cyanobacteria) isolated from wastewater |
Wastewater Air and CO2 (99%) |
– | ~ 99 | ~ 99 | – | 94.3 ± 7.9 mg·L−1·d−1 | – | [52] |
| Chlorella pyrenoidosa and Native bacterial microbial | Municipal wastewater and landfill leachate treatment | 65.8 mg·L−1·d−1 | – | – | – | 1.58 g·L−1 | – | [53] |
| Chlorella PY-ZU1 and bacteria (from anaerobic digestion effluent) |
Undiluted anaerobic digestion effluent of swine manure 15% v/v CO2 |
– | 73%a | 95% | 79% |
4.81 g·L−1 601.2 mg·L−1·d−1 |
35.7–90.0% for Heavy metals | [54] |
|
Chlorella vulgaris Chlorella vulgaris and Ganoderma lucidum Chlorella vulgaris and Activated sludge |
Biogas slurry Biogas (25.27%, 35.08%, 45.36%, 55.17% CO2) |
68.37 79.11 79.06 |
69.12 85.69 84.17 |
66.36 86.17 83.79 |
68.71 86.08 84.28 |
0.174 0.431 0.429 g·L−1·d−1 |
– | [55] |
|
Chlorella sp. and Cupriavidus necator |
Culture medium (with phenol); 1% CO2 | – | – | – | – | 0.45–0.50 g/L | 100% for phenol | [56] |
| Spirulina platensis and H2S-oxidizing bacterial consortium and Activated sludge |
Mineral salt medium; 30% CO2, 69.5–70% N2, 0–5000 ppmv |
95% | – | – | – | 1.2 g·L−1 | 100% for H2S | [57] |
| Chlorella sp. and aerobic sludge |
Mineral salt medium; Biogas |
285 mg CO2·L−1·d−1 | – | – | – | – | > 98% for H2S | [48] |
|
Tree bark consortium; Eukaryotic; Scenedesmus quadricauda |
Aquaculture effluent: Biogas digestate = 90: 10 | 52.958% | 87.227 | 100b | 12.292c | 1.46 g/L | – | [58] |
| 40.497% | 61.346 | 73.154b | 18.455c | 0.86 g/L | – | |||
| 39.097% | 78.693 | 75.391b | − 14.889c | 0.86 g/L | – | |||
|
Tree bark consortium; Eukaryotic; Scenedesmus quadricauda |
Aquaculture effluent: Biogas digestate = 75: 25 | 90.714% | 56.627 | 21.543b | − 3.473c | 0.74 g/L | – | |
| 85.809% | 83.205 | 54.126b | − 8.980c | 0.29 g/L | – | |||
| 89.504% | 52.037 | 1.393b | − 11.366c | 0.19 g/L | – | |||
|
Tree bark consortium; Lake water consortium; Pre-adapted tree bark consortium |
Aquaculture effluent: Biogas digestate = 95: 5 | 55.125% | 91.287 | 100b | − 6.076c | 1.10 g/L | – | |
| 46.293% | 87.626 | 100b | − 52.733c | 1.05 g/L | – | |||
| 55.760% | 90.574 | 100b | − 26.791c | 1.15 g/L | – | |||
|
Tree bark consortium; Lake water consortium; Pre-adapted tree bark consortium |
Aquaculture effluent: Biogas digestate = 90: 10 | 80.446% | 77.987 | 100b | 25.207c | 1.27 g/L | – | |
| 69.592% | 72.122 | 100b | 2.766c | 1.02 g/L | – | |||
| 83.588% | 66.114 | 100b | − 6.334c | 1.30 g/L | ||||
|
Tree bark consortium; Lake water consortium; Pre-adapted tree bark consortium |
Aquaculture effluent: Biogas digestate = 80: 20 | 95.775% | 33.928 | 40.401b | − 1.340c | 0.86 g/L | ||
| 92.653% | 39.702 | 75.353b | − 4.227c | 0.99 g/L | ||||
| 98.466% | 27.637 | 68.861b | − 20.032c | 1.16 g/L | ||||
|
Picochlorum sp. and Halospirulina sp. and Sulphur oxidizing bacteria |
Mineral salt medium Biogas (30% v/v CO2, 0.5% v/v H2S) |
44.5–50.0% | N–NO3−, 52 ~ 55 | P–PO43−, 12 ~ 29 | – | 23–129 mg·L−1·d−1 | 99.5% for H2S | [59] |
|
Chlorella vulgaris–Ganoderma lucidum–endophytic bacteria (S395-2) Scenedusmus obliquus–Pleurotus ostreatus–endophytic bacteria (S395-2) |
Biogas slurry Biogas |
56.29–64.87% 54.79–62.37% |
68.37–79.15; 67.08–75.36 |
74.58–83.65; 70.31–80.43 |
62.27–76.89; 60.27–72.57 |
0.085–0.163 g·L−1·d-1 0.079–0.149 g·L−1·d−1 |
– | [49] |
RCO2, CO2 fixation rate; RN (%), removal rate of nitrogen; RP (%), removal rate of phosphorus; RCOD (%), removal rate of organic carbon; PB, microalgal biomass productivity; Rpol, removal rate of other pollutants in wastewater or gas; a, ammonia nitrogen (NH4+–N); b, orthophosphate (PO43−P); c, dissolved organic carbon (DOC);dw, dry weight
Moreover, the cultivation process of microalgae–bacteria was the only technology capable of simultaneously upgrading biogas by removing CO2 and H2S while recovering nutrients from digestates (in Table 3). The endophytic bacteria S395-1 and S395-2 (different genera) were co-cultivated with Chlorella vulgaris, and the consortium had removal efficiencies of 88.29%, 88.31%, 88.21%, and 68.13% for COD, N, P, and CO2, respectively [46]. Alcantara et al. [47] and Lebrero et al. [48] reported that the CO2 removal by a microalgae–bacteria consortium in pond or bubble column photobioreactors was 55–62%. The performance of biogas slurry purification by Chlorella vulgaris–Ganoderma lucidum–endophytic bacteria (S395-2) symbionts was better than that of biogas slurry purification by Scenedesmus obliquus–Pleurotus ostreatus-S395-2 symbionts [49].
The decrease in CO2 content of biogas (accounting for 25–50% of biogas by volume) will lead to a decrease in transportation costs and an increase in biogas energy content. Although the use of algal–bacterial consortia has achieved promising results, the low CO2 mass transfer rate of this technology limits biogas bioconversion to biomethane [50]. Using natural light as photosynthetic active radiation daily (~ 433 μE·m−2·s−1), cultivating microalgae–bacteria consortium in high-rate ponds can efficiently remove COD within 10 days and remove nutrients within 26 days, without extra cost for CO2 addition [51].
Gaps in applications
Gaps in monoculture of microalgae
Microalgal cultures are grown as monocultures in many studies. An important reason is that the application of microalgal monocultures is easy to conduct in the laboratory and reveal the feasibility of a scheme. Another primary reason is that specific algal strains desired for harvest could obtain a high yield of high-value products. However, it is difficult for microalgae to maintain a pure culture state under natural conditions, and much time and energy are needed for purification and preservation of the microalgae, such as in the disinfection or sterilization of sewage. Moreover, the system stability is poor, and the microalgae are easily killed by foreign species pollution, requiring high culture equipment, which is not conducive to practical application.
Gaps in mixed microalgae and microalgae–bacteria consortium cultivation
Since diversity would improve biomass stability, the ability of large-scale culture systems of mixed microalgae and microalgae–bacteria consortia to resist the mutation of environmental conditions (temperature, illumination, etc.) was higher than that of single microalgae. The use of high-yield microalgal species with different optimal conditions could expand the control range of the culture conditions, thereby reducing the maintenance cost of the culture system.
However, there are several important controversial issues in the applications of mixed microalgae and microalgae–bacteria cultivation. First, it is difficult to obtain a high yield of specific high-value bioproducts, and the microalgal biomass is easily affected by the microalgal species [18]. Second, studies on microalgal biotechnology research investigate already known species. Among the huge variety of natural species—thousands—there is still a wide scope for selecting fast-growing naturally occurring species at specific geographic locations and profiting from their metabolic capabilities. In addition, because of the complexity of the microorganisms in the consortium, the stability is difficult to control, and the pathways of nutrient removal and CO2 fixation in the microalgal–bacterial consortium are easily changed under at different conditions. Ultimately, the efficiency of wastewater treatment and CO2 fixation is influenced [60].
Effective factors
The efficiencies of wastewater treatment and CO2 fixation by microalgae or microalgal–bacterial consortia are easily changed under different conditions. The effective factors can be classified as microorganisms and cultivation conditions in general.
Microorganisms
Microalgae strains with rapid growth rates and dense populations were picked up for production and capturing CO2 from flue gas. As shown in Tables 1–3, microalgae used to fix CO2 from waste gas and purify wastewater are Chlorella sp., Scenedesmus sp., Pediastrum sp., Phormidium sp. etc., and most of strains belong to Chlorophyta and Cyanophyta. The CO2 fixation rates of Chlorophyta and Cyanophyta are higher than those of others, such as Phormidium valderianum BDU 20041.
The bacteria in microalgae–bacteria consortia are mainly from activated sludge, digestion effluent and other specific sewage sources. However, COD concentrations increased when some microalgae–bacteria consortia were utilized inappropriately [58]. Thus, the CO2 fixation and nutrients removal efficiency should be optimized by identifying suitable strains of microalgae and bacteria to be co-culture. Studies on which bacteria in microalgal–bacterial consortia can efficiently fix carbon under different conditions should be conducted systematically.
Nutrient sources
Waste gas
To provide sufficient carbon source for microalgae growth, air and treated waste gas are bubbled as CO2 sources into the algal body of water. As shown in Tables 1–2, the CO2 concentrations from the atmosphere or flue gas used to cultivate microalgae are in range of 0.038–25%. From Table 3, biogas also can be implemented in microalgae–bacteria consortium cultivation, and the applied CO2 concentrations were in the range of 0.038–99%. To adapt high concentrations of CO2 (20%), Chlamydomonas increased the cell concentrations, Nostoc increased cell size, and Chlorella increased both concentrations and size of cells [61]. When microalgae–bacteria consortium was cultivated, the simulated flue gas with 12% CO2 was injected into primary treated sewage at the gas flow rate of 0.025 vvm [44]. The gas flow rate was low, because CO2 fixed by the consortium was not only from external gas but also from bacterial decomposition [44]. Thus, adequate flow rate and concentrations of CO2 are important to cultivate adequate microorganisms.
However, the presence of NOx, SOx and heavy metals (such as Hg) in flue gas may have a negative effect on microalgae growth. For example, the biomass of Chlorella vulgaris grown in wastewater decreased with the increasing of Hg concentrations (10–30 μg·Nm−3) in flue gas [12]. The CO2 fixation rate and growth of Chlorella sp. were improved when the added amount of NOx was appropriate, but they were decreased when the added amount of NOx was excessive [62]. Due to the pH of culture was easily affected by the acidic gases (such as NOx and SO2), Cheng et al. [5] reported that controlling volume flowrate of flue gas is crucial, and the effect of these acidic gases’ components can be neglected if an optimized volume flowrate is applied.
Wastewater
Multiple types of wastewaters, such as municipal wastewater, industrial wastewater, palm oil mill effluent, aquaculture wastewater, steel mill wastewater, ossein effluent, and pharmaceutical wastewater, have been utilized to cultivate single species of microalgae (in Table 1). For mixed microalgae, they have rarely been grown in industrial wastewater or pharmaceutical wastewater (in Table 2). From Tables 1 and 2, the composition of wastewater affects microalgal growth as well as the efficiency of wastewater purification due to the utilization of nutrients and other compounds by microalgae. For different wastewater, the removal rates of N and P can reach 58.1–100% and 37.9–100%, respectively (Tables 1 and 2).
Both common wastewater and biogas slurry have been used to cultivate microalgae–bacteria consortia. According to Table 3, both N and P could be removed with high efficiency (60–100%) in most cultivation systems, but the nutrients removal efficiencies are low when the consortia were grown in biogas digestate. The removal rates of N in 80% aquaculture effluent and 20% biogas digestate by the Tree bark consortium, Lake water consortium, and preadapted tree bark consortium were 27.64–39.70% [58]. The removal rate of P in 80% aquaculture effluent and 20% biogas digestate by the Tree bark consortium, Eukaryotic consortium, and Scenedesmus quadricauda reached 1.39–54.13% [58]. Moreover, the removal efficiencies of COD by microalgae–bacteria consortium were negative [58].
In wastewater, N deficiency directs the carbon flux generated during photosynthesis towards the production of fatty acids, but the cell division is low in this technology which ultimately leads to a decrease in productivity of biomass and fatty acids [63]. When N is too high, it can have toxic effects on the microalgae. Some of the effects caused by P deficiency are similar to those obtained in N-deficient cultures, influencing the cellular content of metabolite production.
Methods to improve the performance
Optimization of microorganisms
Microalgae and microalgae–bacteria consortia composed of more microalgal and bacterial species have been explored, such as the selection of native microalgae and bacteria [18, 44, 45, 52]. The selected native microorganisms can adapt to environment more easily. In general, complex ecosystem containing microalgae, algae, and bacteria from waste source (such as walls of the secondary clarifier, etc.) were collected, and they were passed through a laboratory paper filter to remove filamentous bacteria and zooplankton from reactor [18, 44, 45, 52]. Then, the filtered solution was inoculated into a suitable medium with suitable environment, and the main microalgae or bacterial genus present in the medium were screened after cultivation [18, 44, 45, 52].
Moreover, to achieve improvement of CO2 fixation or a high yield of high-value products, bioengineer and mutation in CBB cycle in microalgae cells have been studied [46]. For example, when a Rubisco activase was induced in Nannochloropsis oceanica, the over expression of Rubisco was elevated, the biomass growth rate and lipid productivity was increased by 32% and 41%, respectively [64]. In Yang et al.’s study, the aldolase gene from Synechocystis sp. PCC 6803 (sFBA) was cloned and fused with cTP sequence to be targeted into the chloroplast of C. vulgaris [65]. The overexpression of gene encoding aldolase in Chlorella vulgaris cells can significantly enhance the efficiency of CO2 fixation [65]. In Kato et al.’s study, in the ISA gene encoding an isoamylase-type starch debranching enzyme of Chlamydomonas sp. KOR1, a 2.0 kb sequence covering the initiation codon through part of the N-terminal early set domain is deleted and substituted by a 0.6 kb sequence [10]. The CO2 fixation rate by starch debranching enzyme-deficient microalgae was improved through the above process, and its CO2 fixation mechanism and carbon partition/repartition model are shown in Fig. 2 [10]. CO2 is fixed through the CBB cycle under light conditions, and CO2 are mainly captured in the form of water-soluble phytoglycogen (Fig. 2). When microalgae were grown in dark, the phytoglycogen were degraded and converted into intermedia metabolites, which in turn serve as substrate for the synthesis of lipid and carotenoid [10].
Fig. 2.
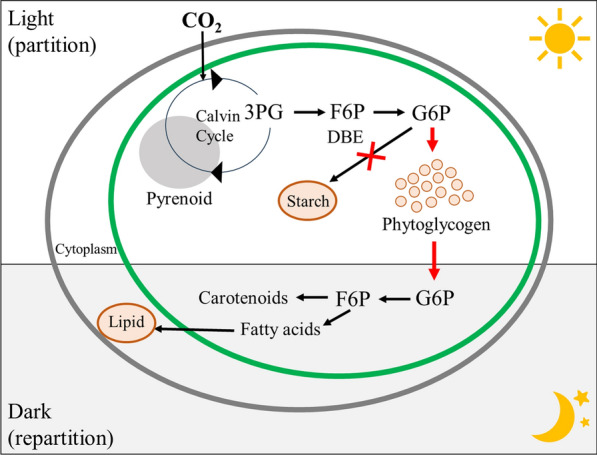
Carbon partition/repartition model for starch debranching enzyme (DBE)-deficient microalgae under light/dark conditions [10]
Optimization of cultivation conditions
Microalgal growth and biochemical composition can be influenced by temperature. For example, increasing temperature can decrease the content of total lipid in microalgae cells while improve the content of neutral lipid [63, 66]. Depending on strain, region and season, microalgae can typically grow in-between 15 and 40 ℃ [63], and the suitable temperature for algal cultivation was in range of 21–30 ℃. However, in industrial application, when microalgae were cultivated in industrial flue gases, the temperature of flue gases can reach up to 70 ℃ [67]. Thus, to prevent the inhibition effect caused by high temperature, the temperature should be decreased through cooling or high temperature dominant microalgal strains should be selected.
Illumination (including illumination time and illumination intensity) is another crucial parameter influencing microalgal growth [63, 68]. In microalgae cells, photons can be absorbed and converted to chemical bound energy instantaneously [63, 68]. Long illumination time is beneficial to cultivating microalgae, but only suitable light intensity is beneficial to reaching up highest biomass productivity and CO2 fixation rate. In Zhang et al.’s study, the NH4+–N removal efficiency is higher for 24 h compared to 6 h illumination time with same other culture conditions [69]. When light intensity beyond optimum level, it resulted in photo inhibition, reduced biomass productivity, CO2 fixation rate as well as the PUFA content of algae [63, 70].
In fact, in addition to temperature and illumination, many other operation parameters should be optimized, such as N concentrations, P concentrations, organic carbon concentrations, to alleviate the limitations or inhibitory effects of unsuitable cultivation conditions on microalgae growth [14]. Tetradesmus obliquus was cultured in municipal wastewater supplemented with 0–100 mg·L−1 NH4Cl, the maximum biomass and maximal CO2 fixation rates were obtained at 100 mg·L−1 NH4Cl [35]. The supplement of organic carbon also promotes microalgal growth and helps in diverting the carbon flow towards the accumulation of lipid or starch [63]. Due to many factors can affect microalgae growth, microalgae culture conditions were optimized through artificial intelligence [71]. For instance, Yew et al. compared the effects of waste molasses and commercial BG-11 medium on microalgae cultivation by the artificial intelligence algorithm, and determined the optimal culture condition [72].
Addition of phytohormone
Phytohormone have been applied in resistance of microalgal cells to stress such as SO2, NOx and heavy metals from complicated waste gas or wastewater. For example, in Wang et al.’s study, to resist the adversity from NO in coal fired flue gas and improve CO2 fixation efficiency by Chlorella sp., 500 μM spermidine was supplied into microalgal culture system [73]. The result showed that Chlorella sp. biomass productivity was increased by 30.5% under 327 ppm NO [73]. Similarly, when Chlorella vulgaris was cultured in 10% CO2 gas with 30 μg·m−3 Hg, indole-3-acetic acid can alleviate the toxicity of Hg on Chlorella vulgaris, ultimately resulting in enhanced chlorophyll synthesis rates and biomass [12]. Zhao et al. also reported that phytohormones aided Tetraselmis cordiformis to enhance their growth under high ammonia stress [74]. In sum, phytohormones can be used to cultivate microalgae under complicated waste water and waste gas.
Mechanism of microalgae and microalgae–bacteria consortia application
Relationships among microorganisms
Cells in microalgal consortia interact with each other through allelopathy, growth resource competition, and cell contact, thereby presenting three relationships: promotion, neutrality, and inhibition [75–77]. Two possible reasons may explain why mixed culture can promote the biomass production of microalgae. On one hand, microalgae in mixed culture can release allelochemicals that aggregated nutrient ions and promote biomass accumulation. On the other hand, the demand of different microalgae for nutrients forms a complementary relationship, and mixed algal species can improve the utilization of nutrient resources.
The interaction between microalgae and bacteria is complex and mainly includes an improvement relationship and an inhibitory relationship. The inhibitory relationship between microalgae and bacteria is caused by their competition for nutrients and toxins released to inhibit their activities [78]. The improvement relationship is the main relationship when CO2 is fixed by microalgae–bacteria consortia, and is mainly manifested in several aspects. Microalgae produce O2 through photosynthesis (Fig. 3), increasing the dissolved oxygen content in the water, which is more conducive to the growth of aerobic bacteria [79]. Meanwhile, bacteria oxidize and decompose organic matter for respiration, and promote microalgal growth by creating a favorable microenvironment and providing CO2, nutrients, vitamins, phytohormones or volatile organic compounds [80–83]. Moreover, microalgae could serve as a habitat for bacteria, protect them from adverse environmental conditions, and release extracellular polymeric substances to promote bacterial growth [84]. The long-term application of microalgae–bacteria consortium may result in gene transfer to promote their growth [83].
Fig. 3.
Interaction in microalgal–bacterial consortia (OC, organic carbon; IOC, inorganic carbon; N, nitrogen; P, phosphorus)
To improve the ability of CO2 fixation with nutrient removal by mixed microalgae or microalgae–bacteria consortia, a promotion relationship among microorganisms is necessary. However, the relationship among cells is impacted by their species. Selection of appropriate microalgae and bacteria for mixed culture can effectively improve the biomass yield and CO2 fixation rate. At the same time, due to the inherent complexity of the water environment, the influence of other organisms cannot be excluded when explaining the interactions, resulting in conflicting results from some experiments.
Mechanism of CO2 fixation
For most species of microalgae, 1,5-diphosphate ribulose carboxylase/oxygenase (Rubisco) enzymes that catalyze CO2 fixation have low affinity for CO2 [85], and they only use CO2 as substrate. These microalgae can actively absorb HCO3− and convert it into CO2 under the catalysis of carbonic anhydrase (CA enzyme) for Rubisco fixation (in Fig. 4a) [85]. To increase the CO2 concentration in cells and adapt to the change in inorganic carbon concentration, the cells would form a CO2 concentration mechanism (CCM), which can increase the CO2 concentration of the carboxylase site to 1000 times that of the surrounding environment and form a local high concentration of CO2 [86].
Fig. 4.
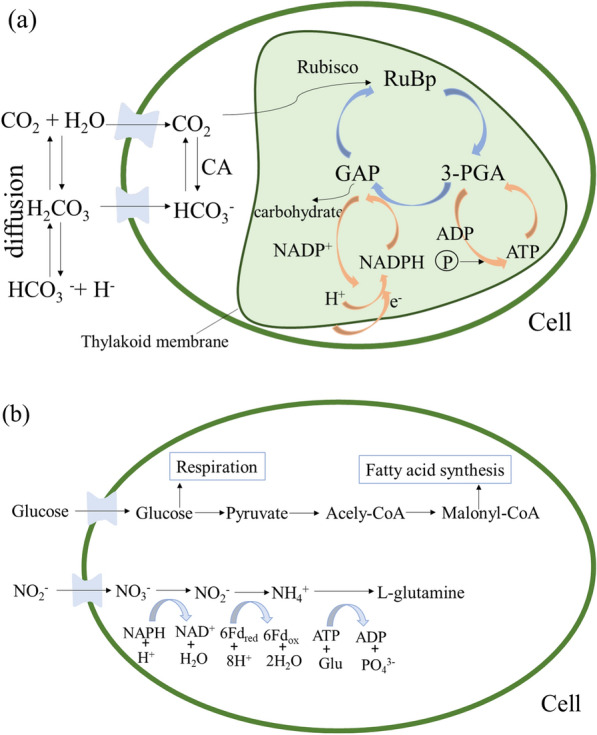
CO2 and phosphorus fixation mechanism of microalgae (a); Mechanism of organic carbon and nitrogen absorbed by microalgae (b)
The Calvin–Benson–Bassham (CBB) cycle (i.e., Calvin cycle) is the best-known pathway for CO2 assimilation in microalgae cells [87–89]. In Fig. 4, CO2 in wastewater enters the cell through an inorganic carbon pump and is transported from the cytoplasm to chlorophyll. Then, under the catalysis of Rubisco, CO2 is combined with pentose sugar to form 3-phosphoglyceride (3-PGA) to achieve carbon fixation during the CBB cycle. In has been found that, during CO2 fixation process, the activity of key enzymes (e.g., Rubisco) or transcription of the cbb gene are expected to be improved [90].
In terms of microalgae–bacteria consortia, the mechanisms of CO2 fixation are also CCM and CBB and mainly occurred in microalgal cells (Fig. 4a). The progress of CO2 fixation is regulated when microalgae are co-cultivated with bacteria [56]. In the study by Yi et al. when Chlorella sp. was cultivated with immobilized Cupriavidus necator, the expression of most genes related to light reactions and encoding antenna proteins were upregulated to varying degrees [56]. Moreover, most enzymes involved in the C3 pathways were also upregulated in Chlorella sp. in the consortium, and it indicates that the fixed CO2 amounts were increased [56].
Mechanism of organic carbon removal
Microalgae are able to directly utilize organic carbon (such as glucose, ethanol, and glycerol) in wastewater as a carbon source through heterotrophy [91]. As illustrated in Fig. 4b, after glucose (as organic carbon) is transported into the cell through the sugar transporter on the algal cell membrane, it can undergo phosphorylation reaction with adenosine triphosphate (ATP) under the catalysis of hexokinase or glucokinase to generate glucose-6-phosphate and ADP [91]. Then, glucose-6-phosphate and ADP enter the glycolysis metabolic pathway, and generate the final product pyruvate. After that, pyruvate is oxidized into CO2 and H2O through the tricarboxylic acid cycle reaction and electron transport chain and generates ATP. Pyruvate flows through acetyl-CoA into the fatty acid elongation reaction. Glycerol enters algal cells through free diffusion, is phosphorylated by ATP to form 3-phosphoglycerate, forms pyruvate through glycolysis, and then enters the TCA cycle [92].
When a microalgae–bacteria consortium was utilized to treat wastewater, the system was generally in mixotrophic mode. As shown in Fig. 3, the bacteria in the consortium degrades the pollutants in wastewater, and the metabolites produced by bacteria during this process would promote microalgae growth. At the same time, microalgae secretions including carbohydrates, proteins, and fats served as main carbon sources for bacteria growth. The photosynthesis of microalgae produces O2, enhances the content of dissolved oxygen in the wastewater, thereby promoting the uptake of organic matter by bacteria and reducing the COD in the wastewater [83]. Microalgae in consortia could also assimilate organic carbon, as described in Figs. 3 and 4a [93]. However, it was noted that this process would decrease the potential for microalgal CO2 fixation. In other words, when microalgae were used to fix CO2, the utilization of organic carbon in wastewater is limited. With the development of detection technologies, such as high-throughput and missing isotopes analyses, it will be possible to build a mature microalgal–bacterial consortium with a cleaner interaction mechanism and more controllable effects.
Mechanism of N removal
N plays a vital role in microalgae photosynthesis, participating in the synthesis of organic N, such as amino acids, chlorophyll, energy transfer molecules (ATP and ADP) and genetic components (DNA and RNA). It has been found that the mechanism of N removal is mainly assimilation by microalgae during the cultivation of either microalgae or microalgae–bacteria consortia [15, 93, 94]. As ammonia oxidizing bacteria, Nitrosomonadaceae in the microalgae–bacteria consortium are responsible for the nitrification process, but the contribution of bacteria in the process of bioremediation of wastewater is only in range of 1–3% [93]. In a study by Choi et al., microalgae were added into media containing nitrifying bacteria, the results showed that the nitrification rate was reduced despite the near complete removal of NH4+–N from the system, which also indicates algae were responsible for the removal of NH4+–N [95].
N in wastewater exists in the form of NO3−, NH4+, urea, etc. The utilization pathway of NH4+ is shorter than other forms of N (such as NO3−, NO2−) and requires less energy, and it is preferentially assimilated by microalgae [96]. Any forms of inorganic nitrogen have to be transported into the cells to consume. The consumption of any inorganic nitrogen source requires it to be transport into the cells, which is mediated by an energy-dependent-specific permease in each case. Microalgae convert inorganic N into NH4+ for utilization through an assimilation process, which is then reduced into two steps by enzymes (Fig. 4b). First, nicotinamide adenine dinucleotide phosphate (NADH) formed by photoreaction is employed as an electron donor to catalyze the transfer of two electrons from NO3− to NO2−, and then NO2− is reduced to NH4+ by nitrite reductase and ferredoxin. Finally, glutamate (Glu), reduced NH4+ and ATP are combined to generate glutamine under the catalysis of glutamine synthase.
The removal of N by the microalgae–bacteria also related to oxidative degradation by bacteria (Fig. 5). As shown in Fig. 5, nitrification is accomplished by adding oxygen from microalgae or gas into water, thereby converting ammonia to nitrate. Under the action of nitrate reductase, NO3−–N in sewage is reduced to NO2−–N, and then further reduced to NH4+–N. NH4+–N is further utilized by microalgae. In contrast, denitrification occurs in an anoxic environment. Some facultative aerobic heterotrophs in the consortium such as Bacillus are added to reduce nitrate and nitrite to nitrogen [80]. Moreover, some metabolites, as enzyme activators, were secreted by co-cultivated bacteria, and a synergistic mechanism between microalgae and bacteria in the enzymology was found [60]. In a study by Wang et al., N-related enzymatic activities in the photosynthesis pathway of Chlorella were detected [60]. The results showed that the activities of nitrate reductase (NR), nitrite reductase (NiR), glutamine synthetase (GS), and glutamate synthetase (GOGAT) were improved by 94.2%, 57.5%, 58.6%, and 79.4% caused by the addition of Exiguobacterium, respectively [60].
Fig. 5.
Nitrification and denitrification process (The blue line is nitrification and the black line is denitrification)
Mechanism of P removal
The process of P removal from wastewater by microalgae is mainly divided into assimilation and chemical precipitation. The assimilation process means that the P absorbed by cells is converted into organic compounds such as nucleic acids, phospholipids and ATP through multiple phosphorylation pathways, such as oxidation, phosphorylation, photosynthesis etc. [97]. In the process, microalgae often preferentially absorb the inorganic ions H2PO4− and HPO42− [97, 98]. As reported, a large amount of assimilated P is applied in the production of ATP from ADP, accompanied by a form of energy input, as indicated in Fig. 4a [94]. In the chloroplast, Pi participates in organic binding during photophosphorylation, as ATPases release proton gradients into the substrate; in the stroma, ATP is consumed through the CBB cycle. Consequently, a sufficient amount of P may be one of the parameters for obtaining higher CO2 fixation rates.
Chemical precipitation is affected by pH and dissolved oxygen in wastewater. P precipitation may occur when the oxygen concentration is high or the pH exceeds 8.0. When algae perform photosynthesis, CO2 is consumed, which increases the wastewater pH. Consequently, in wastewater, the volatilization of NH3 and NH4+ increases, phosphate and calcium ions form calcium phosphate precipitates under high pH conditions, thereby achieving the effective removal of N and P [99]. However, when CO2 gas is injected into wastewater, the pH of wastewater remains relatively low (pH < 6.5), and the effects of pH on the removal rate can be ignored [19].
In terms of microalgae–bacteria consortia in aerobic environments, it is found that Deviosa sp. and Bdellovibrio sp. are the phosphate accumulation bacteria [100]. However, after the wastewater treatment, Deviosa sp. accounts for less than 1% of all microorganisms [93, 101]. Due to the percentage of phosphate accumulating bacteria in microorganisms is small, it could be concluded that most phosphate were removed by the microalgae [93, 102]. Microalgae and bacteria can secret polysaccharides, phosphate as well as phosphate hydrolyzed from organophosphorus can also be adsorbed on the surface by forming hydrogen bonds with extracellular polysaccharides. At the same time, similar to the inorganic form, organophosphorus could be combined with functional groups of extracellular polymers, adsorbed to microalgal–bacterial consortium, and then further transformed.
Harvest and application of microalgal biomass
Harvest of microalgal biomass
Microalgal cells are often in a relatively stable suspended state in a culture system, and the sedimentation rate is low. Thus, microalgae cells are difficult to achieve separation through gravity sedimentation and easily clogging the reactor. The harvesting cost of microalgal cells is high, accounting for 20–30% of their biomass production cost [103, 104]. Currently, the methods of microalgae collection are mainly divided into two types: batch collection (flocculation, flotation/gravity setting) and thickening (centrifugation, filtration) (as depicted in Fig. 6) [105–107]. The collection method should be selected according to the desired moisture level of microalgal paste and product quality [103, 108]. For instance, sedimentation/flocculation was used for producing low-value products from microalgae, while centrifugation is suitable for producing high-value products [103]. The percentage of dry matter content in microalgae paste can reach up to 25% through centrifugation [108].
Fig. 6.
Harvesting and application of microalgae biomass
However, most of these techniques have disadvantages of high operating cost, secondary pollution, and low long-term operating efficiency [109, 110]. For instance, centrifugation and filtration are effective methods for collecting microalgae cells, but the costs are very high. In contrast, bioflocculation followed by gravity sedimentation or screening, is a rapid, simple and cost-effective method for harvesting microalgal biomass in large-scale [111].
Biological flocculation is a process in which microalgal cells flocculate with the assistance of microorganisms or their metabolites [13, 106, 112]. During this period, microorganisms aggregate to form large flocs, which are settled by gravity without the addition of any chemical flocculants [113]. Thus, the use of microalgae–bacteria consortia can increase the sedimentation rate of microalgae in culture system to a rate much higher than that of single microalgae. The flocs that bacteria attach to the surface of microalgal cells play an important role in flocculation, enhancing the floc volume of the microalgal cells, so that the flocs are large enough to settle [33]. At the same time, the flocs could adsorb microalgae cells, prevent them from losing in the reaction process, thereby maintaining the structural stability of the microalgae–bacteria consortium [78]. Chlorella was cultured in unsterilized seafood wastewater, and the flocculation activity was 92.0% ± 6.0%, which was much higher than that of sterilized seafood wastewater (8.7% ± 2.5%) [104].
Application of microalgal biomass
Through biochemical or thermochemical conversion, microalgal biomass can be applied in biofuel, feed, food, fertilizer, biochar and pharmaccutical biocompound.
Biofuel production
Algal biomass is capable to produce biofuels, including biodiesel, biogas, bioalcohols, biohydrogen, biosynfuel, and bio-oils [114, 115]. For example, the lipid content produced by cyanobacteria reached 12.74%, and the obvious dominance of C14 and C18 fatty acids in the total lipid content indicates their applicability as potential biofuels [23]. The residual microalgal biomass after lipid extraction were further processed by anaerobic digestion to produce biogas [116]. Chlamydomonas sp. QWY37 contained high content of carbohydrate in their cells, and the carbohydrate can be transformed to ethanol by applying engineered yeast [117]. Through anaerobic solid-state fermentation and the subsequent light independent fermentation, microalgal biomass was transformed to biohydrogen [118]. Lunprom et al. is reported that this sequential process produced 16.2 mL⋅H2/gvs [118].
Feed and food
Microalgae are rich in nutrients (e.g., vitamins, polysaccharides, mono- and polyunsaturated fatty acids, minerals, etc.) [67]. Therefore, cultured microalgae have been widely used in animal feed [119]. Qureshi et al. incorporated Spirulina platensis into poultry feed and improved the yellowness of the skin and yolk of broilers [120]. Thaakur et al. found that adding Spirulina plantensis to feed can help improve the antioxidant level of animal tissues [121].
Moreover, microalgae have been directly used in complementary food for humans (such as baked food, snacks, beverages, yogurt etc.), and its extracts can be produced as tablets or capsules as functional foods [99]. For example, Spirulina plantensis has much substances with biological activities to achieve antioxidant, antiviral, antibacterial, immune regulation, and cancer suppression. Rhodococcus pluvialis is rich in natural astaxanthin, which has multiple effects, such as anti-aging, relieving fatigue, and preventing cardiovascular and cerebrovascular diseases. Fernando et al. documented that ~ 49.3% TN, ~ 50.9% COD, and ~ 69.4% TP were reduced by Haematococcus pluvialis in industrialized run-off, and ~ 22.43 mg/L of astaxanthin were produced from these Haematococcus pluvialis [122]. It should be noted that microalgae grown in wastewater or waste gas may absorb some pollutants in cells, thereby affecting their usage as feed or food.
Fertilizer
The application of microalgal fertilizer is able to (1) improve the physical and chemical properties of soil, and (2) enhance the quality and yield of crops grown [123, 124]. However, the application of microalgae fertilizers is in the laboratory research stage. Sharma et al. reported that the addition of microbial fertilizers (algae biofilm and algae) increased the chlorophyll concentrations of soil, enhanced the content of polysaccharide and protein in corn as well as the length of cob [123]. Through field experiments, Dineshkumar et al. found that the content of pigment, total soluble sugar, and total free amino acid in onion grown in treatment with the addition of microalgae and cow manure are higher than those in onion grown in control group with only cow manure [124]. In wastewater, the biomass of mixed microalgae (Chlorella vulgaris and Scenedesmus sp.) reached 1.78 g·L−1 [125]. The combination of their residue after extracting oil and inorganic fertilizers in a ratio of 1:1 increased the yield of Solanum lycopersicum by 1.74 times [125].
Biochar
The applications of microalgal biochar have been explored from the following aspects: (1) improve soil fertility for agricultural purpose, (2) remediate wastewater or soil, (3) develop carbon electrode catalyst, and (4) manufacture energy storage [126, 127]. For example, Enteromorpha prolifera biochar was used to repair coastal saline–alkali soil in Wu et al.’s study, the best soil improvement effect was achieved when the addition amount was 1.5% and pyrolysis temperature was 400 ℃ [127]. Khan et al. reported that the selective modification of microalgae biochar can remove the targeted removal of contaminants effectively [128]. Compared to graphite plate electrodes, algal bloom-derived biochar used as an anode has high adsorption and stronger electrochemical response to redox media [129]. Compared with traditional heat treatment to obtain algal biochar, the treatment duration (20 min) of microwave mediated low-temperature treatment is shortened with obtaining 73.3% carbon [130].
Pharmaceutical bio-compounds
Owing to the bioactive nature of carbohydrates in algal cells, many algal strains are applied widely in the pharmaceutic industries, such as Chlorella, Spirulina, Griffithsia, and Diatoms etc. [131, 132]. High-value compounds from these microalgae inhibit antimicrobial, antifungal, anti-cancer, and antiviral activities [132]. For example, several antiviral agents have been extracted from the microalgal biomass. A protein cyanovirin–N derived from Nostoc elipsosporum, a sulfated polysaccharide calcium spirulan obtained from Spirulina platensis, Gigartina skottsbergii synthesized from marine algae, and carrageenan and chitosan polysaccharides from algae have been applied in inhibiting the replication of a wide variety of viruses [114, 133–135].
Challenges and prospects
The application of single microalgae, mixed microalgae and microalgae–bacteria consortia in fixing CO2 coupled with wastewater purification was discussed and compared in detail, as shown in Fig. 7. Different methods should be selected according to the specific goals, cultivation conditions, advantages and disadvantages. The challenges and prospects in the applications and commercialization of these microorganisms are summarized below.
Fig. 7.
Comparison of application of microalgae and microalgae–bacteria consortia in fixing CO2 coupled with wastewater purification
(1) As carbon and nutrients source, the composition of flue gas and wastewater produced at different conditions are different. The reliance of microalgae on the varied composition of flue gas and wastewater was the main challenge hindering their application for microalgae cultivation. To address this challenge, microalgae with strong adaptability to environment and high CO2 fixation ability can be selected. In addition, bacteria that could promote the growth of beneficial microalgae can be screened.
The current genomic, transcriptomic, proteomic and metabolomic knowledge of microalgae would provide key information for the improvement of the biomass production and biotechnology processes. The regulation of the interaction of microalgae and bacteria in the consortia should be investigated at the molecular level to establish synergy among the cultured microorganisms and improve the overall efficiency of CO2 fixation and wastewater treatment.
(2) Because most of these studies were performed in laboratory units, they were not applied in scaled-up conditions with different system capacities and external factors. The application of AI technologies in adjusting microalgal CO2 fixation system is still in initial stage. Future studies will require large-scale outdoor experiments with AI technologies to assess the economic viability and sustainability of these biotechnological applications.
(3) Microalgae CO2 fixation technology is still limited by the high costs of system construction, CO2 gas transportation, microalgae cultivation and biomass harvesting. Therefore, it is important to develop cost-effective and efficient extraction and harvesting technologies. Meanwhile, the export of microalgae products is mainly based on microalgae powder, while the proportion of deeply processed microalgae products is relatively small. Thus, researchers also could delve into potential uses of microalgal biomass and further shorten the processing stage of microalgae in various applications to generate income from microalgae for long-term sustainability and environmental benefits.
Conclusions
Microalgae and microalgae–bacteria consortia have broad prospects in CO2 fixation, nutrient removal, and resource utilization. The current goal is to reduce the gaps between the expanding microalgae studies and the related applications by exploring relevant mechanisms, screening and testing adaptable microalgae and bacteria, adjusting suitable cultivation conditions, and obtaining sufficient meaningful data. The reported work and the emergent challenges in the application of single microalgae, mixed microalgae, and microalgae–bacteria consortia were reviewed. Because specific microalgae strains contain high-value products that are desired for harvest, most cultures of algae are currently grown as monocultures. In contrast, a mixed microalgae and microalgae–bacteria consortium may mitigate environmental risk, obtain high biomass, and improve the efficiency of nutrient removal. The mechanisms of nutrient removal and CO2 fixation by microalgae and microalgae–bacteria consortia were also emphasized, and the importance of microalgae was proven. However, the application of microalgal biomass is still in the exploratory stage. Although there are numerous benefits in cultivation of microalgae–bacteria consortium by waste gas–waste water, their industrialization and commercialization still face some challenging obstacles. This paper provided guidance on future work to support the development of CO2 fixation coupled with nutrient removal by microalgae and microalgae–bacteria consortia.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (52100119, 42177218, 51906058); Joint Funds of the National Natural Science Foundation of China (U20A20302); Open Project of the State Key Laboratory of Freshwater Ecology and Biotechnology (2022FB09); Innovative group projects in Hebei Province (E2021202006); the Key Research and Development Projects in Hebei Province (20373701D).
Author contributions
WWK and BXS conceived the research; WWK and JK wrote the article; BXS, YHB and HHL supervised the manuscript; JK, SF, TTY and LFX edited figures in the manuscript; HHL polished the language of the manuscript. All authors read and approved the final manuscript. All authors agreed to authorship and submission of the manuscript for peer review.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
Declarations
Competing interests
The authors declare no competing financial interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Boxiong Shen, Email: shenbx@hebut.edu.cn.
Yonghong Bi, Email: biyh@ihb.ac.cn.
Honghong Lyu, Email: honghonglyu@hebut.edu.cn.
References
- 1.Liu LN. Advances in the bacterial organelles for CO2 fixation. Trends Microbiol. 2022;30(6):567–580. doi: 10.1016/j.tim.2021.10.004. [DOI] [PubMed] [Google Scholar]
- 2.NOAA global monitoring laboratory, 2023, https://gml.noaa.gov/dv/iadv/graph.php?code=MLO&program=ccgg&type=ts
- 3.Gao C, Fernandez VI, Lee KS, Fenizia S, Pohnert G, Seymour JR, Raina JB, Stocker R. Single-cell bacterial transcription measurements reveal the importance of dimethylsulfoniopropionate (DMSP) hotspots in ocean sulfur cycling. Nat Commun. 2020;11(1):1942. doi: 10.1038/s41467-020-15693-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winayu BNR, Chen DS, Hsueh HT, Lin HP, Chu H. Combination of iron-silicate adsorption and Thermosynechococcus sp. CL-1 cultivation for swine wastewater treatment, CO2 fixation, phycobiliproteins generation. J. Water Process Eng. 2021;44:102406. doi: 10.1016/j.jwpe.2021.102406. [DOI] [Google Scholar]
- 5.Cheng J, Yang ZB, Zhou JH, Cen K. Improving the CO2 fixation rate by increasing flow rate of the flue gas from microalgae in a raceway pond. Korean J Chem Eng. 2018;35(2):498–502. doi: 10.1007/s11814-017-0300-1. [DOI] [Google Scholar]
- 6.Wang YN, Kai Y, Wang L, Tsang YF, Fu XH, Hu JJ, Xie YJ. Key internal factors leading to the variability in CO2 fixation efficiency of different sulfur-oxidizing bacteria during autotrophic cultivation. J Environ Manag. 2020;271:110957. doi: 10.1016/j.jenvman.2020.110957. [DOI] [PubMed] [Google Scholar]
- 7.Yadav G, Dash SK, Sen R. A biorefinery for valorization of industrial waste-water and flue gas by microalgae for waste mitigation, carbon-dioxide sequestration and algal biomass production. Sci Total Environ. 2019;688:129–135. doi: 10.1016/j.scitotenv.2019.06.024. [DOI] [PubMed] [Google Scholar]
- 8.Zeinab AS, Fereidun E, Dariush M. Integrated CO2 capture, nutrients removal and biodiesel production using Chlorella vulgaris. J Environ Chem Eng. 2021;9(1):104763. [Google Scholar]
- 9.Fernandez FGA, Gómez-Serrano C, Fernández-Sevilla JM. Recovery of nutrients from wastewaters using microalgae. Front Sustain Food Syst. 2018;2:59. doi: 10.3389/fsufs.2018.00059. [DOI] [Google Scholar]
- 10.Kato Y, Oyama T, Inokuma K, Vavricka CJ, Mastsuda M, Hidese R, Satoh K, Oono Y, Chang JS, Hasunuma T, Kondo A. Enhancing carbohydrate repartitioning into lipid and carotenoid by disruption of microalgae starch debranching enzyme. Commun Biol. 2021;4:450. doi: 10.1038/s42003-021-01976-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng M, Dai J, Ji X, Li D, He Y, Wang M, Huang J, Chen B. An integrated semi-continuous culture to treat original swine wastewater and fix carbon dioxide by an indigeneous Chlorella vulgaris MBFJNU-1 in an outdoor photobioreactor. Bioresour Technol. 2021;340:125703. doi: 10.1016/j.biortech.2021.125703. [DOI] [PubMed] [Google Scholar]
- 12.Kong WW, Kong J, Lyu HH, Ma J, Wang ZZ, Shen BX, Feng S. Application of indole-3-acetic acid in microalgae cultivation to improve the feasibility of simultaneously purifying wastewater, fixing CO2 and producing fatty acids under Hg stress. J Clean Prod. 2022;356:132028. doi: 10.1016/j.jclepro.2022.132028. [DOI] [Google Scholar]
- 13.Zhu L, Li Z, Hiltunen E. Microalgae Chlorella vulgaris biomass harvesting by natural flocculant: effects on biomass sedimentation, spent medium recycling and lipid extraction. Biotechnol Biofuels. 2018;11:1–10. doi: 10.1186/s13068-018-1183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chia SR, Chew KW, Leong HY, Ho SH, Munawaroh HSH, Show PL. CO2 mitigation and phycoremediation of industrial flue gas and wastewater via microalgae-bacteria consortium: possibilities and challenges. Chem Eng J. 2021;425:131436. doi: 10.1016/j.cej.2021.131436. [DOI] [Google Scholar]
- 15.Qu W, Zhang C, Chen X, Ho SH. New concept in swine wastewater treatment: development of a self-sustaining synergetic microalgae-bacteria symbiosis (ABS) system to achieve environmental sustainability. J Hazard Mater. 2021;418:126264. doi: 10.1016/j.jhazmat.2021.126264. [DOI] [PubMed] [Google Scholar]
- 16.Li D, Liu RQ, Cui XY, He ML, Zheng SY, Du WJ, Gao M, Wang CH. Co-culture of bacteria and microalgae for treatment of high concentration biogas slurry. J Water Process Eng. 2021;41:102014. doi: 10.1016/j.jwpe.2021.102014. [DOI] [Google Scholar]
- 17.Wang YN, Wang L, Tsang YF, Fu X, Hu J, Li H, Le Y. Response of cbb gene transcription levels of four typical sulfur-oxidizing bacteria to the CO2 concentration and its effect on their carbon fixation efficiency during sulfur oxidation. Enzym Microb Technol. 2016;92:31–40. doi: 10.1016/j.enzmictec.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 18.Almomani F, Judd S, Bhosale RR, Shurair M, Aljaml K, Khraisheh M. Intergraded wastewater treatment and carbon bio-fixation from flue gases using Spirulina platensis and mixed algal culture. Process Saf Environ. 2019;124:240–250. doi: 10.1016/j.psep.2019.02.009. [DOI] [Google Scholar]
- 19.Kong WW, Kong J, Ma J, Lyu HH, Feng S, Wang ZZ, Yuan P, Shen BX. Chlorella vulgaris cultivation in simulated wastewater for the biomass production, nutrients removal and CO2 fixation simultaneously. J Environ Manage. 2021;284:112070. doi: 10.1016/j.jenvman.2021.112070. [DOI] [PubMed] [Google Scholar]
- 20.Mousavi S, Najafpour GD, Mohammadi M. CO2 bio-fixation and biofuel production in an airlift photobioreactor by an isolated strain of microalgae Coelastrum sp SM under high CO2 concentrations. Environ Sci Pollut R. 2018;25(30):30139–30150. doi: 10.1007/s11356-018-3037-4. [DOI] [PubMed] [Google Scholar]
- 21.Shen QH, Jiang JW, Chen LP, Cheng LH, Xu XH, Chen HL. Effect of carbon source on biomass growth and nutrients removal of Scenedesmus obliquus for wastewater advanced treatment and lipid production. Bioresour Technol. 2015;190:257–263. doi: 10.1016/j.biortech.2015.04.053. [DOI] [PubMed] [Google Scholar]
- 22.Gonçalves AL, Simoes M, Pires JCM. The effect of light supply on microalgal growth, CO2 uptake and nutrient removal from wastewater. Energ Convers Manag. 2014;85:530–536. doi: 10.1016/j.enconman.2014.05.085. [DOI] [Google Scholar]
- 23.Dineshbabu G, Uma VS, Mathimani T, Deviram G, Ananth DA, Prabaharan D, Uma L. On-site concurrent carbon dioxide sequestration from flue gas and calcite formation in ossein effluent by a marine cyanobacterium Phormidium valderianum BDU 20041. Energ Convers Manag. 2017;141:315–324. doi: 10.1016/j.enconman.2016.09.040. [DOI] [Google Scholar]
- 24.Amit Nayak JK, Ghosh UK. Microalgal remediation of anaerobic pretreated pharmaceutical wastewater for sustainable biodiesel production and electricity generation. J Water Process Eng. 2020;35:101192. doi: 10.1016/j.jwpe.2020.101192. [DOI] [Google Scholar]
- 25.Hariz HB, Takriff MS. Palm oil mill effluent treatment and CO2 sequestration by using microalgae-sustainable strategiess for environmental protection. Environ Sci Pollut Res. 2017;24(25):20209–20240. doi: 10.1007/s11356-017-9742-6. [DOI] [PubMed] [Google Scholar]
- 26.Chaudhary R, Dikshit AK, Tong YW. Carbon-dioxide biofixation and phycoremediation of municipal wastewater using Chlorella vulgaris and Scenedesmus obliquus. Environ Sci Pollut R. 2018;25(21):20399–20406. doi: 10.1007/s11356-017-9575-3. [DOI] [PubMed] [Google Scholar]
- 27.Hu XF, Song CF, Mu HN, Liu ZZ, Kitamura Y. Optimization of simultaneous sobean processing wastewater treatment and flue gas CO2 fixation via chlorella sp. L166 cultivation. J Environ Chem Eng. 2020;8(4):103960. doi: 10.1016/j.jece.2020.103960. [DOI] [Google Scholar]
- 28.Faruque MO, Mohammed KA, Hossain MM, Razzak SA. Influence of elevated CO2 concentrations on growth, nutrient removal, and CO2 biofixation using Chlorella kessleri cultivation. Int J Environ Sci Te. 2021;18:913–926. doi: 10.1007/s13762-020-02909-4. [DOI] [Google Scholar]
- 29.Kuo CM, Jian JF, Lin TH, Chang YB, Wan XH, Lai JT, Chang JS, Lin CS. Simultaneous microalgal biomass production and CO2 fixation by cultivating Chlorella sp. GD with aquaculture wastewater and boiler flue gas. Bioresour Technol. 2016;221:241–250. doi: 10.1016/j.biortech.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Zheng M, Ji XW, He YJ, Li ZF, Wang MZ, Chen BL, Huang J. Simultaneous fixation of carbon dioxide and purification of undiluted swine slurry by culturing Chlorella vulgaris MBFJNU-1. Algal Res. 2020;47:101866. doi: 10.1016/j.algal.2020.101866. [DOI] [Google Scholar]
- 31.Liu XN, Chen GY, Tao Y, Wang J. Application of effluent from WWTP in cultivation of four microalgae for nutrients removal and lipid production under the supply of CO2. Renew Energ. 2020;149:708–715. doi: 10.1016/j.renene.2019.12.092. [DOI] [Google Scholar]
- 32.Çayli D, Uludag-Demirer S, Demirer GN. Coupled nutrient removal from the wastewater and CO2 biofixation from the flue gas of iron and steel manufacturing. Int J Global Warming. 2018;16(2):148–161. doi: 10.1504/IJGW.2018.094554. [DOI] [Google Scholar]
- 33.Shen Y, Gao JQ, Li LS. Municipal wastewater treatment via co-immobilized microalgal-bacterial symbiosis: Microorganism growth and nutrients removal. Bioresource Technol. 2017;243:905–913. doi: 10.1016/j.biortech.2017.07.041. [DOI] [PubMed] [Google Scholar]
- 34.Choix FJ, Polster EP, Corona-González RI, Snell-Castro R, Méndez-Acosta HO. Nutrient composition of culture media induces different patterns of CO2 fixation from biogas and biomass production by the microalga Scenedesmus obliquus U169. Bioprocess Biosyst Eng. 2017;40:1733–1742. doi: 10.1007/s00449-017-1828-5. [DOI] [PubMed] [Google Scholar]
- 35.Kong WW, Kong J, Lyu HH, Ma J, Yuan P, Wang ZZ, Shen BX, Feng S. Integrating municipal wastewater treatment with CO2 fixation and fatty acid production by cultivating Tetradesmus obliquus. J Clean Prod. 2021;320:128916. doi: 10.1016/j.jclepro.2021.128916. [DOI] [Google Scholar]
- 36.Iasimone F, De Felice V, Panico A, Pirozzi F. Experimental study for the reduction of CO2 emissions in wastewater treatment plant using microalgal cultivation. J CO2 Util. 2017;22:1–8. doi: 10.1016/j.jcou.2017.09.004. [DOI] [Google Scholar]
- 37.Honda R, Boonnorat J, Chiemchaisri C, Chiemchaisri W, Yamamoto K. Carbon dioxide capture and nutrients removal utilizing treated sewage by concentrated microalgae cultivation in a membrane photobioreactor. Bioresour Technol. 2012;125:59–64. doi: 10.1016/j.biortech.2012.08.138. [DOI] [PubMed] [Google Scholar]
- 38.Bhakta JN, Lahiri S, Pittman JK, Jana BB. Carbon dioxide sequestration in wastewater by a consortium of elevated carbon dioxide-tolerant microalgae. J CO2 Util. 2015;10:105–112. doi: 10.1016/j.jcou.2015.02.001. [DOI] [Google Scholar]
- 39.Zhu SF, Yin Y, Hu HY. Effects on the growth characteristic by mixed culture of three energy microalgae species using domestic secondary effluent. Ecol Environ Sci. 2014;23(4):642–648. [Google Scholar]
- 40.Johnson KR, Admassu W. Mixed algae cultures for low cost environmental compensation in cultures grown for lipid production and wastewater remediation. J Chem Technol Biot. 2013;88(6):992–998. doi: 10.1002/jctb.3943. [DOI] [Google Scholar]
- 41.Aslam A, Thomas-Hall SR, Manzoor M, Jabeen F, Iqbal M, Uz Zaman Q, Schenk M, Tahir MA. Mixed microalgae consortia growth under higher concentration of CO2 from unfiltered coal fired flue gas: fatty acid profiling and biodiesel production. J Photochem Photobiol. 2018;179:126–133. doi: 10.1016/j.jphotobiol.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Liu X, Wang KJ, Wang JY, Zuo JN, Peng F, Wu J, San EF. Carbon dioxide fixation coupled with ammonium uptake by immobilized Scenedesmus obliquus and its potential for protein production. Bioresour Technol. 2019;289:121685. doi: 10.1016/j.biortech.2019.121685. [DOI] [PubMed] [Google Scholar]
- 43.Abid A, Saidane F, Hamdi M. Feasibility of carbon dioxide sequestration by Spongiochloris sp microalgae during petroleum wastewater treatment in airlift bioreactor. Bioresour Technol. 2017;234:297–302. doi: 10.1016/j.biortech.2017.03.041. [DOI] [PubMed] [Google Scholar]
- 44.Van den Hende S, Vervaeren H, Desmet S, Boon N. Bioflocculation of microalgae and bacteria combined with flue gas to improve sewage treatment. New Biotechnol. 2011;29(1):23–31. doi: 10.1016/j.nbt.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 45.Ma SS, Yu YL, Cui H, Yadav RS, Li J, Feng YJ. Unsterilized sewage treatment and carbohydrate accumulation in Tetradesmus obliquus PF3 with CO2 supplementation. Algal Res. 2020;45:101741. doi: 10.1016/j.algal.2019.101741. [DOI] [Google Scholar]
- 46.Xu M, Xue ZX, Sun SQ, Zhao CZ, Liu JH, Liu J, Zhao YJ. Co-culturing microalgae with endophytic bacteria increases nutrient removal efficiency for biogas purification. Bioresour Technol. 2020;314:123766. doi: 10.1016/j.biortech.2020.123766. [DOI] [PubMed] [Google Scholar]
- 47.Alcantara C, Garcia-Encina PA, Muñoz R. Evaluation of the simultaneous biogas upgrading and treatment of centrates in a high-rate algal pond through C, N and P mass balances. Water Sci Technol. 2015;72(1):150–157. doi: 10.2166/wst.2015.198. [DOI] [PubMed] [Google Scholar]
- 48.Lebrero R, Toledo-Cervantes A, Muñoz R, del Nery V, Foresti E. Biogas upgrading from vinasse digesters: a comparison between an anoxic biotrickling filter and an algal-bacterial photobioreactor. J Chem Technol Biotechnol. 2016;91:2488–2495. doi: 10.1002/jctb.4843. [DOI] [Google Scholar]
- 49.Dong XC, Wei J, Huang J, Zhao CZ, Sun SQ, Zhao YJ. Performance of different microalgae-fungi-bacteria co-culture technologies in photosynthetic and removal performance in response to various GR24 concentrations. Bioresour Technol. 2022;347:126428. doi: 10.1016/j.biortech.2021.126428. [DOI] [PubMed] [Google Scholar]
- 50.Yan C, Muñoz R, Zhu L, Wang Y. The effects of various LED (light emitting diode) lighting strategies on simultaneous biogas upgrading and biogas slurry nutrient reduction by using of microalgae Chlorella sp. Energy. 2016;106:554–561. doi: 10.1016/j.energy.2016.03.033. [DOI] [Google Scholar]
- 51.Robles A, Capson-Tojo G, Gales A, Ruano MV, Sialve B, Ferrer J, Steyer JP. Microalgae-bacteria consortia in high-rate ponds for treating urban wastewater: elucidating the key state indicators under dynamic conditions. J Environ Manage. 2020;261:110244. doi: 10.1016/j.jenvman.2020.110244. [DOI] [PubMed] [Google Scholar]
- 52.González-Camejo J, Barat R, Pachés M, Murgui M, Seco A, Ferrer J. Wastewater nutrient removal in a mixed microalgae-bacteria culture: effect of light and temperature on the microalgae-bacteria competition. Environ Technol. 2018;39(4):503–515. doi: 10.1080/09593330.2017.1305001. [DOI] [PubMed] [Google Scholar]
- 53.Zhao X, Zhou Y, Huang S, Qiu D, Schideman L, Chai X, Zhao Y. Characterization of microalgae-bacteria consortium cultured in landfill leachate for carbon fixation and lipid production. Bioresour Technol. 2014;156:322–328. doi: 10.1016/j.biortech.2013.12.112. [DOI] [PubMed] [Google Scholar]
- 54.Cheng J, Xu J, Huang Y, Li Y, Zhou J, Cen K. Growth optimisation of microalga mutant at high CO2 concentration to purify undiluted anaerobic digestion effluent of swine manure. Bioresour Technol. 2015;177:240–246. doi: 10.1016/j.biortech.2014.11.099. [DOI] [PubMed] [Google Scholar]
- 55.Wang X, Gao S, Zhang Y, Zhao Y, Cao W. Performance of different microalgae-based techenologies in biogas slurry nutrient removal and biogas upgrading in response to various initial CO2 concentration and mixed light-emitting diode light wavelength treatments. J Clean Prod. 2017;166:408–416. doi: 10.1016/j.jclepro.2017.08.071. [DOI] [Google Scholar]
- 56.Yi T, Shan Y, Huang B, Tang T, Wei W, Quinn NWT. An efficient Chlorella sp.-Cupriavidus necator microcosm for phenol degradation and its cooperation mechanism. Sci Total Environ. 2020;743:140775. doi: 10.1016/j.scitotenv.2020.140775. [DOI] [PubMed] [Google Scholar]
- 57.Bahr M, Díaz I, Doming A, Sánchez AG, Muñoz R. Microalgal-biotechnology as a platform for an integral biogas upgrading and nutrient removal from anaerobi effluents. Environ Sci Technol. 2014;48:573–581. doi: 10.1021/es403596m. [DOI] [PubMed] [Google Scholar]
- 58.Wicker R, Bhatnagar A. Application of Nordic microalgal-bacterial consortia for nutrient removal from wastewater. Chem Eng J. 2020;398:125567. doi: 10.1016/j.cej.2020.125567. [DOI] [Google Scholar]
- 59.Franco-Morgado M, Alcántara C, Noyola A, Muñoz R, Sánchez AG. A study of photosynthetic biogas upgrading based on a high rate algal pond under alkaline conditions: influence of the illumination regime. Sci Total Environ. 2017;592:419–425. doi: 10.1016/j.scitotenv.2017.03.077. [DOI] [PubMed] [Google Scholar]
- 60.Wang YY, Wang SY, Sun LQ, Sun ZL, Li D. Screening of a Chlorella-bacteria consortium and research on piggery wastewater purification. Algal Res. 2020;47:101840. doi: 10.1016/j.algal.2020.101840. [DOI] [Google Scholar]
- 61.Lim YA, Khong NMH, Priyawardana SD, Ooi KR, Ilankoon IMSK, Chong MN, Foo SC. Distinctive correlations between cell concentration and cell size to microalgae biomass under increasing carbon dioxide. Bioresour Technol. 2022;347:126733. doi: 10.1016/j.biortech.2022.126733. [DOI] [PubMed] [Google Scholar]
- 62.Kao CY, Chen TY, Chang YB, Chiu TW, Lin HY, Chen CD, Chang JS, Lin CS. Utilization of carbon dioxide in industrial flue gases for the cultivation ofmicroalga Chlorella sp. Bioresour Technol. 2014;166:485–493. doi: 10.1016/j.biortech.2014.05.094. [DOI] [PubMed] [Google Scholar]
- 63.Banerjee S, Banerjee S, Ghosh AK, Das D. Maneuvering the generic and metabolic pathway for improving biofuel production in algae: present status and future prospective. Renew Sust Energ Rev. 2020;133:110155. doi: 10.1016/j.rser.2020.110155. [DOI] [Google Scholar]
- 64.Wei L, Wang Q, Xin Y, Lu Y, Xu J. Enhancing photosynthetic biomass productivity of industrial oleaginous microalgae by overexpression of RuBisCo activase. Algal Res. 2017;27:366–375. doi: 10.1016/j.algal.2017.07.023. [DOI] [Google Scholar]
- 65.Yang B, Liu J, Ma X, Guo B, Liu B, Wu T, Jiang Y, Chen F. Genetic engineering of the Calvin cycle toward enhanced photosynthetic CO2 fixation in microalgae. Biotechnol Biofuels. 2017;10(1):229. doi: 10.1186/s13068-017-0916-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang S, Guarnieri MT, Smolinski S, Ghirardi M, Pienkos PT. De novo transcriptomic analysis of hydrogen production in the green alga Chlamydomonas moewusii through RNA-Seq. Biotechnol Biofuels. 2013;6(1):118. doi: 10.1186/1754-6834-6-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu PL, Li J, Qian J, Wang B, Liu J, Xu R, Chen P, Zhou WG. Recent advances in CO2 fixation by microalgae and its potential contribution to carbon neutrality. Chemosphere. 2023;319:137987. doi: 10.1016/j.chemosphere.2023.137987. [DOI] [PubMed] [Google Scholar]
- 68.Gim GH, Ryu J, Kim MJ, Kim PI, Kim SW. Effects of carbon source and light intensity on the growth and total lipid production of three microalgae under different culture conditions. J Ind Microbiol Biotechnol. 2016;43(5):605–616. doi: 10.1007/s10295-016-1741-y. [DOI] [PubMed] [Google Scholar]
- 69.Zhang B, Wang L, Riddicka BA, Li R, Able JR, Boakye-Boaten NA, Shahbazi A. Sustainable production of algal biomass and biofuels using swine wastewater in North Carolina, US. Sustainability. 2016;8(5):477. doi: 10.3390/su8050477. [DOI] [Google Scholar]
- 70.Markou G, Angelidaki I, Georgakakis D. Microalgal carbohydrates: an overview of the factors influencing carbohydrates production, and of main bioconversion technologies for production of biofuels. Appl Microbiol Biotechnol. 2012;3:631–645. doi: 10.1007/s00253-012-4398-0. [DOI] [PubMed] [Google Scholar]
- 71.Lim HR, Khoo KS, Chia WY, Chew KW, Ho S-H, Show PL. Smart microalgae farming with internet-of-things for sustainable agriculture. Biotechnol Adv. 2022;57:107931. doi: 10.1016/j.biotechadv.2022.107931. [DOI] [PubMed] [Google Scholar]
- 72.Yew GY, Puah BK, Chew KW, Teng SY, Show PL, Nguyen THP. Chlorella vulgaris FSP-E cultivation in waste molasses: photo-to-property estimation by artificial intelligence. Chem Eng J. 2020;402:126230. doi: 10.1016/j.cej.2020.126230. [DOI] [Google Scholar]
- 73.Wang ZY, Chen J, Zhang XD, Chen LC, Liu JZ. Metabolic pathways of Chlorella sp. cells induced by exogenous spermidine against nitric oxide damage from coal-fired flue gas. Bioresour Technol. 2021;328:124827. doi: 10.1016/j.biortech.2021.124827. [DOI] [PubMed] [Google Scholar]
- 74.Zhao PC, Wang YM, Lin ZY, Zhou J, Chai HX, He Q, Li YC, Wang JL. The alleviative effect of exogenous phytohormones on the growth, physiology and gene expression of Tetraselmis cordiformis under high ammonia-nitrogen stress. Bioresour Technol. 2019;282:339–347. doi: 10.1016/j.biortech.2019.03.031. [DOI] [PubMed] [Google Scholar]
- 75.Floerder S, Combuechen A, Pasternak A, Hillebrand H. Competition between pelagic and benthic microalgae for phosphorus and light. Aquat Sci. 2006;68(4):425–433. doi: 10.1007/s00027-006-0824-7. [DOI] [Google Scholar]
- 76.Kuwata A, Miyazaki T. Effects of ammonium supply rates on competition between Microcystis novacekii (Cyanobacteria) and Scenadesmus quadricauda (Chlorophyta): simulation study. Ecol Model. 2000;135(1):81–87. doi: 10.1016/S0304-3800(00)00363-X. [DOI] [Google Scholar]
- 77.Twirler M, Dixon SI, Trick CG. Toxic effects of Heterosigma akashiwo do not appear to be mediated by hydrogen peroxide. Limnol Oceanogr. 2001;46:1400–1405. doi: 10.4319/lo.2001.46.6.1400. [DOI] [Google Scholar]
- 78.Cai ZB, Li HT, Pu SC, Ke J, Wang D, Liu YH, Chen JQ, Guo RX. Development of autotrophic and heterotrophic consortia via immobilized microbial beads for chemical wastewater treatment, using PTA wastewater as an approach. Chemosphere. 2021;281:131001. doi: 10.1016/j.chemosphere.2021.131001. [DOI] [PubMed] [Google Scholar]
- 79.Munoz R, Guieysse B. Algal-bacterial processes for the treatment of hazardous contaiminants: a review. Water Res. 2006;40(15):2799–2815. doi: 10.1016/j.watres.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 80.Saravanan A, Kumar PS, Varjani S, Jeevanantham S, Yaashikaa PR, Thamarai P, Abirmi B, George CS. A review on algal-bacterial symbiotic system for effective treatment of wastewater. Chemosphere. 2021;271:129540. doi: 10.1016/j.chemosphere.2021.129540. [DOI] [PubMed] [Google Scholar]
- 81.Toyama T, Kasuya M, Hanaoka T, Kobayashi N, Tanaka Y, Inoue D, Sei K, Morikawa M, Mori K. Growth promotion of three microalgae, Chlamydomonas reinhardtii, Chlorella vulgaris and Euglena gracilis, by in situ indigenous bacteria in wastewater effluent. Biotechnol Biofuels. 2018;11:176. doi: 10.1186/s13068-018-1174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Unnithan VV, Unc A, Smith GB. Mini-review: a priori considerations for bacteria-algae interactions in algal biofuel systems receiving municipal wastewater. Algal Res. 2014;4:35–40. doi: 10.1016/j.algal.2013.11.009. [DOI] [Google Scholar]
- 83.Xu JR, Alam A, Wang J, Wu WC, Yusof ZNB, Daroch M, Zhang DY, Liu LF, Russel M. Enhanced removal of tetracycline from synthetic wastewater using an optimal ratio of co-culture of Desmodesmus sp. and Klebsiella pneumoniae. Bioresour Technol. 2022;351:127056. doi: 10.1016/j.biortech.2022.127056. [DOI] [PubMed] [Google Scholar]
- 84.Moheimani NR, Isdepsky A, Liscc J, Raes E, Borowitzka MA. Coccolithophorid algae culture in closed photobioreactors. Biotechnol Bioeng. 2011;108(9):2078–2087. doi: 10.1002/bit.23161. [DOI] [PubMed] [Google Scholar]
- 85.Stoll HM, Guitian J, Hernandez-Almeida I, Mejia LM, Phelps S, Polissar P, Rosenthal Y, Zhang HR, Ziveri P. Upregulation of phytoplankton carbon concentrating mechanisms during low CO2 glacial periods and implications for the phytoplankton pCO(2) proxy. Quaternary Sci Rev. 2019;208:1–20. doi: 10.1016/j.quascirev.2019.01.012. [DOI] [Google Scholar]
- 86.Price GD, Badge MR, Woodger FJ, Long BM. Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. J Exp Bot. 2007;59(7):1441–1471. doi: 10.1093/jxb/erm112. [DOI] [PubMed] [Google Scholar]
- 87.Berg IA. Ecological aspects of the distribution of different autotrophic CO2 fixation pathways. Appl Environ Microbiol. 2011;77:1925–1936. doi: 10.1128/AEM.02473-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Choi KR, Ahn YJ, Lee SY. Bacterial conversion of CO2 to organic compounds. J CO2 Util. 2022;58:101929. doi: 10.1016/j.jcou.2022.101929. [DOI] [Google Scholar]
- 89.Tourova TP, Kovaleva OL, Sorokin DY, Muyzer G. Ribulose-1,5-bisphosphate carboxylase/oxygenase genes as a functional marker for chemolithoautotrophic halophilic sulfur-oxidizing bacteria in hypersaline habitats. Microbiology. 2010;156:2016–2025. doi: 10.1099/mic.0.034603-0. [DOI] [PubMed] [Google Scholar]
- 90.Toyoda K, Ishii M, Arai H. Function of three rubisco enzymes under different CO2 conditions in Hydrogenovibrio marinus. J Biosci Bioeng. 2018;126:730–735. doi: 10.1016/j.jbiosc.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 91.Mohan SV, Rohit MV, Chiranjeevi P, Chandra R, Navaneeth B. Heterotrophic microalgae cultivation to synergize biodiesel production with waste remediation: progress and perspectives. Bioresour Technol. 2015;184:169–178. doi: 10.1016/j.biortech.2014.10.056. [DOI] [PubMed] [Google Scholar]
- 92.Perez-Garcia O, Escalante FME, De-Bashan LE, Bashan Y. Heterotrophic cultures of microalgae: metabolism and potential products. Water Res. 2011;45(1):11–36. doi: 10.1016/j.watres.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 93.Udaiyappan AFM, Hasan HA, Takriff MS, Abdullah SRS, Maeda T, Mustapha NA, Yasin NHM, Hakimi NIN. Microalgae-bacteria interaction in palm oil mill effluent treatment. J Water Process Eng. 2020;35:101203. doi: 10.1016/j.jwpe.2020.101203. [DOI] [Google Scholar]
- 94.Liu JZ, Wu YH, Wu CX, Muylaet K, Vyverman W, Yu HQ, Munoz R, Rittmann B. Advanced nutrient removal from surface water by a consortium of attached microalgae and bacteria: a review. Bioresour Technol. 2017;241:1127–1137. doi: 10.1016/j.biortech.2017.06.054. [DOI] [PubMed] [Google Scholar]
- 95.Choi O, Das A, Yu CP, Hu Z. Nitrifying bacterial growth inhibition in the presence of algae and cyanobacteria. Biotechnol Bioeng. 2010;107(6):1004–1011. doi: 10.1002/bit.22860. [DOI] [PubMed] [Google Scholar]
- 96.Cai T, Park SY, Li YB. Nutrient recovery from wastewater streams by microalgae: status and prospects. Renew Sust Energ Rev. 2013;19:360–369. doi: 10.1016/j.rser.2012.11.030. [DOI] [Google Scholar]
- 97.Solovchenko A, Verschoor AM, Jablonowski ND, Nedbal L. Phosphorus from wastewater to crops: An alternative path involving microalgae. Biotechnol Adv. 2016;34(5):550–564. doi: 10.1016/j.biotechadv.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 98.Martínez ME, Jimenez JM, El Yousfi F. Influence of phosphorus concentration and temperature on growth and phosphorus uptake by the microalga Scenedesmus obliquus. Bioresour Technol. 1999;67(3):233–240. doi: 10.1016/S0960-8524(98)00120-5. [DOI] [Google Scholar]
- 99.Fallahi A, Rezvani F, Asgharnejad H, Nazloo EK, Hajinajaf N, Higgins B. Interactions of microalgae-bacteria consortia for nutrient removal from wastewater: a review. Chemosphere. 2021;272:129878. doi: 10.1016/j.chemosphere.2021.129878. [DOI] [PubMed] [Google Scholar]
- 100.Zuo N, He J, Ma X, Peng Y, Li X. Phosphorus removal performance and population structure of phosphorus-accumulating organisms in HA-A/A-MCO sludge reduction process. Bioengineered. 2016;7(5):327–333. doi: 10.1080/21655979.2016.1197026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mohd Nor MN, Sabaratnam V, Tan GYA. Devosia elais sp. Nov., isolated from oil palm rhizospheric soil. Int J Evol Microbiol. 2017;67(4):851–855. doi: 10.1099/ijsem.0.001683. [DOI] [PubMed] [Google Scholar]
- 102.Tarayre C, Nguyen HT, Brognaux A, Delepierre A, Clercq LD, Charlier R, Michels E, Meers E, Delvigne F. Characterisation of phosphate accumulating organisms and techniques for polyphosphate detection: a review. Sensors. 2016;16:797–810. doi: 10.3390/s16060797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Forján E, Navarro F, Cuaresma M, Vaquero I, Ruíz-domínguez MC, Gojkovic Ž, Vázquez M, Márquez M, Mogedas B, Bermejo E, Girlich S, Domínguez MJ, Vílchez C, Garbayo JMI. Microalgae: fast-growth sustainable green factories. Crit Rev Environ Sci Technol. 2015;45(16):1705–1755. doi: 10.1080/10643389.2014.966426. [DOI] [Google Scholar]
- 104.Nguyen TDP, Le TVA, Show PL, Nguyen TT, Tran MH, Tran TNT, Lee SY. Bioflocculation formation of microalgae-bacteria in enhancing microalgae harvesting and nutrient removal from wastewater effluent. Bioresour Technol. 2018;272:34–39. doi: 10.1016/j.biortech.2018.09.146. [DOI] [PubMed] [Google Scholar]
- 105.Matter IA, Hoang Bui VK, Jung M, Seo JY, Kim YE, Lee YC, Oh YK. Flocculation harvesting techniques for microalgae: a review. Appl Sci. 2019;9(15):3069. doi: 10.3390/app9153069. [DOI] [Google Scholar]
- 106.Rodero MDR, Muñoz R, Lebrero R, Verfaillie A, Blockx J, Thielemans W, Muylaer K, Praveenkumar R. Harvesting microalgal-bacterial biomass from biogas upgrading process and evaluating the impact of flocculants on their growth during repeated recycling of the spent medium. Algal Res. 2020;48:101915. doi: 10.1016/j.algal.2020.101915. [DOI] [Google Scholar]
- 107.Singh G, Patidar SK. Microalgae harvesting techniques: a review. J Environ Manag. 2018;217:499–508. doi: 10.1016/j.jenvman.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 108.Mata TM, Martins AA, Caetano NS. Microalgae for biodiesel production and other applications: a review. Renew Sust Energ Rev. 2010;14:217–232. doi: 10.1016/j.rser.2009.07.020. [DOI] [Google Scholar]
- 109.Cho DH, Ramanan R, Heo J, Kang Z, Kim BH, Ahn CY, Oh HM, Kim HS. Organic carbon, influent microbial diversity and temperature strongly influence algal diversity and biomass in raceway ponds treating raw municipal wastewater. Bioresour Technol. 2015;191:481–487. doi: 10.1016/j.biortech.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 110.Gerardo ML, Van den Hende S, Vervaeren H, Coward T, Skill SC. Harvesting of microalgae with a biorefinery approach: a review of the developments and case studies from pilot-plants. Algal Res. 2015;11:248–262. doi: 10.1016/j.algal.2015.06.019. [DOI] [Google Scholar]
- 111.Gutiérrez R, Passos F, Ferrer I, Uggetti E, García J. Harvesting microalgae from wastewater treatment systems with natural flocculants: effect on biomass settling and biogas production. Algal Res. 2015;9:204–211. doi: 10.1016/j.algal.2015.03.010. [DOI] [Google Scholar]
- 112.Lee J, Cho DH, Ramanan R, Kim BH, Oh HM, Kim HS. Microalgae-associated bacteria play a key role in the flocculation of Chlorella vulgaris. Bioresour Technol. 2013;131:195–201. doi: 10.1016/j.biortech.2012.11.130. [DOI] [PubMed] [Google Scholar]
- 113.Malik S, Khan F, Atta Z, Habib N, Haider MN, Wang N, Alam A, Jambi EJ, Gull M, Mehmood MA, Zhu H. Microalgal flocculation: global research progress and prospects for algal biorefinery. Biotechnol Appl Bioc. 2020;67(1):52–60. doi: 10.1002/bab.1828. [DOI] [PubMed] [Google Scholar]
- 114.Wu H, Li J, Wang C, Liao Q, Fu Q, Liu Z. Sequent production of proteins and biogas from Chlorella sp. via CO2 assisted hydrothermal treatment and anaerobic digestion. J Clean Prod. 2020;277:123563. doi: 10.1016/j.jclepro.2020.123563. [DOI] [Google Scholar]
- 115.Yap JK, Sankaran R, Chew KW, Munawaroh HSH, Ho SH, Banu JR. Advancement of green technologies: a comprehensive review on the potential application of microalgae biomass. Chemosphere. 2021;281:130886. doi: 10.1016/j.chemosphere.2021.130886. [DOI] [PubMed] [Google Scholar]
- 116.Behera S, Singh R, Arora R, Sharma NK, Shukla M, Kumar S. Scope of algae as third generation biofuels. Front Bioeng Biotechnol. 2015;2:90. doi: 10.3389/fbioe.2014.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Qu W, Show PL, Hasunuma T, Ho SH. Optimizing real swine wastewater treatment efficiency and carbohydrate productivity of newly microalga Chlamydomonas sp. QWY37 used for cell-displayed bioethanol production. Bioresour Technol. 2020;305:123072. doi: 10.1016/j.biortech.2020.123072. [DOI] [PubMed] [Google Scholar]
- 118.Lunprom S, Phanduang O, Salakkam A, Liao Q, Reungsang A. A sequential process of anaerobic solid-state fermentation followed by dark fermentation for bio-hydrogen production from Chlorella sp. Int J Hydrogen Energy. 2019;4(6):3306–3316. doi: 10.1016/j.ijhydene.2018.06.012. [DOI] [Google Scholar]
- 119.Camacho F, Macedo A, Malcata F. Potential industrial applications and commercialization of microalgae in the functional food and feed industries: a short review. Mar Drugs. 2019;17(6):312. doi: 10.3390/md17060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Qureshi MA, Garlich JD, Kidd MT. Dietary Spirulina platensis enhances humoral and cell-mediated immune functions in chickens. Immunopharm Immunot. 1996;18(3):465–476. doi: 10.3109/08923979609052748. [DOI] [PubMed] [Google Scholar]
- 121.Thaakur SR, Jyothi B. Effect of Spirulina maxima on the haloperidol induced tardive dyskinesia and oxidative stress in rats. J Neural Transm. 2007;114(9):1217–1255. doi: 10.1007/s00702-007-0744-2. [DOI] [PubMed] [Google Scholar]
- 122.Fernando JSR, Premaratne M, Dinalankara DMSD, Perera GLNJ, Ariyadasa TU. Cultivation of microalgae in palm oil mill effluent (POME) for astaxanthin production and simultaneous phycoremediation. J Environ Chem Eng. 2021;9:105375. doi: 10.1016/j.jece.2021.105375. [DOI] [Google Scholar]
- 123.Sharma V, Prasanna R, Hossain F, Muthusamy V, Nain L, Shivay YS, Kumar S. Cyanobacterial inoculation as resource conserving options for improving the soil nutrient availability and growth of maize genotypes. Arch Microbiol. 2021;203(5):2393–2409. doi: 10.1007/s00203-021-02223-8. [DOI] [PubMed] [Google Scholar]
- 124.Dineshkumar R, Subramanian J, Arumugam A, Rasheeq AA, Sampathkumar P. Exploring the microalgae biofertilizer effect on onion cultivation by field experiment. Waste Biomass Valori. 2018;11(1):77–87. doi: 10.1007/s12649-018-0466-8. [DOI] [Google Scholar]
- 125.Silambarasan S, Logeswari P, Sivaramakrishnan R, Incharoensakdi A, Cornejo P, Kamaraj B, Chi NTL. Removal of nutrients from domestic wastewater by microalgae coupled to lipid augmentation for biodiesel production and influence of deoiled algal biomass as biofertilizer for Solanum lycopersicum cultivation. Chemosphere. 2021;268:129323. doi: 10.1016/j.chemosphere.2020.129323. [DOI] [PubMed] [Google Scholar]
- 126.Khoo KS, Chia WY, Chew KW, Show PL. Microalgal-bacterial consortia as future prospect in wastewater bioremediation, environmental management and bioenergy production. Indian J Microbiol. 2021;61(3):262–269. doi: 10.1007/s12088-021-00924-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wu D, Sun P, Lu PZ, Chen YY, Guo JM, Liu M, Wang L, Zhang CJ. Effect and approach of Enteromorpha prolifera biochar to improve coastal saline soil. Environ Sci. 2020;41(4):1941–1949. doi: 10.13227/j.hjkx.201909044. [DOI] [PubMed] [Google Scholar]
- 128.Khan AA, Gul J, Naqvi SR, Ali I, Farooq W, Liaqat R, AlMohamdi H, Štĕpanec L, Juchelková D. Recent progress in microalgae-derived biochar for the treatment of texile industry wastewater. Chemosphere. 2022;306:135565. doi: 10.1016/j.chemosphere.2022.135565. [DOI] [PubMed] [Google Scholar]
- 129.Singh A, Sharma R, Pant D, Malaviya P. Engineered algal biochar for contaminant remediation and electrochemical applications. Sci Total Environ. 2021;774:1456786. doi: 10.1016/j.scitotenv.2021.145676. [DOI] [Google Scholar]
- 130.Cao L, Yu IKM, Cho DW, Wang D, Tsang DCW, Zhang S, Ding S, Wang L, Ok YS. Microwave-assisted low-temperature hydrothermal treatment of red seaweed (Gracilaria lemaneiformis) for production of levulinic acid and algae hydrochar. Bioresour Technol. 2019;273:251–258. doi: 10.1016/j.biortech.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 131.Ullah S, Khalil AA, Shaukat F, Song Y. Sources, extraction and biomedical properties of polysaccharides. Foods. 2019;8:304. doi: 10.3390/foods8080304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ahirwar A, Kesharwani K, Deka R, Muthukumar S, Khan MJ, Rai A, Vinayak V, Varjani S, Joshi KB, Morjaria S. Microalgal drugs: A promising therapeutic reserve for the future. J Biotechnol. 2022;349:32–46. doi: 10.1016/j.jbiotec.2022.03.012. [DOI] [PubMed] [Google Scholar]
- 133.Nowruzi B, Sarvari G, Blanco S. Applications of Cyanobacteria in Biomedicine. Handbook of Algal Science, Technology and Medicine. Elsevier. 2020: 441–453. 10.1016/B978-0-12-818305-2.00028-0
- 134.Rizwan M, Mujtaba G, Memon SA, Lee K, Rashid N. Exploring the potential of microalgae for new biotechnology applications and beyond: a review. Renew Sustain Energy Rev. 2018;92:394–404. doi: 10.1016/j.rser.2018.04.034. [DOI] [Google Scholar]
- 135.Gopu M, Selvam K. Polysaccharides from marine red algae Amphiroa rigida and their biomedical potential: an in-vitro study. Biocatal Agric Biotechnol. 2020;29:101769. doi: 10.1016/j.bcab.2020.101769. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.