SUMMARY
Glaucoma is a leading cause of irreversible blindness worldwide, caused by the gradual degeneration of retinal ganglion cells and their axons. While glaucoma is primarily considered a genetic and age-related disease, some inflammatory conditions, such as uveitis and viral-induced anterior segment inflammation, cause secondary or uveitic glaucoma. Viruses are predominant ocular pathogens and can impose both acute and chronic pathological insults to the human eye. Many viruses, including herpes simplex virus, varicella-zoster virus, cytomegalovirus, rubella virus, dengue virus, chikungunya virus, Ebola virus, and, more recently, Zika virus (ZIKV) and severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), have been associated with sequela of either primary or secondary glaucoma. Epidemiological and clinical studies suggest the association between these viruses and subsequent glaucoma development. Despite this, the ocular manifestation and sequela of viral infections are not well understood. In fact, the association of viruses with glaucoma is considered relatively uncommon in part due to underreporting and/or lack of long-term follow-up studies. In recent years, literature on the pathological spectrum of emerging viral infections, such as ZIKV and SARS-CoV-2, has strengthened this proposition and renewed research activity in this area. Clinical studies from endemic regions as well as laboratory and preclinical investigations demonstrate a strong link between an infectious trigger and development of glaucomatous pathology. In this article, we review the current understanding of the field with a particular focus on viruses and their association with the pathogenesis of glaucoma.
KEYWORDS: glaucoma, ocular infections, uveitis, optic neuritis, virus, Zika, dengue, Ebola, SARS-CoV-2, HSV, CMV, VZV
INTRODUCTION
Glaucoma is a group of optic neuropathies characterized by the progressive degeneration of retinal ganglion cells (RGCs) and the optic nerve, leading to vision loss. It affects approximately 70 million people worldwide and is the leading cause of irreversible blindness globally (1). The incidence of glaucoma increases exponentially with age, and ~5%–8% of patients are diagnosed with glaucoma by the age of 75–80, whereas 10% of patients are blinded bilaterally by this disease (2). Although the pathogenesis of glaucoma is unclear, multiple risk factors, such as intraocular pressure (IOP), ocular perfusion pressure, ocular blood flow, myopia, central corneal thickness, and optic disc hemorrhage, have been shown to contribute to the pathogenesis of glaucoma. Systemic risk factors, such as age, smoking, race/ethnicity, genetic factors, diabetes, and obesity, have also contributed to glaucoma pathogenesis (3–5). Although elevated IOP is the only modifiable risk factor in glaucoma, approximately one-third of the patients have IOP levels in the normal range and have shown glaucomatous neurodegeneration. Glaucoma is classified into two broad categories: open-angle and angle-closure glaucomas. Primary open-angle glaucoma (POAG) is the most common form of the disease, particularly in African and Western countries. Two major theories, mechanical and vascular theories, have been proposed as initiating mechanisms for POAG. The mechanical theory hypothesizes that elevated IOP causes compression in and around the optic nerve, leading to disruption of axonal transport that ultimately results in the death and loss of RGC and their axons. The vascular theory proposes that elevated IOP and reduced ocular blood flow due to systemic blood pressure or vasospasm result in insufficient ocular blood supply, ultimately leading to glaucoma (4). Both open-angle and angle-closure glaucomas can be a primary disease; however, many risk factors such as viral infection, inflammation, ocular trauma, corticosteroid usage, tumors, and advanced cases of cataracts or diabetes have been shown to cause secondary glaucoma (SG) (5, 6).
Conventionally, glaucoma is considered a genetic and age-related disease until recent Zika virus (ZIKV) epidemics in the Americas. Babies born from ZIKV-infected mothers showed congenital, bilateral glaucoma with open-angle and high IOP (7–9). Moreover, many viruses, including herpes simplex virus (HSV), varicella-zoster virus (VZV), cytomegalovirus (CMV), rubella virus (RUBV), dengue virus (DENV), chikungunya virus (CHIKV), West Nile virus (WNV), Ebola virus (EBOV), Japanese encephalitis virus, Kyusnur forest disease virus, Epstein-Barr virus (EBV), and, more recently, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), have been associated with the pathogenesis of glaucoma (Table 1) (10–16).
TABLE 1.
Percent (%) prevalence of ocular viral infections and glaucoma (G) in various reported cohorts
| HSV (G) | VZV (G) | CMV (G) | RUBV (G) | DENV (G) | ZIKV (G) | CHIKV (G) | WNV (G) | EBOV (G) | MUVc (G) | Overall glaucoma incidence (%) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Shirahama et al. (17) (Japan) n = 170 |
18 (16) | 38 (9) | 44 (72) | − | − | − | − | − | − | − | 37.64 |
| Hoeksema et al. (18) (The Netherlands) n = 73 (100% unilateral) |
74 (17) | 26 (11) | − | − | − | − | − | − | − | 15 | |
| Shimizu et al. (19) (Japan) n = 105 (141 eyes) |
6.6 (6.6) | 1.9 (1–9) | − | − | − | − | − | − | − | − | 9.73 |
| Pohlmann et al. (20) (Germany) n = 270 (all open angle) |
29 (6.49) | 17 (6.66) | 21 (56.14) | 29 (14.28) | − | − | − | − | − | 5 (7.14) | 19 |
| Wensing et al. (21) (The Netherlands) n = 106 |
36.8 (22) | 10.6 (23) | − | 53.77 (17) | − | − | − | − | − | − | 21 |
| Miserocchi et al. (22) (USA) n = 64 (68 eyes) |
62.5 (23) | 37.5 (25) | − | − | − | − | − | − | − | − | 51 |
| Miserocchi et al. (24) (Italy) n = 241 |
78.4 (38.1) | 18.7 (27) | 2.9 (28.6) | − | − | − | − | − | − | − | 38 |
| Khieu et al. (10) (Thailand) n = 31 (PCR confirmed) | 10 (0) | 3 (0) | 19 (17) | − | − | − | − | − | − | − | 41.93 |
| van Boxtel et al. (25) (The Netherlands) n = 7 (case series) |
− | − | 100 (100) | − | − | − | − | − | − | − | 100 |
| Leleu et al. (26) (France) n = 38 (PCR or GWC confirmed) |
− | − | 100 (50) | − | − | − | − | − | − | − | 50 |
| Engelhard et al. (27) (USA) n = 77 (92 eyes) |
51.326 (NA)d | 43.6 (NA) | 2.56 (NA) | − | − | − | − | − | − | − | 23.37 |
|
aTugal-Tutkun et al. (28) (Turkey) n = 111 (114 eyes) |
− | − | − | − | − | − | − | − | − | − | 1.8 |
| Sungur et al. (29) (Turkey) n = 76 |
76 (12) | 24 (2) | − | − | − | − | − | − | − | − | 13.1 |
|
bYepez et al. (7) (South America) n = 43 |
− | − | − | − | − | NA (12) | − | − | − | − | 12 |
| Groen-Hakan et al. (30) (The Netherlands) n = 127 (144 eyes) |
− | − | − | 38 (28) | − | − | − | − | − | − | 28 |
| Thean et al. (31) (Australia) n = 34 | − | 100 (56) | − | − | − | − | − | − | − | − | 56 |
| Chee and Jap (32) (Singapore) n = 102 (103 eyes) |
− | − | 38.83 (12.62) | − | − | − | − | − | − | − | 25.24 |
| Fan et al. (33) n = 60 |
− | 41.66 (41.66) | 21.66 (21.66) | − | − | − | − | − | − | − | 100 |
| de Visser et al. (34) (The Netherlands) n = 30 |
− | − | − | 100 (23.33) | − | − | − | − | − | − | 23.3 |
| Shantha et al. (35) n = 23 (pediatric cohort) |
− | − | − | − | − | − | − | − | 56.5 (6.5) | − | 6.5 |
No PCR or Goldman-Witmer coefficient (GWC) analysis data were reported. Diagnosis was established based on clinical presentation.
CZS cohort.
MUV, multiple viruses.
NA, not applicable.
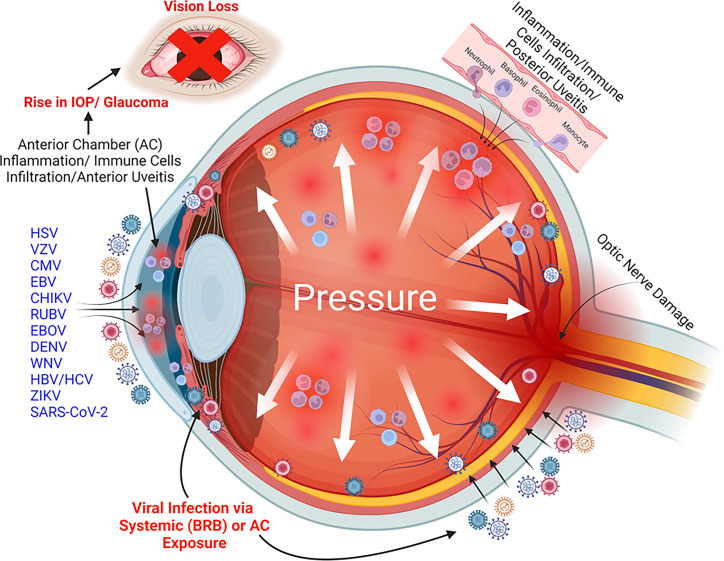
On one end, the eye is continuously exposed to the outside environment, while on the other, it is protected by the blood-retinal barrier (BRB) from systemic breaches. The eye being an immune-privileged organ also predisposes it to become a niche for many viral and bacterial pathogens. Glaucoma is a major cause of disability and blindness, and associated viruses add to its toll on the quality of human lives. Up to 20% of all cases of glaucoma are of infectious origin, and viruses share a significant portion of this burden. Viruses can enter the eye via direct exposure to the outside environment or can permeate through systemic circulation due to a breach in the BRB (Fig. 1). The virus-induced uveitis that breaches the blood-aqueous barrier paves the way for inflammatory molecules and immune cells to infiltrate the eye (36). In addition, macrophages, polymorphonuclear leukocytes (PMNs), and T cells have been known to contribute to uveitic inflammation with additional roles of inflammatory cellular effectors such as prostaglandins (36).
FIG 1.
A schematic depicting the role of viruses in the pathogenesis of glaucoma. Many oculotropic viruses (e.g., HSV, VZV, CMV, EBV, CHIKV, RUBV, EBOV, DENV, WNV, HBV, HCV, ZIKV, and SARS-CoV-2) cause immune cell-mediated ocular inflammation, called uveitis, that results into elevated IOP. This virus-induced IOP often leads to the development of glaucomatous pathology resulting in vision loss. Image created with BioRender.com.
In general, the introduction of viral pathogens in the eye triggers inflammatory and effector immune responses to confine the infection. This virus-induced ocular inflammation is referred to as uveitis. Anterior chamber inflammation or anterior uveitis (AU) is a common manifestation of viral infections in the eye (11–13, 37). The prevalence of AU is noted to be between 24.5% and 52.3% in a variety of settings (38). AU caused by several viruses has been reported to be associated with the development of glaucomatous pathology or uveitic glaucoma (UG) (Fig. 1; Table 1) (8, 9, 17–20, 39). In addition, some viruses, such as ZIKV, have been shown to cause congenital glaucoma in newborns, independent of AU (7, 8). In the USA, 20% of all the patients diagnosed with any form of uveitis develop glaucoma irrespective of the intraocular region involved (40). Virus-associated uveitis primarily affects the anterior segment of the eye and is therefore called viral anterior uveitis (VAU) (20). VAU can either be acute/recurrent (e.g., HSV and VZV) or chronic (RUBV-associated uveitis), or both (e.g., CMV, causing both acute and chronic sequela of the infection) (41). VAU, if left untreated or clinically mismanaged, leads to the development of glaucoma. Apart from anterior segment inflammation, the classic feature of viral uveitis is elevated IOP combined with iris atrophy and keratic precipitate formation (19, 36). Many viruses, such as CMV and EBOV, can also cause posterior segment pathology, mainly in immunocompromised individuals (11, 42). Herpetic viruses, including CMV, VZV, and HSV, are together responsible for VAU in 5%–10% of all cases (20). A retrospective study on 927 patients with chronic ocular inflammation determined anterior chamber uveitis as one of the predominant ocular manifestations and a cause of severe vision-threatening uveitic sequela in 28.5% of patients (43). In this cohort, 31% of the cases were linked with herpetic viral etiology, which underlines the need to acknowledge virus-associated uveitis and subsequent secondary morbidities.
Trabeculitis, inflammation of trabecular meshwork (TM), has been proposed as one of the prime mechanisms behind increased IOP in VAU (36, 44, 45). Studies from our laboratory and many others have demonstrated the permissivity of TM toward viruses with a concomitant increase in IOP in animal models (45–47). Some viruses, such as EBOV, have been shown to remain in the eye for a more extended period and cause hypertensive uveitis despite clearance of systemic viremia (42). In addition to pathogens, infiltration of inflammatory immune cells such as PMNs, macrophages, and T cells has been shown to obstruct the TM and Schlemm’s canal either by direct cytotoxicity or by releasing proinflammatory cytokines, enzymes, and reactive oxygen species, leading to glaucomatous pathologies (20, 36, 48).
In this review, we emphasize the role of common and emerging viral infections and their association with the pathophysiology of glaucoma. We also highlight cases where viruses have been shown to cause glaucoma independent of VAU.
HERPESVIRUSES AND GLAUCOMA
Herpesviruses are among the most frequent causes of VAU worldwide. Members of the Herpesviridae family, such as HSV, VZV, and CMV, have lifelong latency after primary infection. Herpesviruses are prevalent ocular pathogens infecting 60%–90% of the population globally, and the clinical spectrum predominantly involves the anterior segment of the eye. Glaucoma is one of the common complications of herpesvirus ocular infection. In the following sections, we provide an overview of different herpesviruses known to cause ocular infections and their association with the pathogenesis of glaucoma.
Herpes simplex virus
HSV is a successful parasite in humans that remains associated with its host throughout life. There are two different strains of HSV: HSV-1 and HSV-2, and these are members of the human Herpesviridae family. Among HSVs, HSV-1 is responsible for the majority of VAU cases in developed countries (41). Within the USA, 50% of the population by the age of 30 and 100% by the age of 60 are estimated to be infected by HSV-1 (49). The common clinical manifestation of HSV-1 includes AU, skin blisters, and neuralgia in the first branch of the trigeminal nerve (41, 50). Following primary infection, HSV establishes latent infection in the trigeminal ganglion or the sensory nerve ganglia (51). It then reaches neural axons, causing inflammation and disease in the periocular, corneal, and intraocular areas (41, 51, 52). HSV ocular infection is usually unilateral, but in some cases, it may present bilaterally (11, 12, 50, 51). The common ocular manifestation of HSV includes ocular hypertension (increased IOP), severe eye pain, blepharitis, conjunctivitis, keratitis, tearing, photophobia, blurring of vision, diffuse stellate keratic precipitates, and iris atrophy with or without keratitis (12, 41, 52) (Table 2). The corneal involvement in HSV infection ranges between 33% and 41%, and it can present as active keratitis (epithelial, stromal, interstitial, and disciform), corneal scar, endotheliitis, neurotrophic keratopathy, and reduced corneal sensation (12, 41, 49). Most of the patients with ocular herpes infection presented with IOP elevation had stromal keratitis (96–100%) or metaherpetic ulcer (4%) (53). In addition, HSV-1 and HSV-2 can present with retinitis as acute retinal necrosis (ARN) (49).
TABLE 2.
Differential pathological characteristics of various viral ocular manifestations
| HSV | VZV | CMV | EBV | RUBV | DENV | ZIKV | CHIKV | WNV | EBOV | HBV | SARS-CoV-2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Presence of corneal scars/hypoesthesia/sector iris atrophy | + | + | − | − | + | − | − | − | − | − | − | − |
| Patchy or diffuse iris atrophy | − | − | + | − | + | − | − | − | − | − | − | − |
| No posterior changes/synechiae | − | − | + | − | + | − | − | − | − | − | − | − |
| Posterior sub-capsular cataract formation | − | − | − | − | + | − | − | − | − | − | − | − |
| Vitritis | − | − | − | − | + | − | − | − | − | − | − | − |
| Retinal vascular leakage/disc hyperfluorescence in angiography | – | – | − | − | + | − | − | − | − | − | − | − |
| Diffuse/fine/stellate keratic precipitates/keratitis | + | − | − | + | + | − | − | + | + | + | − | − |
| Macular pigment mottling | − | − | − | − | − | + | + | + | − | − | − | − |
| Foveal reflex loss | − | − | − | − | − | − | + | − | − | − | − | − |
| Macular neuroretinal atrophy/fundoscopic alterations | − | − | − | − | − | + | + | − | + | + | − | − |
| Conjunctivitis | − | − | − | − | − | + | + | − | − | + | − | + |
| Neuritis | − | − | + | − | − | − | + | + | + | − | − | + |
| Retinitis | − | + | − | − | − | + | − | + | + | + | − | − |
| Endotheliitis | + | − | + | − | − | − | − | − | − | − | − | − |
| Uveitis/iridocyclitis | + | + | + | − | − | + | + | + | + | + | + | + |
| Cataract | − | − | + | − | + | − | − | − | − | − | − | − |
| Episcleritis | − | − | − | + | − | − | − | + | − | − | − | + |
| Mononucleosis | − | − | − | + | − | − | − | − | − | − | − | − |
| Choroiditis | − | − | + | + | − | − | − | + | + | − | − | − |
| Glaucoma | + | + | + | − | + | + | + | − | − | + | + | + |
The pathogenesis of HSV-induced uveitic glaucoma has been debated, and some studies suggest that increased IOP was not secondary to inflammation but rather developed due to corticosteroid therapy (53). However, many studies, including animal studies, suggest that increased IOP in HSV-induced glaucoma is linked to trabecular blockade and trabeculitis (53). Interestingly, mutations in a host protein and an autophagy receptor optineurin have been implicated in the development of glaucoma of non-infectious origin in a race-specific manner (23, 54). A recent study described the role of optineurin in an ocular HSV-1 infection model indicating optineurin is essential in controlling HSV-1 infection and associated neuronal hyperinflammation and consequent neurodegenerative pathology (54). In light of these findings, it will be interesting to dissect the role of optineurin in HSV-1 and other virus-linked glaucomas.
Overall, HSV-induced AU is one of the well-known causes of SG. Recently, a retrospective study on 270 patients from Germany reported that almost one-third (29%) of all VAU cases were caused by HSV, as revealed by Goldman-Witmer coefficient (GWC) analysis (20). Out of a total of 77 HSV-induced AU cases in this cohort, 9.6% went on to develop SG. Interestingly, only 4% of them have shown elevated IOP (20)
In another retrospective study, Shimizu et al. reported close to 10% prevalence of UG in an analysis of retrospective data from 141 eyes of 105 uveitis patients (19). Of these, 6.7% were HSV-associated UG cases with IOP of >21 mm Hg. In another cohort from Japan, SG was reported in 16% of patients diagnosed with HSV-AU (17). The study by Hoeksema et al. of 73 patients from The Netherlands reported the development of SG in 17% of HSV patients with major risk factor of elevated IOP (18). In this study, most of the patients with elevated IOP (91%) had an IOP increase at the beginning of one or more uveitis episodes.
In a cohort of 241 uveitic patients from Italy, the share of HSV-associated AU was reported to be 18.5%. In this cohort, a total 38.1% of the HSV-infected patients developed glaucoma irrespective of the specific ocular tissues involved (24). In another retrospective, observational study of 30 years of data from uveitis patients (n = 491) at the University of Virginia, USA, a prevalence rate of 15.7% was noted for infectious origin uveitis (27). Among infectious uveitis, 48.1% of AU was caused by herpetic viruses, with 26% by HSV. Out of these, 23.4% of subjects developed glaucoma and underwent either glaucoma topical therapy (18.2%) or surgery (5.2%) (27). In another cohort from the USA, 54% and 38% prevalence of SG was found in patients who presented with HSV- and VZV-associated uveitis, respectively (22).
The existing literature and clinical presentations establish a strong link between HSV infection as a significant cause of anterior chamber uveitis and the consequent development of uveitic glaucoma. As HSV is a foremost viral pathogen carried in >90% of adults and frequently infects the eyes, control strategies aimed at curbing its pathognomy will be vital to prevent its ocular complications including glaucoma.
Varicella zoster virus
VZV is another Herpesvirus family member involved in causing uveitic syndrome and often leads to the development of glaucoma as a secondary manifestation. VZV is known to cause 9%–16% of all herpes zoster cases known as herpes zoster ophthalmicus (HZO), among which 43%–53% of patients have been reported to develop AU (22, 55). As opposed to HSV uveitis, which has an acute recurrent course, VZV uveitis exhibits a chronic course (22). VZV-associated uveitis is usually reported in older individuals due to reactivation of the viral reservoir in the latently infected carriers (22, 52). However, in some reports, it has been shown to affect young individuals as well (11, 56–58). Most of these cases were either caused by vaccine strain or wild-type virus reactivation episodes. Similar to HSV, VZV almost always manifests as unilateral disease with granulomatous keratic precipitates. In a comparative account of five different PCR-proven cohorts, 100% of VZV cases were found to be unilaterally involved (50).
VZV usually presents as a lifelong latent infection at the ophthalmic division of the trigeminal nerve (sensory ganglia), which reactivates at a later age (11, 41). Facial lesions, including vesicles, rashes, and blebs, were described as exclusive signatures of VZV infection (16). Some cases of VZV uveitis are misdiagnosed due to lack of cutaneous eruptions; therefore, aqueous PCR diagnosis is essential to differentiate among herpetic uveitis cases (33, 55). VZV-associated uveitis usually presents with a higher mean IOP than HSV (36). Other VZV-related consequences include ARN and progressive outer retinal necrosis. ARN is severe necrotizing retinitis that causes unilateral vision loss, photophobia, floaters, and pain, but one-third of patients acquire bilateral disease (38, 49).
VZV is frequently reported to cause anterior chamber uveitis and consequent uveitic SG. In two separate cohorts from Japan, 1.9% (19) and 9.0% (17) of uveitic glaucoma cases were found to have involvement of VZV and associated anterior chamber inflammation. In a cohort from Italy with herpetic ocular infection, 40% of patients with VZV infection were reported to develop glaucoma (24). Similarly, an unusually high prevalence (56%) of SG was found in an HZO-affected cohort (n = 34 patients) from Victoria, Australia (31). In this study, all the cases of SG presented with uveitis, consequent to VZV reactivation. Using a Dutch cohort of herpetic AU, Hoeksema and colleagues reported a prevalence rate of 42% of VZV-associated AU, of which 11% of patients developed SG (18). In an earlier study by Miserocchi and colleagues of a cohort from Massachusetts, USA, the prevalence of SG due to VZV-associated uveitis was reported to be 38% (22). Wensing et al. also reported SG prevalence of 30% in VZV-affected eyes with AU, where 50% of patients demonstrated an IOP of >30 mm Hg (21). Engelhard et al. reported the prevalence of VZV (HZO)-associated anterior uveitis in 43.6% of patients from Virginia. In this study, 18.2% and 5.2% underwent therapeutic and surgical interventions, respectively, to manage SG (27). However, the study did not discuss the exact share of the etiological agent in the progression to SG.
Evidence related to VZV-associated secondary complications presents a clearer picture of VZV etiology and associated uveitic glaucoma. It also emphasizes the need to study the virus in greater detail and to find accurate diagnoses and possible interventional strategies to curb VZV-imposed short- and long-term ocular morbidities.
Cytomegalovirus
CMV is a third member of the Herpesvirus family that has been reported to be found in 50% of the adult population and 2.5% of newborns in developed countries (59). CMV, which has rarely been a cause of concern in immunocompetent individuals, has lately presented as a frequent cause of ocular pathologies in otherwise healthy individuals (60–62). CMV classically has been known to cause ocular infections, often accompanied by glaucoma, in immunodeficient individuals including those that are HIV positive (63–66). An association between CMV and clinical eye syndromes including Posner-Schlossman syndrome (PSS) and Fuchs heterochromic iridocyclitis or Fuchs uveitic syndrome (FUS) has been established (12, 32, 36, 67). In addition, CMV is a major cause of hypertensive AU in immune-competent populations (11, 25, 44, 60, 68–70).
CMV may manifest as an acute, recurrent AU with elevated IOP or a chronic AU with endotheliitis (71). Acute, recurrent AU coupled with ocular hypertension is often referred to as PSS, which may be aggravated by glaucoma (11, 32). The anterior chamber inflammation is mild in PSS AU-affected eyes, without any posterior synechiae or posterior segment changes (11). The CMV-induced acute recurrent AU episodes are more common in middle-aged individuals and often present with unilateral eye pain and blurring of vision associated with halos and ipsilateral headaches (72). CMV-associated AU is often misdiagnosed with HSV and VZV AU because of overlapping clinical signs.
Patients between their fifth and seventh decades of life, with an average age of 65, are more likely to develop chronic CMV AU, which can cause ocular pain and blurred vision. Based on observations in mouse models, CMV has been suspected of establishing latency in ocular tissues (12). It has been noted to persist in the eye despite getting cleared systemically (73). The clinical manifestations of chronic CMV AU between Eastern and Western populations differ significantly (41). CMV has been described as a leading pathogen causing FUS in east Asian populations (12, 60, 68, 70, 72). However, in Western countries, patients often present with less distinguishable characteristics that usually have overlapping features with both PSS and FUS (72) and manifest as mild, unilateral, recurrent, or chronic iridocyclitis (44, 60, 68).
In CMV cases, IOP usually exceeds 50 mm Hg on average, and 8% of eyes develop glaucomatous optic neuropathy (11). Glaucomatous optic neuropathy has been observed to be present in acute (23%) or chronic (36%) CMV-infected patients (32). Elevated IOP, either in acute or chronic CMV AU, is common and often leads to glaucomatous damage and consequent uveitic glaucoma (25, 44, 60). In acute CMV AU episodes, trabeculitis has been linked to recurrent ocular hypertension, while chronically increased IOP could result from restricted aqueous outflow due to irreversible damages to trabecular meshwork following chronic inflammation (44).
Evidence from several demographically distinct cohorts is available to attest the role of CMV AU in the development of glaucoma. In a series of case reports from seven patients in The Netherlands, three (43%) developed secondary glaucoma with iris abnormalities, three (43%) without iris atrophy, and one (14%) with mild IOP without iris involvement (25). In an Italian cohort of 241 subjects with herpes infection, 7 patients (2.9%) presented with CMV infection, among which 28.6% developed glaucoma as well as cataract (24). A relatively high prevalence of SG (72%) has been recorded among patients with CMV AU in a study by Shirahama and colleagues in a Japanese cohort (17). A study from Thailand reported the development of glaucoma in one (17%) out of six PCR-positive CMV AU patients (10). Interestingly, the study failed to detect PCR positivity for any known etiology in two-thirds of the cases (n = 21) in the cohort, of which 12 (57%) went on to develop glaucoma with a greater rate and underwent glaucoma surgery (10). The one limitation of the study was that PCR examination was only conducted on suspected CMV AU cases and not GWC assay, which is a more accurate way to detect active or persistent infection based on antibody production. Leleu et al. have also reported a mean prevalence of glaucoma in 50% of subjects in a multicenter, retrospective study in Paris (France) of a diverse pool of African-, Asian-, and Caucasian-descent patients affected with CMV AU (26). In another cohort from Germany, 61.5% of patients with CMV AU co-presented with glaucoma/uveitic glaucoma (20). In a study by Chee and Jap of a cohort from Singapore, almost 23% of CMV PCR positive with coincident PSS eyes developed optic neuropathy characteristic of glaucomatous damage and cataract (32). Interestingly, an isolated case of CMV-associated endotheliitis and AU coupled with SG has been wrongly construed as HLA-B27-linked idiopathic uveitis (74).
Altogether, these studies are indicative of the potential of CMV to cause AU with subsequent development of secondary glaucoma. This reinforces the need for greater surveillance and accurate detection of CMV accompanied by aggressive therapeutic management strategies to minimize the pathological burden conferred by CMV AU.
Epstein-Barr virus
EBV, also known as human Herpesvirus-4, is another member of the Herpesvirus family. Almost 95% of the world population is reportedly exposed to this cosmopolitan virus (75). Like other herpesviruses, EBV establishes a lifelong latent infection with periodic reactivation, usually during an immunocompromised state or in immune deficiencies (76). Despite limited tissue tropism, EBV is known to replicate and establish latency in all major immune cells, including B, T, and NK cells, and is a causative agent of multiple lymphoproliferative disorders, especially B-cell lymphomas (75, 77) and immunopathological/autoimmune diseases including infectious mononucleosis, X-linked lymphoproliferative disease/X-linked inhibitor of apoptosis protein disease and multiple sclerosis (MS). A recent study found 32 fold higher risk of developing MS in US veterans exposed to EBV historically than unexposed subjects (78). Besides immune cells, EBV infects and transforms epithelial cells, leading to the development of malignancies, mainly nasopharyngeal and gastric carcinoma (79).
Ocular manifestations of EBV have been reported with a wide range of clinical signs, including infectious mononucleosis, oculoglandular syndrome, dry eye syndrome, dacryoadenitis, conjunctivitis, episcleritis, keratitis, uveitis, choroiditis, retinitis, retinal vasculitis, and papillitis involving almost all segments of the eye (80) (Table 2). Although the role and manifestation of EBV are controversial in the pathogenesis of anterior uveitis, many cases of AU have been reported with EBV infection (81). A case of EBV AU associated with tubulo-interstitial nephritis syndrome has been reported by Grefer et al.(82). Basiaga et al. have reported a chronic severe recalcitrant bilateral AU in a 6-year-old male patient with EBV infection (83). Unlike other herpesviruses, EBV-induced uveitis is neither hypertensive nor granulomatous. Despite many reports of EBV-associated AU, elevation in IOP is not very common. Kelly et al. reported elevated IOP (40 mm Hg) in a 17-year-old male patient with lymphadenopathy with multiple ocular complications with EBV infection (84). Similarly, Rohrbach et al. reported elevated IOP (40 mm Hg) in a 7-year-old male patient with a polymorphic post-transplantation lymphoproliferative disorder with EBV infection (85). Recently, Aldaas et al. have reviewed some of these cases, implicating the association of elevated IOP with EBV infection as a cause of glaucoma development (59). It is plausible that EBV reactivation during an immunocompromised state may cause ocular inflammation, which, if unresolved, can cause glaucomatous pathology. Further studies with an appropriate clinical diagnosis and focused pathological investigations are required to establish an association of EBV-induced AU with the development of glaucoma.
TOGAVIRUSES AND GLAUCOMA
Chikungunya virus
CHIKV is an enveloped single-stranded RNA virus that belongs to the genus Alphavirus of the Togaviridae family. It causes an acute febrile illness called chikungunya fever as a result of being bitten by an infected Aedes mosquito vector. CHIKV is endemic to Africa, South and Southeast Asia, India, and South and Central America; nonetheless, it has been identified all over the world because of travel-associated transmission. Between 2005 and 2006, several CHIKV epidemics were reported in the Indian Ocean islands of Mayotte, Madagascar, Comoros, Seychelles, Mauritius, and La Réunion (86). The outbreak on the island of La Réunion in 2005 affected almost half of the population, and currently, >85% population is seropositive (87, 88). Similarly, CHIKV outbreaks were reported in India between 2005 and 2007 and in the Americas and the USA between 2013 and 2016 (89, 90). The CHIKV epidemics in Europe were linked to international tourists who brought the virus back from endemic zones, mainly India (90, 91). At present, CHIKV fever is present in >40 countries, and in 2008, the National Institute of Allergy and Infectious Diseases listed CHIKV as a category C priority pathogen (92).
Following the CHIKV outbreak in India in 2005, the most common ocular manifestation reported was AU (93–95). In a study from southern India, Mittal et al. reported AU in 18 eyes from 15 CHIKV-infected patients. In another study from India comprising 37 cases of CHIKV-related ocular sequela, Lalitha et al. reported the development of AU in 27% of patients and panuveitis in 13.5% (94). CHIKV-associated uveitis could be either granulomatous or non-granulomatous, unilateral, or bilateral, and sometimes it is linked to high intraocular pressure in the 40s. A study by Mahendradas et al. reported CHIKV-induced retinitis and iridocyclitis with increased IOP in the range of 27–42 mm Hg (93). The use of anti-glaucoma medication in combination with steroids and other cycloplegic agents has been shown to alleviate CHIKV-associated IOP elevation and inflammation respectively (93).
In addition to AU, CHIKV could also present as posterior and intermediate uveitis (89, 94). Retinitis, choroiditis, and neuroretinitis are also possible symptoms of CHIKV infection. In CHIKV patients, neuroretinitis is characterized by exudative hemorrhagic lesions that are mainly limited to the posterior pole and are associated with macular edema (93, 96). CHIKV-associated optic neuritis accounts for about 10% of all cases of ocular involvement (94). In a study, cases of optic neuritis were reported in 19 eyes from 14 patients who presented with CHIKV infection following the 2005 outbreak in India (97). Rose et al. reported the development of optic neuritis in 13 eyes from 10 patients following CHIKV infection (98). Bilateral macular choroiditis has also been reported in the CHIKV outbreak from India (99). In the acute phase, photophobia, conjunctival hyperemia, and retro-orbital discomfort are other common manifestations, and they can occur without any further ocular involvement (86). Keratitis, scleritis, episcleritis, and oculomotor palsies are examples of other ocular complications in CHIKV patients (94, 97, 100–102) (Table 2). CHIKV was found to be present in the cornea during the viremic phase, leading to a ban on corneal donation in endemic locations (103). Few reports of mother-to-child trans-placental transfer were without any substantial ocular involvement (104, 105).
Based on existing literature, despite symptoms of elevated IOP, CHIKV usually is not linked to the development of infection-triggered uveitic glaucoma. However, given the incidents of AU with elevated IOP and several optic nerve abnormalities, the possibility of its involvement in the development of glaucoma cannot be ruled out entirely. Further studies are required to determine any association between CHIKV infection and glaucoma pathogenesis.
Rubella virus
Rubella virus, also called German measles, is the only known member of the genus Rubivirus, belonging to the family Matonaviridae that causes infection in humans (106). It is an enveloped, positive sense, single-stranded RNA virus. RUBV causes congenital rubella syndrome (CRS) in infants born to exposed mothers, and its manifestations include microcephaly, hearing loss, cataracts, glaucoma, retinopathy, microphthalmia, and congenital heart disease. It usually spreads through human-to-human transmission via nasopharyngeal secretions, and carriers other than humans have not yet been identified (106).
RUBV commonly affects younger age groups, and positive patients usually present with unilateral involvement and a combination of three to four defining symptoms including keratic precipitates, iris atrophy, vitreous opacities, and cataracts (34). Quentin and Reiber first described the association between RUBV and the development of Fuchs heterochromic cyclitis, also known as FUS (107). Many other studies then followed, presented similar findings, and reported the association between RUBV and FUS by detecting either RUBV-specific intraocular antibodies or viral RNA or a combination of both (21, 34, 108–112). Although RUBV is most commonly implicated in the pathogenesis of FUS, the FUS associated with RUBV has steeply declined in the USA since the introduction of the rubella vaccine (113). However, the virus is still a cause of concern among the unvaccinated and in endemic areas (30). In Europe, the majority of FUS cases have been associated with RUBV (107, 108).
The contribution of rubella to AU and SG is comparatively less than herpetic viruses; however, the mechanism of glaucoma in RUBV is complex (21). RUBV-induced glaucoma is often associated with dysgenesis of the anterior chamber angle. In addition, inflammatory components, early extraction of cataracts, and necrosis of the ciliary body and TM are other contributing factors for RUBV-induced IOP and glaucoma (59). de Visser et al. have reported the development of SG in 23% and 5% of PCR or GWC analysis-confirmed RUBV-positive patients at first presentation or after a year, respectively (34). However, the rate of glaucoma development was not significantly different among virus-negative patients with AU, which suggests the role of either virus-independent mechanisms behind the development of glaucoma in those patients or limitations associated with an accurate diagnosis. Wensing et al. have also reported the development of glaucoma in 22% of patients with RUBV AU in a Dutch cohort that included patients of Belgian and Slovenian origin (21). In another study, Pohlmann et al. reported the prevalence of uveitic glaucoma in 21.2% of RUBV-associated AU cases (20).
Recently, Groen-Hakan and colleagues tried to deconstruct the idea that FUS and RUBV AU are superimposable ocular pathologies (30). The authors suggest that FUS and RUBV AU are not exchangeable. They emphasize the need to see them as two distinct syndromes as RUBV AU presented with a broader spectrum of characteristics, including vitritis (without posterior synechiae and CME) with the early development of cataracts and glaucoma. In this cohort, 38% of patients were presented with AU; among these, 28%, 80%, and 89% ended up developing glaucoma, cataract, and vitritis, respectively. Their study also supported the possible existence of a latent intraocular reservoir of RUBV as none of the patients were vaccinated, and all had cleared systemic viremia (30). In addition, CRS has been reported in 40% (26/65) of children aged 0–59 months from a south Indian population (114). The viral genome was detected in 92% of cases with confirmed serodiagnosis of rubella, which shows significant ocular tropism of the virus in children under age 5.
FILOVIRUS AND GLAUCOMA
Ebola virus
The West African Ebola virus disease (EVD) outbreak, which lasted from 2013 to 2016, killed more than 11,000 people and affected >28,500, with a high mortality rate of 38% (115). Similarly, another EVD outbreak in the Democratic Republic of Congo during 2017–2020 resulted in 3,200 cases and over 2,000 deaths (116). Since the first documented outbreak of the Ebola virus in 1976 and the West African epidemics in 2013, 23 outbreaks have been reported, resulting in 1,546 deaths and 2,345 confirmed cases (117). Although the bat is assumed to be the EBOV reservoir, the virus can also spread from person to person or from animal to animal. The Ebola virus causes a hemorrhagic fever with a variety of symptoms such as fever, chills, headache, maculopapular rash, myalgia, abdominal pain, diarrhea, and respiratory symptoms within 3 weeks of exposure (118, 119). The case fatality rate in the West African outbreak was as high as 74%. In contrast, those medically evacuated to Europe and the USA had a mortality rate of around 20% (118). The persistence of the live Ebola virus in immune-privileged sites such as the eye, reproductive organs, placenta, and central nervous system poses a substantial public health threat. Survivors often develop complications during convalescence and could present with severe symptoms such as uveitis and other complex ocular disorders, lethargy, myalgia, arthralgia, gastrointestinal pain, auditory, and mental health disorders (120). In an intriguing example, a physician who was exposed and diagnosed with EVD while on duty in Sierra Leone, medically evacuated, and treated in the USA showed cleared viremia on the day of discharge in his blood and urine; however, live EBOV was isolated from his semen sample. This patient eventually developed chorioretinal scars, photophobia, keratic precipitates, leukocyte infiltration, and protein (flare) in the anterior chamber, with a diagnosis of AU and ocular hypertension with elevated IOP (44 mm Hg) as a sequela of EVD after a month of his discharge (42).
EBOV ophthalmic consequences are known from recent and previous epidemics. Prevalence of uveitis ranged between 18% and 34% in survivors of the recent West African outbreak, and more than one-third of these uveitic patients became blind (119). There were isolated cases of AU and intermediate uveitis, in addition to the cases of posterior uveitis, which have been described to be associated with the development of retinal lesions following EVD (121, 122). EBOV-induced ocular complications included optic neuropathy and other neuro-ophthalmic issues in some people and phthisis in others (119, 123) (Table 2). Twenty percent of the convalescent patients have reportedly developed hypertensive uveitis, which is characterized by ocular pain, photophobia, hyperacrimation, foreign body sensation, red eye, and progressive vision loss after being asymptomatic for up to 2 months (124). The size and shape of Ebola retinal lesions varied, but they all had prominent linear borders with strong angulations. On fundus photography, lesions appeared light gray and were mostly non-pigmented (121).
In a study among EVD survivors from Sierra Leone, ocular complications were reported in 57% of survivors, and uveitis was identified as the most common ophthalmic manifestation (61.7%) (120). The EBOV-induced uveitis was either bilateral (59%), anterior (62%), or panuveitis (21%). One case each of glaucoma, cataract with glaucoma, and corneal scar with glaucoma was presented among these EVD survivors with uveitis (3/21 = 14%). Another large retrospective study from Siera Leone reported the presence of AU (46%), posterior uveitis (26%), panuveitis (25%), and intermediate uveitis (3%) in EVD survivors (123). Similarly, a prospective study from Guinea reported the development of anterior, posterior, and panuveitis in 48%, 28%, and 8.7% of EVD survivors, respectively (125). Shantha and co-workers, using 23 pediatric EVD survivors, reported ocular complications in 47.8% of cases, with the development of glaucoma in 6.5% of these patients (35).
Thus, like all other oculotropic viruses, EBOV causes significant and long-delayed pathology in exposed survivors. Although uveitis is identified as the most common pathology, EVD survivors may present late with a spectrum of ocular manifestations that eventually lead to blindness. Therefore, proper clinical management and understanding of the chronic pathogenesis of these infection-triggered pathologies are crucial to enhancing the quality of life of those affected.
FLAVIVIRUSES AND GLAUCOMA
Dengue virus
DENV belongs to the genus Flavivirus and is among one of the most commonly distributed Arboviruses worldwide. DENV is an enveloped, positive sense, single-stranded RNA virus. It causes febrile to severe illness in affected individuals bitten by Aedes aegypti or Aedes albopictus mosquito carrying any one of the four known serotypes DENV 1–4. According to the Centers for Disease Control and Prevention, around half of the world’s population lives in DENV-endemic areas and is at risk of getting DENV infection (126). While nearly 75% of people infected with DENV remain asymptomatic, the symptomatic patients generally present with fever, headache, myalgia, arthralgia, nausea, vomiting, and rash (127). Dengue hemorrhagic fever (DHF), dengue shock syndrome, and Guillain-Barré syndrome are severe consequences of DENV infection. With DHF, the mortality rate could be as high as 10%–15% in the lack of emergency supportive treatment and platelet replacement (128–130). Reinfection with different serotypes of DENV generally leads to an increased disease severity due to defective antibody binding and virus neutralization called antibody-dependent enhancement (131).
DENV-related ocular complications are generally overlooked; however, many recent reports confirm increasing incidences of ocular manifestation. DENV-induced ocular complications range from blurred vision, paracentral scotoma, choroiditis, retinitis, and maculopathy in the posterior segment to hemorrhagic conjunctivitis/hyposphagma, panopthalmitis, retinal pigment epithelium (RPE) mottling, AU, and increased IOP (38, 130, 132–137) (Table 2). Visual impairment in DENV patients has been noted to occur simultaneously with the nadir of serum thrombocytopenia (138). Although the occurrence of AU has been documented less often in patients with DENV infection, it is an important viral pathology with vision-threatening implications, including the development of glaucoma. While reports on DENV-specific uveitic glaucoma are sparse in the field, multiple case reports have shown the incidences of bilateral acute angle-closure glaucoma with DENV infection (139–141). A case study from Finland in 1976 described the development of acute glaucoma in three patients with hemorrhagic fever with renal syndrome (nephropathia epidemica) resembling DENV infection with unknown other etiology (59, 142).
These reports are indicative of the prominent pathological role of DENV in ocular infections and pathology. Despite the paucity of reports on DENV-induced uveitic glaucoma, it is a potential viral agent with ocular tropism and needs to be studied further for a possible role in anterior chamber inflammation and subsequent glaucomatous pathology.
West Nile virus
WNV is another ubiquitous Arbovirus of the RNA virus family, Flaviviridae. It has a single-stranded positive-sense RNA genome. WNV was isolated for the first time in 1937 in Uganda’s West Nile district (143). WNV is the most prevalent mosquito-borne disease in the continental USA, which presented dramatically in New York City at the end of the 20th century and subsequently speared across the North American continent. Two different lineages of WNV have been described; lineage 1 is present globally, with the highest concentrations in North America, Eastern Europe, the Middle East, West Africa, and Australia. African enzootic strains comprise lineage 2 (144). Currently, no vaccines or medications are available to prevent or treat WNV infection in humans. The majority of those infected with WNV are asymptomatic. Twenty-five percent of the infected develop a febrile illness called West Nile fever, and ~1% develop a serious or fatal neuroinvasive illness (145). The first human WNV infection was identified in the USA in August 1999, during a meningoencephalitis outbreak of uncertain etiology in New York City with 62 positive cases and seven deaths (146). In 2003, during the largest outbreak to date in the USA, 9,858 human cases were reported, including 262 deaths (147). From 1999 to 2021, a total of 55,443 positive cases with 27,857 neuroinvasive diseases and 2,683 deaths were documented alone in the USA (145).
The most common way to contract WNV is through the bite of an infected Culex mosquito. WNV infection is classified clinically into three sub-categories: asymptomatic, West Nile fever, or West Nile meningoencephalitis. WNV fever is typically characterized by the abrupt onset of a high-grade fever (greater than 39°C), headache, myalgias, and gastrointestinal symptoms. Additionally, pharyngitis, arthralgias, tiredness, and a maculopapular rash on the chest, back, and lower extremities have also been recorded. Aseptic meningitis, encephalitis, meningoencephalitis, myelitis, polyradiculitis, or optic neuritis are all possible manifestations of WNV (Table 2). Meningitis or encephalitis was diagnosed in 29% of the 9,858 cases in the USA in 2003. Additionally, reduced deep tendon reflexes, proximal muscular weakness, flaccid paralysis, and respiratory failure may occur. There have been reports of movement problems such as tremors, myoclonus, and parkinsonism (148). The common ocular manifestations caused by WNV include chorioretinitis, uveitis, occlusive retinal vasculitis, congenital chorioretinal scarring, and optic neuritis (137, 147, 149, 150). Bilateral multifocal chorioretinitis is the most frequent ocular presentation in WNV cases (151). In WNV-affected individuals, active chorioretinal lesions present as multifocal, deep, flat, white, or yellowish lesions with a 200- to 1,000-micron diameter on fundoscopic examination (86). Cases of AU and optic neuropathy have also been presented in WNV-infected individuals (147, 152, 153). WNV infection has been associated with focal neuro-ophthalmologic symptoms such as diplopia (154). Ocular involvement is more common during the acute phase of the disease, although it can also occur as a result of trans-placental infection (152).
Despite the known involvement of WNV in ocular complications, there have been sporadic case reports. In one study from India, 37 out of 52 (71%) subjects developed retinitis, arteritis, phlebitis, and retinal hemorrhage with or without a macular star. These patients also demonstrated retinal inflammation with indistinct borders and vascular and optic disc leakage. All these patients were positive for WNV infection by serological testing, reverse transcription-PCR (RT-PCR), reverse transcriptase loop mediated isothermal amplification (RT-LAMP), and nucleotide sequencing (155). In another case study, a 56-year-old female with multiple co-morbidities has been shown to develop anterior chamber inflammation, bilateral nongranulomatous uveitis, and keratic precipitates with increased IOP in both eyes (156). This patient tested positive for WNV by ELISA and plaque reduction neutralization assay. Her ocular symptoms resolved with topical steroid treatment. This study also postulates that WNV-affected patients with photophobia, conjunctival hyperemia, and ocular pain may have uveitis but are usually misdiagnosed by ophthalmologists due to lack of detailed examination (156).
Although few reports demonstrate the link between WNV infection and the development of glaucoma, it has been implicated in a range of ocular manifestations, including AU (153, 156). This necessitates a detailed ophthalmic investigation of WNV-infected patients and further experimental and epidemiological studies to explore the possible role of WNV in the development of uveitis and associated glaucomatous pathology.
Hepatitis virus
Among Flaviviral diseases, viral hepatitis is a major global health problem. Various serotypes of hepatitis, including hepatitis A (HAV), hepatitis B (HBV), and hepatitis C (HCV), are known to cause serious infections, among which HBV and HCV can be fatal. Multiple epidemiological studies and case reports have documented the prevalence of uveitis among hepatitis-exposed subjects (157–160).
In a large-scale population-based study by Kridin et al., a significant association was found between HBV infection and the development of uveitis, but not with HCV (157). In another large cohort from Taiwan, co-infection of HBV and HCV was linked to a higher risk of uveitis (159). Previously, 13% and 2% seropositivity for HBV were found in cohorts of uveitis-affected patients from Switzerland and England, respectively (161, 162). In addition, a case report described the development of bilateral AU in a 35-year-old male patient as a manifestation of HAV infection (160). Seven cases of uveitis (3 = anterior, 1 = intermediate, and 3 = panuveitis) were documented in a cohort of acute autoimmune hepatitis with glaucoma in three out of seven subjects (158). Some cases of glaucoma associated with hepatitis are linked to the interferon therapy used to treat viral infection. A case report of a 15-year-old boy reported that he developed glaucoma following interferon-α therapy treatment of chronic hepatitis B (163). Similarly, a case of neovascular glaucoma with high IOP (40 mm Hg) was reported in a 56-year-old man due to interferon-α therapy used to treat hepatitis C infection (164). Recently, Player et al. identified candidate genes associated with glaucoma development by retrospectively analyzing the transcriptomics data set from hepatitis C-infected human peripheral blood mononuclear cells (PBMCs) stimulated with TLR3 agonist poly I:C (165). This study also reported a correlation between chronic inflammation in HCV and the dysregulated expression of genes involved in glaucoma progression (165).
These reports are suggestive of ocular manifestations, including AU arising due to hepatitis infection and its potential role in the development of glaucomatous pathology in affected individuals. However, the available evidence is not robust and requires more in-depth investigations to determine the precise role of hepatitis viruses in the development of glaucoma.
Zika virus
ZIKV is one of the re-emerging Arboviruses belonging to the Flavivirus family with a single-stranded RNA genome (37). It was first isolated from the blood of the captive sentinel rhesus monkey in 1947 from Uganda’s Zika forest (166). In 1952, the first human case was registered in Nigeria (167). The first incidence of ZIKV infection outside of Africa or Asia was reported in 2007 on Yap Island of Micronesia (168). In 2013, a major outbreak was observed on the French Polynesian islands, and the first local cases were recorded in Brazil in May 2015 (169, 170). ZIKV is primarily transmitted by Aedes aegypti mosquito bites (171). In addition, direct human-to-human transmission has also been documented through mother-to-fetus during pregnancy, breastfeeding, blood transfusion, sexual contact, and organ transplantation (168–170). Based on the epidemiology and geographical origin of ZIKV, two major lineages—African and Asian—have been identified (172).
ZIKV induces very mild symptoms in adults, which include fever, arthralgia, maculopapular rash, conjunctivitis, headache, thrombocytopenia, and leukopenia (37, 168). However, gestational ZIKV infection leads to congenital Zika syndrome (CZS), with the most dramatic manifestation being microcephaly. Guillain-Barré syndrome is another fatal pathology linked with ZIKV infection (104, 137). Following the 2015 Brazilian ZIKV outbreak, a 20-fold increment in microcephaly cases was recorded with concomitant vision-threatening ophthalmological alterations in around one-third of cases. Many recent studies also indicate that ZIKV can also cause severe ocular abnormalities without microcephaly (173–176). During the ZIKV outbreak in the Americas, a significant proportion of the infants born from infected mothers reportedly showed ocular pathologies that included unilateral microcornea, iris coloboma, chorioretinal atrophy, hypoplasia, focal pigmented mottling, RPE mottling, retinal focal spots, severe retinal vessel attenuation, macular pigmentation alterations including pallor, optic nerve atrophy, optic disc anomalies, and congenital glaucoma (7, 8, 45, 137, 177–187) (Table 2). Approximately 35% of infants born with microcephaly during the ZIKV epidemic in Brazil showed vision-threatening lesions (175). Children of the female sex were reportedly more affected, and bilateral findings were presented in 85% of all cases (38, 175). Studies from our laboratory and other investigators have also experimentally demonstrated involvement of ZIKV in the development of pathological eye conditions, including panuveitis (188), chorioretinal atrophy (178, 179), and glaucoma (45, 189). It was recently shown that the African strain of ZIKV induces a more robust innate anti-viral response in iPSC derived RPE cells than the Asian strain of ZIKV (180). Interestingly, this strong inflammation was associated with greater disruption of tissue homeostasis accompanied by substantial destruction of RPE cells, ultimately leading to dysregulated inflammation, tissue pathology, and poor virus control (180). On the other hand, epidemic Asian strains negatively modulate type-1 IFN response (190) and induce immunosuppressive non-classical monocytes permissive to ZIKV (191). This ability to cause low-intensity infection and inflammation has been suggested to confer the Asian ZIKV strain with a persistent survival advantage in placental tissues with the potential to cause post-natal defects (192). The ability of epidemic ZIKV strains to persist chronically can be corroborated with the long-term visual impairments and ocular pathology reported in seropositive children and adults from various clinical reports and retrospective epidemiological studies (179, 183, 185, 193–196).
Studies from ZIKV epidemics have shown a strong association between ZIKV and glaucoma with or without uveitis. A retrospective case study of 43 infants from Colombia and Venezuela with CZS reported congenital glaucoma in 12% of infants, whereas 88% had optic nerve abnormalities (7). All infants with glaucoma from this study showed extremely high IOP (mean of 57.4; range, 49.8–76.1 mm Hg) with open angle and buphthalmia without symptoms of AU. Another follow-up study of ZIKV patients reported bilateral AU prevalence in 48% of subjects with concomitant ocular hypertension in 83% (197). De Moraes et al. presented three cases of ocular abnormalities secondary to ZIKV infection in seropositive patients (9). In this case series, a 49-year-old woman presented with bilateral optic neuritis; a 4-year-old boy presented retrobulbar uveitis; and a 17-day-old infant presented with CZS and underwent glaucoma correction surgery (9). Most recently, in a meta-analysis of 13 pregnancy cohorts/studies from epidemic hit Brazil, 4% of offspring displayed significant ophthalmic abnormalities, confirming ZIKV ability to cause ocular damage (198). However, this study does not explain or typify the underlined pathological spectrum in the ocular tissues.
In contrast to several reports of ZIKV-induced congenital glaucoma, De Oliveira et al. did not find any incidence of glaucoma as per definitions established in the Ninth Consensus Report of the World Glaucoma Association in a study of 188 eyes from 107 children born with presumed CZS in Brazil (199). Instead, the authors reported increased vertical cup-to-disk ratio (CDR), IOP, horizontal corneal diameter, and myopia related to increased axial length, etc., as attributes in the 2.5% of CZS-qualified subjects that may lead to misdiagnosis of glaucoma (199). The authors of this study speculate that CDR criteria for glaucoma may not be reliable for CZS, as the increased CDR could be related to ZIKV neurotropism and not due to elevated IOP. In addition, the incidence of congenital glaucoma has also been suspected to be a function of maternal genetic susceptibility due to single-nucleotide variations in adenylate cyclase genes that influence the outcomes of CZS (200).
ZIKV-associated ocular comorbidities in infants and anterior (8, 86, 201, 202) and posterior chamber uveitis (181–183, 194) in adults have been evident from several other studies. A case of AU with PCR-confirmed ZIKV etiology was reported by Furtado et al. from Brazil (202). Fontes also reported one isolated case of a ZIKV-infected physician who presented with bilateral hypertensive iridocyclitis (201). In a recent retrospective analysis of the cohort of 469 infants (938 eyes) with CZS from the 2015 to 2016 Brazilian ZIKV outbreak, 31.6% of patients (28.7% eyes) were found to have ocular complications. The predominant ocular pathological features were optic nerve pallor (13% eyes), focal pigment mottling (12% eyes), and chorioretinal scars (11% eyes) (185). Similar findings have been reported from Costa Rica from a cohort of 11 confirmed and 11 probable (total 22) perinatal ZIKV-exposed infants. In this cohort, 41% of infants presented ocular manifestations with chorioretinal scarring and optic nerve anomaly in 27% and 23% of cases, respectively (195). Further, a first-of-its-kind study from North America (US Virgin Islands) reported the development of post-natal visual impairment in more than a quarter of infants exposed antenatally to ZIKV with a median age of 9 months (196). The rate of visual impairment was even greater in those with confirmed ZIKV etiology (38.5%). Interestingly, the prevalence of visual impairment was unusually high in children despite any evident neurological, developmental, or ophthalmological anomaly at the time of examination (196).
These observations are a serious indicator of the pathogenic ability of ZIKV to be able to not only transmit vertically but also cause delayed and long-lasting ocular abnormalities. While these studies offer fresh insights and motivation to carefully work out the role of ZIKV in causing ocular complications and congenital glaucoma, the threat is still open to affect the exposed population in endemic regions. Thus, it is imperative to establish vigorous experimental, epidemiological, and long-term follow-up investigations.
CORONAVIRUSES AND GLAUCOMA
Experimental evidence on the ocular involvement of coronaviruses is long studied in mouse models. A member of the murine coronavirus, mouse hepatitis virus (MHV), is known to cause degenerative retinopathy in two different mouse models, BALB/c and CD1 mice (203, 204). The MHV establishes infection in both the anterior and posterior chamber in susceptible BALB/c mice during the acute phase of infection (5–7 days). After 7 days, the virus was not detected in the anterior chamber but persisted in the retina (until 60 days) and caused severe degenerative pathology (204). These early experimental studies suggest the ocular tropism of coronaviruses. In addition, the prominent endemic members of the coronavirus family are well known to cause human infections. Infections with HCoV-NL63, SARS, and, more recently, SARS-CoV-2 have been shown to cause numerous ophthalmological manifestations in affected individuals (205–210).
SARS-CoV-2
SARS-CoV-2 is an enveloped RNA Coronavirus that caused the recent pandemic of COVID-19. The global COVID-19 pandemic started with a glaucoma patient when an ophthalmologist warned about a strange virus, later identified as SARS-CoV-2. This ophthalmologist passed away due to exposure to SARS-CoV-2 from his patient. In the beginning of the pandemic, SARS-CoV-2 was linked to causing conjunctivitis, and few studies reported the presence of viral RNA/protein in a cadaver’s conjunctival tissue (205). Later, many studies reported the ocular involvement of SARS-CoV-2. The most common ocular manifestation includes dry eye or foreign body sensation, redness, tearing, itching, ocular pain and discharge, hordeolum, pingueculitis, keratitis, episcleritis, keratoconjunctivitis, and anterior uveitis (206–208, 211–216) (Table 2). Aldaas et al. recently reviewed the evidence on the ocular manifestations of COVID-19 and found conjunctivitis is the main ocular sequalae with a prevalence rate of 8.0%–88.8% (59). Although reports of glaucoma due to SARS-CoV-2 are sparse, few isolated cases have shown the association of SARS-CoV-2 with glaucoma. Barosco et al. presented a case of bilateral primary angle-closure glaucoma in a 64-year-old male with COVID-19 (217). Similarly, Nerlikar et al. reported bilateral angle-closure glaucoma development in a 53-year-old male after prone position ventilation for COVID-19 pneumonia (218). Özmen and coworkers reported three cases of acute angle-closure glaucoma, all with high IOP (35, 44, and 40 mm Hg, respectively) in the setting of hyponatremia due to SARS-CoV-2 infection (219). Soman et al. has described a case of neovascular glaucoma with high IOP (44 mm Hg) in a 50-year-old male with COVID-19-related retinopathy (220). All these cases were treated symptomatically in combination with anti-glaucoma medication to control the IOP. It is not known if the angle-closure patients were at risk before the intensive treatment for COVID infection. Many cases of optic neuropathies, such as optic neuritis, have been shown to be caused either by SARS-CoV-2 or its vaccine (216, 221–227).
Based on the currently available literature, it is evident that SARS-CoV-2 and other coronaviruses can cause significant ocular manifestations, including uveitis, optic neuropathies, and possible glaucoma. The neuro-ophthalmological complications related to SARS-CoV-2 can be severe, and therefore patients should be monitored continuously, along with serological, immunological, and ocular imaging. Additionally, further in-depth investigations are warranted to study the role and molecular mechanisms of SARS-CoV-2-mediated ocular manifestations and glaucoma.
ANTI-VIRALS AND GLAUCOMA
Conventionally, glaucoma is managed by topical corticosteroids, cycloplegics, and IOP-lowering medication, and in some cases, surgical interventions are necessary. However, glaucoma management in the setting of viral infections depends on multiple factors, such as treating the underlying condition, treating the virus-induced inflammation, and controlling the elevated IOP. The treatment regimen for virus-associated glaucoma usually includes systemic or topical anti-viral agents combined with IOP-lowering eye drops. However, anti-viral therapy does not always control IOP, and some patients do not tolerate systemic anti-viral treatment because of their side effects. It is also crucial to treat viral-associated inflammation to reduce the risk of long-term damage to the TM. In viral-associated SG, a common mistake is undertreating the inflammatory response for fear of a corticosteroid-induced IOP elevation. Therefore, vigilant monitoring of steroid dosage and frequency is required to manage viral-induced uveitis and associated glaucomatous pathology.
Some recent studies have indicated disproportionately higher incidences of glaucoma in patients with anti-viral treatments. A study on Australian cohorts has shown patients on anti-retroviral therapy have increased incidence of use of anti-glaucoma medications (2). Similarly, a few clinical case studies have reported the development of bilateral acute angle-closure glaucoma with the use of oseltamivir, a common anti-viral used to treat influenza (228, 229). A case study with CMV AU reported ganciclovir treatment in combination with anti-inflammatory and anti-glaucoma medication controlled the CMV burden but failed to reduce the IOP without surgical intervention (230). Another anti-viral drug, cidofovir, used to treat CMV and HSV infections, has been shown to develop anterior uveitis (231). In addition to anti-viral medication, some vaccines have also demonstrated the development of anterior uveitis and/or panuveitis. Measles, mumps, and rubella vaccine has been shown to cause uveitis and panuveitis in a few isolated cases (232, 233). Similarly, SARS-CoV-2 vaccine has been shown to cause optic neuropathies in some cases (225, 234–236). Although no clear evidence indicates how anti-virals lead to a higher incidence of glaucoma, a plausible hypothesis suggests it could be due to drug-induced toxicity, ocular hypertension, or idiosyncratic drug reaction (2, 228). An anti-viral drug, ritonavir, a protease inhibitor, has shown visual field defects in 2% or less and retinal impairments in SARS-CoV-2 patients (2, 237, 238). Similarly, anti-virals with nucleoside reverse transcriptase inhibitors have shown mitochondrial injury that may affect the pathogenesis of glaucoma and optic neuropathy (239).
Despite a few contrary reports, systemic (acyclovir, ganciclovir, valacyclovir, or famciclovir) or topical (trifluridine or ganciclovir eye drops/ gels) anti-viral agents, in combination with corticosteroids and IOP-lowering drops, is a standard treatment in managing viruses-associated glaucoma. The anti-viral therapy should be personalized for glaucoma management in patients with viral infection based on their immunocompetent vs immunocompromised state. In some cases, glaucoma filtration surgery or a tube shunt is essential to control the IOP.
CONCLUSION AND FUTURE DIRECTIONS
Viruses are well known to cause a wide range of ocular manifestations, either as primary infection of ocular tissue or secondary complications consequent to perturbed tissue homeostasis. Glaucoma is the commonest optic neuropathy, characterized by RGC degeneration and vision loss. Viruses are a significant cause of elevated intraocular pressure and the development of glaucoma (Fig. 1). Although viral glaucoma is often preceded by sequela of uveitis, some viruses can cause glaucoma independent of uveitis. Uveitis is characterized by inflammation in any part of the eye due to the infiltration of immune cells to clear the infection (Fig. 1). This either clears out or forces the infection to hide in latent form while also posing significant collateral damage to the tissue involved. This perturbed tissue homeostasis often leads to the development of secondary complications, including glaucoma. Many viruses including those in the herpesvirus family (HSV, VZV, CMV, and EBV), members of the Flavivirus family, (e.g., ZIKV, DENV, and WNV), Filovirus (EBOV), Togavirus (RUBV and CHIKV), and, most recently, SARS-CoV-2, are shown to be associated with the development of glaucoma. Despite a large body of evidence available regarding the role of viruses in glaucoma pathogenesis, studies deciphering molecular mechanisms involved in its pathophysiology are scarce. Studies aimed at dissecting molecular pathways and mechanistic insights behind the development of glaucoma are much needed to understand the pathophysiology of the disease and to develop precise therapeutic modalities. Infectious glaucoma is different from hereditary or genetic glaucoma and can be managed with therapeutic and surgical interventions. However, timely identification of the infectious etiology is crucial for prioritizing and managing the disease. Traditional symptom-targeted therapies for viral glaucoma management involve the usage of steroids beta blockers, and carbonic anhydrase inhibitors alongside anti-viral agents. Therapies aimed at inhibiting or fine-tuning intraocular inflammation and inhibiting specific immune cell infiltration could be the next-generation choice of treatment for managing virus-induced ocular pathologies.
ACKNOWLEDGMENTS
This study is supported by the National Institute of Health/National Eye Institute grant R01EY032495 and research start-up funds from the University of Missouri School of Medicine. The authors are grateful to other laboratory members for their helpful discussion.
To the best of our knowledge, we have made every effort to cite appropriate and topic-related references. The authors would like to apologize to the groups whose references were overlooked during the literature search and writing of this review article.
Conceptualization: P.K.S.; writing (manuscript draft preparation): F.A. and P.K.S.; writing (manuscript review and editing): F.A., N.D., A.W., S.J., A.S., R.M., F.F., and P.K.S.; supervision: P.K.S.; funding acquisition: P.K.S. All authors read and agreed to publish the final version of the manuscript.
Biographies

Faraz Ahmad, Ph.D., is currently a Postdoctoral Fellow at the Ocular Infectious Disease and Glaucoma Lab, Department of Ophthalmology, University of Missouri School of Medicine, Columbia, USA. He acquired a Ph.D. degree from Aligarh Muslim University, Aligarh, India, with a specialization in Molecular Immunology and Vaccine Development against Infectious Diseases. Post Ph.D., he served as a Research Associate at the Indian Council of Medical Research-National Institute of Pathology, New Delhi, India. Dr. Ahmad's research interests are in the field of infectious diseases and developing novel prophylactic and host-directed therapeutic interventions for their management. In his current research endeavors, he is studying the role of innate and adaptive arms of the immune system in the pathogenesis of flavivirus infections with a prime focus on the Zika virus and associated congenital ocular abnormalities, including glaucoma.

Nikhil Deshmukh is an undergraduate student at the University of Missouri-Columbia, USA, majoring in Biology and Statistics and minoring in Computational Neuroscience. He is currently working as a research assistant at the Ocular Infectious Disease and Glaucoma lab at the University of Missouri School of Medicine, Columbia, USA. His research interest includes host-pathogen interaction, innate immunity, neuro-ophthalmic complications, and infectious diseases. He is currently studying the role of the Zika virus in the pathophysiology of ocular infections and screening small molecule inhibitors against the Zika virus for therapeutic development.

Aaron Webel, M.D., is an Assistant Professor of clinical ophthalmology and a glaucoma specialist at the University of Missouri-Columbia, USA, where he is director of ophthalmology education for the medical school. His research is focused on ophthalmic medical device research and development, with an aim to improve eye care through innovative ophthalmic diagnostic and surgical devices. His work is primarily focused on traditional incisional glaucoma surgery. His areas of interest include ocular fibrosis following glaucoma surgery and developing technologies and techniques to mitigate this fibrotic response to improve surgical outcomes. He has been awarded a Mentoring for the Advancement of Physician Scientists (MAPS) grant by the American Glaucoma Society (AGS) to develop a glaucoma drainage device sulcus tube insertion system.

Sandra M. Johnson, M.D., is a Clinical Professor of Ophthalmology at the University of Missouri-Columbia, USA. She attended college at Dartmouth and then went on to medical school at Boston University, USA. Dr. Johnson did ophthalmology residency training at the University of South Carolina, then a clinical glaucoma fellowship at the Harvard University Massachusetts Eye and Ear Infirmary, where she had the appointment of Clinical Instructor. She was later a faculty at the University of North Carolina, Dartmouth, and the University of Virginia. She practices clinical glaucoma and is broadly interested in topics pertaining to her clinical area of expertise.

Ayman Suleiman, M.D., is an Assistant Professor and Neuro-ophthalmology physician at the University of Missouri-Columbia, USA. Dr. Suleiman obtained his M.D. at the Medical University of Varna, Bulgaria. Following his immigration to the U.S., he completed his fellowships in numerous ophthalmology specialties, including clinical research in Glaucoma at Wills Eye Hospital, Philadelphia, PA. He then completed another fellowship in neuro-ophthalmology at Michigan State University, Michigan, followed by a one-year fellowship under the supervision of the renowned neuro-ophthalmologist Dr. Andrew G. Lee at the Methodist Hospital, Houston, Texas. After two years of specializing in neuro-ophthalmology, Dr. Suleiman completed an additional two years of fellowship training in pediatric ophthalmology at the University of Tennessee-Memphis. Currently, he is serving as the head of neuro-ophthalmology at the University of Missouri and the Mason Eye Institute.

Rajiv R. Mohan, Ph.D., is a Curators' Distinguished Professor and Endowed Chair of Ophthalmology and Molecular Medicine at the University of Missouri, Columbia, Missouri, USA. He is also a Senior Research Career Scientist at the Truman VA Medical Center in Columbia, Missouri. Dr. Mohan's research is focused on studying mechanisms causing blindness and developing novel gene therapies and nanomedicine approaches to treat corneal blindness and restore vision using state-of-the-art multimodal in vivo eye imaging systems, molecular techniques, in vivo preclinical rodent, rabbit, and pig and human in vitro cells and organ culture models. His research is continually funded through NIH, VA, and foundation grants. He has coauthored >180 peer-reviewed journal articles, 10 book chapters, >400 meeting abstracts and received many prestigious national/international awards. Dr. Mohan routinely serves on grant review panels, the editorial board of journals, and professional eye research societies. He has organized several eye research conferences, chaired/co-chaired scientific sessions, and delivered >120 invited lectures in 30+ countries.

Frederick Fraunfelder, M.D., MBA, is the Roy E. Mason and Elizabeth Patee Mason Distinguished Professor and Chair of Ophthalmology and the Assistant Dean for Faculty Affairs. Dr. Fraunfelder is a cornea specialist with expertise in external disease, ocular oncology, and refractive surgery. He is one of the few physicians in Missouri to perform two specialized eye surgeries, DMEK and DSEK. Dr. Fraunfelder is an active surgeon and physician-scientist with a robust clinical practice and ongoing research projects. He is the author of over 125 published articles, book chapters, and books, including the recently revised edition of the book Drug-Induced Ocular Side Effects. Dr. Fraunfelder's research interests include the development of methods for improving the storage of donor corneal tissue, the mechanisms of and risk factors for corneal graft rejection, corneal scarring, and fibrosis, drug- and vaccine-induced ocular side effects, and the use of liquid nitrogen cryotherapy for eradiating lesions of the eye surface. Dr. Fraunfelder is board certified by the American Board of Ophthalmology and is a member of the prestigious American Ophthalmological Society.

Pawan Kumar Singh, Ph.D., is an Assistant Professor of Ophthalmology at Mason Eye Institute at the University of Missouri School of Medicine, Columbia, Missouri. He is also a director of the Ocular Infectious Disease and Glaucoma lab. Dr. Singh completed his Ph.D. in Biotechnology at Defense Research and Development Organization/ Jiwaji University in India. Following his Ph.D., he served as Research Associate at one of India's premier immunology research institutions, the National Institute of Immunology, New Delhi. After completing his postdoctoral fellowship at Wayne State University in Detroit, USA, Dr. Singh started his independent laboratory at the University of Missouri in 2021. Dr. Singh's training and research background is in virology, immunology, and microbiology. His research focuses on understanding the role of innate and adaptive immunity, host-pathogen interaction, and molecular signaling mechanisms of retinal cell death in glaucoma and ocular infectious diseases and discovering therapeutic molecules to protect retinal cells from damage. In one of his NIH-funded projects, Singh's lab is investigating the role of the Zika virus and maternal immunity in ocular complications and congenital glaucoma. In addition, using the omics approaches, his lab is working on the discovery of biomarkers and immunomodulatory/ neuroprotective therapies for glaucoma. Dr. Singh has coauthored >30 publications in peer-reviewed journals, >50 abstracts, and delivered several talks. He serves as a reviewer and editorial board member for many international journals. He also serves on NIH-NIAID and NEI study sections. Dr. Singh has received many prestigious awards, including the Young Scientist Award by the M.P. Council of Science and Technology, India, and the Young Investigator Award in Microbiology by the Raniyah Ramadan Foundation, USA.
Contributor Information
Pawan Kumar Singh, Email: pksfcq@health.missouri.edu.
Jennifer Dien Bard, Children's Hospital Los Angeles, Los Angeles, California, USA.
REFERENCES
- 1. Shalaby WS, Ahmed OM, Waisbourd M, Katz LJ. 2022. A review of potential novel glaucoma therapeutic options independent of intraocular pressure. Surv Ophthalmol 67:1062–1080. doi: 10.1016/j.survophthal.2021.12.003 [DOI] [PubMed] [Google Scholar]
- 2. Lee W-S, Parsons S, Cugley D, Rogers S, Lim LL, Hall A. 2020. Increased incidence of glaucoma medication usage in middle-aged Australian males taking antiretroviral medication – a population-based study. J Ophthalmic Inflamm Infect 10:30. doi: 10.1186/s12348-020-00218-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grzybowski A, Och M, Kanclerz P, Leffler C, Moraes CGD. 2020. Primary open angle glaucoma and vascular risk factors: a review of population based studies from 1990 to 2019. J Clin Med 9:761. doi: 10.3390/jcm9030761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yanagi M, Kawasaki R, Wang JJ, Wong TY, Crowston J, Kiuchi Y. 2011. Vascular risk factors in glaucoma: a review. Clin Exp Ophthalmol 39:252–258. doi: 10.1111/j.1442-9071.2010.02455.x [DOI] [PubMed] [Google Scholar]
- 5. Weinreb RN, Aung T, Medeiros FA. 2014. The pathophysiology and treatment of glaucoma: a review. JAMA 311:1901–1911. doi: 10.1001/jama.2014.3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Greco A, Rizzo MI, De Virgilio A, Gallo A, Fusconi M, de Vincentiis M. 2016. Emerging concepts in glaucoma and review of the literature. Am J Med 129:1000. doi: 10.1016/j.amjmed.2016.03.038 [DOI] [PubMed] [Google Scholar]
- 7. Yepez JB, Murati FA, Pettito M, Peñaranda CF, de Yepez J, Maestre G, Arevalo JF, Johns Hopkins Zika Center . 2017. Ophthalmic manifestations of congenital Zika syndrome in Colombia and Venezuela. JAMA Ophthalmol 135:440–445. doi: 10.1001/jamaophthalmol.2017.0561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Paula Freitas B, Ko AI, Khouri R, Mayoral M, Henriques DF, Maia M, Belfort Jr R. 2017. Glaucoma and congenital Zika syndrome. Ophthalmology 124:407–408. doi: 10.1016/j.ophtha.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 9. De Moraes CG, Pettito M, Yepez JB, Sakuntabhai A, Simon-Loriere E, Zaidi MB, Prot M, Ruffie C, Kim SS, Allikmets R, Terwilliger JD, Lee JH, Maestre GE. 2018. Optic neuropathy and congenital glaucoma associated with probable Zika virus infection in Venezuelan patients. JMM Case Rep 5:e005161. doi: 10.1099/jmmcr.0.005161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khieu C, Kongyai N, Pathanapitoon K, Van Der Eijk AA, Rothova A. 2020. Causes of hypertensive anterior uveitis in Thailand. Ocul Immunol Inflamm 28:559–565. doi: 10.1080/09273948.2019.1678651 [DOI] [PubMed] [Google Scholar]
- 11. Jap A, Chee S-P. 2011. Viral anterior uveitis. Curr Opin Ophthalmol 22:483–488. doi: 10.1097/ICU.0b013e32834be021 [DOI] [PubMed] [Google Scholar]
- 12. Chan N-W, Chee S-P. 2019. Demystifying viral anterior uveitis: a review. Clin Exp Ophthalmol 47:320–333. doi: 10.1111/ceo.13417 [DOI] [PubMed] [Google Scholar]
- 13. Singh S, Kumar A. 2018. Ocular manifestations of emerging flaviviruses and the blood-retinal barrier. Viruses 10:530. doi: 10.3390/v10100530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Groen-Hakan F, Van Der Eijk AA, Rothova A. 2020. The usefulness of aqueous fluid analysis for Epstein–Barr virus in patients with uveitis. Ocul Immunol Inflamm 28:126–132. doi: 10.1080/09273948.2018.1543709 [DOI] [PubMed] [Google Scholar]
- 15. Smit D, Meyer D, Maritz J, de Groot-Mijnes JDF. 2019. Polymerase chain reaction and Goldmann-Witmer coefficient to examine the role of Epstein-Barr virus in uveitis. Ocul Immunol Inflamm 27:108–113. doi: 10.1080/09273948.2017.1370653 [DOI] [PubMed] [Google Scholar]
- 16. Keorochana N, Treesit I, Funarunart P. 2020. Characteristics and clinical outcomes of hypertensive anterior uveitis. Ocul Immunol Inflamm 28:538–548. doi: 10.1080/09273948.2019.1587471 [DOI] [PubMed] [Google Scholar]
- 17. Shirahama S, Kaburaki T, Takada S, Nakahara H, Tanaka R, Komae K, Fujino Y, Kawashima H, Aihara M. 2020. Comparison of visual field defect progression in secondary glaucoma due to anterior uveitis caused by three types of herpes viruses. Graefes Arch Clin Exp Ophthalmol 258:639–645. doi: 10.1007/s00417-019-04559-w [DOI] [PubMed] [Google Scholar]
- 18. Hoeksema L, Jansonius NM, Los LI. 2017. Risk factors for secondary glaucoma in herpetic anterior uveitis. Am J Ophthalmol 181:55–60. doi: 10.1016/j.ajo.2017.06.013 [DOI] [PubMed] [Google Scholar]
- 19. Shimizu A, Maruyama K, Yokoyama Y, Tsuda S, Ryu M, Nakazawa T. 2014. Characteristics of uveitic glaucoma and evaluation of its surgical treatment. Clin Ophthalmol 8:2383–2389. doi: 10.2147/OPTH.S72383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pohlmann D, Pahlitzsch M, Schlickeiser S, Metzner S, Lenglinger M, Bertelmann E, Maier A-K, Winterhalter S, Pleyer U. 2020. Virus-associated anterior uveitis and secondary glaucoma: diagnostics, clinical characteristics, and surgical options. PLoS One 15:e0229260. doi: 10.1371/journal.pone.0229260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wensing B, Relvas LM, Caspers LE, Valentincic NV, Stunf S, de Groot-Mijnes JDF, Rothova A. 2011. Comparison of rubella virus- and herpes virus-associated anterior uveitis: clinical manifestations and visual prognosis. Ophthalmology 118:1905–1910. doi: 10.1016/j.ophtha.2011.03.033 [DOI] [PubMed] [Google Scholar]
- 22. Miserocchi E, Waheed NK, Dios E, Christen W, Merayo J, Roque M, Foster CS. 2002. Visual outcome in herpes simplex virus and varicella zoster virus uveitis: a clinical evaluation and comparison. Ophthalmology 109:1532–1537. doi: 10.1016/s0161-6420(02)01113-2 [DOI] [PubMed] [Google Scholar]
- 23. Grinage E, Shukla D. 2022. Optineurin in ocular herpes infection. Exp Eye Res 219:109059. doi: 10.1016/j.exer.2022.109059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miserocchi E, Fogliato G, Bianchi I, Bandello F, Modorati G. 2014. Clinical features of ocular herpetic infection in an Italian referral center. Cornea 33:565–570. doi: 10.1097/ICO.0000000000000129 [DOI] [PubMed] [Google Scholar]
- 25. van Boxtel LAA, van der Lelij A, van der Meer J, Los LI. 2007. Cytomegalovirus as a cause of anterior uveitis in immunocompetent patients. Ophthalmology 114:1358–1362. doi: 10.1016/j.ophtha.2006.09.035 [DOI] [PubMed] [Google Scholar]
- 26. Leleu I, Jhanji V, Touhami S, Westcott M, Angi M, Titah C, Rousseau A, Hamard P, Brasnu E, Manicom T, Blumen-Ohana E, Rozenberg F, Vauloup-Fellous C, Deback C, Labetoulle M, Sahel J-A, Bodaghi B, Merabet L, Kobal A, Brignole-Baudouin F, Errera M-H. 2021. Clinical features and diagnosis of anterior segment inflammation related to cytomegalovirus in immunocompetent African, Asian, and caucasian patients. Ocul Immunol Inflamm 29:160–168. doi: 10.1080/09273948.2019.1662059 [DOI] [PubMed] [Google Scholar]
- 27. Engelhard SB, Haddad Z, Bajwa A, Patrie J, Xin W, Reddy AK. 2015. Infectious uveitis in Virginia. Clin Ophthalmol 9:1589–1594. doi: 10.2147/OPTH.S86578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tugal-Tutkun I, Otük-Yasar B, Altinkurt E. 2010. Clinical features and prognosis of herpetic anterior uveitis: a retrospective study of 111 cases. Int Ophthalmol 30:559–565. doi: 10.1007/s10792-010-9394-8 [DOI] [PubMed] [Google Scholar]
- 29. Sungur GK, Hazirolan D, Yalvac IS, Ozer PA, Aslan BS, Duman S. 2010. Incidence and prognosis of ocular hypertension secondary to viral uveitis. Int Ophthalmol 30:191–194. doi: 10.1007/s10792-009-9305-z [DOI] [PubMed] [Google Scholar]
- 30. Groen-Hakan F, van de Laar S, van der Eijk-Baltissen AA, Ten Dam-van Loon N, de Boer J, Rothova A. 2019. Clinical manifestations, prognosis, and vaccination status of patients with rubella virus–associated uveitis. Am J Ophthalmol 202:37–46. doi: 10.1016/j.ajo.2019.02.002 [DOI] [PubMed] [Google Scholar]
- 31. Thean JH, Hall AJ, Stawell RJ. 2001. Uveitis in herpes zoster ophthalmicus. Clin Exp Ophthalmol 29:406–410. doi: 10.1046/j.1442-9071.2001.d01-29.x [DOI] [PubMed] [Google Scholar]
- 32. Chee S-P, Jap A. 2008. Presumed fuchs heterochromic iridocyclitis and posner-schlossman syndrome: comparison of cytomegalovirus-positive and negative eyes. Am J Ophthalmol 146:883–889. doi: 10.1016/j.ajo.2008.09.001 [DOI] [PubMed] [Google Scholar]
- 33. Fan X, Li Z, Zhai R, Sheng Q, Kong X. 2022. Clinical characteristics of virus-related uveitic secondary glaucoma: focus on cytomegalovirus and varicella zoster virus. BMC Ophthalmol 22:130. doi: 10.1186/s12886-022-02348-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Visser L, Braakenburg A, Rothova A, de Boer JH. 2008. Rubella virus–associated uveitis: clinical manifestations and visual prognosis. Am J Ophthalmol 146:292–297. doi: 10.1016/j.ajo.2008.04.011 [DOI] [PubMed] [Google Scholar]
- 35. Shantha JG, Canady D, Hartley C, Cassedy A, Miller C, Angeles-Han ST, Harrison-Williams LCM, Vandy MJ, Weil N, Bastien G, Yeh S. 2022. Ophthalmic sequelae and psychosocial impact in pediatric Ebola survivors. EClinicalMedicine 49:101483. doi: 10.1016/j.eclinm.2022.101483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sng CCA, Ang M, Barton K. 2015. Uveitis and glaucoma: New insights in the pathogenesis and treatment. Prog Brain Res 221:243–269. doi: 10.1016/bs.pbr.2015.06.008 [DOI] [PubMed] [Google Scholar]
- 37. Khairallah M, Mahendradas P, Curi A, Khochtali S, Cunningham ET. 2019. Emerging viral infections causing anterior uveitis. Ocul Immunol Inflamm 27:219–228. doi: 10.1080/09273948.2018.1562080 [DOI] [PubMed] [Google Scholar]
- 38. Lee JH, Agarwal A, Mahendradas P, Lee CS, Gupta V, Pavesio CE, Agrawal R. 2017. Viral posterior uveitis. Surv Ophthalmol 62:404–445. doi: 10.1016/j.survophthal.2016.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rao A, Gawas L. 2021. Atypical associations of viral anterior uveitis with glaucoma-a series of challenging scenarios with review of literature. Semin Ophthalmol 36:605–613. doi: 10.1080/08820538.2021.1890789 [DOI] [PubMed] [Google Scholar]
- 40. Siddique SS, Suelves AM, Baheti U, Foster CS. 2013. Glaucoma and uveitis. Surv Ophthalmol 58:1–10. doi: 10.1016/j.survophthal.2012.04.006 [DOI] [PubMed] [Google Scholar]
- 41. Babu K, Konana VK, Ganesh SK, Patnaik G, Chan NSW, Chee S-P, Sobolewska B, Zierhut M. 2020. Viral anterior uveitis. Indian J Ophthalmol 68:1764–1773. doi: 10.4103/ijo.IJO_928_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Varkey JB, Shantha JG, Crozier I, Kraft CS, Lyon GM, Mehta AK, Kumar G, Smith JR, Kainulainen MH, Whitmer S, Ströher U, Uyeki TM, Ribner BS, Yeh S. 2015. Persistence of Ebola virus in ocular fluid during convalescence. N Engl J Med 372:2423–2427. doi: 10.1056/NEJMoa1500306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bodaghi B, Cassoux N, Wechsler B, Hannouche D, Fardeau C, Papo T, Huong DL, Piette JC, LeHoang P. 2001. Chronic severe uveitis: etiology and visual outcome in 927 patients from a single center. Medicine 80:263–270. doi: 10.1097/00005792-200107000-00005 [DOI] [PubMed] [Google Scholar]
- 44. de Schryver I, Rozenberg F, Cassoux N, Michelson S, Kestelyn P, Lehoang P, Davis JL, Bodaghi B. 2006. Diagnosis and treatment of cytomegalovirus iridocyclitis without retinal necrosis. Br J Ophthalmol 90:852–855. doi: 10.1136/bjo.2005.086546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Singh PK, Kasetti RB, Zode GS, Goyal A, Juzych MS, Kumar A. 2019. Zika virus infects trabecular meshwork and causes trabeculitis and glaucomatous pathology in mouse eyes. mSphere 4:1–14. doi: 10.1128/mSphere.00173-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Choi JA, Kim J-E, Noh S-J, Kyoung Kim E, Park CK, Paik S-Y. 2017. Enhanced cytomegalovirus infection in human trabecular meshwork cells and its implication in glaucoma pathogenesis. Sci Rep 7:43349. doi: 10.1038/srep43349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Amano S, Oshika T, Kaji Y, Numaga J, Matsubara M, Araie M. 1999. Herpes simplex virus in the trabeculum of an eye with corneal endotheliitis. Am J Ophthalmol 127:721–722. doi: 10.1016/s0002-9394(99)00018-5 [DOI] [PubMed] [Google Scholar]
- 48. Rao NA. 1990. Role of oxygen free radicals in retinal damage associated with experimental uveitis. Trans Am Ophthalmol Soc 88:797–850. [PMC free article] [PubMed] [Google Scholar]
- 49. Valerio GS, Lin CC. 2019. Ocular manifestations of herpes simplex virus. Curr Opin Ophthalmol 30:525–531. doi: 10.1097/ICU.0000000000000618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Terada Y, Kaburaki T, Takase H, Goto H, Nakano S, Inoue Y, Maruyama K, Miyata K, Namba K, Sonoda K-H, Kaneko Y, Numaga J, Fukushima M, Horiguchi N, Ide M, Ehara F, Miyazaki D, Hasegawa E, Mochizuki M. 2021. Distinguishing features of anterior uveitis caused by herpes simplex virus, varicella-zoster virus, and cytomegalovirus. Am J Ophthalmol 227:191–200. doi: 10.1016/j.ajo.2021.03.020 [DOI] [PubMed] [Google Scholar]
- 51. Young RC, Hodge DO, Liesegang TJ, Baratz KH. 2010. Incidence, recurrence, and outcomes of herpes simplex virus eye disease in Olmsted County, Minnesota, 1976-2007: the effect of oral antiviral prophylaxis. Arch Ophthalmol 128:1178–1183. doi: 10.1001/archophthalmol.2010.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Van der Lelij A, Ooijman FM, Kijlstra A, Rothova A. 2000. Anterior uveitis with sectoral iris atrophy in the absence of keratitis: a distinct clinical entity among herpetic eye diseases. Ophthalmology 107:1164–1170. doi: 10.1016/s0161-6420(00)00115-9 [DOI] [PubMed] [Google Scholar]
- 53. Jones R, Pasquale LR, Pavan-Langston D. 2007. Herpes simplex virus: an important etiology for secondary glaucoma. Int Ophthalmol Clin 47:99–107. doi: 10.1097/IIO.0b013e3180377632 [DOI] [PubMed] [Google Scholar]
- 54. Ames J, Yadavalli T, Suryawanshi R, Hopkins J, Agelidis A, Patil C, Fredericks B, Tseng H, Valyi-Nagy T, Shukla D. 2021. OPTN is a host intrinsic restriction factor against neuroinvasive HSV-1 infection. Nat Commun 12:5401. doi: 10.1038/s41467-021-25642-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stavrou P, Mitchell SM, Fox JD, Hope-Ross MW, Murray PI. 1994. Detection of varicella-zoster virus DNA in ocular samples from patients with uveitis but no cutaneous eruption. Eye (Lond) 8 ( Pt 6):684–687. doi: 10.1038/eye.1994.169 [DOI] [PubMed] [Google Scholar]
- 56. Lin P, Yoon MK, Chiu CS. 2009. Herpes zoster keratouveitis and inflammatory ocular hypertension 8 years after varicella vaccination. Ocul Immunol Inflamm 17:33–35. doi: 10.1080/09273940802491892 [DOI] [PubMed] [Google Scholar]
- 57. Esmaeli-Gutstein B, Winkelman JZ. 1999. Uveitis associated with varicella virus vaccine. Am J Ophthalmol 127:733–734. doi: 10.1016/s0002-9394(99)00059-8 [DOI] [PubMed] [Google Scholar]
- 58. Naseri A, Good WV, Cunningham ET. 2003. Herpes zoster virus sclerokeratitis and anterior uveitis in a child following varicella vaccination. Am J Ophthalmol 135:415–417. doi: 10.1016/s0002-9394(02)01957-8 [DOI] [PubMed] [Google Scholar]
- 59. Aldaas K, Challa P, Weber DJ, Fleischman D. 2022. Infections and glaucoma. Surv Ophthalmol 67:637–658. doi: 10.1016/j.survophthal.2021.08.009 [DOI] [PubMed] [Google Scholar]
- 60. Chee S-P, Bacsal K, Jap A, Se-Thoe S-Y, Cheng CL, Tan BH. 2008. Clinical features of cytomegalovirus anterior uveitis in immunocompetent patients. Am J Ophthalmol 145:834–840. doi: 10.1016/j.ajo.2007.12.015 [DOI] [PubMed] [Google Scholar]
- 61. Chee SP, Jap A. 2012. Treatment outcome and risk factors for visual loss in cytomegalovirus endotheliitis. Graefes Arch Clin Exp Ophthalmol 250:383–389. doi: 10.1007/s00417-011-1813-7 [DOI] [PubMed] [Google Scholar]
- 62. Koizumi N, Suzuki T, Uno T, Chihara H, Shiraishi A, Hara Y, Inatomi T, Sotozono C, Kawasaki S, Yamasaki K, Mochida C, Ohashi Y, Kinoshita S. 2008. Cytomegalovirus as an etiologic factor in corneal endotheliitis. Ophthalmology 115:292–297. doi: 10.1016/j.ophtha.2007.04.053 [DOI] [PubMed] [Google Scholar]
- 63. de Smet MD. 1992. Differential diagnosis of retinitis and choroiditis in patients with acquired immunodeficiency syndrome. Am J Med 92:S17–S21. doi: 10.1016/0002-9343(92)90332-6 [DOI] [PubMed] [Google Scholar]
- 64. Goldberg DE, Freeman WR. 2002. Uveitic angle closure glaucoma in a patient with inactive cytomegalovirus retinitis and immune recovery uveitis. Ophthalmic Surg Lasers 33:421–425. [PubMed] [Google Scholar]
- 65. Meige P, Cohen H, Morin B, Athlani A. 1989. [Acute bilateral glaucoma in an LAV-positive subject]. Bull Soc Ophtalmol Fr 89:449–454. [PubMed] [Google Scholar]
- 66. Ullman S, Wilson RP, Schwartz L. 1986. Bilateral angle-closure glaucoma in association with the acquired immune deficiency syndrome. Am J Ophthalmol 101:419–424. doi: 10.1016/0002-9394(86)90639-2 [DOI] [PubMed] [Google Scholar]
- 67. Bloch-Michel E, Dussaix E, Cerqueti P, Patarin D. 1987. Possible role of cytomegalovirus infection in the etiology of the Posner-Schlossmann syndrome. Int Ophthalmol 11:95–96. doi: 10.1007/BF00136737 [DOI] [PubMed] [Google Scholar]
- 68. Woo JH, Lim WK, Ho SL, Teoh SC. 2015. Characteristics of cytomegalovirus uveitis in immunocompetent patients. Ocul Immunol Inflamm 23:378–383. doi: 10.3109/09273948.2014.950384 [DOI] [PubMed] [Google Scholar]
- 69. Teoh S-B, Thean L, Koay E. 2005. Cytomegalovirus in aetiology of Posner–Schlossman syndrome: evidence from quantitative polymerase chain reaction. Eye (Lond) 19:1338–1340. doi: 10.1038/sj.eye.6701757 [DOI] [PubMed] [Google Scholar]
- 70. Park SW, Yu HG. 2013. Association of cytomegalovirus with idiopathic chronic anterior uveitis with ocular hypertension in Korean patients. Ocul Immunol Inflamm 21:192–196. doi: 10.3109/09273948.2012.754908 [DOI] [PubMed] [Google Scholar]
- 71. Anshu A, Tan D, Chee S-P, Mehta JS, Htoon HM. 2017. Interventions for the management of CMV-associated anterior segment inflammation. Cochrane Database Syst Rev 8:CD011908. doi: 10.1002/14651858.CD011908.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chan N-W, Chee S-P, Caspers L, Bodaghi B. 2018. Clinical features of CMV-associated anterior uveitis. Ocul Immunol Inflamm 26:107–115. doi: 10.1080/09273948.2017.1394471 [DOI] [PubMed] [Google Scholar]
- 73. Bale JFJ, O’Neil ME, Hogan RN, Kern ER. 1984. Experimental murine cytomegalovirus infection of ocular structures. Arch Ophthalmol 102:1214–1219. doi: 10.1001/archopht.1984.01040030984032 [DOI] [PubMed] [Google Scholar]
- 74. Tendolkar S, Murthy SI, Bhatia P, Senthil S. 2021. Cytomegalovirus endotheliitis with recurrent anterior uveitis and secondary glaucoma misdiagnosed as HLA-B27 uveitis. BMJ Case Rep 14:e240061. doi: 10.1136/bcr-2020-240061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ansari MA, Singh VV, Dutta S, Veettil MV, Dutta D, Chikoti L, Lu J, Everly D, Chandran B. 2013. Constitutive interferon-inducible protein 16-inflammasome activation during Epstein-Barr virus latency I, II, and III in B and epithelial cells. J Virol 87:8606–8623. doi: 10.1128/JVI.00805-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Taylor GS, Long HM, Brooks JM, Rickinson AB, Hislop AD. 2015. The immunology of Epstein-Barr virus-induced disease. Annu Rev Immunol 33:787–821. doi: 10.1146/annurev-immunol-032414-112326 [DOI] [PubMed] [Google Scholar]
- 77. Cunningham ET, Zierhut M. 2020. Epstein-Barr virus and the eye. Ocul Immunol Inflamm 28:533–537. doi: 10.1080/09273948.2020.1760549 [DOI] [PubMed] [Google Scholar]
- 78. Bjornevik K, Cortese M, Healy BC, Kuhle J, Mina MJ, Leng Y, Elledge SJ, Niebuhr DW, Scher AI, Munger KL, Ascherio A. 2022. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 375:296–301. doi: 10.1126/science.abj8222 [DOI] [PubMed] [Google Scholar]
- 79. Wong Y, Meehan MT, Burrows SR, Doolan DL, Miles JJ. 2022. Estimating the global burden of Epstein–Barr virus-related cancers. J Cancer Res Clin Oncol 148:31–46. doi: 10.1007/s00432-021-03824-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Arus VA. 2021. Ocular manifestations in Epstein Barr virus infection. Intech Open. doi: 10.5772/intechopen.87415 [DOI] [Google Scholar]
- 81. Alba-Linero C, Rocha-de-Lossada C, Rachwani-Anil R, Sainz-de-la-Maza M, Sena-Corrales G, Romano V, Rodríguez-Calvo-de-Mora M. 2022. Anterior segment involvement in Epstein–Barr virus: a review. Acta Ophthalmol 100:e1052–e1060. doi: 10.1111/aos.15061 [DOI] [PubMed] [Google Scholar]
- 82. Grefer J, Santer R, Ankermann T, Faul S, Nölle B, Eggert P. 1999. Tubulointerstitial nephritis and uveitis in association with Epstein-Barr virus infection. Pediatr Nephrol 13:336–339. doi: 10.1007/s004670050621 [DOI] [PubMed] [Google Scholar]
- 83. Basiaga ML, Weiss PF, Behrens EM. 2015. BIRC4 mutation: an important rare cause of uveitis. J Clin Rheumatol 21:444–447. doi: 10.1097/RHU.0000000000000327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kelly SP, Rosenthal AR, Nicholson KG, Woodward CG. 1989. Retinochoroiditis in acute Epstein-Barr virus infection. Br J Ophthalmol 73:1002–1003. doi: 10.1136/bjo.73.12.1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rohrbach JM, Kröber SM, Teufel T, Kortmann RD, Zierhut M. 2004. EBV-induced polymorphic lymphoproliferative disorder of the iris after heart transplantation. Graefes Arch Clin Exp Ophthalmol 242:44–50. doi: 10.1007/s00417-003-0751-4 [DOI] [PubMed] [Google Scholar]
- 86. Merle H, Donnio A, Jean-Charles A, Guyomarch J, Hage R, Najioullah F, Césaire R, Cabié A. 2018. Ocular manifestations of emerging arboviruses: dengue fever, chikungunya, zika virus, West Nile virus, and yellow fever. J Fr Ophtalmol 41:e235–e243. doi: 10.1016/j.jfo.2018.05.002 [DOI] [PubMed] [Google Scholar]
- 87. Gérardin P, Guernier V, Perrau J, Fianu A, Le Roux K, Grivard P, Michault A, de Lamballerie X, Flahault A, Favier F. 2008. Estimating chikungunya prevalence in La Réunion Island outbreak by serosurveys: two methods for two critical times of the epidemic. BMC Infect Dis 8:99. doi: 10.1186/1471-2334-8-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lemant J, Boisson V, Winer A, Thibault L, André H, Tixier F, Lemercier M, Antok E, Cresta MP, Grivard P, Besnard M, Rollot O, Favier F, Huerre M, Campinos JL, Michault A. 2008. Serious acute chikungunya virus infection requiring intensive care during the reunion island outbreak in 2005-2006. Crit Care Med 36:2536–2541. doi: 10.1097/CCM.0b013e318183f2d2 [DOI] [PubMed] [Google Scholar]
- 89. Oliver GF, Carr JM, Smith JR. 2019. Emerging infectious uveitis: chikungunya, dengue, Zika and Ebola: a review. Clin Exp Ophthalmol 47:372–380. doi: 10.1111/ceo.13450 [DOI] [PubMed] [Google Scholar]
- 90. Mahendradas P, Avadhani K, Shetty R. 2013. Chikungunya and the eye: a review. J Ophthalmic Inflamm Infect 3:35. doi: 10.1186/1869-5760-3-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Staples JE, Breiman RF, Powers AM. 2009. Chikungunya fever: an epidemiological review of a re-emerging infectious disease. Clin Infect Dis 49:942–948. doi: 10.1086/605496 [DOI] [PubMed] [Google Scholar]
- 92. Thiboutot MM, Kannan S, Kawalekar OU, Shedlock DJ, Khan AS, Sarangan G, Srikanth P, Weiner DB, Muthumani K. 2010. Chikungunya: a potentially emerging epidemic? PLoS Negl Trop Dis 4:e623. doi: 10.1371/journal.pntd.0000623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mahendradas P, Ranganna SK, Shetty R, Balu R, Narayana KM, Babu RB, Shetty BK. 2008. Ocular manifestations associated with chikungunya. Ophthalmology 115:287–291. doi: 10.1016/j.ophtha.2007.03.085 [DOI] [PubMed] [Google Scholar]
- 94. Lalitha P, Rathinam S, Banushree K, Maheshkumar S, Vijayakumar R, Sathe P. 2007. Ocular involvement associated with an epidemic outbreak of chikungunya virus infection. Am J Ophthalmol 144:552–556. doi: 10.1016/j.ajo.2007.06.002 [DOI] [PubMed] [Google Scholar]
- 95. Mittal A, Mittal S, Bharathi JM, Ramakrishnan R, Sathe PS. 2007. Uveitis during outbreak of chikungunya fever. Ophthalmology 114:1798. doi: 10.1016/j.ophtha.2007.03.045 [DOI] [PubMed] [Google Scholar]
- 96. Scripsema NK, Sharifi E, Samson CM, Kedhar S, Rosen RB. 2015. Chikungunya-associated uveitis and exudative retinal detachment: a case report. Retin Cases Brief Rep 9:352–356. doi: 10.1097/ICB.0000000000000232 [DOI] [PubMed] [Google Scholar]
- 97. Mittal A, Mittal S, Bharati MJ, Ramakrishnan R, Saravanan S, Sathe PS. 2007. Optic neuritis associated with chikungunya virus infection in South India. Arch Ophthalmol 125:1381–1386. doi: 10.1001/archopht.125.10.1381 [DOI] [PubMed] [Google Scholar]
- 98. Rose N, Anoop TM, John AP, Jabbar PK, George KC. 2011. Acute optic neuritis following infection with chikungunya virus in southern rural India. Int J Infect Dis 15:e147–50. doi: 10.1016/j.ijid.2010.10.004 [DOI] [PubMed] [Google Scholar]
- 99. Chanana B, Azad RV, Nair S. 2007. Bilateral macular choroiditis following chikungunya virus infection. Eye 21:1020–1021. doi: 10.1038/sj.eye.6702863 [DOI] [PubMed] [Google Scholar]
- 100. Hayek S, Rousseau A, Bouthry E, Prat CM, Labetoulle M. 2015. Chikungunya virus infection and bilateral stromal keratouveitis. JAMA Ophthalmol 133:849–850. doi: 10.1001/jamaophthalmol.2015.0698 [DOI] [PubMed] [Google Scholar]
- 101. Martínez-Pulgarín DF, Chowdhury FR, Villamil-Gomez WE, Rodriguez-Morales AJ, Blohm GM, Paniz-Mondolfi AE. 2016. Ophthalmologic aspects of chikungunya infection. Travel Med Infect Dis 14:451–457. doi: 10.1016/j.tmaid.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 102. Benzekri R, Hage R, Merle H. 2016. Case report: third cranial nerve palsy in the setting of chikungunya virus infection. Am J Trop Med Hyg 95:180–181. doi: 10.4269/ajtmh.15-0547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Couderc T, Gangneux N, Chrétien F, Caro V, Le Luong T, Ducloux B, Tolou H, Lecuit M, Grandadam M. 2012. Chikungunya virus infection of corneal grafts. J Infect Dis 206:851–859. doi: 10.1093/infdis/jis296 [DOI] [PubMed] [Google Scholar]
- 104. Karesh JW, Mazzoli RA, Heintz SK. 2018. Ocular manifestations of mosquito-transmitted diseases. Mil Med 183:450–458. doi: 10.1093/milmed/usx183 [DOI] [PubMed] [Google Scholar]
- 105. Fritel X, Rollot O, Gerardin P, Gauzere BA, Bideault J, Lagarde L, Dhuime B, Orvain E, Cuillier F, Ramful D, Samperiz S, Jaffar-Bandjee MC, Michault A, Cotte L, Kaminski M, Fourmaintraux A, Chikungunya-Mere-Enfant Team . 2010. Chikungunya virus infection during pregnancy, Réunion, France, 2006. Emerg Infect Dis 16:418–425. doi: 10.3201/eid1603.091403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kleinschmidt‐DeMasters BK. 2020. Rubella virus, p 105–111. In Infections of the central nervous system. Wiley. doi: 10.1002/9781119467748 [DOI] [Google Scholar]
- 107. Quentin CD, Reiber H. 2004. Fuchs heterochromic cyclitis: rubella virus antibodies and genome in aqueous humor. Am J Ophthalmol 138:46–54. doi: 10.1016/j.ajo.2004.02.055 [DOI] [PubMed] [Google Scholar]
- 108. de Groot-Mijnes JDF, de Visser L, Rothova A, Schuller M, van Loon AM, Weersink AJL. 2006. Rubella virus is associated with Fuchs heterochromic iridocyclitis. Am J Ophthalmol 141:212–214. doi: 10.1016/j.ajo.2005.07.078 [DOI] [PubMed] [Google Scholar]
- 109. Ruokonen PC, Metzner S, Ucer A, Torun N, Hofmann J, Pleyer U. 2010. Intraocular antibody synthesis against rubella virus and other microorganisms in Fuchs’ heterochromic cyclitis. Graefes Arch Clin Exp Ophthalmol 248:565–571. doi: 10.1007/s00417-009-1239-7 [DOI] [PubMed] [Google Scholar]
- 110. Suzuki J, Goto H, Komase K, Abo H, Fujii K, Otsuki N, Okamoto K. 2010. Rubella virus as a possible etiological agent of Fuchs heterochromic iridocyclitis. Graefes Arch Clin Exp Ophthalmol 248:1487–1491. doi: 10.1007/s00417-010-1434-6 [DOI] [PubMed] [Google Scholar]
- 111. Stunf S, Petrovec M, Žigon N, Hawlina M, Kraut A, de Groot-Mijnes JDF, Valentinčič NV. 2012. High concordance of intraocular antibody synthesis against the rubella virus and fuchs heterochromic uveitis syndrome in Slovenia. Mol Vis 18:2909–2914. [PMC free article] [PubMed] [Google Scholar]
- 112. Winchester SA, Varga Z, Parmar D, Brown KE. 2013. Persistent intraocular rubella infection in a patient with Fuchs’ uveitis and congenital rubella syndrome. J Clin Microbiol 51:1622–1624. doi: 10.1128/JCM.03239-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Birnbaum AD, Tessler HH, Schultz KL, Farber MD, Gao W, Lin P, Oh F, Goldstein DA. 2007. Epidemiologic relationship between Fuchs heterochromic iridocyclitis and the United States rubella vaccination program. Am J Ophthalmol 144:424–428. doi: 10.1016/j.ajo.2007.05.026 [DOI] [PubMed] [Google Scholar]
- 114. Rajasundari TA, Sundaresan P, Vijayalakshmi P, Brown DWG, Jin L. 2008. Laboratory confirmation of congenital rubella syndrome in infants: an eye hospital based investigation. J Med Virol 80:536–546. doi: 10.1002/jmv.21097 [DOI] [PubMed] [Google Scholar]
- 115. Gulland A. 2016. Ebola outbreak in west Africa is officially over. BMJ 352:i243. doi: 10.1136/bmj.i243 [DOI] [PubMed] [Google Scholar]
- 116. Shantha JG, Crozier I, Kraft CS, Grant DG, Goba A, Hayek BR, Hartley C, Barnes KG, Uyeki TM, Schieffelin J, Garry RF, Bausch DG, Farmer PE, Mattia JG, Vandy MJ, Yeh S, EVICT Study Investigators . 2021. Implementation of the Ebola virus persistence in ocular tissues and fluids (EVICT) study: lessons learned for vision health systems strengthening in Sierra Leone. PLoS One 16:e0252905. doi: 10.1371/journal.pone.0252905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Van Kerkhove MD, Bento AI, Mills HL, Ferguson NM, Donnelly CA. 2015. A review of epidemiological parameters from Ebola outbreaks to inform early public health decision-making. Sci Data 2:150019. doi: 10.1038/sdata.2015.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Uyeki TM, Mehta AK, Davey RT, Liddell AM, Wolf T, Vetter P, Schmiedel S, Grünewald T, Jacobs M, Arribas JR, Evans L, Hewlett AL, Brantsaeter AB, Ippolito G, Rapp C, Hoepelman AIM, Gutman J, Working Group of the U.S.–European Clinical Network on Clinical Management of Ebola Virus Disease Patients in the U.S. and Europe . 2016. Clinical management of Ebola virus disease in the United States and Europe. N Engl J Med 374:636–646. doi: 10.1056/NEJMoa1504874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Shantha JG, Yeh S, Nguyen QD. 2016. Ebola virus disease and the eye. Curr Opin Ophthalmol 27:538–544. doi: 10.1097/ICU.0000000000000313 [DOI] [PubMed] [Google Scholar]
- 120. Tiffany A, Vetter P, Mattia J, Dayer J-A, Bartsch M, Kasztura M, Sterk E, Tijerino AM, Kaiser L, Ciglenecki I. 2016. Ebola virus disease complications as experienced by survivors in Sierra Leone. Clin Infect Dis 62:1360–1366. doi: 10.1093/cid/ciw158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Steptoe PJ, Momorie F, Fornah AD, Komba SP, Emsley E, Scott JT, Harding SP, Vandy MJ, Sahr F, Beare NAV, Semple MG. 2018. Multimodal imaging and spatial analysis of Ebola retinal lesions in 14 survivors of Ebola virus disease. JAMA Ophthalmol 136:689–693. doi: 10.1001/jamaophthalmol.2018.1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Steptoe PJ, Scott JT, Baxter JM, Parkes CK, Dwivedi R, Czanner G, Vandy MJ, Momorie F, Fornah AD, Komba P, Richards J, Sahr F, Beare NAV, Semple MG. 2017. Novel retinal lesion in Ebola survivors, Sierra Leone, 2016. Emerg Infect Dis 23:1102–1109. doi: 10.3201/eid2307.161608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Mattia JG, Vandy MJ, Chang JC, Platt DE, Dierberg K, Bausch DG, Brooks T, Conteh S, Crozier I, Fowler RA, Kamara AP, Kang C, Mahadevan S, Mansaray Y, Marcell L, McKay G, O’Dempsey T, Parris V, Pinto R, Rangel A, Salam AP, Shantha J, Wolfman V, Yeh S, Chan AK, Mishra S. 2016. Early clinical sequelae of Ebola virus disease in Sierra Leone: a cross-sectional study. Lancet Infect Dis 16:331–338. doi: 10.1016/S1473-3099(15)00489-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kibadi K, Mupapa K, Kuvula K, Massamba M, Ndaberey D, Muyembe-Tamfum JJ, Bwaka MA, De Roo A, Colebunders R. 1999. Late ophthalmologic manifestations in survivors of the 1995 Ebola virus epidemic in Kikwit, Democratic Republic of the Congo. J Infect Dis 179 Suppl 1:S13–S14. doi: 10.1086/514288 [DOI] [PubMed] [Google Scholar]
- 125. Hereth-Hebert E, Bah MO, Etard JF, Sow MS, Resnikoff S, Fardeau C, Toure A, Ouendeno AN, Sagno IC, March L, Izard S, Lama PL, Barry M, Delaporte E, Ayouba A, Baize S, Barry M, Camara A, Cissé M, Delaporte E, Delfraissy J-F, Delmas C, Desclaux A, Diallo SB, Diallo MS, Étard JF, Etienne C, Granouillac B, Izard S, Kassé D, Keita AK, Keita S, Hébert EH, Koivugui L, Kpamou C, Lacarabaratz C, Leroy S, Marchal CL, Levy Y, Magassouba N, March L, Peeters M, Raoul H, Savané I, Sow MS, Taverne B, Touré A, Yazdanpanah Y. 2017. Ocular complications in survivors of the Ebola outbreak in Guinea. Am J Ophthalmol 175:114–121. doi: 10.1016/j.ajo.2016.12.005 [DOI] [PubMed] [Google Scholar]
- 126. CDC (USA) . 2023. Dengue. Available from: https://www.cdc.gov/dengue. Retrieved 12 Apr 2022.
- 127. de Andrade GC, Ventura CV, Mello Filho P de A, Maia M, Vianello S, Rodrigues EB. 2017. Arboviruses and the eye. Int J Retina Vitreous 3:4. doi: 10.1186/s40942-016-0057-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Boo YL, Aris MAM, Chin PW, Sulaiman WAW, Basri H, Hoo FK. 2016. Guillain–Barré syndrome complicating dengue fever: two case reports. Tzu Chi Med J 28:157–159. doi: 10.1016/j.tcmj.2015.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Chan DPL, Teoh SCB, Tan CSH, Nah GKM, Rajagopalan R, Prabhakaragupta MK, Chee CKL, Lim TH, Goh KY, The Eye Institute Dengue-Related Ophthalmic Complications Workgroup . 2006. Ophthalmic complications of dengue. Emerg Infect Dis 12:285–289. doi: 10.3201/eid1202.050274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Yip V-H, Sanjay S, Koh YT. 2012. Ophthalmic complications of dengue fever: a systematic review. Ophthalmol Ther 1:2. doi: 10.1007/s40123-012-0002-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Dejnirattisai W, Jumnainsong A, Onsirisakul N, Fitton P, Vasanawathana S, Limpitikul W, Puttikhunt C, Edwards C, Duangchinda T, Supasa S, Chawansuntati K, Malasit P, Mongkolsapaya J, Screaton G. 2010. Cross-reacting antibodies enhance dengue virus infection in humans. Science 328:745–748. doi: 10.1126/science.1185181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Gupta A, Srinivasan R, Setia S, Soundravally R, Pandian DG. 2009. Uveitis following dengue fever. Eye 23:873–876. doi: 10.1038/eye.2008.124 [DOI] [PubMed] [Google Scholar]
- 133. Lim W-K, Mathur R, Koh A, Yeoh R, Chee S-P. 2004. Ocular manifestations of dengue fever. Ophthalmology 111:2057–2064. doi: 10.1016/j.ophtha.2004.03.038 [DOI] [PubMed] [Google Scholar]
- 134. Su D-W, Bacsal K, Chee S-P, Flores JVP, Lim W-K, Cheng B-L, Jap A-E, Dengue Maculopathy Study Group . 2007. Prevalence of dengue maculopathy in patients hospitalized for dengue fever. Ophthalmology 114:1743–1747. doi: 10.1016/j.ophtha.2007.03.054 [DOI] [PubMed] [Google Scholar]
- 135. Saranappa S B S, Sowbhagya HN. 2012. Panophthalmitis in dengue fever. Indian Pediatr 49:760–760. doi: 10.1007/s13312-012-0142-1 [DOI] [PubMed] [Google Scholar]
- 136. Tabbara K. 2012. Dengue retinochoroiditis. Ann Saudi Med 32:530–533. doi: 10.5144/0256-4947.2012.30.4.1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Abroug N, Khairallah M, Zina S, Ksiaa I, Amor HB, Attia S, Jelliti B, Khochtali S, Khairallah M. 2021. Ocular manifestations of emerging arthropod-borne infectious diseases. J Curr Ophthalmol 33:227–235. doi: 10.4103/joco.joco_134_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Teoh SCB, Chan DPL, Nah GKM, Rajagopalan R, Laude A, Ang BSP, Barkham T, Chee CKL, Lim TH, Goh KY. 2006. A re-look at ocular complications in dengue fever and dengue haemorrhagic fever. Dengue Bull 30:184–190. [Google Scholar]
- 139. Aragão R de, Barreira IMA, Lima LNC, Rabelo LP, Pereira FBA. 2010. Neurite óptica bilateral após infecção viral por dengue: relato de casos. Arq Bras Oftalmol 73:175–178. doi: 10.1590/S0004-27492010000200015 [DOI] [PubMed] [Google Scholar]
- 140. Pierre Filho P de T, Carvalho Filho JP, Pierre ÉTL. 2008. Bilateral acute angle closure glaucoma in a patient with dengue fever: case report. Arq Bras Oftalmol 71:265–268. doi: 10.1590/S0004-27492008000200025 [DOI] [PubMed] [Google Scholar]
- 141. Levaggi ND, Lucas AN, Barletta JÁE. 2017. Bilateral acute angle closure in a patient with dengue fever: a case report. Arq Bras Oftalmol 80:266–267. doi: 10.5935/0004-2749.20170065 [DOI] [PubMed] [Google Scholar]
- 142. Saari KM. 1976. Acute glaucoma in hemorrhagic fever with renal syndrome (nephropathia epidemica). Am J Ophthalmol 81:455–461. doi: 10.1016/0002-9394(76)90301-9 [DOI] [PubMed] [Google Scholar]
- 143. Smithburn KC, Hughes TP, Burke AW, Paul JH. 1940. A neurotropic virus isolated from the blood of a native of Uganda. Am J Trop Med Hyg s1-20:471–492. doi: 10.4269/ajtmh.1940.s1-20.471 [DOI] [Google Scholar]
- 144. Campbell GL, Marfin AA, Lanciotti RS, Gubler DJ. 2002. West Nile virus. Lancet Infect Dis 2:519–529. doi: 10.1016/s1473-3099(02)00368-7 [DOI] [PubMed] [Google Scholar]
- 145. CDC . 2023. West Nile virus. https://www.cdc.gov/westnile/index.html.
- 146. Nash D, Mostashari F, Fine A, Miller J, O’Leary D, Murray K, Huang A, Rosenberg A, Greenberg A, Sherman M, Wong S, Layton M, 1999 West Nile Outbreak Response Working Group . 2001. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med 344:1807–1814. doi: 10.1056/NEJM200106143442401 [DOI] [PubMed] [Google Scholar]
- 147. Garg S, Jampol LM. 2005. Systemic and intraocular manifestations of West Nile virus infection. Surv Ophthalmol 50:3–13. doi: 10.1016/j.survophthal.2004.10.001 [DOI] [PubMed] [Google Scholar]
- 148. Sejvar JJ, Haddad MB, Tierney BC, Campbell GL, Marfin AA, Van Gerpen JA, Fleischauer A, Leis AA, Stokic DS, Petersen LR. 2003. Neurologic manifestations and outcome of West Nile virus infection. JAMA 290:511–515. doi: 10.1001/jama.290.4.511 [DOI] [PubMed] [Google Scholar]
- 149. Shaikh S, Trese MT. 2004. West Nile virus chorioretinitis. Br J Ophthalmol 88:1599–1560. doi: 10.1136/bjo.2004.049460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Khairallah M, Kahloun R. 2013. Ocular manifestations of emerging infectious diseases. Curr Opin Ophthalmol 24:574–580. doi: 10.1097/ICU.0b013e3283654e09 [DOI] [PubMed] [Google Scholar]
- 151. Rousseau A, Haigh O, Ksiaa I, Khairallah M, Labetoulle M. 2020. Ocular manifestations of West Nile virus. Vaccines (Basel) 8:641. doi: 10.3390/vaccines8040641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Alpert SG, Fergerson J, Noël LP. 2003. Intrauterine West Nile virus: ocular and systemic findings. Am J Ophthalmol 136:733–735. doi: 10.1016/s0002-9394(03)00452-5 [DOI] [PubMed] [Google Scholar]
- 153. Bains HS, Jampol LM, Caughron MC, Parnell JR. 2003. Vitritis and chorioretinitis in a patient with West Nile virus infection. Arch Ophthalmol 121:205–207. doi: 10.1001/archopht.121.2.205 [DOI] [PubMed] [Google Scholar]
- 154. Miller AH, Liang IEH. 2003. Diplopia: a focal neurologic presentation of West Nile meningoencephalitis. Ann Emerg Med 42:413–416. doi: 10.1067/mem.2003.322 [DOI] [PubMed] [Google Scholar]
- 155. Sivakumar RR, Prajna L, Arya LK, Muraly P, Shukla J, Saxena D, Parida M. 2013. Molecular diagnosis and ocular imaging of West Nile virus retinitis and neuroretinitis. Ophthalmology 120:1820–1826. doi: 10.1016/j.ophtha.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 156. Kuchtey RW, Kosmorsky GS, Martin D, Lee MS. 2003. Uveitis associated with West Nile virus infection. Arch Ophthalmol 121:1648–1649. doi: 10.1001/archopht.121.11.1648 [DOI] [PubMed] [Google Scholar]
- 157. Kridin M, Zloto O, Kridin K, Cohen AD, Mann O, Weinstein O. 2023. The association of uveitis with hepatitis B and hepatitis C viruses: a large-scale population-based study. Eye 37:720–724. doi: 10.1038/s41433-022-02037-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Lim LL, Scarborough JD, Thorne JE, Graham E, Kempen JH, Mackensen F, Nguyen QD, Prabriputaloong T, Read RW, Suhler EB, Schwartz JM, Smith JR. 2009. Uveitis in patients with autoimmune hepatitis. Am J Ophthalmol 147:332–338. doi: 10.1016/j.ajo.2008.08.019 [DOI] [PubMed] [Google Scholar]
- 159. Tien P-T, Lin C-J, Tsai Y-Y, Chen H-S, Hwang D-K, Muo C-H, Lin J-M, Chen W-L. 2016. Relationship between uveitis, different types of viral hepatitis, and liver cirrhosis: a 12-year nationwide population-based cohort study. Retina 36:2391–2398. doi: 10.1097/IAE.0000000000001103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Azimi A, Shirvani M, Hosseini S, Bazojoo V, Masihpoor N, Mohaghegh S, Sadeghi SM. 2020. Acute bilateral granulomatous anterior uveitis as an extra-hepatic manifestation of hepatitis A virus (HAV) infection: a case report. J Ophthalmic Inflamm Infect 10:18. doi: 10.1186/s12348-020-00210-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Grob PJ, Martenet AC, Witmer R. 1976. Nonspecific immune parameters and hepatitis B antigens in patients with uveitis. Mod Probl Ophthalmol 16:254–258. [PubMed] [Google Scholar]
- 162. Murray PI, Prasad J, Rahi AH. 1983. Status of hepatitis B virus in the aetiology of uveitis in Great Britain. Br J Ophthalmol 67:685–687. doi: 10.1136/bjo.67.10.685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Kwon YS, Choe YH, Chin HS. 2001. Development of glaucoma in the course of interferon Alpha therapy for chronic hepatitis B. Yonsei Med J 42:134–136. doi: 10.3349/ymj.2001.42.1.134 [DOI] [PubMed] [Google Scholar]
- 164. Ayaki M. 1994. Development of neovascular glaucoma in the course of interferon alfa therapy for hepatitis type C. Br J Ophthalmol 78:238. doi: 10.1136/bjo.78.3.238-a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Player JK, Riordan SM, Duncan RS, Koulen P. 2022. Analysis of glaucoma associated genes in response to inflammation, an examination of a public data set derived from peripheral blood from patients with hepatitis C. Clin Ophthalmol 16:2093–2103. doi: 10.2147/OPTH.S364739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Dick GWA, Kitchen SF, Haddow AJ. 1952. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg 46:509–520. doi: 10.1016/0035-9203(52)90042-4 [DOI] [PubMed] [Google Scholar]
- 167. Macnamara FN. 1954. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg 48:139–145. doi: 10.1016/0035-9203(54)90006-1 [DOI] [PubMed] [Google Scholar]
- 168. Duffy MR, Chen T-H, Hancock WT, Powers AM, Kool JL, Lanciotti RS, Pretrick M, Marfel M, Holzbauer S, Dubray C, Guillaumot L, Griggs A, Bel M, Lambert AJ, Laven J, Kosoy O, Panella A, Biggerstaff BJ, Fischer M, Hayes EB. 2009. Zika virus outbreak on Yap Island, federated states of Micronesia. N Engl J Med 360:2536–2543. doi: 10.1056/NEJMoa0805715 [DOI] [PubMed] [Google Scholar]
- 169. Tognarelli J, Ulloa S, Villagra E, Lagos J, Aguayo C, Fasce R, Parra B, Mora J, Becerra N, Lagos N, Vera L, Olivares B, Vilches M, Fernández J. 2016. A report on the outbreak of Zika virus on Easter Island, South Pacific, 2014. Arch Virol 161:665–668. doi: 10.1007/s00705-015-2695-5 [DOI] [PubMed] [Google Scholar]
- 170. Schuler-Faccini L, Ribeiro EM, Feitosa IML, Horovitz DDG, Cavalcanti DP, Pessoa A, Doriqui MJR, Neri JI, Neto J de P, Wanderley HYC, Cernach M, El-Husny AS, Pone MVS, Serao CLC, Sanseverino MTV, Brazilian Medical Genetics Society–Zika Embryopathy Task Force . 2016. Possible association between Zika virus infection and microcephaly - Brazil, 2015. MMWR Morb Mortal Wkly Rep 65:59–62. doi: 10.15585/mmwr.mm6503e2 [DOI] [PubMed] [Google Scholar]
- 171. WHO . 2022. Zika virus. Available from: https://www.who.int/news-room/fact-sheets/detail/zika-virus
- 172. Haddow AD, Schuh AJ, Yasuda CY, Kasper MR, Heang V, Huy R, Guzman H, Tesh RB, Weaver SC. 2012. Genetic characterization of zika virus strains: geographic expansion of the asian lineage. PLoS Negl Trop Dis 6:e1477. doi: 10.1371/journal.pntd.0001477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Portnoi Baran LC, Fernades da Costa M, Summer Vidal K, Damico FM, Telles Salgueiro Barboni M, da Silva Lima D, de Cássia Rodrigues de Matos França V, Gomes Martins CM, Segundo Tabares H, Leonardo Dias S, Aparecido Silva L, Decleva D, Hamer RD, Zatz M, A P Bertozzi AP, Gazeta RE, Duarte Passos S, Fix Ventura D. 2019. Alterations in visual acuity and visual development in infants 1-24 months old either exposed to or infected by Zika virus during gestation, with and without microcephaly. J AAPOS 23:215. doi: 10.1016/j.jaapos.2019.03.005 [DOI] [PubMed] [Google Scholar]
- 174. Tsui I, Moreira MEL, Rossetto JD, Vasconcelos Z, Gaw SL, Neves LM, Zin OA, Haefeli L, Silveira Filho JCB, Gomes SC, Adachi K, Pone M da S, Pone SM, Pereira JP, Belfort R, Arumugaswami V, Brasil P, Nielsen-Saines K, Zin AA. 2018. Eye findings in infants with suspected or confirmed antenatal Zika virus exposure. Pediatrics 142:e20181104. doi: 10.1542/peds.2018-1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Ventura CV, Maia M, Ventura BV, Linden VVD, Araújo EB, Ramos RC, Rocha MAW, Carvalho M, Belfort R, Ventura LO. 2016. Ophthalmological findings in infants with microcephaly and presumable intra-uterus Zika virus infection. Arq Bras Oftalmol 79:1–3. doi: 10.5935/0004-2749.20160002 [DOI] [PubMed] [Google Scholar]
- 176. Ventura CV, Maia M, Dias N, Ventura LO, Belfort R. 2016. Zika: neurological and ocular findings in infant without microcephaly. Lancet 387:2502. doi: 10.1016/S0140-6736(16)30776-0 [DOI] [PubMed] [Google Scholar]
- 177. Sanjay S, Agrawal S, Mahendradas P, Kawali A, Gupta A, Shetty R. 2020. Post fever uveoretinal manifestations in an immunocompetent individual. EMJ Allergy Immunol:91–105. doi: 10.33590/emjallergyimmunol/20-00092 [DOI] [Google Scholar]
- 178. Singh PK, Guest J-M, Kanwar M, Boss J, Gao N, Juzych MS, Abrams GW, Yu F-S, Kumar A. 2017. Zika virus infects cells lining the blood-retinal barrier and causes chorioretinal atrophy in mouse eyes. JCI Insight 2:e92340. doi: 10.1172/jci.insight.92340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Manangeeswaran M, Kielczewski JL, Sen HN, Xu BC, Ireland DDC, McWilliams IL, Chan C-C, Caspi RR, Verthelyi D. 2018. ZIKA virus infection causes persistent chorioretinal lesions article. Emerg Microbes Infect 7:96. doi: 10.1038/s41426-018-0096-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Simonin Y, Erkilic N, Damodar K, Clé M, Desmetz C, Bolloré K, Taleb M, Torriano S, Barthelemy J, Dubois G, Lajoix AD, Foulongne V, Tuaillon E, Van de Perre P, Kalatzis V, Salinas S. 2019. Zika virus induces strong inflammatory responses and impairs homeostasis and function of the human retinal pigment epithelium. EBioMedicine 39:315–331. doi: 10.1016/j.ebiom.2018.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Parke DW, Almeida DRP, Albini TA, Ventura CV, Berrocal AM, Mittra RA. 2016. Serologically confirmed Zika-related unilateral acute maculopathy in an adult. Ophthalmology 123:2432–2433. doi: 10.1016/j.ophtha.2016.06.039 [DOI] [PubMed] [Google Scholar]
- 182. Panday A, Sandy S, King D, Ramdeen S. 2017. A case of suspected symptomatic Zika neuroretinitis. IDCases 9:104–105. doi: 10.1016/j.idcr.2017.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Henry CR, Al-Attar L, Cruz-Chacón AM, Davis JL. 2017. Chorioretinal lesions presumed secondary to Zika virus infection in an immunocompromised adult. JAMA Ophthalmol 135:386–389. doi: 10.1001/jamaophthalmol.2017.0098 [DOI] [PubMed] [Google Scholar]
- 184. Ventura CV, Maia M, Travassos SB, Martins TT, Patriota F, Nunes ME, Agra C, Torres VL, van der Linden V, Ramos RC, Rocha MÂW, Silva PS, Ventura LO, Belfort R. 2016. Risk factors associated with the ophthalmoscopic findings identified in infants with presumed Zika virus congenital infection. JAMA Ophthalmol 134:912–918. doi: 10.1001/jamaophthalmol.2016.1784 [DOI] [PubMed] [Google Scholar]
- 185. Ventura CV, Zin A, Paula Freitas B de, Ventura LO, Rocha C, Costa F, Nery N, De Senna TCR, Lopes Moreira ME, Maia M, Belfort R. 2021. Ophthalmological manifestations in congenital Zika syndrome in 469 Brazilian children. J AAPOS 25:158. doi: 10.1016/j.jaapos.2021.01.009 [DOI] [PubMed] [Google Scholar]
- 186. de Paula Freitas B, de Oliveira Dias JR, Prazeres J, Sacramento GA, Ko AI, Maia M, Belfort R. 2016. Ocular findings in infants with microcephaly associated with presumed Zika virus congenital infection in Salvador, Brazil. JAMA Ophthalmol 134:529–535. doi: 10.1001/jamaophthalmol.2016.0267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Costa C da C, Freitas D. 2022. Ocular findings of congenital Zika virus infection with microcephaly. Int Ophthalmol 42:3117–3127. doi: 10.1007/s10792-022-02311-8 [DOI] [PubMed] [Google Scholar]
- 188. Miner JJ, Sene A, Richner JM, Smith AM, Santeford A, Ban N, Weger-Lucarelli J, Manzella F, Rückert C, Govero J, Noguchi KK, Ebel GD, Diamond MS, Apte RS. 2016. Zika virus infection in mice causes panuveitis with shedding of virus in tears. Cell Rep 16:3208–3218. doi: 10.1016/j.celrep.2016.08.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Camargos VN, Foureaux G, Medeiros DC, da Silveira VT, Queiroz-Junior CM, Matosinhos ALB, Figueiredo AFA, Sousa CDF, Moreira TP, Queiroz VF, Dias ACF, Santana KTO, Passos I, Real A, Silva LC, Mourão FAG, Wnuk NT, Oliveira MAP, Macari S, Silva T, Garlet GP, Jackman JA, Soriani FM, Moraes MFD, Mendes E, Ribeiro FM, Costa GMJ, Teixeira AL, Cho N-J, Oliveira ACP, Teixeira MM, Costa VV, Souza DG. 2019. In-depth characterization of congenital Zika syndrome in immunocompetent mice: antibody-dependent enhancement and an antiviral peptide therapy. EBioMedicine 44:516–529. doi: 10.1016/j.ebiom.2019.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Xia H, Luo H, Shan C, Muruato AE, Nunes BTD, Medeiros DBA, Zou J, Xie X, Giraldo MI, Vasconcelos PFC, Weaver SC, Wang T, Rajsbaum R, Shi PY. 2018. An evolutionary NS1 mutation enhances Zika virus evasion of host interferon induction. Nat Commun 9:414. doi: 10.1038/s41467-017-02816-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Foo S-S, Chen W, Chan Y, Bowman JW, Chang L-C, Choi Y, Yoo JS, Ge J, Cheng G, Bonnin A, Nielsen-Saines K, Brasil P, Jung JU. 2017. Asian Zika virus strains target CD14+ blood monocytes and induce M2-skewed immunosuppression during pregnancy. Nat Microbiol 2:1558–1570. doi: 10.1038/s41564-017-0016-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Sheridan MA, Yunusov D, Balaraman V, Alexenko AP, Yabe S, Verjovski-Almeida S, Schust DJ, Franz AW, Sadovsky Y, Ezashi T, Roberts RM. 2017. Vulnerability of primitive human placental trophoblast to Zika virus. Proc Natl Acad Sci U S A 114:E1587–E1596. doi: 10.1073/pnas.1616097114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Zaidi MB, De Moraes CG, Petitto M, Yepez JB, Sakuntabhai A, Simon-Loriere E, Prot M, Ruffie C, Kim SS, Allikmets R, Terwilliger JD, Lee JH, Maestre GE. 2018. Non-congenital severe ocular complications of Zika virus infection. JMM Case Rep 5:e005152. doi: 10.1099/jmmcr.0.005152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Kodati S, Palmore TN, Spellman FA, Cunningham D, Weistrop B, Sen HN. 2017. Bilateral posterior uveitis associated with Zika virus infection. Lancet 389:125–126. doi: 10.1016/S0140-6736(16)32518-1 [DOI] [PubMed] [Google Scholar]
- 195. Benavides-Lara A, la Paz Barboza-Arguello M de, González-Elizondo M, Hernández-deMezerville M, Brenes-Chacón H, Ramírez-Rojas M, Ramírez-Hernández C, Arjona-Ortegón N, Godfred-Cato S, Valencia D, Moore CA, Soriano-Fallas A. 2021. Zika virus-associated birth defects, Costa Rica, 2016-2018. Emerg Infect Dis 27:360–371. doi: 10.3201/eid2702.202047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Prakalapakorn SG, Bonafede L, Lawrence L, Lattin D, Kim N, House RD, Hillman B, de Wilde L, Harrison C, Fehrenbach N, Godfred-Cato S, Reynolds MR, Ellis EM. 2021. Ocular findings and visual function in children examined during the Zika health brigade in the us Virgin islands, March 2018. Trop Med Infect Dis 6:66. doi: 10.3390/tropicalmed6020066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Troumani Y, Touhami S, Jackson TL, Ventura CV, Stanescu-Segall DM, Errera M-H, Rousset D, Bodaghi B, Cartry G, David T, Beral L. 2021. Association of anterior uveitis with acute Zika virus infection in adults. JAMA Ophthalmol 139:95–102. doi: 10.1001/jamaophthalmol.2020.5131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. de Alencar Ximenes RA, Miranda-Filho D de B, Brickley EB, Barreto de Araújo TV, Montarroyos UR, Abtibol-Bernardino MR, Mussi-Pinhata MM, Duarte G, Coutinho CM, Biason de Moura Negrini SF, Costa Alecrim M das G, Albuquerque de Almeida Peixoto L de F, Lopes Moreira ME, Zin A, Pereira Júnior JP, Nielsen-Saines K, Turchi Martelli CM, Rodrigues LC, de Souza WV, Ventura LO, de Oliveira CS, de Matos H, Furtado Serra EM, Souza Gomes LT, Nogueira ML, Estofolete C, Vaz-Oliani DC, Passos SD, Moron A, Duarte Rodrigues MM, Pereira Sarmento SG, Turchi MD, Pela Rosado LE, de Sene Amâncio Zara AL, Franco Gomes MB, Schuler-Faccini L, Herrero-Silva J, Amorim MM, Melo AO, Ledo Alves da Cunha AJ, Prata-Barbosa A, Amim J, Rezende-Filho J, Calcagno JI, Júnior Alcântara LC, de Almeida BL, Hofer CB, Machado ES, de Siqueira IC, Martinez-Espinoza FE, Brasil P. 2023. Risk of adverse outcomes in offspring with RT-PCR confirmed prenatal Zika virus exposure: an individual participant data meta-analysis of 13 cohorts in the Zika Brazilian Cohorts Consortium. Lancet Reg Health Am 17:100395. doi: 10.1016/j.lana.2022.100395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. de Oliveira DR, Ventura CV, Esporcatte BLB, Gracitelli CPB, Dias N de C, Ventura LO, Belfort R, Rolim-de-Moura C. 2019. Glaucoma workup in congenital Zika syndrome. J Glaucoma 28:313–317. doi: 10.1097/IJG.0000000000001150 [DOI] [PubMed] [Google Scholar]
- 200. Rossi ÁD, Faucz FR, Melo A, Pezzuto P, de Azevedo GS, Schamber-Reis BLF, Tavares JS, Mattapallil JJ, Tanuri A, Aguiar RS, Cardoso CC, Stratakis CA. 2019. Variations in maternal adenylate cyclase genes are associated with congenital Zika syndrome in a cohort from Northeast, Brazil. J Intern Med 285:215–222. doi: 10.1111/joim.12829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Fontes BM. 2016. Zika virus-related hypertensive iridocyclitis. Arq Bras Oftalmol 79:63. doi: 10.5935/0004-2749.20160020 [DOI] [PubMed] [Google Scholar]
- 202. Furtado JM, Espósito DL, Klein TM, Teixeira-Pinto T, da Fonseca BA. 2016. Uveitis associated with Zika virus infection. N Engl J Med 375:394–396. doi: 10.1056/NEJMc1603618 [DOI] [PubMed] [Google Scholar]
- 203. Detrick B, Lee MT, Chin MS, Hooper LC, Chan C-C, Hooks JJ. 2008. Experimental coronavirus retinopathy (ECOR): retinal degeneration susceptible mice have an augmented interferon and chemokine (CXCL9, CXCL10) response early after virus infection. J Neuroimmunol 193:28–37. doi: 10.1016/j.jneuroim.2007.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Komurasaki Y, Nagineni CN, Wang Y, Hooks JJ. 1996. Virus RNA persists within the retina in coronavirus-induced retinopathy. Virology 222:446–450. doi: 10.1006/viro.1996.0442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Sawant OB, Singh S, Wright RE, Jones KM, Titus MS, Dennis E, Hicks E, Majmudar PA, Kumar A, Mian SI. 2021. Prevalence of SARS-CoV-2 in human post-mortem ocular tissues. Ocul Surf 19:322–329. doi: 10.1016/j.jtos.2020.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Lim LW, Tan GS, Yong V, Anderson DE, Lye DC, Young B, Agrawal R. 2020. Acute onset of bilateral follicular conjunctivitis in two patients with confirmed SARS-CoV-2 infections. Ocul Immunol Inflamm 28:1280–1284. doi: 10.1080/09273948.2020.1821901 [DOI] [PubMed] [Google Scholar]
- 207. Shantha J, Reddy AK, Sura A, Tsang A, Moussa K, Acharya N, Gonzales J, Doan T. 2022. Bilateral anterior and intermediate uveitis in SARS-CoV-2 associated multisystem inflammatory syndrome in a pediatric patient. Pediatr Rheumatol Online J 20:59. doi: 10.1186/s12969-022-00712-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Mazzotta C, Giancipoli E. 2020. Anterior acute uveitis report in a SARS-CoV-2 patient managed with adjunctive topical antiseptic prophylaxis preventing 2019-ncov spread through the ocular surface route. Int Med Case Rep J 13:513–520. doi: 10.2147/IMCRJ.S260252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY, Freedberg DE, Kirtane AJ, Parikh SA, Maurer MS, Nordvig AS, Accili D, Bathon JM, Mohan S, Bauer KA, Leon MB, Krumholz HM, Uriel N, Mehra MR, Elkind MSV, Stone GW, Schwartz A, Ho DD, Bilezikian JP, Landry DW. 2020. Extrapulmonary manifestations of COVID-19. Nat Med 26:1017–1032. doi: 10.1038/s41591-020-0968-3 [DOI] [PubMed] [Google Scholar]
- 210. Invernizzi A, Torre A, Parrulli S, Zicarelli F, Schiuma M, Colombo V, Giacomelli A, Cigada M, Milazzo L, Ridolfo A, Faggion I, Cordier L, Oldani M, Marini S, Villa P, Rizzardini G, Galli M, Antinori S, Staurenghi G, Meroni L. 2020. Retinal findings in patients with COVID-19: results from the SERPICO-19 study. EClinicalMedicine 27:100550. doi: 10.1016/j.eclinm.2020.100550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Scalinci SZ, Trovato Battagliola E. 2020. Conjunctivitis can be the only presenting sign and symptom of COVID-19. IDCases 20:e00774. doi: 10.1016/j.idcr.2020.e00774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Wu P, Duan F, Luo C, Liu Q, Qu X, Liang L, Wu K. 2020. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol 138:575–578. doi: 10.1001/jamaophthalmol.2020.1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Méndez Mangana C, Barraquer Kargacin A, Barraquer RI. 2020. Episcleritis as an ocular manifestation in a patient with COVID-19. Acta Ophthalmol 98:e1056–e1057. doi: 10.1111/aos.14484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Otaif W, Al Somali AI, Al Habash A. 2020. Episcleritis as a possible presenting sign of the novel coronavirus disease: a case report. Am J Ophthalmol Case Rep 20:100917. doi: 10.1016/j.ajoc.2020.100917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Zhou S, Jones-Lopez EC, Soneji DJ, Azevedo CJ, Patel VR. 2020. Myelin oligodendrocyte glycoprotein antibody-associated optic neuritis and myelitis in COVID-19. J Neuroophthalmol 40:398–402. doi: 10.1097/WNO.0000000000001049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Sawalha K, Adeodokun S, Kamoga G-R. 2020. COVID-19-induced acute bilateral optic neuritis. J Investig Med High Impact Case Rep 8:2324709620976018. doi: 10.1177/2324709620976018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Barosco G, Morbio R, Chemello F, Tosi R, Marchini G. 2022. Bilateral angle-closure during hospitalization for coronavirus disease-19 (COVID-19): a case report. Eur J Ophthalmol 32:NP75–NP82. doi: 10.1177/11206721211012197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Nerlikar RR, Palsule AC, Vadke S. 2021. Bilateral acute angle closure glaucoma after prone position ventilation for COVID-19 pneumonia. J Glaucoma 30:e364–e366. doi: 10.1097/IJG.0000000000001864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Özmen S, Özkan Aksoy N, Çakır B, Alagöz G. 2023. Acute angle-closure glaucoma concurrent with COVID 19 infection; case report. Eur J Ophthalmol 33:NP42–NP45. doi: 10.1177/11206721221113201 [DOI] [PubMed] [Google Scholar]
- 220. Soman M, Indurkar A, George T, Sheth JU, Nair U. 2022. Rapid onset neovascular glaucoma due to COVID-19-related retinopathy. J Curr Glaucoma Pract 16:136–140. doi: 10.5005/jp-journals-10078-1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Abdul-Salam State S-E, Sfredel V, Mocanu CL, Albu CV, Bălășoiu A-T. 2022. Optic neuropathies post-Covid 19 - review. Rom J Ophthalmol 66:289–298. doi: 10.22336/rjo.2022.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Katayama H, Itoh K, Hashimoto M. 2022. Bilateral optic neuritis after COVID-19 mRNA vaccination. Case Rep Ophthalmol 13:578–583. doi: 10.1159/000525938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Sachdeva V. 2023. Optic neuritis following COVID-19 vaccination. Oman J Ophthalmol 16:4–5. doi: 10.4103/ojo.ojo_22_23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Fu R, Du Y. 2022. Optic neuritis after COVID-19 vaccination. J Neuroophthalmol. doi: 10.1097/WNO.0000000000001711 [DOI] [PubMed] [Google Scholar]
- 225. Lee W-J. 2022. COVID-19 vaccine-associated optic neuritis. QJM 115:683–685. doi: 10.1093/qjmed/hcac208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Jossy A, Jacob N, Sarkar S, Gokhale T, Kaliaperumal S, Deb AK. 2022. COVID-19-associated optic neuritis - A case series and review of literature. Indian J Ophthalmol 70:310–316. doi: 10.4103/ijo.IJO_2235_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. García-Estrada C, Gómez-Figueroa E, Alban L, Arias-Cárdenas A. 2022. Optic neuritis after COVID-19 vaccine application. Clin Exp Neuroimmunol 13:72–74. doi: 10.1111/cen3.12682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Yazdani S, Esfandiari H, Safi S, Fatemi A. 2017. Oseltamivir (Tamiflu)-induced bilateral ciliochoroidal effusion and angle closure glaucoma: what type of idiosyncratic reaction? J Ophthalmic Vis Res 12:434–436. doi: 10.4103/jovr.jovr_200_15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Lee JW, Lee JE, Choi HY, Lee JS. 2014. Oseltamivir (Tamiflu)-induced bilateral acute angle closure glaucoma and transient myopia. Indian J Ophthalmol 62:1165–1167. doi: 10.4103/0301-4738.109531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Xi L, Zhang L, Fei W. 2018. Cytomegalovirus-related uncontrolled glaucoma in an immunocompetent patient: a case report and systematic review. BMC Ophthalmol 18:259. doi: 10.1186/s12886-018-0917-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Ambati J, Wynne KB, Angerame MC, Robinson MR. 1999. Anterior uveitis associated with intravenous cidofovir use in patients with cytomegalovirus retinitis. Br J Ophthalmol 83:1153–1158. doi: 10.1136/bjo.83.10.1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Monowarul Islam SM, El-Sheikh HF, Tabbara KF. 2000. Anterior uveitis following combined vaccinationa for measles, mumps and rubella (MMR): a report of two cases. Acta Ophthalmol Scand 78:590–592. doi: 10.1034/j.1600-0420.2000.078005590.x [DOI] [PubMed] [Google Scholar]
- 233. Sedaghat M, Zarei-Ghanavati S, Shokoohi S, Ghasemi A. 2007. Panuveitis and dermal vasculitis following MMR vaccination. East Mediterr Health J 13:470–474. [PubMed] [Google Scholar]
- 234. Keikha M, Zandhaghighi M, Zahedani SS. 2023. Optic neuritis associated with COVID-19-related vaccines. Vacunas 24:158–159. doi: 10.1016/j.vacun.2022.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Haseeb A, Elhusseiny AM, Chauhan MZ, Elnahry AG. 2022. Optic neuropathy after COVID-19 vaccination: case report and systematic review. Neuroimmunology Reports 2:100121. doi: 10.1016/j.nerep.2022.100121 [DOI] [Google Scholar]
- 236. Elnahry AG, Al-Nawaflh MY, Gamal Eldin AA, Solyman O, Sallam AB, Phillips PH, Elhusseiny AM. 2022. COVID-19 vaccine-associated optic neuropathy: a systematic review of 45 patients. Vaccines (Basel) 10:1758. doi: 10.3390/vaccines10101758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Cozzupoli GM, Savastano MC, Falsini B, Savastano A, Rizzo S. 2020. Possible retinal impairment secondary to Ritonavir use in SARS-CoV-2 patients: a narrative systematic review. J Ophthalmol 2020:5350494. doi: 10.1155/2020/5350494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Tu Y, Poblete RJ, Freilich BD, Zarbin MA, Bhagat N. 2016. Retinal toxicity with Ritonavir. Int J Ophthalmol 9:640–642. doi: 10.18240/ijo.2016.04.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Lee EY, Srinivasan Y, de Anda J, Nicastro LK, Tükel Ç, Wong GCL. 2020. Functional reciprocity of amyloids and antimicrobial peptides: rethinking the role of supramolecular assembly in host defense, immune activation, and inflammation. Front Immunol 11:1629. doi: 10.3389/fimmu.2020.01629 [DOI] [PMC free article] [PubMed] [Google Scholar]