Abstract
INTRODUCTION
We aimed to evaluate informal caregivers’ attitudes toward undergoing and future implementation of blood‐based biomarkers (BBBM) testing for Alzheimer's disease (AD).
METHODS
We explored caregivers’ perspectives, by combining an online survey (n = 107) with a subsequent focus group (n = 7). We used descriptive statistics and thematic content analysis to identify common themes in answers to open‐ended survey questions and focus group data.
RESULTS
Most caregivers (72.0%) favored BBBM for AD diagnosis. Provided with hypothetical scenarios, confidence in a normal result decreased significantly if experienced symptoms were more severe (mild: 78.5% vs. severe: 48.6%). Caregivers’ attitudes toward BBBM for screening purposes significantly improved with prospect of treatment (53.3% vs. 92.5%). Concerns toward BBBM testing included treatment unavailability, increased/prolonged distress, and AD‐related stigma. Potential benefits were actionability, explanation for symptoms, and opportunities for better care and future treatment.
DISCUSSION
Emerging AD treatment and reduction of AD‐related stigma could profoundly increase public interest in BBBM testing for AD.
Highlights
Most informal caregivers would want blood‐based biomarker (BBBM) testing for Alzheimer's disease (AD) diagnosis.
Perceived (dis)advantages were related to diagnosing AD early.
With severe symptoms, there was less confidence in normal BBBM results.
Treatment availability would significantly increase interest in BBBM testing for AD.
Informal caregivers showed uncertainty regarding the meaning of the term “AD.”
Keywords: Alzheimer's disease, blood‐based biomarkers, caregivers, dementia, public patient involvement, public perspective
1. BACKGROUND
Alzheimer's disease (AD) is the most common cause of dementia posing a high personal, social, and economic burden. 1 , 2 This burden is thought to further increase, as the number of people with dementia is expected to triple by 2050. 3 To allow for optimal patient care, a timely diagnosis is needed. 4 It previously has been shown that intervention at early disease stages is crucial. 5 Recently, the first medical treatments targeting the underlying pathophysiology of AD have been approved by the Food and Drug Administration (FDA) in the United States. 6 , 7 Future personalized prevention approaches for AD could involve tailored combinations of disease‐modifying and lifestyle interventions. 8 The possible availability of such personalized interventions further reinforces the importance of a timely and accurate AD diagnosis.
In recent years, research in low‐invasive fluid biomarkers has accelerated, indicating the utility of blood‐based biomarkers (BBBM) for detection of AD pathology. 4 , 9 , 10 , 11 With further advancements and validation, BBBM could become part of the diagnostic work‐up for AD. The cost‐effective and low‐invasive nature of BBBM would allow for implementation in the primary care setting. 8 , 12 This could enable more refined detection of those with indicative signs of AD pathology and those patients whose symptoms do not stem from AD, resulting in fewer specialist referrals and thereby reducing wait times for consultations as well as mitigating the current diagnostic delay. 13 In addition to using BBBM testing in the diagnostic work‐up for AD, a long‐term goal could be to implement BBBM testing as a routine screening tool for the general public before onset of symptoms, although this is not yet envisioned in the near future. 14
To smooth the implementation of novel diagnostic tests like BBBM testing in practice, it is particularly important to know how such tests are being perceived and which hurdles need to be overcome for acceptance by relevant stakeholders. In the AD context, previous research has examined the perspectives of patients, (informal) caregivers, and cognitively unimpaired research participants regarding early disclosure of amyloid positron emission tomography (PET) imaging results, indicating increased risk of AD dementia. These studies have shown that individuals are often interested in knowing their risk, value getting information about disease etiology, and that disclosure of such information is safe in terms of short‐term psychological impact. 15 , 16 , 17 , 18
However, scarcely any data are available on stakeholder perspectives regarding BBBM testing for AD 19 , 20 , 21 , 22 and recent advancements in AD‐drug developments also raise the question how the public perspective is influenced by the availability of disease‐modifying treatment. In this study, we aimed to explore the perspectives of informal caregivers toward BBBM testing in the context of the diagnostic work‐up for AD and for the purpose of screening among the general public in the distant future.
2. METHODS
2.1. Study design and ethics
We conducted a mixed‐methods study to explore the attitudes of informal caregivers toward BBBM tests and their possible implementation in practice, combining an online survey with a focus group.
Participant recruitment was initiated through (a panel of) the Dutch patient organization “Alzheimer Nederland” (AN; www.alzheimer‐nederland.nl) and all participants provided written informed consent.
2.2. Sample and recruitment
The AN panel consists of 1579 registered individuals with an interest in dementia research and willingness to participate in scientific research. We randomly selected and invited 500 informal caregivers (those with lived experience caring for a loved one with dementia), registered in this panel, via e‐mail to participate in an online survey. A total of 111 informal caregivers completed the survey, of which four participants did not give consent for their data to be used for scientific purposes, resulting in the current study sample of 107 informal caregivers. At the end of the survey, 25 participants indicated their interest for participating in a focus group. We included seven participants based on their availability. Demographic data were available through the AN panel database.
2.3. Survey
We developed a survey (Supplement A in supporting information) consisting of 12 questions, structured into four subsections: (1) personal situation and experience with AD, (2) knowledge about BBBM, (3) openness toward and confidence in BBBM tests as part of the diagnostic work‐up for AD, and (4) BBBM tests as a screening tool among the general public. Information sections were inserted before Subsections 2.1, 3, and 4 to ensure basic knowledge of the participants about AD and BBBM tests. To assess confidence in normal BBBM test results, participants were provided with hypothetical scenarios and asked to imagine experiencing mild (Scenario 1) or severe (Scenario 2) memory complaints and to report their confidence in the BBBM test result if it came back normal in both scenarios.
2.4. Focus group
To aid interpretation of findings from the online survey, a focus group was organized at the AN head office. The duration of the focus group was set to 90 minutes. Group discussions were facilitated by authors Hester Blok and Rob B. M. Groot Zwaaftink, and background information was presented by author Katharina Bolsewig. The focus group guide, developed based on preliminary survey results, included five discussion topics that we introduced by means of open‐ended questions: participants’ opinions on the necessity to change the current diagnostic process, (dis)advantages of a timely diagnosis through BBBM testing, openness toward BBBM testing with severe symptoms, factors contributing to or hindering confidence in BBBM test results, and perspectives on BBBM testing as a screening tool.
2.5. Data analysis
Statistical analyses were performed using R version 4.0.3 23 with a significance level of α = 0.05. Descriptive statistics were used to report participants’ characteristics and categorical group differences were determined by a chi‐square test. The audio recording of the focus group session was transcribed manually by authors Katharina Bolsewig and Rob B. M. Groot Zwaaftink. All qualitative data were analyzed by two authors using content analysis: after familiarization with the data, authors Katharina Bolsewig and Hester Blok categorized answers to open‐ended survey questions and Katharina Bolsewig and Leonie N. C. Visser categorized focus‐group data, generating initial codes emerging from the data, using MaxQDA software. Coding differences between authors were discussed until consensus was reached. Next, Katharina Bolsewig and Leonie N. C. Visser reviewed all codes to find patterns and generate overarching themes. Katharina Bolsewig then drafted a first version of the result section that included these themes, and illustrative quotes. The result section was finalized in a process of reviewing and revising against the initial codes, a process in which Katharina Bolsewig, Hester Blok, and Leonie N. C. Visser were involved.
RESEARCH IN CONTEXT
Systematic review: Traditional sources (e.g. PubMed) were used to review literature on attitudes of the general public toward timely Alzheimer's disease (AD) diagnosis, through (blood‐based) biomarkers (BBBM). While studies with this specific focus are scarce, many studies have analyzed perspectives toward timely AD diagnosis by using amyloid positron emission tomography (PET) imaging. These studies have been cited in relevant sections.
Interpretation: Our findings indicate that the attitudes of informal caregivers toward BBBM testing for AD are mainly motivated by getting clarity about symptoms and actionability. Concerns regarding AD‐related stigma and negative feelings are in line with previous research on the (perceived) impact of sharing amyloid PET scan results with individuals without dementia. This underlines that perceived advantages and worries are more related to diagnosing AD in early stages, rather than specifically to BBBM testing as a novel diagnostic procedure.
Future directions: Identified concerns toward timely diagnosis should be addressed in the general public to increase acceptance of BBBM for AD.
3. RESULTS
3.1. Participant characteristics
Survey participants (n = 107) were on average 64.3 (standard deviation [SD] = 11.4) years old and 69.2% (n = 74) were female (Table 1). Most of the participants (have) had a parent (n = 56, 52.3%) and/or a partner (n = 44, 41.1%) with dementia. Seven of the participants (6.5%) were a parent, a sibling, other family member, friend, acquaintance, or colleague of someone with dementia, and one participant did not specify the relation. Most of the participants (n = 67, 62.6%) indicated they were not experiencing any memory complaints/symptoms themselves, while 12 participants (11.2%) reported they were experiencing memory complaints and 28 participants (26.2%) were unsure. A total of 32 (29.9%) participants were worried about their own brain health, but no effects on survey results could be observed (P > .07).
TABLE 1.
Demographics.
| Characteristics | Survey participants (n = 107) | Focus group participants (n = 7) |
|---|---|---|
| Age in years (mean [SD]) | 64.3 (11.4) * | 58.0 (9.9) |
| Sex (n [%]) | ||
| Female | 74 (69.2) | 5 (71.4%) |
| Male | 32 (29.9) | 2 (28.6%) |
| Other | 1 (0.9) | 0 (0.0%) |
| Relation with dementia (n [%]) | ||
| Child of someone with dementia | 56 a (52.3) | 6 (85.7%) |
| Partner of someone with dementia | 44 a (41.1) | 1 (14.3%) |
| Other family member of someone with dementia | 1 (0.9) | 0 (0.0%) |
| Parent of someone with dementia | 1 (0.9) | 0 (0.0%) |
| Friend, acquaintance, colleague of someone with dementia | 3 (2.7) | 0 (0.0%) |
| Sibling of someone with dementia | 2 (1.8) | 0 (0.0%) |
| Not specified | 1 (0.9) | 0 (0.0%) |
Abbreviation: SD, standard deviation.
n = 99.
One participant was the partner and child of someone with dementia.
Focus group participants (n = 7) were on average 58.0 (SD = 9.9) years old and 71.4% (n = 5) were female. Six (85.7%) of the focus group participants were a child and one participant (14.3%) was a partner of someone with dementia.
3.2. Previous experience with the current, non–blood‐based diagnostic process
Many participants evaluated their experience with the diagnostic work‐up for AD as “neutral” concerning the overall experience (n = 48, 44.9%), organization (n = 45, 42.1%), duration (n = 62, 57.9%), and amount of information received (n = 63, 58.9%). However, a considerable proportion of participants was more negative, evaluating the overall experience as unpleasant (n = 42, 39.3%), the duration as too long (n = 28, 26.2%), and the amount of received information as too little (n = 38, 35.5%). Many participants also reported the process as stressful (n = 57, 53.3%) and the diagnostic tests as emotionally burdensome (n = 59, 55.1%), unpleasant (n = 25, 23.4%), or stressful (n = 38, 35.5%). Although most evaluated the provided information as understandable (n = 58, 54.2%) or neutral (n = 35, 32.7%), some indicated the information was too complicated (n = 14, 13.1%).
Focus group participants expressed a wish for a shorter, more empathetic diagnostic process, with sensitive tests that could detect the disease at early stages. Some added that BBBM tests could help achieve these wishes and could lower the burden of testing on individuals (Quotes 1–3, Table 2).
TABLE 2.
Informal caregivers’ attitudes toward and experiences with current diagnostic process for Alzheimer's disease.
| Quote 1 (focus group) | “[…] How can it [the diagnostic process] be done in a more empathetic way?” |
| Quote 2 (focus group) | “I think the tests were being performed too late. […] I think only very late [in the disease process] people cannot draw a clock anymore. I think the neuropsychological tests really come in too late. […] For you to fail such a test, you're already so far in [the disease process].” |
| Quote 3 (focus group) | “But with such a scan you simply go in, you get pictures and that is actually giving you a hard outcome. I would find that more accessible than [answering] hundreds of questions about how many brothers and sisters you have first and drawing a clock. […] Availability of a blood test could be even more accessible. In my opinion you would burden the person less that way” |
3.3. Awareness and attitudes toward BBBM tests in diagnostic work‐up for AD
Most participants were not aware of ongoing efforts to develop and implement BBBM for AD (n = 74, 69.2%), 26 participants (24.3%) had heard about BBBM tests for AD, and 7 participants (6.5%) were unsure. Presented with only Scenario 1 (mild memory complaints), most participants (n = 77, 72.0%) were in favor of getting a blood test done. Of these participants, 71 (92.2%) indicated wanting to know as early as possible if their symptoms are indicative of AD. Six participants (7.8%) stated various reasons (see Section 3.5). A total of 13 participants (12.1%) would not want a BBBM test, of which 10 participants (76.9%) would not want to know if they have AD when only experiencing mild complaints. Three participants (23.1%) stated various reasons for not wanting a BBBM test (see Section 3.5). A total of 17 participants (15.9%) were unsure if they would want to get a BBBM test done in this scenario.
Participants’ openness toward BBBM testing upon experiencing severe memory complaints (Scenario 2) was discussed only in the focus group. Participants expressed different opinions: on the one hand, participants mentioned arguments favoring BBBM testing, such as the possibility to rule out or confirm AD as the underlying cause of their symptoms, to allow access to and better arrangement of care, and the need for a definite diagnosis for legal/financial purposes. On the other hand, one participant vocalized perceiving BBBM testing at this stage as too late, and therefore as unnecessary. This participant focused on acceptance of the experienced symptoms and did not want to put extra burden on the health‐care system through more testing.
In the survey, we additionally assessed the level of confidence that participants would have in normal BBBM test results, in response to both scenarios. In Scenario 1, most participants would feel reassured by a normal result of the blood test (n = 84, 78.5%), while 6 participants (5.6%) would still feel doubtful and 17 participants (15.9%) were unsure whether they would trust the test result. In Scenario 2, the number of participants feeling reassured by the test result significantly decreased to 52 (48.6%, P < .001) and the number of participants still feeling doubtful significantly increased to 29 (27.1%, P < .001), while the number of participants being unsure about whether they trust the test did not change significantly (n = 26, 24.3%, Figure 1).
FIGURE 1.
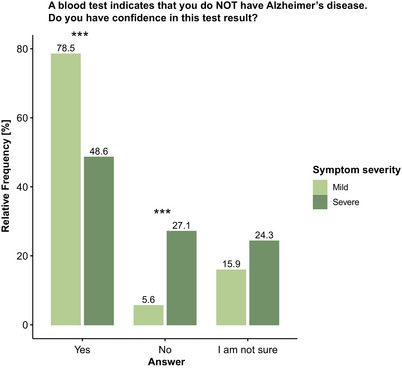
Influence of experienced symptom severity on confidence in normal blood‐based biomarkers (BBBM) test results. Confidence in a normal BBBM test result significantly decreased with increased severity of experienced complaints/symptoms. Group differences were assessed with a chi‐square test. *** P < .001
3.4. Attitudes toward BBBM tests for AD for screening purposes
More than half of participants (n = 67, 62.6%) would want to get screened for AD in the absence of any symptoms using BBBM. A total of 21 (19.7%) participants would not be interested and 19 participants (17.8%) were unsure about their willingness to participate in BBBM screening for AD.
Without defined context, most participants (n = 60, 56.1%) would be open to regular AD screening (e.g., each year), while 16 participants would not consider getting screened regularly. Of these participants, 1 (0.9%) stated that blood collection is too invasive, 13 (12.1%) considered it unnecessary, and 2 (1.9%) found it too time consuming. Twenty‐one participants (19.6%) were unsure if they would want to participate in regular AD screening and 10 participants (9.3%) chose the answer option “other,” subsequently indicating their motivations (Section 3.5).
When defining the context for screening, specifying no treatment availability for AD, 57 participants (53.3%) would consider getting screened, while 21 participants (19.6%) would not consider AD screening, and 29 participants (27.1%) were unsure. Specifying that a treatment option would be available, the number of participants willing to get screened for AD in the future significantly increased to 99 (92.5%, P < .001), and the number of participants not wanting to get screened or being unsure significantly decreased to 1 (0.9%, P < .001) and 7 (6.5%, P < .001), respectively (Figure 2).
FIGURE 2.
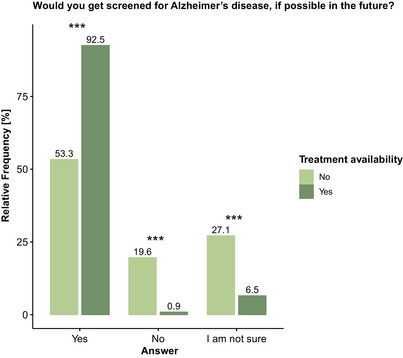
Influence of treatment availability on openness toward blood‐based biomarkers (BBBM)–based screening for Alzheimer's disease (AD). Openness toward BBBM screening significantly increased with the availability of treatment for AD. Percentages may not total 100 due to rounding. Group differences were assessed with a chi‐square test. *** P < .001
3.5. Advantages, disadvantages, and additional considerations of BBBM testing
In answers to the open‐ended survey questions and focus group discussion, some rationales shaping the participants’ attitudes toward BBBM testing were mentioned repeatedly. Notably, participants reported similar rationales/reasons for (not) wanting to undergo BBBM testing in the context of diagnostic work‐up for AD/dementia and population‐based screening, and seemed to focus most on (dis)advantages of early AD diagnosis, independent of the context of testing.
Key rationales favoring timely BBBM testing for AD were the possibility to take action in time (Table 3, Quote 1), referring to both administrative aspects (e.g., preparing a will) as well as enjoying life to the fullest (e.g., working off one's bucket list), getting clarity by understanding the cause of experienced symptoms, future possibility of treatment options, and contributing to scientific research. Participants also considered the presence of AD risk factors, such as comorbidities or a family history of AD, as a reason to get tested for AD.
TABLE 3.
Informal caregivers’ perceived (dis)advantages and additional considerations regarding blood‐based biomarkers (BBBM) testing for Alzheimer's disease (AD).
| Quote 1 (survey) | “So I can take measures into my own hands and make the right decisions on time.” |
| Quote 2 (survey) | “I would not like to know already now that I am developing young‐onset dementia […], because there is no treatment yet.” |
| Quote 3 (focus group) | “As long as there is no cure, this [population‐based screening for AD] is a nightmare for society! So much guidance and support would be needed. If you would offer [BBBM testing] to everyone at 55 years or 60 years of age, then ten thousands of people will have this protein and get a death sentence. How are you going to solve that for so many people?” |
| Quote 4 (focus group) | “The danger is that if you get sick, getting sick is becomes your own fault.” |
The most important rationales against timely BBBM testing for AD were treatment unavailability (Table 3, Quote 2), AD‐related stigma possibly leading to early incapacitation, and (a prolonged time period in which someone might experience) negative feelings of helplessness, fear, and insecurity. Participants who were against BBBM testing or in doubt, stated that they would want to enjoy life without worries and that they would not see an added value of getting an early diagnosis without treatment availability.
Some considerations were specifically mentioned in the context of population‐based screening. These included a generally positive view on population‐based screening, which could allow for taking preventive measures (e.g., adopting a healthier diet) to delay symptom onset or decrease the risk of developing dementia. Mentioned disadvantages were that (health‐care) resources used for testing could be used in a different/better way and that the health‐care system is not yet equipped to manage and provide support to so many people, years prior to experiencing any symptoms (Table 3, Quote 3). Some participants believed AD screening should only be offered under certain circumstances, for example, based on treatment availability or increased risk factors (e.g., family history of AD or being of older age [> 60 years]). One participant believed screening should be offered to everyone and start as early as pathological processes could take place, that is, ≈ 20 years before the average symptom onset. Other considerations regarded testing frequency (e.g., annually or bi‐annually), and clarification about who would bear the costs. Participants further mentioned addressing the uncertainty surrounding the test result, referring to meaning and certainty of an abnormal test result. Ethical considerations were also addressed, for example, the consequences of an abnormal test result and concerns about blaming individuals for developing the disease if they fail to sufficiently reduce AD‐related risk factors, for example, through lifestyle changes (Table 3, Quote 4).
3.6. Confusion regarding the definition of AD
Although all participants had prior experience with AD and/or dementia, we observed certain confusion regarding the definition of AD. Participants were not sure what the current definition of AD entails and as a result, what an abnormal BBBM test result would mean, and which legal consequences (in regard to, for example, incapacitation or withdrawal of driving license) it could have (Quotes 1 and 2, Table 4).
TABLE 4.
Informal caregivers’ confusion regarding definition of Alzheimer's disease.
| Quote 1 (focus group) | “Alzheimer's, is there actually a clear definition? Because to me it is not very clear.” |
| Quote 2 (focus group) | “If you have mild complaints and you did not do the testing, then that [making a will/arranging legal things] might still be possible. But when you have done blood testing and get the label Alzheimer or that you have dementia, you can probably forget about it!” |
| Quote 3 (focus group) | “Maybe we need to detach it [the ‐result of‐ screening by means of BBBM testing] from the term Alzheimer's.” |
| Quote 4 (focus group) | “The disease is there. What can we do to get another image of the disease, so that you do not feel like: I got this diagnosis and now it's all over?” |
Abbreviation: BBBM, blood‐based biomarker.
Focus group participants suggested a different picture of the disease needs to be created to avoid stigma and the detrimental feeling that your life is ending upon receiving an abnormal BBBM test result. Further, some participants suggested that, to avoid negative (legal/social) consequences in early/presymptomatic stages, BBBM testing should be detached from the terminology “Alzheimer's” and “diagnosis” (Quotes 3 and 4, Table 4).
4. DISCUSSION
In this mixed‐methods study we have explored the perspectives of (former) informal caregivers of people with (AD) dementia toward a timely AD diagnosis by means of BBBM testing. Most participants had a positive attitude toward BBBM testing (72.0%), which would further increase with access to disease‐modifying treatment, also in the context of population‐based screening (53.3% without medication vs. 92.5% with prospect of medication). Confidence in normal BBBM test results depended on the severity of experienced memory complaints. Rationales shaping participants’ attitudes toward BBBM testing were directed toward an early diagnosis in general.
While earlier studies have focused on views of patients and caregivers regarding the disclosure of PET imaging results, mostly in research settings, 18 , 24 and interest in medical (screening) tests for early AD diagnosis without a likely availability of such tests in the near future, 25 , 26 attitudes toward novel BBBM for AD have only scarcely been assessed. 19 For a smooth implementation, it is relevant to understand the perception of these novel tests by relevant stakeholders. Previously, the perception of BBBM testing for AD was analyzed in Americans in a population‐based cohort, 19 in which no previous experience with AD or the diagnostic process thereof was necessary for participation. In contrast, we included Dutch informal caregivers, with lived experiences with the disease and the current diagnostic work‐up for AD/dementia. This population is aware of the impact receiving the diagnosis without the availability of disease‐modifying treatment had on their loved ones, themselves, and various aspects of their life (e.g., legal, financial, emotional). These insights allowed for a more informed perspective toward the use of BBBM tests. Additionally, participants were not only characterized by their lived experience with AD/dementia, and could therefore become a target population for BBBM testing in the future, these cognitively unimpaired individuals were also Dutch citizens. Our findings thus extend the existing literature by focus on novel BBBM tests and choosing a specific study population with previous experience with AD/dementia.
A considerable proportion of participants evaluated the current diagnostic process negatively, concerning its duration, emotional impact, and the current diagnostic tests detecting the disease at too advanced stages. From the perspective of these informal caregivers, low‐cost tests that detect the disease at early stages, like BBBM testing, would be beneficial to reduce the duration and burden of this process. Participants also mentioned that objective measures, such as BBBM, would be perceived more accessible and acceptable than extensive neuropsychological testing. However, the currently used diagnostic tests are not going to be replaced by BBBM testing, but BBBM tests will rather be used to support and refine the diagnosis and possibly aid in an earlier detection of the disease (i.e., before onset of symptoms). Still, the addition of BBBM tests to, for example, neuropsychological tests, could increase confidence in the diagnosis due to a more intuitive interpretation for patients and family members. We found that the confidence in normal BBBM test results was significantly lower in a hypothetical scenario with more severe experienced symptoms compared to mild symptoms. This indicates that BBBM testing in early disease stages would be most optimal, but could also imply that memory complaints are commonly attributed to AD/dementia and other causes are less known. The latter would emphasize the need for education and counseling in terms of other potential causes, and potential follow‐up exams of normal BBBM test results.
While advancements in BBBM research are very promising, suggesting their implementation in memory clinic settings in the near future, there are still several challenges that need to be overcome. Among others, the best (combination of) BBBM and platform to measure them need to be established and validated in real‐life populations, potential confounders need to be identified, and tools for the interpretation of BBBM test results need to be developed. 27 The majority of our study participants were not familiar with BBBM, but most of them would be interested in BBBM testing for a timely diagnosis. Interestingly, mentioned rationales were seemingly directed toward an early diagnosis of AD in general rather than the method of testing or the novelty of BBBM tests for AD being a point of concern. This notion is supported by similar motivations identified in studies on disclosure of PET imaging results and early AD diagnosis. 28 , 29 , 30 These findings highlight that BBBM testing as a tool for a timely diagnosis could become more widely accepted if general concerns toward a timely diagnosis are addressed.
(Un)availability of disease‐modifying treatment particularly affected the openness toward BBBM population‐wide screening, being significantly higher with the prospect of disease‐modifying treatment compared to a situation without such availability. Participants mentioned concerns regarding the considerable emotional burden that could be caused by a pre‐symptomatic diagnosis without access to disease‐modifying treatment for a large proportion of the general population, and the burden that it would put upon the health‐care system. Previous research has shown that early disclosure of increased AD risk did not increase risk for depression and anxiety. 15 , 31 Nevertheless, it will be important to prepare the health‐care system and care facilities for AD‐specific care approaches to allow for optimal patient care and support, if BBBM testing is implemented. 8 Despite their experience with the disease, we noticed certain uncertainty and confusion concerning the understanding of the term “AD” within our study population, which was shown before in the general public. 32 This confusion might stem from the rather recent switch from a clinical–biological to a purely biological definition of AD, 2 , 33 which is highly relevant for treatments targeting AD pathology specifically. Even though disease‐modifying treatments have not yet become available in Europe, 34 , 35 our findings highlight that to increase receptiveness of novel diagnostic tests and timely diagnosis of AD, more awareness should be brought to the general public about developments in AD definition and terminology.
Furthermore, AD‐related stigma in society and fear of accompanying incapacitation were among the most commonly mentioned factors for a negative view on early AD diagnosis, thereby negatively affecting acceptance of BBBM testing. A similar trend has been observed in previous studies not only in patients experiencing public stigma and internalization thereof, but also showing in physicians’ reluctance toward diagnosis of AD at early stages. 36 , 37 , 38 To protect individuals from stigmatization, implementation of novel BBBM tests should address the ethical considerations and take into account possible consequences of finding out about the presence of AD pathology at early stages. As proposed previously, legal guidelines focusing on confidentiality and defining that a diagnosis of AD at early stages does not imply disability that could lead to incapacitation, should be considered. 39
A strength of this study is the inclusion of a large population of informal caregivers that have lived experiences with AD and are familiar with the current diagnostic work‐up for AD/dementia. Combining a survey with a subsequent focus group allowed us to get a deep, faceted understanding of caregivers’ perspectives regarding BBBM testing. While most studies have focused on the general population's attitude toward more invasive diagnostic tools, like cerebrospinal fluid testing or PET imagining, we here focus on attitudes toward low‐invasive, state‐of‐the‐art BBBM.
The specific study population is also a limitation to our study. First, it needs to be considered that informal caregivers’ attitudes might differ from those experiencing symptoms, as has been shown previously in the context of cancer patients and related caregivers. 40 It also has been shown before that interest in an early medical test for AD was higher in people with experience with the disease compared to people without such knowledge. 25 For a smooth implementation of BBBM in the clinic, perspectives of physicians should also be inventoried. Second, inclusion of a specific Dutch population and the nature of the selected study population with lived experiences with AD and dementia could limit the generalizability of our findings. It has been shown that willingness to undergo BBBM testing for AD varies between different population groups 19 and other factors (e.g., diagnostic process, availability of care services, perceived stigma) can vary among countries, illustrating the need for replication in different (international) study populations.
5. CONCLUSION
This study gives important insights into what hampers or contributes to acceptance of BBBM testing as part of the diagnostic work‐up and population‐based screening for AD. Participants were mostly positive toward BBBM testing and stated rationales in agreement with earlier findings: actionability, clarity about experienced symptoms, treatment availability, and AD‐related stigma. Our findings highlight how the availability of disease‐modifying treatment would increase openness toward timely diagnosis by means of BBBM and could potentially help to reduce stigmatization. Meanwhile, we should increase public knowledge and awareness about AD, dementia, and BBBM testing, and prepare society and health‐care systems for a future with personalized AD treatment.
CONFLICT OF INTEREST STATEMENT
Leonie Visser (LNCV) has been an invited speaker for the Schwabe Group and her research has been funded by ZonMW, Health Holland, the Amsterdam Public Health research institute, Alzheimer Nederland, and private partners, including Eisai. All fees and funding are paid to her institution. Author disclosures are available in the supporting information.
CONSENT STATEMENT
All participants provided written informed consent for their data to be used for scientific research.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
This project received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska‐Curie grant agreement No 860197, “MIRIADE.” EMAS, CET, and LNCV are recipients of ABOARD, which is a public–private partnership (PPP) receiving funding from ZonMW (#73305095007) and Health∼Holland, Topsector Life Sciences & Health (PPP allowance; #LSHM20106). More than 30 partners participate in ABOARD. ABOARD also receives funding from Edwin Bouw Fonds and Gieskes‐Strijbisfonds.
Bolsewig K, Blok H, Willemse EAJ, et al. Caregivers’ attitudes toward blood‐based biomarker testing for Alzheimer's disease. Alzheimer's Dement. 2024;16:e12549. 10.1002/dad2.12549
REFERENCES
- 1. 2020 Alzheimer's disease facts and figures. Alzheimers Dement. 2020;16: 391‐46. [DOI] [PubMed] [Google Scholar]
- 2. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Collaborators GBDDF. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7:e105‐e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mattke S, Cho SK, Bittner T, Hlavka J, Hanson M. Blood‐based biomarkers for Alzheimer's pathology and the diagnostic process for a disease‐modifying treatment: projecting the impact on the cost and wait times. Alzheimers Dement. 2020;12:e12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fan DY, Wang YJ. Early intervention in Alzheimer's disease: how early is early enough? Neurosci Bull. 2020;36:195‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dhillon S. Aducanumab: first approval. Drugs. 2021;81:1437‐1443. [DOI] [PubMed] [Google Scholar]
- 7. Hoy SM. Lecanemab: first Approval. Drugs. 2023;83:359‐365. [DOI] [PubMed] [Google Scholar]
- 8. van der Flier WM, de Vugt ME, Smets EMA, Blom M, Teunissen CE. Towards a future where Alzheimer's disease pathology is stopped before the onset of dementia. Nat Aging. 2023;3:494‐505. [DOI] [PubMed] [Google Scholar]
- 9. Palmqvist S, Janelidze S, Stomrud E, et al. Performance of fully automated plasma assays as screening tests for Alzheimer disease‐related beta‐amyloid status. JAMA Neurol. 2019;76:1060‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hansson O. Biomarkers for neurodegenerative diseases. Nat Med. 2021;27:954‐963. [DOI] [PubMed] [Google Scholar]
- 11. Hansson O, Blennow K, Zetterberg H, Dage J. Blood biomarkers for Alzheimer's disease in clinical practice and trials. Nat Aging. 2023;3:506‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sabbagh MN, Boada M, Borson S, et al. Early detection of mild cognitive impairment (MCI) in primary care. J Prev Alzheimers Dis. 2020;7:165‐170. [DOI] [PubMed] [Google Scholar]
- 13. Europe A. European Carers’ Report 2018: carers’ experiences of diagnosis in five European countries. Alzheimer Europe; 2018. [Google Scholar]
- 14. Teunissen CE, Verberk IMW, Thijssen EH, et al. Blood‐based biomarkers for Alzheimer's disease: towards clinical implementation. Lancet Neurol. 2022;21:66‐77. [DOI] [PubMed] [Google Scholar]
- 15. Grill JD, Raman R, Ernstrom K, et al. Short‐term psychological outcomes of disclosing amyloid imaging results to research participants who do not have cognitive impairment. JAMA Neurol. 2020;77:1504‐1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Largent EA, Harkins K, van Dyck CH, Hachey S, Sankar P, Karlawish J. Cognitively unimpaired adults' reactions to disclosure of amyloid PET scan results. PLoS One. 2020;15:e0229137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lim YY, Maruff P, Getter C, Snyder PJ. Disclosure of positron emission tomography amyloid imaging results: a preliminary study of safety and tolerability. Alzheimers Dement. 2016;12:454‐458. [DOI] [PubMed] [Google Scholar]
- 18. Vanderschaeghe G, Vandenberghe R, Dierickx K. Stakeholders' views on early diagnosis for Alzheimer's disease, clinical trial participation and amyloid PET disclosure: a focus group study. J Bioeth Inq. 2019;16:45‐59. [DOI] [PubMed] [Google Scholar]
- 19. Li M, Li Y, Schindler SE, et al. Design and feasibility of an Alzheimer's disease blood test study in a diverse community‐based population. Alzheimers Dement. 2023;19(12):5387‐5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van der Schaar J, Visser LNC, Ket JCF, et al. Impact of sharing Alzheimer's disease biomarkers with individuals without dementia: a systematic review and meta‐analysis of empirical data. Alzheimers Dement. 2023. [DOI] [PubMed] [Google Scholar]
- 21. Lingler JH, Roberts JS, Kim H, et al. Amyloid positron emission tomography candidates may focus more on benefits than risks of results disclosure. Alzheimers Dement (Amst). 2018;10:413‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arias JJ, Cummings J, Grant AR, Ford PJ. Stakeholders' perspectives on preclinical testing for Alzheimer's disease. J Clin Ethics. 2015;26:297‐305. [PubMed] [Google Scholar]
- 23. R: A Language and Environment for Statistical Computing [computer program]: R Foundation for Statistical Computing; 2020. [Google Scholar]
- 24. Armstrong MJ, Gronseth GS, Day GS, Rheaume C, Alliance S, Mullins CD. Patient stakeholder versus physician preferences regarding amyloid PET testing. Alzheimer Dis Assoc Disord. 2019;33:246‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wikler EM, Blendon RJ, Benson JM. Would you want to know? Public attitudes on early diagnostic testing for Alzheimer's disease. Alzheimers Res Ther. 2013;5:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gooblar J, Roe CM, Selsor NJ, Gabel MJ, Morris JC. Attitudes of research participants and the general public regarding disclosure of Alzheimer disease research results. JAMA Neurol. 2015;72:1484‐1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hansson O, Edelmayer RM, Boxer AL, et al. The Alzheimer's association appropriate use recommendations for blood biomarkers in Alzheimer's disease. Alzheimers Dement. 2022;18:2669‐2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grill JD, Cox CG, Kremen S, et al. Patient and caregiver reactions to clinical amyloid imaging. Alzheimers Dement. 2017;13:924‐932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vanderschaeghe G, Schaeverbeke J, Vandenberghe R, Dierickx K. Amnestic MCI patients' perspectives toward disclosure of amyloid PET results in a research context. Neuroethics. 2017;10:281‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van der Schaar J, Visser LNC, Bouwman FH, et al. Considerations regarding a diagnosis of Alzheimer's disease before dementia: a systematic review. Alzheimers Res Ther. 2022;14:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wake T, Tabuchi H, Funaki K, et al. The psychological impact of disclosing amyloid status to Japanese elderly: a preliminary study on asymptomatic patients with subjective cognitive decline. Int Psychogeriatr. 2018;30:635‐639. [DOI] [PubMed] [Google Scholar]
- 32. Huisman M, Stijn J, Biltereyst D. The Alzheimer case: perceptions, knowledge and the acquisition of information about Alzheimer's disease by middle‐aged and older adults in flanders. Ageing & Soc. 2022;42:918‐937. [Google Scholar]
- 33. Jack CR Jr, Bennett DA, Blennow K, et al. NIA‐AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Walsh S, Merrick R, Richard E, Nurock S, Brayne C. Lecanemab for Alzheimer's disease. BMJ. 2022;379:o3010. [DOI] [PubMed] [Google Scholar]
- 35. Mahase E. Aducanumab: European agency rejects Alzheimer's drug over efficacy and safety concerns. BMJ. 2021;375:n3127. [DOI] [PubMed] [Google Scholar]
- 36. Gauthier S, Leuzy A, Racine E, Rosa‐Neto P. Diagnosis and management of Alzheimer's disease: past, present and future ethical issues. Prog Neurobiol. 2013;110:102‐113. [DOI] [PubMed] [Google Scholar]
- 37. Vernooij‐Dassen MJ, Moniz‐Cook ED, Woods RT, et al. Factors affecting timely recognition and diagnosis of dementia across Europe: from awareness to stigma. Int J Geriatr Psychiatry. 2005;20:377‐386. [DOI] [PubMed] [Google Scholar]
- 38. Stites SD, Gill J, Largent EA, et al. The relative contributions of biomarkers, disease modifying treatment, and dementia severity to Alzheimer's stigma: a vignette‐based experiment. Soc Sci Med. 2022;292:114620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Karlawish J. Addressing the ethical, policy, and social challenges of preclinical Alzheimer disease. Neurology. 2011;77:1487‐1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van der Velden NCA, Smets EMA, Hagedoorn M, et al. Patient‐Caregiver Dyads' prognostic information preferences and perceptions in advanced cancer. J Pain Symptom Manage. 2023;65:442‐455 e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


