Abstract
Charcot–Marie–Tooth disease (CMT) is a group of inherited peripheral neuropathies related to variants in the mitochondrial transfer RNA (mt-tRNAval) gene. Here, we report a Chinese family harboring the m.1661A>G variant in the mt-tRNAval gene. Clinical evaluation, neuroelectrodiagnostic testing, and nerve biopsy were performed on four affected family members. Weakness, spasms, and pain in the limbs (especially in the lower limbs) were the main complaints of the proband. Physical examination revealed atrophy and weakness in the distal limbs, increased muscle tone, and hyperreflexia in four limbs. Neuroelectrodiagnostic tests and nerve biopsy supported an axonal polyneuropathy. This study furthers the understanding of phenotype diversity caused by variants in the mt-tRNAval gene in CMT.
Keywords: Charcot–Marie–Tooth disease (CMT), the mitochondrial transfer RNA (mt-tRNAval) gene, the m.1661A>G variant, neuroelectrodiagnostic testing, peripheral neuropathies
Introduction
Charcot–Marie–Tooth disease (CMT) is a group of inherited peripheral neuropathies characterized by motor and sensory symptoms (1, 2). The onset is usually in childhood or early adulthood, the typical clinical features are distal muscle weakness and atrophy (3). Based on neurophysiological examination, CMT can be divided into three forms: (1) demyelinating form caused by Schwann cell dysfunction, (2) axonal form caused by degeneration of peripheral nerve axons, and (3) intermediate form (4–6).
Most CMT are found to be monogenic disease, and genetic patterns include autosomal dominant, autosomal recessive, X-linked and maternal (mitochondrial) inheritance (7). In recent years, more than 100 genes have been identified as genetic causes of CMT due to the rapid development of next generation sequencing (NGS) technologies (1, 8, 9). However, there are few reports on mitochondrial mutations related to CMT.
Mitochondria are a crucial organelle in nerve cells, whose primary function is to produce ATP and provide energy for various cellular activities. Due to the long axons, peripheral nerves have peculiar energetic requirements to maintain complex metabolic mechanisms. Therefore, correct mitochondrial function is crucial for maintaining energy metabolism. Many subtypes of CMT are associated with abnormality in mitochondrial function. Pathogenic mutations in the mt-tRNAval gene may impair mitochondrial translation, leading to CMT (10, 11).
Here, we report the variant of m.1661A>G in a Chinese family with CMT with a mitochondrial inheritance pattern. Furthermore, we describe in detail the clinical and neurophysiological studies data and nerve biopsy findings of the recruited family members. This study furthers the understanding of phenotype diversity caused by variants in the mt-tRNAval gene in CMT.
Materials and methods
Participants and neurophysiological examination
The research was approved by the Ethics Committee of Sichuan Provincial People’s Hospital. All participants signed the informed consent and agreed to undergo a detailed clinical examination, including assessment of medical history and physical examination performed by neurologists.
All participants underwent standard neurophysiological examination via Sierra Summit (Cadwell industries, Washington, America). Sensory conduction velocity, sensory nerve action potential (SNAP), motor conduction velocity (MCV), compound muscle action potential (CMAP), distal motor latency (DML), and F-wave were recorded from median, ulnar, peroneal, and tibial nerves. Electromyography (EMG) was performed on the distal and proximal muscles of the limbs.
Sural nerve biopsy analysis
Hematoxylin–eosin staining and pathological microscopes were used to examine the proband’s sural nerves. Immunohistochemistry staining was performed to examine the expression of human leukocyte antigen DR (HLA-DR), myelin basic protein (MBP), leukocyte common antigen (LCA), and KP1. Images were captured at a magnification of 40×.
Genetic analyses
Whole genome sequencing (WGS) was performed on two affected members (IV:2 and III:1) and one unaffected member (IV:1) via the Illumina platform (CIPHER, Beijing, China). Then, Sanger sequencing was used to validate the candidate variants and co-segregation between the genotype and the CMT phenotype in the family.
Results
Pedigree
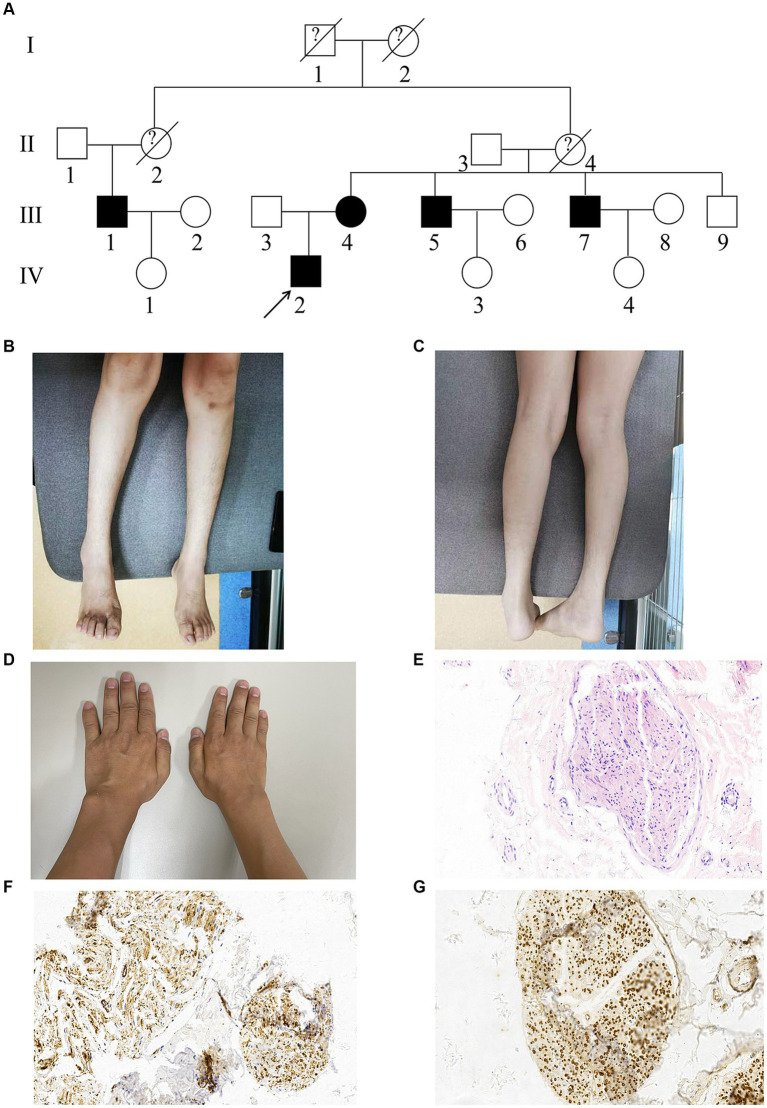
A Chinese family with CMT was recruited (Figure 1A). Participants included five affected members (III:1, III:4, III:5, III:7, and IV:2) and four unaffected members (III:3, IV:1, IV:3, and IV:4). All descendants of male members with the CMT phenotype were unaffected.
Figure 1.
Pedigree, clinical and nerve biopsy findings of the proband (IV:2). (A) Pedigrees of the CMT family. Affected individuals are denoted in Black. The arrow indicates the proband. (B,C) The proband exhibited obvious atrophy in tibialis anterior and gastrocnemius of lower limbs. (D) The proband exhibited moderate atrophy in dorsal interosseous muscles of upper limbs. (E) Hematoxylin–eosin staining (HE staining) of the proband’s sural nerves. (F,G) Immunohistochemistry showed HLA-DR (positive) and MBP (positive).
Clinical investigations
Patient 1 (proband, IV:2)
Patient 1 was a 36 years-old man. At 20 years of age, he complained of weakness, pain, and spasms in his lower limbs after walking or sitting for a long time. Later, his sleep quality was affected by continuous spasms and pain, and he woke more than a dozen times every night. He was also diagnosed with hereditary spastic paraplegia and was treated with baclofen. Since the age of 33, the pain in the outer and posterior sides of the right leg, buttock, and coccyx has progressively worsened and caused a severe sleep disorder. Finally, after a nerve conduction study (NCS), EMG and sural nerve biopsy, he was diagnosed with hereditary motor and sensory neuropathy type 2.
Neurological examination revealed obvious atrophy in the tibialis anterior and gastrocnemius of the lower limbs (Figures 1B,C), moderate atrophy in the dorsal interosseous muscles of the upper limbs (Figure 1D), increased muscle tone, weakness in the four distal limbs through manual muscle tests (MMT 3/5 in the lower limbs and 4/5 in the upper limbs), and hyperreflexia in the four limbs. Pinprick sensations were symmetrically decreased in the distal lower limbs.
NCS (Table 1) showed reduced amplitude of CMAPs in the bilateral tibial, peroneal, and right ulnar nerves. There was a slight slowing of MCVs in the left peroneal nerve and some prolonging of DMLs in bilateral tibial nerves and the left peroneal nerve. SNAPs could not be evoked in any sampled nerve. F-wave could not be evoked in the right tibial nerve, and F-wave latency was extended in the left tibial nerve.
Table 1.
Electrophysiological data.
| Nerve patient | Patient 1 (IV:2) | Patient 2 (III:4) | Patient 3 (III:5) | Patient 4 (III:7) | ||||
|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | Left | Right | |
| Median nerve | ||||||||
| MCV (m/s) | 55 | 59 | 45 | 50 | 56 | 54 | 55.1 | 59.4 |
| Distal latency (ms) | 3.33 | 3.44 | 4.58 | 4.06 | 3.70 | 3.49 | 3.1 | 3.5 |
| CMAP amplitude (mV) | 11.8 | 7.9 | 10.9 | 10.9 | 13.2 | 16.9 | 10.5 | 10.7 |
| SCV (m/s) | NR | NR | NR | NR | NR | NR | 52.2 | 55 |
| SNAP amplitude | NR | NR | NR | NR | NR | NR | 20.6 | 14.9 |
| F mean latency (ms) | 28.1 | 29.5 | 31.2 | NR | 25.9 | 25.8 | 24.9 | / |
| F% | 100 | 90 | 30 | 0 | 100 | 100 | 100 | / |
| Ulnar nerve | ||||||||
| MCV (m/s) | 59 | 55 | 35 | 46 | 65 | 52 | 60.9 | 60.5 |
| Distal latency (ms) | 3.23 | 3.02 | 2.81 | 3.54 | 3.54 | 3.23 | 2.7 | 2.8 |
| CMAP amplitude (mV) | 7.8 | 6.4 | 3.5 | 5.2 | 8.6 | 4.4 | 10.8 | 13.4 |
| SCV (m/s) | NR | NR | NR | NR | NR | NR | 54.8 | 56.2 |
| SNAP amplitude | NR | NR | NR | NR | NR | NR | 12.9 | 13.8 |
| F mean latency (ms) | 30.7 | 30.0 | 30.1 | 30 | 27.7 | 27.8 | 49.3 | 49.7 |
| F% | 100 | 80 | 60 | 90 | 100 | 80 | 100 | 100 |
| Tibial nerve | ||||||||
| MCV (m/s) | 43 | 46 | NR | NR | 35 | NR | 48.9 | 48.3 |
| Distal latency (ms) | 6.25 | 4.58 | NR | NR | 7.60 | NR | 4.0 | 4.0 |
| CMAP amplitude (mV) | 4.6 | 2.0 | NR | NR | 0.6 | NR | 12.7 | 16.0 |
| Sural nerve | ||||||||
| SCV (m/s) | NR | NR | NR | NR | NR | NR | 56.3 | 51.2 |
| SNAP amplitude | NR | NR | NR | NR | NR | NR | 5.6 | 2.1 |
| Superficial peroneal nerve | ||||||||
| SCV (m/s) | NR | NR | NR | NR | NR | NR | 42.9 | 44.5 |
| SNAP amplitude | NR | NR | NR | NR | NR | NR | 4.9 | 2.5 |
MCV, motor conduction velocity; CMAP, compound motor action potential; SCV, sensory conduction velocity; SNAP, sensory nerve action potential; NR, not recordable.
EMG revealed chronic neurogenic lesions with reinnervated MUAPs and decreased recruitment in the right tibialis anterior, left quadriceps medialis, first interosseous dorsal muscle, and right biceps.
Sural nerve biopsy
The biopsy of the six bundles of sural nerves from the proband showed a medium depletion of myelinated nerve fibers, especially of the large-diameter fibers. Schwann cells exhibited mild proliferation with degeneration. Some fiber axonal microfilaments increased and aggregated, while a few unmyelinated fiber axonal microfilaments degenerated. Immunohistochemistry (Figures 1E–G) showed that the samples were positive for HLA-DR and MBP and negative for LCA and KP1. These results suggested that the histopathological profile of the patient was mainly axonal degeneration with nerve damage.
Patient 2 (III:4)
Patient 2 was a 59 years-old woman who was the mother of the proband. At an age of around 18 years, she began to feel slight pain in her knee, which she initially thought was due to physical labor; however, the pain gradually worsened over time. She also complained of weakness in her lower limbs.
NCS showed decreased CMAPs in bilateral ulnar nerves. CMAPs could not be evoked in any nerve of the lower limbs, and SNAPs could not be evoked in any sampled nerve. F-wave could not be evoked in the right median nerve, and F-wave latencies extended in the nerves of the upper limbs.
Patient 3 (III:5)
Patient 3 was a 58 years-old man who was the uncle of the proband. At the age of around 20 years, he began to feel weakness after long-distance walking and climbing hills. The symptoms gradually and slowly worsened. Until the age of 40, he felt very weak when walking, but did not have any pain.
Similar to his nephew and elder sister, SNAPs could not be evoked in any sampled nerve, and CMAPs could not be evoked in the nerves of the lower limbs. CMAPs were decreased in the left tibial and bilateral ulnar nerves. F-waves could not be evoked in bilateral tibial nerves.
The EMGs of two patients (III:4 and III:5) showed chronic neurogenic damage to the muscles of the upper and lower limbs. The results of three patients (III:4, III:5, and IV:2) indicated a length-dependent motor and sensory axonal polyneuropathy.
Patient 4 (III:7)
Patient 4 (proband’s uncle) was a 56 years-old man who did not complain of any weakness or pain. However, Sanger sequencing revealed that he carried the variant m.1661A>G. It was unclear why the patient did not show any symptoms of the disease. Therefore, NCS was performed on him, which revealed a slight impairment of the sensory fibers of the peripheral nerves in his four limbs, particularly in the lower limbs.
Genetic findings
WGS sequencing of mtDNA was performed on three family members (IV:2, III:1 and IV:1). Patients IV:2 and III:1, but not patient IV:1, carried the genes, which were filtered to identify a potential causal mutation. Considering a mitochondrial inheritance pattern, genes located in mitochondria were focused on. Finally, a homoplasmic variant (m.1661A>G) in the mt-tRNAval gene was identified in the proband (IV:2) and proband’s uncle (III:1) using WGS.
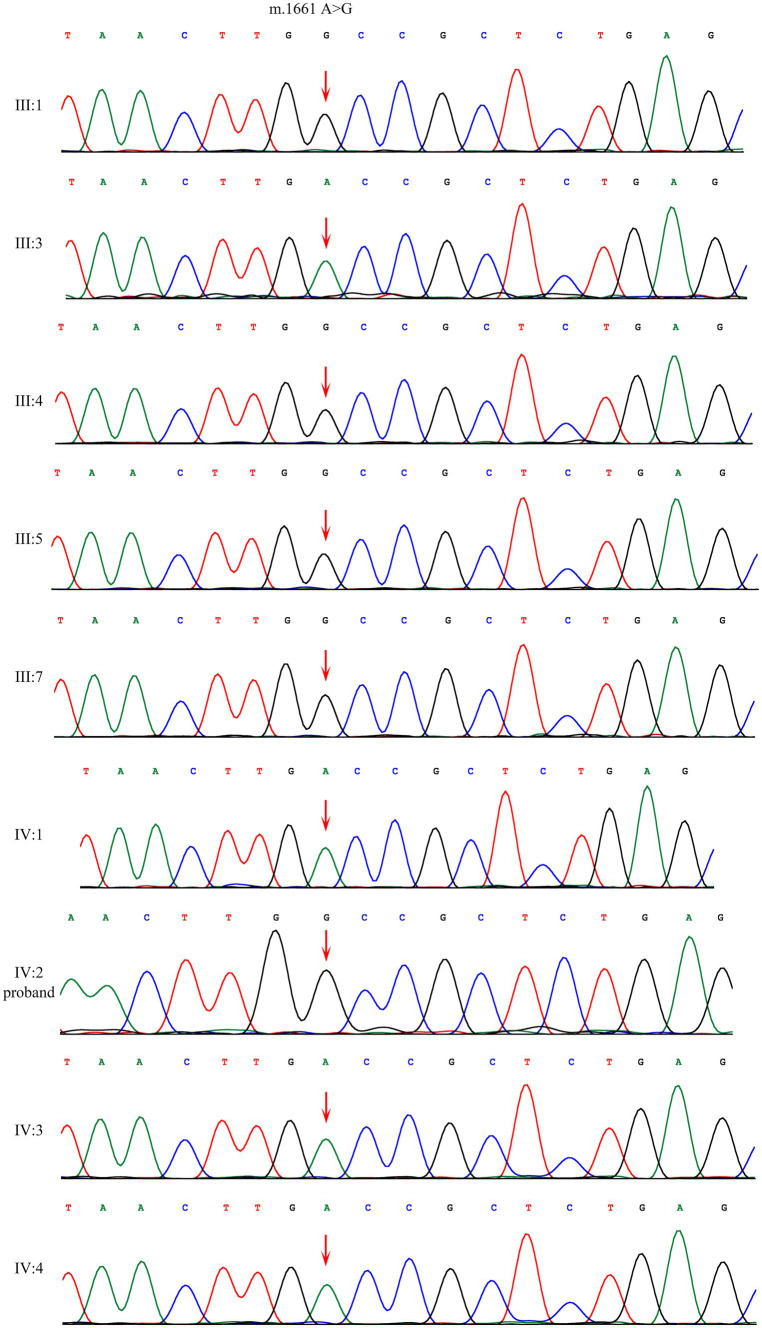
Sanger sequencing showed that the homoplasmic variant: m.1661 A>G was identified among four affected family members (III:1, III:4, III:5, IV:2) and one family member that denied any symptoms (III:7). This variant is absent in four unaffected individuals (III:3, IV:1, IV:3, IV:4) (Figure 2).
Figure 2.
Sanger sequencing of the m.1661A>G variant in mt-tRNAval gene showed that the variant were present among patients and absent among normal individuals.
Discussion
Here, we reported a Chinese family with a length-dependent motor and sensory neuropathy based on axonal lesions and shown that the family members harbored the m.1661A>G variant in the mt-tRNAval gene. Weakness of the limbs, especially the lower limbs, was the main symptom of most of the patients. In addition, continuous severe spasms and pain in the lower limbs were the other symptoms in this family, especially in the proband, which was different from the previous reports.
Although there were indeed some cases who presented Spastic paresis and Babinski reflex in the previously reported family from Venezuela (12), the damage of pyramidal tracts seems to be more prominent (severe spasmodic paresis and pain, which requires long-term use of a baclofen pump to relieve) in our study especially in the proband, and leading to clinical diagnosis of HSP in different hospitals. However, genetic tests either for HSP or for CMT2 did not show any positive results. Then, pedigree analysis of this family showed a mitochondrial inheritance pattern, and m. 1661A>G variant in the mt-RNAval gene via WGS sequencing was identified among affected family members, which can also explain the phenotypic heterogeneity in this family. Given the severity of peripheral nerve and vertebral tract damage in this family, it may be more appropriate to refer to it as a complex neurological syndrome with overlap between spinal paresis and axial sensorimotor polyneuropathy. Electrodiagnostic results indicated significant axonal lesions in four limbs, especially in the lower limbs, which were confirmed by decreased or absent CMAPs and SNAPs, while conduction velocities and distal latencies were normal or just slightly slowing. Sensory fibers were more severely damaged than motor fibers. EMG showed reduced recruitment and reinnervated MUAPs without any abnormal spontaneous potential, indicating a chronic stage of axonal loss lesions. The sural nerve biopsy of the proband showed axonal degeneration with nerve damage, which was consistent with electrodiagnostic results.
Notably, patient 4 (III:7) denied any symptoms of sensation and movement in the limbs. However, his genetic and neurophysiological examination showed abnormalities. He carried the variant m.1661A>G and showed a slight impairment of the sensory fibers of the peripheral nerves in his four limbs, particularly in the lower limbs. The subtle nature of the changes, according to the EMG, may explain why the patient denied having any symptoms.
To identify the gene related to phenotype, we considered modes of inheritance other than mitochondrial transmission, but no candidate mutations that that segregate with the disease phenotype have been identified, through WGS of two affected members (IV:2 and III:1) and one unaffected member (IV:1). All descendants of male members with the CMT phenotype were unaffected, which led us to focus on mitochondrial genetics. Finally, the m.1661A>G variant in the mt-tRNAval gene was identified, which is homoplasmic in blood. The main function of mt-tRNAval is to bring charged valine into the ribosome, in order to translate the valine codon during mitochondrial protein synthesis. In addition, it is also a structural component of the human mitochondrial ribosome (13). The m.1661A>G mutation is in the stem region of the tRNA T-loop, which may interfere with mitochondrial function in any cell with high energy metabolism. This may explain the reason why mt-tRNAval mutations lead to CMT.
Our study indicated that the phenotypes of tRNAval-related CMT are complicated and diverse. The variable and overlapping clinical phenotypes between syndromes may explain the proband’s past clinical diagnosis. These Chinese family members were unable to receive proper medical care for many years due to the complexity of the diagnosis, and they underwent many genetic tests. Finally, they were diagnosed with CMT caused by the homoplasmic m.1661A>G variant in the mt-tRNAval gene via WGS sequencing. This variant was previously reported in a Venezuelan family (12). To the best of our knowledge, this study is the first to report this variant in a Chinese family. The study expands the phenotype diversity and furthers the understanding of CMT caused by mitochondrial gene mutations in different ethnic groups.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Sichuan Provincial People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LC: Investigation, Methodology, Validation, Writing – original draft. JeY: Investigation, Methodology, Validation, Writing – original draft. XZ: Data curation, Writing – original draft. XW: Data curation, Writing – original draft. ZC: Data curation, Writing – original draft. ST: Project administration, Resources, Supervision, Writing – review & editing. JnY: Project administration, Resources, Supervision, Writing – review & editing.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Sichuan Province Research Fund for Transfer of Scientific and Technological Achievements (No. 2022JDZH0029, to JnY), the Special Fund for Clinical Research and Translational Medicine from Chinese Academy of Medical Sciences (No. 2022-I2M-C&T-B-117, to JnY).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1337065/full#supplementary-material
References
- 1.Laurá M, Pipis M, Rossor A, Reilly M. Charcot–Marie–Tooth disease and related disorders: an evolving landscape. Curr Opin Neurol. (2019) 32:641–50. doi: 10.1097/wco.0000000000000735, PMID: [DOI] [PubMed] [Google Scholar]
- 2.Masingue M, Fernández-Eulate G, Debs R, Tard C, Labeyrie C, Leonard-Louis S, et al. Strategy for genetic analysis in hereditary neuropathy. Rev Neurol. (2023) 179:10–29. doi: 10.1016/j.neurol.2022.11.007, PMID: [DOI] [PubMed] [Google Scholar]
- 3.Jani-Acsadi A, Ounpuu S, Pierz K, Acsadi G. Pediatric Charcot–Marie–Tooth disease. Pediatr Clin N Am. (2015) 62:767–86. doi: 10.1016/j.pcl.2015.03.012 [DOI] [PubMed] [Google Scholar]
- 4.Pareyson D, Scaioli V, Laurà M. Clinical and electrophysiological aspects of Charcot–Marie–Tooth disease. NeuroMolecular Med. (2006) 8:3–22. doi: 10.1385/nmm:8:1-2:3, PMID: [DOI] [PubMed] [Google Scholar]
- 5.McCray B, Scherer S. Axonal Charcot–Marie–Tooth disease: from common pathogenic mechanisms to emerging treatment opportunities. Neurotherapeutics. (2021) 18:2269–85. doi: 10.1007/s13311-021-01099-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murakami T, Sunada Y. Schwann cell and the pathogenesis of Charcot–Marie–Tooth disease. Adv Exp Med Biol. (2019) 1190:301–21. doi: 10.1007/978-981-32-9636-7_19 [DOI] [PubMed] [Google Scholar]
- 7.Jung N, Kwon H, Nam D, Tamanna N, Lee AJ, Kim SB, et al. Peripheral myelin protein 22 gene mutations in Charcot–Marie–Tooth disease type 1E patients. Genes. (2022) 13:1219. doi: 10.3390/genes13071219, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okamoto Y, Takashima H. The current state of Charcot–Marie–Tooth disease treatment. Genes. (2023) 14:1391. doi: 10.3390/genes14071391, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavalcanti E, Leal R, Marques Junior W, Nascimento O. Charcot–Marie–Tooth disease: from historical landmarks in Brazil to current care perspectives. Arq Neuropsiquiatr. (2023) 81:913–21. doi: 10.1055/s-0043-1770348, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pareyson D, Saveri P, Sagnelli A, Piscosquito G. Mitochondrial dynamics and inherited peripheral nerve diseases. Neurosci Lett. (2015) 596:66–77. doi: 10.1016/j.neulet.2015.04.001, PMID: [DOI] [PubMed] [Google Scholar]
- 11.Zhang H, Zhou Z, Sun L. Aminoacyl-tRNA synthetases in Charcot–Marie–Tooth disease: a gain or a loss? J Neurochem. (2021) 157:351–69. doi: 10.1111/jnc.15249, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fay A, Garcia Y, Margeta M, Maharjan S, Jürgensen C, Briceño J, et al. A mitochondrial tRNA mutation causes axonal CMT in a large Venezuelan family. Ann Neurol. (2020) 88:830–42. doi: 10.1002/ana.25854, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amunts A, Brown A, Toots J, Scheres S, Ramakrishnan VJS. Ribosome. The structure of the human mitochondrial ribosome. Science. (2015) 348:95–8. doi: 10.1126/science.aaa1193, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.