Abstract
Salmonella enterica poxA mutants exhibit a pleiotropic phenotype, including reduced pyruvate oxidase activity; reduced growth rate; and hypersensitivity to the herbicide sulfometuron methyl, α-ketobutyrate, and amino acid analogs. These mutants also failed to grow in the presence of the host antimicrobial peptide, protamine. In this study, PoxA− mutants of S. enterica serovar Typhimurium (S. typhimurium) were found to be 10,000-fold attenuated in orally inoculated BALB/c mice and 1,000-fold attenuated in intraperitoneally inoculated BALB/c mice, compared to wild-type S. typhimurium UK-1. In addition, poxA mutants were found to be capable of colonizing the spleen, mesenteric lymph nodes, and Peyer’s patches; to induce strong humoral immune responses; and to protect mice against a lethal wild-type Salmonella challenge. A 2-kb DNA fragment was isolated from wild-type S. typhimurium UK-1 based on its ability to complement an isogenic poxA mutant. The nucleotide sequence of this DNA fragment revealed an open reading frame of 325 amino acids capable of encoding a polypeptide of 36.8 kDa that was confirmed in the bacteriophage T7 expression system. Comparison of the translated sequence to the available databases indicated high homology to a family of lysyl-tRNA synthetases. Our results indicate that a mutation of poxA has an attenuating effect on Salmonella virulence. Further, poxA mutants are immunogenic and could be useful in designing live vaccines with a variety of bacterial species. To our knowledge, this is the first report on the effect of poxA mutation on bacterial virulence.
The pyruvate oxidase of Escherichia coli is a peripheral membrane protein that catalyzes the oxidative decarboxylation of pyruvate to acetate and CO2 (19). Under laboratory conditions, this enzyme is not essential and conversion of pyruvate to acetate is considered a waste of energy, compared with its conversion to acetyl coenzyme A (18). Pyruvate oxidase has been of interest primarily as a model for studying protein-lipid interaction. The enzyme is a water-soluble tetramer of identical 62-kDa subunits (18). It requires thiamine pyrophosphate, flavin adenine dinucleotide, and Mg2+ as cofactors (2, 3, 19). In the presence of the substrate and cofactors, the enzyme undergoes conformational changes and binds to E. coli membrane vesicles and to phospholipid vesicles (38, 43). This peripheral membrane binding is necessary for the terminal transfer of electrons to ubiquinone-8, which is dissolved in the lipid bilayer (20, 28).
To study protein-lipid interactions by genetic means, mutations in two genes affecting pyruvate oxidase activity have been identified. The structural gene for pyruvate oxidase, poxB, has been located at 18.7 min on the E. coli genetic map (5), and a regulatory gene, poxA, has been located at 94 min (4). Enzymatic and immunological data indicated that mutations in poxA result in a 6- to 10-fold decrease in pyruvate oxidase levels (4, 5). Chang and Cronan reported that poxA mutants grew more slowly than the isogenic parent in both minimal and rich media, while poxB mutants exhibited normal growth.
In their efforts to elucidate the mechanism of inhibition of acetolactate synthase II by the herbicide sulfometuron methyl (SM) {N-[(4,6-dimethylpyrimidin-2-yl)aminocarbonyl]-2-methoxycarbonyl-benzenesulfonamide} in Salmonella enterica serovar Typhimurium (S. typhimurium), Van Dyk and LaRossa (48) isolated 15 mutant strains sensitive to SM, following Tn10 mutagenesis. Among these SM-hypersensitive mutations, a poxA mutation was identified and mapped to the 94-min region of the S. typhimurium genetic map (49), a location analogous to that of poxA in E. coli. Like the E. coli counterpart, the S. typhimurium poxA mutant exhibited reduced pyruvate oxidase activity and growth rate (49). Furthermore, the E. coli and S. typhimurium poxA mutants shared several additional phenotypes including hypersensitivity to SM; α-ketobutyrate (AKB); and a wide range of bacterial growth inhibitors such as antibiotics, amino acid analogs, and dyes (49). Since mutants defective in poxB did not exhibit these phenotypes, it was concluded that a mutation within the poxA regulatory locus of E. coli and S. typhimurium would result in pleiotropic effects not due solely to decreased poxB expression.
Although the poxA gene was identified 16 years ago, molecular and functional data on the gene and gene product are lacking. In this study, we cloned and determined the nucleotide sequence of the poxA gene and characterized the poxA gene product of S. typhimurium. Our results show for the first time that S. typhimurium mutants with deletions of the poxA gene are attenuated and immunogenic.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains used in this study and their sources are listed in Table 1. Bacteriophage P22HTint was used for transduction of markers into Salmonella strains (41). Strains were grown in Luria broth (LB), Lennox medium (32), or antibiotic no. 2 (AB2) agar (Difco, Detroit, Mich.). When required, antibiotics were added to the growth media at the following concentrations: kanamycin, 50 μg/ml; ampicillin, 100 μg/ml; streptomycin, 100 μg/ml; tetracycline, 12.5 μg/ml.
TABLE 1.
Bacterial strains used in this study
| Strain | Relevant genotype | Reference or source |
|---|---|---|
| S. typhimurium LT2 strain SMS401 | poxA401::Tn10 | 49 |
| S. typhimurium UK-1 | ||
| χ3761 | Wild-type UK-1 | R. Curtiss’ collection |
| MGN-791 | poxA401::Tn10 | This work |
| MGN-816 | ΔpoxA402 | This work |
| MGN-939 | ΔpoxA402 (pMEG-250) | This work |
| MGN-1036 | ΔpoxA270 | This work |
| MGN-1154 | ΔpoxA270 (pMEG-274) | This work |
| E. coli | ||
| BL21(DE3) | F−ompT [lon] hsdS (r− m−; an E. coli B strain) with DE3, λ T7 RNA polymerase | 46 |
| MGN-617 | SM10 λpir derivative, thi thr leu tonA lacY supE λpir recA::RP4-2-Tc::Mu (Kmr) ΔasdA1 | 37a |
| CC118 λpir | araD139 Δ(ara-leu)7697 ΔlacX74 galK ΔphoA20 galE recA1 rpsE argE(Am) rpoB thi λpir | 10 |
Recombinant DNA, genetic techniques, and nucleotide sequencing.
Recombinant DNA techniques were performed according to standard procedures (40). Total DNAs from Salmonella, Shigella, Klebsiella, Pseudomonas, Pasteurella, and Borrelia species were isolated according to a published protocol (34). Total DNAs of Mycobacterium tuberculosis and Erysipelothrix rhusiopathiae were kindly provided by J. Clark-Curtiss and T. K. Ball, respectively. Total DNA was digested with ClaI or EcoRV and resolved on a 0.8% agarose gel. Southern blotting and hybridization were performed according to standard procedures (40). DNA probes were fluorescein labeled (Amersham, Arlington Heights, Ill.) according to the manufacturer’s instructions. Transformation of plasmid DNA into E. coli and Salmonella strains by electroporation was carried out as described elsewhere (36) by using an E. coli Gene Pulser apparatus (Bio-Rad Laboratories, Richmond, Calif.). The poxA401::Tn10 insertion (48, 49) was moved into S. typhimurium UK-1 by P22HTint transduction as previously described (42). Expression and [35S]methionine labeling of plasmid-encoded polypeptides in a bacteriophage T7 expression system were carried out as described elsewhere (47), with E. coli BL21(DE3) as host (46) for the expression vectors derived from plasmid pBluescript II (Stratagene, La Jolla, Calif.). Nucleotide sequence determination was performed by ACGT, Inc. (Northbrook, Ill.), with double-stranded DNA as the template, and both strands were sequenced. Nucleotide sequence analyses were performed with the MacVector software package (version 5). Comparison of translated and nontranslated nucleotide sequences with those in the available databases was carried out with the BLAST program at the server of the National Center for Biotechnology Information at the National Library of Medicine (1).
Construction of ΔpoxA270 defined deletion strains.
A defined deletion in poxA was constructed by removing the last 270 amino acids of PoxA encoded by a 1,018-bp BstBI-XhoI fragment of pMEG-273, filling in the termini with the large fragment of DNA polymerase I, and religating, yielding plasmid pMEG-279. A 1-kb XhoI-XbaI fragment of pMEG-279, carrying the mutated ΔpoxA270 allele and flanking sequences, was cloned into the SalI-XbaI sites of the R6K-derived replicon pKNG101 (24) to give the suicide plasmid pMEG-280. The defined mutation ΔpoxA270 was subsequently recombined into the S. typhimurium UK-1 chromosome by bacterial mating according to the procedure described elsewhere (23) with the following modification: 100 μl of fresh overnight culture of the universal donor E. coli MGN-617 (Table 1) harboring the suicide plasmid pMEG-280 and 100 μl of the recipient S. typhimurium χ3761 were mixed in 5 ml of buffered saline gelatin. The solution was filtered through a 0.45-μm-pore-size-filter, which was then placed on the surface of an L-agar plate containing 100 μg of diaminopimelic acid per ml and incubated at 37°C for at least 8 h. Bacteria bound to the filter were then resuspended in 5 ml of buffered saline gelatin, and 10-fold dilutions were plated onto LB agar containing 100 μg of streptomycin per ml. Single recombinants that had the plasmid integrated into the chromosome were grown in LB in the absence of antibiotic selection, and 10-fold dilutions were plated on AB2 agar medium containing 5% sucrose to select for strains that had undergone resolution of the cointegrate by recombination. Double-crossover recombinants were tested for streptomycin sensitivity due to loss of the suicide plasmid, for the PoxA− phenotype on AB2 plates, and for sensitivity to 1.25 mg of protamine sulfate per ml.
Animal experiments.
Oral and intraperitoneal inoculations of 6- to 7-week-old female BALB/c mice with the different S. typhimurium strains were carried out as previously described (17).
Analysis of the humoral immune responses.
The humoral immune responses to S. typhimurium poxA mutants were assessed by enzyme-linked immunosorbent assay (ELISA). Microtiter plates were coated with 250 ng of S. typhimurium lipopolysaccharide (LPS) (Sigma, St. Louis, Mo.) per well in 0.2% trichloroacetic acid (pH 7.4) for 2 h at 37°C. Free binding sites were blocked with 3% bovine serum albumin and 0.1% Tween in phosphate-buffered saline (PBS) for 30 min at room temperature. Washes were performed with PBS-Tween between incubations. Plates were incubated with mouse sera diluted in PBS-Tween for 1 h at 37°C, followed by a 1-h incubation at 37°C with the secondary antibody goat anti-mouse immunoglobulin A (IgA)-, IgM-, or IgG-conjugated alkaline phosphatase (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) diluted 1:500 in PBS-Tween. Plates were developed with p-nitrophenylphosphate (Sigma), and the optical density at 405 nm was measured after a 30-min incubation at 37°C.
Nucleotide sequence accession number.
The nucleotide sequence described in this paper has been deposited in GenBank under accession no. AF001831.
RESULTS
Construction of S. typhimurium poxA mutants and cloning of the poxA gene by genetic complementation.
The poxA401::Tn10 allele was introduced into wild-type S. typhimurium UK-1 strain χ3761 by P22 transduction (see Materials and Methods) by using a P22 lysate grown on S. typhimurium SMS401, to generate strain MGN-791 (Table 1). Although poxA mutants grow slower than the parent (4), this differential growth rate does not appear to be a strong phenotype. Unlike the LT2 derivative strain SMS401, which exhibits hypersensitivity to AKB (48, 49), we found that S. typhimurium UK-1 poxA401::Tn10 showed only marginal sensitivity to AKB (data not shown). Therefore, we screened a range of bacteriological media for substantial difference in growth rates between poxA mutants and the isogenic parent. We found that MGN-791, a poxA401::Tn10 derivative of S. typhimurium UK-1, produced microcolonies on MacConkey and AB2 agar, compared to the parental strain, which produced large colonies. A fusaric-acid-resistant derivative of MGN-791 was selected following Tn10 deletion to generate MGN-816, which also produced microcolonies on AB2 agar (Fig. 1). Under the same conditions, the parental strain χ3761 produced normal-size colonies on both AB2 agar and LB agar plates.
FIG. 1.
Phenotype of S. typhimurium poxA mutants on AB2 agar medium. χ3761, wild-type UK-1; MGN-816, ΔpoxA402; MGN-939, MGN-816(pMEG-250). The plate was photographed after 17 h of incubation at 37°C.
The poxA gene was cloned based upon its ability to restore normal growth to a poxA-defective strain, MGN-816, on AB2 agar medium. A genomic library of wild-type S. typhimurium UK-1 strain χ3761 in a pUC19-derived vector, pNEB193, was used to select a clone expressing PoxA. This library was introduced into S. typhimurium MGN-816 by electroporation and selection on an AB2 agar plate containing ampicillin. Among ∼5,000 ampicillin-resistant microcolonies, one transformant, MGN-939, exhibited a large colony morphology (Fig. 1), indicating that strain MGN-939 had acquired a DNA fragment capable of fully complementing the growth defect of the poxA mutant. The plasmid, designated pMEG-250, was purified and introduced into E. coli CC118 λpir. A partial restriction map of the 2-kb DNA insert in pMEG-250 was established (Fig. 2). To ensure that the complementation was due solely to cloned poxA and not to an unknown secondary mutation acquired during the selection, pMEG-250 was reintroduced into MGN-816. All transformants were complemented to the large colony phenotype on AB2 agar, suggesting that the 2-kb DNA fragment of UK-1 in pMEG-250 encodes an active PoxA protein. Alternatively, the DNA fragment could also encode a suppressor of poxA, as the complementing pMEG-250 is a high-copy-number plasmid. To address that possibility, we performed a Southern blot analysis of ClaI-digested total DNA from the parental strain χ3761 and the transposon Tn10 insertion in poxA strain MGN-791, with the labeled 2-kb insert from pMEG-250 as the probe. The data in Fig. 3 show that the probe hybridized to a single 7.5-kb ClaI fragment in the parent (lane 2) and to two ClaI fragments (7.5 and 9.5 kb) in poxA401::Tn10 (lane 1). These data are consistent with the size of transposon Tn10 (9.3 kb) and the internal ClaI site (27). The results confirmed that the 2-kb DNA fragment of pMEG-250 encodes the poxA gene.
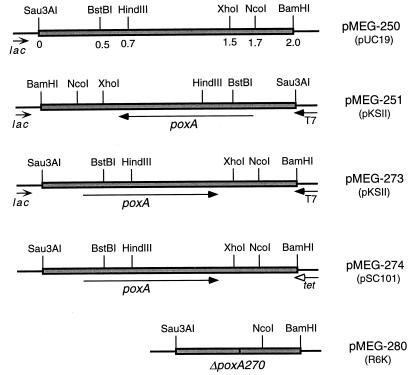
FIG. 2.
Partial restriction endonuclease maps of the poxA insert on relevant plasmids. The positions of relevant restriction endonucleases are shown. The direction of transcription of the poxA gene is indicated by the arrow. The plasmid replicon is indicated in parentheses.
FIG. 3.

Southern blot analyses of S. typhimurium poxA transposon insertion mutant. Chromosomal DNA from mutant and isogenic wild-type strains was digested with ClaI or EcoRV and transferred to a GeneScreen Plus nylon membrane. The blot was hybridized to a fluorescein-labeled 2-kb DNA probe containing the poxA gene. Std, 1-kb DNA ladder; lane 1, ClaI-digested MGN-791 (poxA401::Tn10); lane 2, ClaI-digested χ3761 (parent); lane 3, EcoRV-digested MGN-791 (poxA401::Tn10); lane 4, ClaI-digested pMEG-250.
We next wondered whether the cloned poxA gene was expressed from its natural promoter. The poxA-encoding fragment was cloned in both orientations with respect to the plasmid-encoded lac promoter, generating pMEG-251 and pMEG-273 (Fig. 2). Both pMEG-251 and pMEG-273 were able to complement poxA401::Tn10 mutation (data not shown), indicating that the 2-kb DNA fragment carries a promoter sequence necessary for the expression of poxA. In the absence of antibiotic selection, the high-copy-number plasmids, pMEG-250, pMEG-251, and pMEG-273, were unstable and were lost at a high frequency (data not shown). Therefore, the poxA gene was subcloned into the low-copy-number pLG339 vector (45), a pSC101 derivative, to give pMEG-274 (Fig. 2). On this plasmid, poxA was in opposite orientation in regard to the tet promoter. This construct was stable in S. typhimurium and was used in subsequent complementation experiments.
Nucleotide sequence of the poxA gene: poxA encodes the second lysyl-tRNA synthetase in Salmonella.
The 2-kb DNA fragment of plasmid pMEG-250 was sequenced on both strands (see Materials and Methods). The nucleotide sequence (GenBank accession no. AF001831) was analyzed with MacVector 5.0 software. Searches for homology in the available databases were performed with the BLAST program at the National Center for Biotechnology Information (1). At the nucleotide level, the 2,008 bp showed 81% identity to the 94-min region of the E. coli chromosome, including yjeA and yjeM, and 61% identity to a segment in the Haemophilus influenzae chromosome including the yjeA and yjbM genes. This is in agreement with the map position of poxA401::Tn10 determined in S. typhimurium. yjeA and yjeM/yjbM were identified during the automated sequencing of the genome of these organisms. No function has been assigned to their corresponding gene products. One complete open reading frame (ORF) and one truncated ORF were found in the 2,008-bp sequence. The first ORF, preceded by a putative Shine-Dalgarno sequence, starts at nucleotide 345 and encodes a polypeptide of 325 amino acids with a predicted pI of 5.1 and molecular mass of 36.8 kDa. This complete ORF was assigned to the PoxA protein. Hydrophobicity analysis using the algorithm of Kyte and Doolittle (30) indicated that PoxA does not have a signal sequence or membrane-spanning domain, suggesting that PoxA is a cytoplasmic protein. PoxA showed 91 and 65% identity to GenX (YjeA) of E. coli (29) and H. influenzae, respectively. GenX (YjeA) has not been characterized at the molecular level, and no function has been ascribed to this protein in either organism. However, PoxA and GenX appear to belong to a family of class II lysyl-tRNA synthetases. This family of enzymes is characterized by two motifs: signature 1, FRNEEMGRHHNPEFTMLE, and signature 2, ALGVDRLVML. The second ORF, preceded by a putative Shine-Dalgarno sequence, starts at nucleotide 1548 and was truncated at nucleotide 2,008. This ORF was found to be homologous to E. coli YjeM (88% identity and 96% similarity). Although the transposon Tn10 insertion has not been mapped, it is reasonable based upon the genetic complementation and defined deletion (see below) to state that the insertion took place in the poxA coding region. The complementation of the poxA401::Tn10 mutation by pMEG-274 suggests that the downstream gene does not have a role in the phenotypes ascribed to poxA in this study.
Expression of poxA in a bacteriophage T7 RNA polymerase expression system.
The 2-kb DNA fragment capable of complementing the poxA mutation was cloned in both orientations under the control of the bacteriophage T7 promoter in the vector pKSII, to generate pMEG-251 and pMEG-273 (Fig. 2). Plasmid-encoded polypeptides were examined as previously described (47). Cell lysates of E. coli BL21(DE3) carrying pMEG-251 (Fig. 4, lane 2) showed a polypeptide with a molecular mass of about 36 kDa which was absent from lysates of cells carrying either pMEG-273 (Fig. 4, lane 3) or the vector alone (Fig. 4, lane 1). The size of the expressed polypeptide is in complete agreement with the predicted size of PoxA. A 15-kDa polypeptide corresponding to the truncated ORF 2 was not detected under these experimental conditions.
FIG. 4.
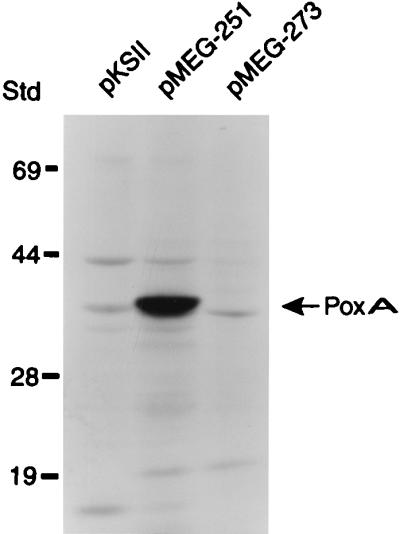
Identification of poxA gene product by using the T7 expression system. poxA was cloned in both orientations under the control of the bacteriophage T7 promoter in the vector pKSII and introduced into E. coli BL21(DE3), which carries a bacteriophage T7 RNA polymerase gene under the control of the lac promoter. After induction, whole-cell lysate proteins were separated on an SDS-polyacrylamide gel as described in Materials and Methods. Numbers on the left indicate the positions of molecular mass standards (in kilodaltons). The arrow indicates a polypeptide of about 36 kDa specifically encoded by pMEG-251, the putative gene product of poxA. pMEG-251, poxA under the T7 promoter; pMEG-273, poxA in opposite orientation from the T7 promoter; pKSII, vector control.
Construction of ΔpoxA270 defined deletion strain of S. typhimurium UK-1.
A defined deletion in poxA was constructed in S. typhimurium UK-1 as described in Materials and Methods, resulting in strain MGN-1036 (Table 1). In this strain, DNA coding for the last 270 amino acids of PoxA was deleted. MGN-1036 gave rise to microcolonies on AB2 agar medium and reduced growth rate in LB as expected. The deletion was confirmed by Southern blot analysis with the 2-kb DNA fragment encoding poxA as the probe (data not shown). Moreover, the defined ΔpoxA270 deletion was fully complemented by plasmid pMEG-274 (poxA+) for normal growth rate and for virulence (see below). It has been reported elsewhere that S. typhimurium mutants sensitive to antimicrobial cationic peptides such as defensins and protamine also show reduced virulence (21). MGN-1036 failed to grow in the presence of 1.25 mg of protamine sulfate per ml, while the isogenic parent exhibited normal growth at that concentration. This phenotype was complementable by pMEG-274. MGN-1036 was characterized biochemically with API strips. The results showed no biochemical difference between the parent and the poxA mutant. The growth rate of the defined deletion strain MGN-1036 was identical to that of the previous poxA mutants MGN-791 and MGN-816. Taken together, the ΔpoxA270 defined mutant was phenotypically identical to the transposon-generated strains.
S. typhimurium poxA mutants are attenuated in virulence in mice.
In S. typhimurium and Salmonella typhi, mutations in several global regulatory loci including cya/crp (9, 26), phoPQ (17, 35), rpoS (8, 13, 37, 50), and ompR (11) have been associated with reduced virulence, without loss of immunogenicity in mice. poxA exhibits pleiotropic effects due to its global regulatory nature (49). To confirm a role of the poxA mutation in virulence, we compared the poxA mutant derivatives of S. typhimurium with the parental strain in the mouse typhoid model. Similar results were obtained in two independent studies. The data below are from the experiment conducted with ΔpoxA270 defined deletion strain MGN-1036, MGN-1154 (MGN-1036 carrying the poxA-complementing plasmid pMEG-274), and the χ3761 parental strain. Treatment groups of 7-week-old female BALB/c mice were orally inoculated with the doses indicated in Table 2. Eight days postinoculation, three mice were removed from the treatment group inoculated with 3.2 × 109 CFU of MGN-1036 and euthanized to determine the level of colonization in the spleen, mesenteric lymph nodes, and Peyer’s patches. The results show on average 1.4 × 105 CFU in the spleen, 7.7 × 104 CFU in the mesenteric lymph nodes, and 2.2 × 105 CFU in the Peyer’s patches. These data indicate that the ΔpoxA270 defined mutant of S. typhimurium UK-1 was capable of colonizing deep tissues in mice after oral inoculation. Following immunization, mice were monitored daily for 21 days. The oral and intraperitoneal 50% lethal doses (LD50) of wild-type S. typhimurium UK-1 are 7.1 × 104 and ∼10 CFU, respectively (25a). All mice orally infected with 2.2 × 105 CFU of wild-type bacteria died within 10 days. In the MGN-1036 treatment groups, mice did not develop any clinical signs of typhoid fever (e.g., scruffiness) such as were seen in wild-type Salmonella-infected mice. Even mice receiving the highest oral dose, 3.2 × 109 CFU, of the defined ΔpoxA270 strain survived. Moreover, mice inoculated with the ΔpoxA270-complementing strain, MGN-1154, died within 10 days irrespective of the doses (Table 2). These results confirmed that the attenuation of S. typhimurium virulence was due to the mutation in the poxA gene. In addition, this phenotype could be reversed to the parental virulence by using the low-copy-number plasmid, pMEG-274, carrying the functional poxA gene.
TABLE 2.
Attenuation of S. typhimurium ΔpoxA270 mutant MGN-1036 and complementation in 7-week-old female BALB/c mice
| Strain | Immunizing dose (CFU) | No. of survivor mice/total no. following:
|
|
|---|---|---|---|
| Immunizationa | Wild-type challengeb | ||
| χ3761 | 2.2 × 105 | 0/4 | NDc |
| MGN-1036 | 3.2 × 108 | 4/4 | 4/4 |
| MGN-1036 | 3.2 × 109 | 4/4 | 4/4 |
| None | ND | ND | 0/4 |
| MGN-1154 | 2.2 × 105 | 0/4 | ND |
| MGN-1154 | 2.2 × 106 | 0/4 | ND |
| MGN-1154 | 2.2 × 107 | 0/4 | ND |
| MGN-1154 | 2.2 × 108 | 0/4 | ND |
Survivors were recorded 21 days postimmunization. All mice infected with 2.2 × 105 CFU of wild-type strain χ3761 or MGN-1154, a poxA-complemented strain, died within 10 days.
Control and immunized mice were orally challenged with 2.6 × 108 CFU of wild-type UK-1 strain χ3761 (∼10,000 × LD50), 35 days postimmunization. Survival was assessed for an additional 16 days postchallenge. Nonimmunized control mice died within 10 days.
ND, not done.
We determined whether the S. typhimurium ΔpoxA270 mutant could protect the immunized mice against lethal wild-type Salmonella challenge. Control and immunized mice were orally challenged with 2.6 × 108 CFU of virulent UK-1 strain χ3761 (∼10,000 × LD50), 35 days postimmunization. All control mice died within 10 days following challenge, and the experiment was terminated 16 days postchallenge. During that time, none of the vaccinated mice showed any clinical signs of disease, and all mice had survived ∼10,000 × LD50 of wild-type Salmonella challenge after a single oral immunization (Table 2). Mice were then euthanized, and the spleen of each was examined for the presence of the immunizing and challenge strains. No bacteria were detected, suggesting that S. typhimurium ΔpoxA270 mutants were capable of protecting mice from the wild-type colonization of visceral organs.
Immunogenicity of S. typhimurium poxA mutants in mice.
The immunogenicity of the defined deletion strain MGN-1036 was assessed, with the results for individual mice presented in Table 3. Humoral immune responses were measured by ELISA with purified Salmonella LPS as the coating antigen (see Materials and Methods). Sera were collected 29 days after a single oral immunization and 16 days postchallenge. High IgG, IgA, and, to a lesser extent, IgM titers were detected in orally immunized animals, indicating that the ΔpoxA270 mutant derivative of S. typhimurium was very immunogenic in mice. Mice receiving the highest oral dose, 3.2 × 109 CFU, were found to have higher IgG titers than mice receiving a log less bacteria (Table 3). However, both groups of mice were found to respond well to challenge as indicated by IgG titers postchallenge.
TABLE 3.
Immunogenicity of S. typhimurium ΔpoxA270 mutant MGN-1036 in BALB/c mice
| Immunizing dose (CFU) | Mouse | Titer in seruma
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IgG
|
IgM
|
IgA
|
||||||||
| After immunizationb | After challengec | Fold increase | After immunization | After challenge | Fold increase | After immunization | After challenge | Fold increase | ||
| 3.2 × 108 | 1 | 1:100 | 1:204,800 | 2,048 | 1:800 | 1:1,600 | 2 | 1:800 | 1:6,400 | 8 |
| 3.2 × 108 | 2 | 1:200 | 1:409,600 | 2,048 | 1:200 | 1:800 | 4 | <1:100 | 1:1,600 | 16 |
| 3.2 × 108 | 3 | 1:800 | 1:102,400 | 128 | 1:1,600 | 1:800 | 0.5 | 1:100 | 1:1,600 | 16 |
| 3.2 × 108 | 4 | ND | 1:102,400 | ND | ND | 1:800 | ND | ND | 1:1,600 | ND |
| 3.2 × 109 | 1 | 1:12,800 | 1:204,800 | 16 | 1:200 | 1:3,200 | 16 | 1:100 | 1:6,400 | 64 |
| 3.2 × 109 | 2 | 1:400 | 1:102,400 | 256 | 1:400 | 1:800 | 2 | <1:100 | 1:400 | 4 |
| 3.2 × 109 | 3 | 1:6,400 | 1:12,800 | 2 | 1:1,600 | 1:1,600 | 1 | 1:200 | 1:400 | 2 |
| 3.2 × 109 | 4 | 1:6,400 | 1:25,600 | 4 | 1:200 | 1:100 | 0.5 | <1:100 | <1:100 | 1 |
Serum IgG, IgM, and IgA titers were measured by ELISA with purified Salmonella LPS as coating antigen at 2.5 μg/ml. The titer is determined as the last serial dilution to have an optical density at 405 nm at or above 0.1. Negative control sera (from nonimmunized mice) had an optical density at 405 nm of <0.1 throughout the study. ND, not determined.
Sera were collected at 29 days postimmunization with MGN-1036.
Sera were collected at 16 days postchallenge with χ3761.
The data from this animal study demonstrate that S. typhimurium ΔpoxA270 mutants are attenuated and immunogenic. Moreover, poxA mutants are capable of protecting mice against a wild-type lethal challenge and colonization of deep tissues. Nearly identical results were obtained in another study using the Tn10 deletion strain MGN-816 (data not shown). Furthermore, mice inoculated by the intraperitoneal route with 104 CFU of this ΔpoxA402 mutant, a dose equivalent to ∼1,000 × LD50 of the parent, remained alive throughout the study (data not shown).
The poxA gene is present in other pathogenic microorganisms.
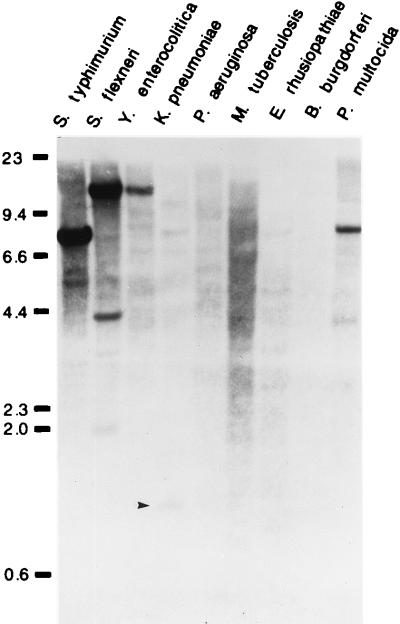
Lastly, we questioned whether poxA was present in the genome of other bacterial species in addition to Salmonella, H. influenzae, and E. coli. To address this possibility, total DNA was purified from S. typhimurium, Shigella flexneri, Yersinia enterocolitica, Klebsiella pneumoniae, Pseudomonas aeruginosa, Pasteurella multocida, Borrelia burgdorferi, M. tuberculosis, and E. rhusiopathiae. Ten micrograms of ClaI-digested genomic DNA was subjected to Southern blot analysis (see Materials and Methods), with a labeled 1-kb BstBI-XhoI internal fragment of poxA as a probe. Despite the stringent hybridization and washing conditions (50°C, 1× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 0.1% sodium dodecyl sulfate [SDS]), the results in Fig. 5 show that strong signals were detected in S. flexneri (three bands), Y. enterocolitica (one band), K. pneumoniae (one band), and P. multocida (two bands). Multiple hybridization bands in S. flexneri and P. multocida indicate the presence of other lysyl-tRNA synthetases (LysS and/or LysU) in these bacterial species. No hybridization bands were detected in M. tuberculosis, B. burgdorferi, P. aeruginosa, and E. rhusiopathiae (Fig. 5). These results indicate that poxA is present in other organisms in addition to Salmonella, E. coli, and H. influenzae.
FIG. 5.
Detection of the poxA gene in other bacterial species. Total DNA from S. typhimurium (5 μg), S. flexneri (10 μg), Y. enterocolitica (10 μg), K. pneumoniae (3 μg), P. aeruginosa (10 μg), M. tuberculosis (10 μg), E. rhusiopathiae (10 μg), B. burgdorferi (10 μg), and P. multocida (10 μg) was digested with the restriction enzyme ClaI. DNA fragments were separated on a 0.8% agarose gel and transferred to a GeneScreen Plus nylon membrane. The blot was hybridized to a fluorescein-labeled 1-kb BstBI-XhoI internal fragment of poxA as a probe. The hybridization was carried out at 50°C with stringent washes in 1× SSC–0.1% SDS. The limited amount of genomic DNA available from K. pneumoniae could account for the weak signal. Numbers at left show molecular mass in kilodaltons.
DISCUSSION
We introduced a poxA mutation in S. typhimurium UK-1 by P22 transduction from an LT2 strain carrying a Tn10 insertion in the gene (48). A clone from an S. typhimurium library was found to complement the effect of the poxA mutation and to hybridize to the poxA locus by Southern blot analysis (Fig. 3). Analysis of the 2-kb DNA sequence revealed one ORF capable of encoding a 36.8-kDa polypeptide, PoxA, which was confirmed in a bacteriophage T7 RNA polymerase expression system (Fig. 4). A homology search in the available databases showed that the poxA gene and encoded polypeptide were homologous to a family of class II lysyl-tRNA synthetases from several organisms. In bacteria, each of the 20 amino acids requires a specific cognate amino acyl-tRNA synthetase, except lysine. Two genes encoding functional lysyl-tRNA synthetases have been identified and characterized at the molecular level in E. coli K-12. lysS and lysU encode the constitutive LysS (25) and temperature-regulated LysU (22) lysyl-tRNA synthetases, respectively. Although mutations in lysU have no detectable phenotype, an alteration of lysS results in growth restriction at temperatures below 30°C (25). E. coli LysS and LysU have strong similarity over their entire length and migrate at similar positions on a two-dimensional electrophoresis gel (12, 33). Kong and colleagues (29) reported that genX, a gene located at min 94 on the E. coli genetic map, encodes a polypeptide with similarity to the carboxy terminus of LysS and LysU (7). It appears then that E. coli encodes three lysyl-tRNA synthetases, LysS, LysU, and GenX. Although the lysS gene has been mapped at 66.2 min on the Salmonella chromosome, a location similar to that of the E. coli counterpart (12), the lysU gene is absent from the Salmonella genome (39). To our knowledge, this is the first report showing that PoxA is the second putative lysyl-tRNA synthetase in Salmonella (the first being LysS) and that genX and poxA are allelic. We detected the presence of the poxA sequence in several bacterial species including S. flexneri, Y. enterocolitica, K. pneumoniae, and P. multocida, by Southern blot analysis (Fig. 5).
The role of PoxA as a lysyl-tRNA synthetase in S. typhimurium remains unclear. In a preliminary study, we observed that the poxA mutation increases the levels of the Sip (Ssp) proteins in the culture supernatant. These proteins are secreted through the S. typhimurium type III protein secretion apparatus encoded in pathogenicity island I. The amount of culture supernatant proteins was partially reduced to the wild-type level when a functional poxA copy was introduced in the poxA mutant. In addition, we also observed that several foreign antigens are produced at much higher levels in poxA mutants compared to other attenuating mutations such as cya/crp and phoP, with the same promoter. These observations are somewhat intriguing, since the analysis of PoxA structure did not reveal the presence of a DNA binding motif or homology to known transcription factors. It is therefore unlikely that the effects of a poxA mutation could be at the transcription level. Based upon the homology of PoxA to lysyl-tRNA synthetases, the effect of the gene alteration is most likely at the translation level. Two codons (AAA and AAG) are associated with lysyl-tRNA. Therefore, it is possible that the two lysyl-tRNA synthetases, LysS and PoxA, constantly compete for substrates with different affinities. A mutation abolishing PoxA production would then result in a high translation of some mRNAs such as those encoding the Sips (Ssps) and low translation of others exemplified by PoxB (4, 5).
S. typhimurium poxA mutants were found to be attenuated for virulence in chicks (data not shown) and mice, although they retained the ability to colonize deep tissues and induce strong humoral immune responses. All immunized mice were protected against Salmonella lethal challenge, suggesting that the poxA mutation could be useful in designing live vaccines from Salmonella and possibly from other organisms. The exact mechanisms by which the poxA mutation is attenuated will require more investigation. It is known, however, that the poxA mutation has pleiotropic effects in S. typhimurium (49) including hypersensitivity to the herbicide SM and to AKB. The mutation reduces the levels of acetolactate synthase, an enzyme involved in the biosynthesis of branched amino acids such as valine and isoleucine. This results in a low turnover of AKB and hypersensitivity to both SM and AKB (49). SM hypersensitivity due to the lack of acetolactate synthase I has been reported for several other mutations in S. typhimurium. These include ilvB and relA genes (14, 31) and genes encoding integration host factor, adenylate synthase, and catabolic activator protein (15, 16). Van Dyk and LaRossa (48) reported that poxA mutants were also hypersensitive to a wide range of compounds of various hydrophobicities and molecular weights that inhibit many different cellular processes. These authors suggested that this phenotype was due to an alteration of membrane permeability. This in turn could lead to a constitutive production and export of Sip (Ssp) proteins in vivo, resulting in attenuation of virulence. However, the absence of immune responses to Sips (Ssps) in mice (unpublished results) argues against that hypothesis. Alternatively, the attenuation of virulence could be the result of reduced growth rate of S. typhimurium poxA mutants, compared to the wild-type bacteria. However, our recent study using other S. typhimurium SM-hypersensitive mutants (48) does not support this theory. Indeed, although some of these SM-hypersensitive mutants exhibit the same reduced growth rate as the poxA strains, they retain full virulence in mice. Therefore, it is unlikely that the attenuation of virulence is the sole result of reduced growth rate of poxA mutants compared to the parental strain.
PoxA controls the expression of poxB, the gene for pyruvate oxidase structural enzyme (4). Mutations in poxA result in a 6- to 10-fold decrease in pyruvate oxidase levels (4, 5), presumably due to an inefficient translation of PoxB mRNA. Although the effect of the poxB mutation on virulence has not been evaluated in Salmonella, it is noteworthy that the PoxB homolog in Streptococcus pneumoniae has been shown to be a virulence determinant in this organism (44). In addition to PoxA, the expression of poxB is also regulated by RpoS and cyclic AMP/cyclic AMP receptor protein (6), two global regulators with established roles in Salmonella virulence. Regulation of pyruvate oxidase by RpoS, cyclic AMP/cyclic AMP receptor protein, and PoxA strongly suggests that they belong to similar but yet different regulons.
We are currently investigating the mechanism of attenuation by poxA mutation by several approaches. In two-dimensional electrophoresis, several proteins are produced only in the ΔpoxA270 strain and not in the parental or the ΔpoxA270-complemented strains when grown in LB. Such proteins produced only in the poxA strain could significantly contribute to the attenuation of bacterial virulence. The identification and characterization of genes encoding these proteins should provide a better understanding of the poxA phenotype, including the attenuation of Salmonella virulence. P. multocida poxA and S. typhimurium poxB mutants are also being investigated for reduced virulence in our laboratory. We are also investigating the humoral, cellular, and mucosal immune responses to foreign antigens expressed in the S. typhimurium ΔpoxA270 defined deletion strain, as well as to antigens in the carrier strain.
ACKNOWLEDGMENTS
We thank Virginia Miller and William Picking for providing Yersinia and Shigella strains, respectively; Josephine Clark-Curtiss and T. K. Ball for providing M. tuberculosis and E. rhusiopathiae genomic DNA, respectively; Suzanna Zahn, Mark Campbell, and Jennifer Moody for technical assistance in ELISA experiments; and Brian Morrow and Christos Stathopoulos for technical help with T7 expression experiments. We acknowledge Donata Sizemore, Kenneth Roland, and Steve Tinge for critical review of the manuscript.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Bertagnolli B L, Hager L P. Minimum requirements for protease activation of flavin pyruvate oxidase. Biochemistry. 1991;30:8131–8137. doi: 10.1021/bi00247a006. [DOI] [PubMed] [Google Scholar]
- 3.Bertagnolli B L, Hager L P. Role of flavin in acetoin production by two bacterial pyruvate oxidases. Arch Biochem Biophys. 1993;300:364–371. doi: 10.1006/abbi.1993.1049. [DOI] [PubMed] [Google Scholar]
- 4.Chang Y Y, Cronan J E., Jr Mapping nonselectable genes of Escherichia coli by using transposon Tn10: location of a gene affecting pyruvate oxidase. J Bacteriol. 1982;151:1279–1289. doi: 10.1128/jb.151.3.1279-1289.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang Y Y, Cronan J E., Jr Genetic and biochemical analyses of Escherichia coli strains having a mutation in the structural gene (poxB) for pyruvate oxidase. J Bacteriol. 1983;154:756–762. doi: 10.1128/jb.154.2.756-762.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang Y Y, Wang A Y, Cronan J E., Jr Expression of Escherichia coli pyruvate oxidase (PoxB) depends on the sigma factor encoded by the rpoS(katF) gene. Mol Microbiol. 1994;11:1019–1028. doi: 10.1111/j.1365-2958.1994.tb00380.x. [DOI] [PubMed] [Google Scholar]
- 7.Clark R L, Neidhardt F C. Roles of the two lysyl-tRNA synthetases of Escherichia coli: analysis of nucleotide sequences and mutant behavior. J Bacteriol. 1990;172:3237–3243. doi: 10.1128/jb.172.6.3237-3243.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coynault C, Robbe-Saule V, Norel F. Virulence and vaccine potential of Salmonella typhimurium mutants deficient in the expression of the RpoS (sigma S) regulon. Mol Microbiol. 1996;22:149–160. doi: 10.1111/j.1365-2958.1996.tb02664.x. [DOI] [PubMed] [Google Scholar]
- 9.Curtiss R, III, Kelly S M. Salmonella typhimurium deletion mutants lacking adenylate cyclase and cyclic AMP receptor protein are avirulent and immunogenic. Infect Immun. 1987;55:3035–3043. doi: 10.1128/iai.55.12.3035-3043.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Lorenzo V, Herrero M, Jakubzik U, Timmis K N. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J Bacteriol. 1990;172:6568–6572. doi: 10.1128/jb.172.11.6568-6572.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorman C J, Chatfield S, Higgins C F, Hayward C, Dougan G. Characterization of porin and ompR mutants of a virulent strain of Salmonella typhimurium: ompR mutants are attenuated in vivo. Infect Immun. 1989;57:2136–2140. doi: 10.1128/iai.57.7.2136-2140.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emmerich R V, Hirshfield I N. Mapping of the constitutive lysyl-tRNA synthetase gene of Escherichia coli K-12. J Bacteriol. 1987;169:5311–5313. doi: 10.1128/jb.169.11.5311-5313.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang F C, Libby S J, Buchmeier N A, Loewen P C, Switala J, Harwood J, Guiney D G. The alternative sigma factor katF (rpoS) regulates Salmonella virulence. Proc Natl Acad Sci USA. 1992;89:11978–11982. doi: 10.1073/pnas.89.24.11978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friden P, Newman T, Freundlich M. Nucleotide sequence of the ilvB promoter-regulatory region: a biosynthetic operon controlled by attenuation and cyclic AMP. Proc Natl Acad Sci USA. 1982;79:6156–6160. doi: 10.1073/pnas.79.20.6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friden P, Tsui P, Okamoto K, Freundlich M. Interaction of cyclic AMP receptor protein with the ilvB biosynthetic operon in E. coli. Nucleic Acids Res. 1984;12:8145–8160. doi: 10.1093/nar/12.21.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedman D I, Olson E J, Carver D, Gellert M. Synergistic effect of himA and gyrB mutations: evidence that him functions control expression of ilv and xyl genes. J Bacteriol. 1984;157:484–489. doi: 10.1128/jb.157.2.484-489.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galán J E, Curtiss R., III Virulence and vaccine potential of phoP mutants of Salmonella typhimurium. Microb Pathog. 1989;6:433–443. doi: 10.1016/0882-4010(89)90085-5. [DOI] [PubMed] [Google Scholar]
- 18.Gennis R B, Stewart V. Respiration. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. I. Washington, D.C: ASM Press; 1996. pp. 217–261. [Google Scholar]
- 19.Gennis R B, Hager L P. Pyruvate oxidase. Vol. 2. New York, N.Y: Plenum Publishing Corp.; 1976. [Google Scholar]
- 20.Grabau C, Cronan J E., Jr In vivo function of Escherichia coli pyruvate oxidase specifically requires a functional lipid binding site. Biochemistry. 1986;25:3748–3751. doi: 10.1021/bi00361a003. [DOI] [PubMed] [Google Scholar]
- 21.Groisman E A, Parra-Lopez C, Salcedo M, Lipps C J, Heffron F. Resistance to host antimicrobial peptides is necessary for Salmonella virulence. Proc Natl Acad Sci USA. 1992;89:11939–11943. doi: 10.1073/pnas.89.24.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hassani M, Pincus D H, Bennett G N, Hirshfield I N. Temperature-dependent induction of an acid-inducible stimulon of Escherichia coli in broth. Appl Environ Microbiol. 1992;58:2704–2707. doi: 10.1128/aem.58.8.2704-2707.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaniga K, Bossio J C, Galán J E. The Salmonella typhimurium invasion genes invF and invG encode homologues of the AraC and PulD family of proteins. Mol Microbiol. 1994;13:555–568. doi: 10.1111/j.1365-2958.1994.tb00450.x. [DOI] [PubMed] [Google Scholar]
- 24.Kaniga K, Delor I, Cornelis G R. A wide-host-range suicide vector for improving reverse genetics in gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene. 1991;109:137–141. doi: 10.1016/0378-1119(91)90599-7. [DOI] [PubMed] [Google Scholar]
- 25.Kawakami K, Ito K, Nakamura Y. Differential regulation of two genes encoding lysyl-tRNA synthetases in Escherichia coli: lysU-constitutive mutations compensate for a lysS null mutation. Mol Microbiol. 1992;6:1739–1745. doi: 10.1111/j.1365-2958.1992.tb01346.x. [DOI] [PubMed] [Google Scholar]
- 25a.Kelly, S. Unpublished data.
- 26.Kelly S M, Bosecker B A, Curtiss R., III Characterization and protective properties of attenuated mutants of Salmonella choleraesuis. Infect Immun. 1992;60:4881–4890. doi: 10.1128/iai.60.11.4881-4890.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleckner N, Bender J, Gottesman S. Uses of transposons with emphasis on Tn10. Methods Enzymol. 1991;204:139–180. doi: 10.1016/0076-6879(91)04009-d. [DOI] [PubMed] [Google Scholar]
- 28.Koland J G, Miller M J, Gennis R B. Reconstitution of the membrane-bound, ubiquinone-dependent pyruvate oxidase respiratory chain of Escherichia coli with the cytochrome d terminal oxidase. Biochemistry. 1984;23:445–453. doi: 10.1021/bi00298a008. [DOI] [PubMed] [Google Scholar]
- 29.Kong L, Fromant M, Blanquet S, Plateau P. Evidence for a new Escherichia coli protein resembling a lysyl-tRNA synthetase. Gene. 1991;108:163–164. doi: 10.1016/0378-1119(91)90503-4. [DOI] [PubMed] [Google Scholar]
- 30.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 31.LaRossa R A, Smulski D R. ilvB-encoded acetolactate synthase is resistant to the herbicide sulfometuron methyl. J Bacteriol. 1984;160:391–394. doi: 10.1128/jb.160.1.391-394.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lennox E S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955;1:190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- 33.Lévèque F, Plateau P, Dessen P, Blanquet S. Homology of lysS and lysU, the two Escherichia coli genes encoding distinct lysyl-tRNA synthetase species. Nucleic Acids Res. 1990;18:305–312. doi: 10.1093/nar/18.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marmur J. A procedure for the isolation of deoxyribonucleic acid from micro-organisms. J Mol Biol. 1961;3:208–218. [Google Scholar]
- 35.Miller S I, Kukral A M, Mekalanos J J. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci USA. 1989;86:5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Callaghan D, Charbit A. High efficiency transformation of Salmonella typhimurium and Salmonella typhi by electroporation. Mol Gen Genet. 1990;223:156–158. doi: 10.1007/BF00315809. [DOI] [PubMed] [Google Scholar]
- 37.Robbe-Saule V, Coynault C, Norel F. The live oral typhoid vaccine Ty21a is a rpoS mutant and is susceptible to various environmental stresses. FEMS Microbiol Lett. 1995;126:171–176. doi: 10.1111/j.1574-6968.1995.tb07412.x. [DOI] [PubMed] [Google Scholar]
- 37a.Roland, K. Unpublished data.
- 38.Russell P, Hager L P, Gennis R B. Characterization of the proteolytic activation of pyruvate oxidase. Control by specific ligands and by the flavin oxidation-reduction state. J Biol Chem. 1977;252:7877–7882. [PubMed] [Google Scholar]
- 39.Saluta M V, Hirshfield I N. The occurrence of duplicate lysyl-tRNA synthetase gene homologs in Escherichia coli and other procaryotes. J Bacteriol. 1995;177:1872–1878. doi: 10.1128/jb.177.7.1872-1878.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 41.Schmieger H. Phage P22-mutants with increased or decreased transduction abilities. Mol Gen Genet. 1972;119:75–88. doi: 10.1007/BF00270447. [DOI] [PubMed] [Google Scholar]
- 42.Schmieger H, Backhaus H. The origin of DNA in transducing particles in P22-mutants with increased transduction-frequencies (HT-mutants) Mol Gen Genet. 1973;120:181–190. doi: 10.1007/BF00267246. [DOI] [PubMed] [Google Scholar]
- 43.Schrock H L, Gennis R B. Specific ligand enhancement of the affinity of E. coli pyruvate oxidase for dipalmitoyl phosphatidylcholine. Biochim Biophys Acta. 1980;614:215–220. doi: 10.1016/0005-2744(80)90182-5. [DOI] [PubMed] [Google Scholar]
- 44.Spellerberg B, Cundell D R, Sandros J, Pearce B J, Idanpaan-Heikkila I, Rosenow C, Masure H R. Pyruvate oxidase, as a determinant of virulence in Streptococcus pneumoniae. Mol Microbiol. 1996;19:803–813. doi: 10.1046/j.1365-2958.1996.425954.x. [DOI] [PubMed] [Google Scholar]
- 45.Stoker N G, Fairweather N F, Spratt B G. Versatile low-copy-number plasmid vectors for cloning in Escherichia coli. Gene. 1982;18:335–341. doi: 10.1016/0378-1119(82)90172-x. [DOI] [PubMed] [Google Scholar]
- 46.Studier F W, Moffatt B A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- 47.Tabor S, Richardson C C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci USA. 1985;82:1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Dyk T K, LaRossa R A. Sensitivity of a Salmonella typhimurium aspC mutant to sulfometuron methyl, a potent inhibitor of acetolactate synthase II. J Bacteriol. 1986;165:386–392. doi: 10.1128/jb.165.2.386-392.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Dyk T K, Smulski D R, Chang Y Y. Pleiotropic effects of poxA regulatory mutations of Escherichia coli and Salmonella typhimurium, mutations conferring sulfometuron methyl and alpha-ketobutyrate hypersensitivity. J Bacteriol. 1987;169:4540–4546. doi: 10.1128/jb.169.10.4540-4546.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilmes-Riesenberg M R, Foster J W, Curtiss R., III An altered rpoS allele contributes to the avirulence of Salmonella typhimurium LT2. Infect Immun. 1997;65:203–210. doi: 10.1128/iai.65.1.203-210.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]