Abstract
Resistance profiles of the two Bordetella species B. bronchiseptica and B. pertussis against various antimicrobial peptides were determined in liquid survival and agar diffusion assays. B. bronchiseptica exhibited significantly higher resistance against all tested peptides than B. pertussis. The most powerful agents acting on B. bronchiseptica were, in the order of their killing efficiencies, cecropin P > cecropin B > magainin-II-amide > protamine > melittin. Interestingly, for B. bronchiseptica, the resistance level was significantly affected by phase variation, as a bvgS deletion derivative showed an increased sensitivity to these peptides. Tn5-induced protamine-sensitive B. bronchiseptica mutants, which were found to be very susceptible to most of the cationic peptides, were isolated. In two of these mutants, the genetic loci inactivated by transposon insertion were identified as containing genes highly homologous to the wlbA and wlbL genes of B. pertussis that are involved in the biosynthesis of lipopolysaccharide (LPS). In agreement with this finding, the two peptide-sensitive mutants revealed structural changes in the LPS, resulting in the loss of the O-specific side chains and the prevalence of the LPS core structure. This demonstrates that LPS plays a major role in the resistance of B. bronchiseptica against the action of antimicrobial peptides and suggests that B. pertussis is much more susceptible to these peptides due to the lack of the highly charged O-specific sugar side chains.
Bordetella pertussis and Bordetella bronchiseptica are highly related pathogens causing infections of the upper respiratory tract in humans and various mammalian species, respectively (8, 49, 50). These organisms produce a variety of virulence factors such as several adhesins, including the filamentous hemagglutinin, pertactin, and fimbriae, as well as the cytotoxic factor adenylate cyclase toxin (20, 29, 38, 48). The expression of these virulence factors is coordinately regulated by the BvgAS two-component system (6, 45, 48) in response to certain environmental stimuli (49, 50). Due to the genetic instability of the bvgAS locus, so-called phase variants, which do not produce virulence factors due to mutations in the bvg locus, arise with a strain-dependent frequency (20, 32). Phase variants are avirulent in animal models and cannot initiate colonization of the ciliated epithelium of the respiratory tract (21, 50).
Although very closely related, Bordetella species show several significant differences regarding their virulence properties. Whereas B. pertussis, the causative agent of whooping cough, is an obligate human pathogen (50), B. bronchiseptica has a broader host range and causes respiratory infections in several mammalian species but only occasionally in humans (52). Some virulence factors possibly involved in differences of the pathogenic potential of the two Bordetella species for different hosts have been identified. For example, only B. pertussis is able to produce a tracheal colonization factor (16) and the pertussis toxin which ADP-ribosylates GTP-binding proteins in the cell membrane of eukaryotic cells (20). There are also interesting variations in the lipopolysaccharide (LPS) structure between the two species, although the contribution of LPS to Bordetella virulence is not yet clear (12, 37). Both organisms also show remarkable differences in their interactions with eukaryotic cells. Bvg-activated factors are required for invasion of B. pertussis in epithelial cells (13, 26, 41), whereas these factors are not required by B. bronchiseptica (22, 40, 41), which in contrast to B. pertussis has a very significant intracellular survival potential in epithelial cells and macrophages (5, 18).
To unravel putative virulence-relevant features which differ for the two species, we analyzed their susceptibilities to various antimicrobial peptides. Cationic peptides may be encountered by these pathogens after engulfment by professional phagocytic cells or during colonization of the epithelium of the upper respiratory tract (14, 19, 33). Indeed, in mammalian airway epithelia, such peptides, including the human β-defensin 1 (hBD1) and the bovine tracheal antimicrobial peptide, have been recently identified (7, 11, 23, 31). The fact that the relatively high salt concentrations present in the lungs of cystic fibrosis patients cause the inactivation of the defensin hBD1 and thereby apparently contribute to the successful colonization of Pseudomonas aeruginosa indicates that such peptides also constitute an important part of the natural defense system in the upper airways (17). Cationic host defense peptides are very widespread in nature and are produced by organisms as different as insects (e.g., cecropins), frogs (e.g., magainin), and mammals (e.g., defensins). Since these peptides possess some similar features, such as their cationic properties, the ability to form amphipathic structures, their size, and possibly a similar mode of action, some commercially available peptides derived from insects and amphibia are frequently used as model substances to characterize the effect of cationic peptides on microorganisms. It is believed that they interact with anionic phospholipids of the target cell and destabilize the cytoplasmic membrane (30).
Preliminary reports indicated a certain degree of resistance of the two Bordetella species against antimicrobial peptides of various origins, but neither a direct comparison of the two species nor an attempt to characterize the molecular basis of resistance has been undertaken so far (15, 28, 42). Here we show that B. bronchiseptica is very resistant to various peptides of different origin, whereas B. pertussis exhibits a much higher sensitivity towards these agents. Furthermore, phase variants and transposon-induced LPS mutants of B. bronchiseptica are much more susceptible to these peptides than the wild-type strain. Possible implications of these results for the virulence of members of the genus Bordetella are discussed.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and media.
The strains and plasmids used are described in Table 1. The B. pertussis Tohama I wild type, its derivative BP347, which carries a Tn5 insertion in the bvgS gene, the B. bronchiseptica wild-type strain BB7865, and its bvg mutant derivative BB7866, which contains a 241-bp deletion in the bvgS gene, have already been described (41, 49). Escherichia coli K-12 DH5α was used as a control strain for various studies throughout this report. Bordetella strains were grown on Bordet-Gengou (BG) agar plates (Difco Inc.) (8) supplemented with 1% glycerol and 20% defibrinated horse blood (Oxoid Inc.), on charcoal agar plates (Difco Inc.), or in SS liquid medium (44).
TABLE 1.
Bacterial strains and plasmids used in this study
| Bacterial strains and plasmids | Description | Source and/or reference |
|---|---|---|
| B. bronchiseptica BB7865 | Wild type but Strr | I.R.I.S., Siena, Italy; Culture Collection, University of Göteborg; reference 41 |
| B. bronchiseptica BB-PS1 | Derivative of BB7865 but wlbA::Tn5 | This study |
| B. bronchiseptica BB-PS2 | Derivative of BB7865 containing an unmapped TnphoA insertion on the chromosome | This study |
| B. bronchiseptica BB-PS3 | Derivative of BB7865 but wlbL::Tn5 | This study |
| B. bronchiseptica BB7866 | As BB7865 but ΔbvgS | I.R.I.S., Siena, Italy; reference 41 |
| B. pertussis Tohama I | Wild type | 49 |
| B. pertussis BP347 | As Tohama I but bvgS::Tn5 | 49 |
| E. coli DH5α | High-efficiency transformation | GIBCO |
| pUC18 | Cloning vector | Pharmacia |
| pSS-TN | Derivative of pSS1129 carrying TnphoA | D. Beier |
Radial diffusion and liquid killing assay.
The radial diffusion assay was performed as described by Lehrer et al. (27) with some modifications as recently described (15). Briefly, bacteria grown on BG agar plates were harvested and resuspended in modified SS liquid medium to a final optical density at 600 nm of 0.2. Two-tenths milliliter of this suspension was added to 10 ml of melted 1% low-electroendosmosis agarose type I (Sigma, Deisenhofen, Germany) in SS medium containing supplements and 0.15% bovine serum albumin. The agarose was dispensed into a petri dish and allowed to solidify. Holes (diameter, 3 mm) were made with an aspirator punch (ICN Biomedicals), and 5 μg of the various peptides (Sigma) diluted in H2O (1 μg/μl) were placed therein. After incubation for 4 h at room temperature, the plates were overlaid with 10 ml of sterile SS agarose. After incubation at 37°C, the resulting inhibition zones were measured with a metric scale under a stereomicroscope.
The liquid killing assay was performed as follows. Serial dilutions of cationic peptides were prepared in phosphate-buffered saline, and 50 μl of each dilution was transferred to a 96-well microtiter plate (final peptide concentrations ranging from 20 μg/ml to 1 mg/ml). Bordetellae were grown to mid-log phase in SS liquid medium; E. coli DH5α was grown in SS liquid medium containing 0.5% glucose. The bacteria were then diluted in SS liquid medium, and 50 μl of the bacteria was added to each well in the microtiter plate to a final concentration of 5 × 104 CFU/ml. After 1 h of incubation at 37°C, 50 μl of each sample was diluted in 450 μl of SS liquid medium and the number of surviving bacteria was determined by plating 10-fold serial dilutions on BG or LB agar plates. All experiments were carried out three times in duplicate, and the Student t test was used to analyze the data for their statistical significance.
Transposon mutagenesis and screening for peptide-sensitive mutants.
For transposon mutagenesis, a derivative of the suicide vector pSS1129 (45) carrying the Tn5phoA transposon (pSS-TN) was used. The vector was introduced into B. bronchiseptica by conjugation as described previously (20). Transposon mutants were selected on BG agar plates containing 75 μg of kanamycin per ml and 100 μg of streptomycin per ml. Protamine-sensitive clones were identified after replica plating the bacteria on BG agar plates containing 1.5 mg of protamine sulfate per ml (34).
Cloning of the transposon integration sites by inverse PCR.
Chromosomal DNA of the transposon mutants was isolated as described previously (18). Aliquots of the chromosomal DNA were digested with PstI in the case of B. bronchiseptica mutant BB-PS1 or PvuII in the case of mutant BB-PS3 and religated with T4 ligase. After precipitation, aliquots were used in a PCR (40 cycles of 1 min at 94°C, 1 min at 53°C, and 80 s at 72°C). The region adjacent to one end of the transposon insertion in BB-PS1 was amplified with the oligonucleotides TninvL (5′-GCTAAGAGAAGCTTGCAGAGCGGCAG-3′) and PstinvL (5′-CGGTCTGTGATCTA-GAAGCCGATATTC-3′), resulting in the amplification of a 850-bp fragment. In the case of BB-PS3, the oligonucleotides TninvR (5′-GTTATCATGAAGCTTACCATGTTAGGA-3′) and PvuIIinvR (5′-ATGGCGATATCTAGACTGGGCGGTT-3′) were used for PCR, resulting in a 280-bp fragment. The two PCR products were cloned into pUC18 and sequenced by using the oligonucleotides applied for amplification as primers in accordance with standard procedures (39). The DNA sequences were subjected to homology searches in the GenEMBL database by using the FASTA program (10).
Preparation and gel electrophoresis of the LPS of Bordetella species.
The preparation of LPS from Bordetella species was carried out as described elsewhere (35). Briefly, bacteria grown on BG agar plates were harvested in phosphate-buffered saline and diluted to a final optical density at 540 nm of 0.3. The bacteria were centrifuged for 10 min at 10,000 × g, resuspended in 100 μl of Laemmli solubilization buffer, and boiled for 5 min (24). Then, 10 μg of proteinase K was added and the samples were incubated at 60°C for 2 h with intermittent vortexing. After the samples were cooled to room temperature, the LPS was precipitated by the addition of 9 volumes of acetone and incubation on ice for 1 h. After centrifugation at 10,000 × g for 10 min, the pellet was resuspended in 150 μl of Laemmli solubilization buffer and boiled for 5 min. The LPS samples were separated on discontinuous sodium dodecyl sulfate–15% polyacrylamide gels (24). The gels were fixed and silver stained in accordance with the protocol of Tsai and Frasch (46).
RESULTS AND DISCUSSION
Susceptibility of Bordetella species to the bactericidal action of cationic peptides.
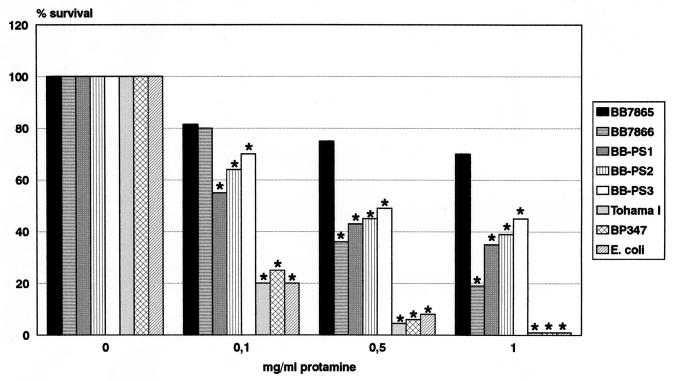
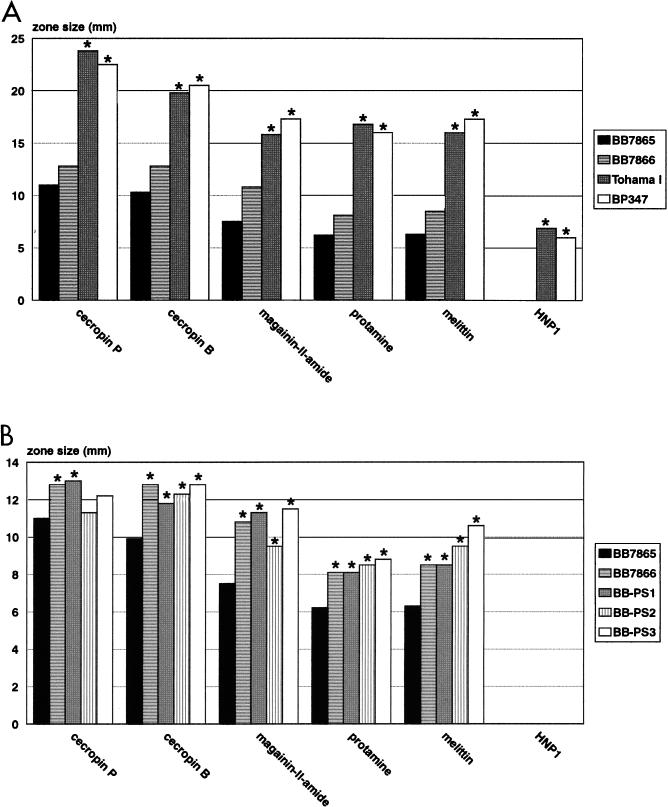
In the present paper, we compared the susceptibilities of B. pertussis and B. bronchiseptica strains to antimicrobial peptides. The bactericidal potential of several peptides was tested in liquid survival and radial diffusion assays. In both assay systems, B. bronchiseptica BB7865 was found to be far more resistant to these peptides than B. pertussis Tohama I (Fig. 1 and 2). According to the results of the radial diffusion assay, in the case of B. bronchiseptica, the analyzed peptides could be ranked in decreasing potency as follows: cecropin P > cecropin B > magainine-II-amide > protamine > melittin, whereas the β-defensin HNP1 did not affect viability of the bacteria. Similarly, cecropin P was most efficient against B. pertussis, followed by cecropin B, protamine, magainin-II-amide, and melittin. In contrast to the case with B. bronchiseptica, HNP1 had a significant inhibitory effect on B. pertussis. The very pronounced resistance of B. bronchiseptica to antimicrobial peptides belonging to various subclasses is in agreement with that described in previous publications, which showed that in contrast to other tested bacteria, including Listeria monocytogenes, Staphylococcus aureus, Streptococcus pneumoniae, and Pseudomonas aeruginosa, a B. bronchiseptica strain was highly resistant to cationic peptides derived from rabbit lung macrophages or from rabbit peritoneal granulocytes (28, 42).
FIG. 1.
Susceptibility of Bordetella strains to the action of protamine as determined in a liquid bactericidal assay. Stars above the bars indicate statistically significant differences in survival of the various strains at different protamine concentrations in comparison to that of the B. bronchiseptica wild-type strain, BB7865 (P < 0.01).
FIG. 2.
Susceptibility of B. bronchiseptica and B. pertussis wild-type and mutant strains to various cationic peptides as determined in radial diffusion assays. (A) Comparison of wild-type B. bronchiseptica BB7865, B. pertussis Tohama I, and their bvg mutant derivatives, BB7866 and BP347, respectively. Stars above the bars indicate statistically significant differences in growth inhibition of the various strains in comparison to that of the B. bronchiseptica phase-variant strain BB7866 (P < 0.01). (B) Comparison of wild-type B. bronchiseptica BB7865, its phase variant BB7866, and transposon-induced protamine-sensitive mutants (BB-PS1 to BB-PS3) in radial diffusion assays. Stars above the bars indicate statistically significant differences in growth inhibition of the various strains in comparison to that of the B. bronchiseptica wild-type strain, BB7865 (P < 0.01).
Interestingly, genetic inactivation of the bvg locus in B. bronchiseptica (strain BB7866) resulted in a significant increase in susceptibility to all tested peptides, with the exception of HNP1 (Fig. 2). This is in contrast to the case with B. pertussis, in which genetic inactivation of the virulence regulatory bvg locus (strain BP347) generally resulted in much milder effects on peptide resistance and a peptide-specific pattern of increase or decrease of susceptibility (Fig. 2) (15). The fact that B. bronchiseptica, a pathogen exhibiting a relatively broad host range, is significantly more resistant to the action of antimicrobial peptides than the obligate human pathogen B. pertussis may indicate that it encounters different cationic peptides during infection as part of the innate immunity of various mammalian hosts. In contrast, the specialization of B. pertussis to a single host may have allowed the loss of protection against a broad range of antimicrobial peptides, which may not be encountered anymore in humans. However, in the future it will be important to analyze the resistance profile of B. pertussis to cationic peptides of human origin such as the β-defensin hBD1 (7, 31, 53).
Isolation of B. bronchiseptica transposon mutants with increased peptide susceptibility.
To elucidate the molecular basis of peptide resistance in B. bronchiseptica, we generated transposon-induced mutants and screened them for increased susceptibility to protamine, which similar to other antimicrobial peptides exhibits a destabilizing effect on the cytoplasmic membrane (4, 34). For this purpose, Tn5 was delivered to B. bronchiseptica BB7865 after conjugation with the suicide vector pSS-TN. Transconjugants containing Tn5 on their chromosome were selected on kanamycin-containing BG agar plates. About 15,000 transconjugants were replica plated on SS agar plates containing 1.5 mg of protamine per ml. Twenty clones unable to grow on the protamine-containing plates were selected and further analyzed for their growth properties and resistance patterns against protamine in a liquid survival assay. Several mutants were impaired in their growth characteristics in SS broth and were not considered for further investigations (data not shown). Three mutants (BB-PS1 to BB-PS3) could not be distinguished from the wild-type strain in their growth properties and were significantly more sensitive to the action of protamine. These three mutants were further characterized. The integration of a single copy of Tn5 into their chromosome was confirmed by Southern blotting (data not shown). As in the case of the wild type, the β-defensin HNP1 did not affect growth of any of the mutant bacteria (Fig. 2). All three mutants showed significantly increased sensitivities to the various cationic peptides, with the exception of cecropin P (Fig. 2).
Cloning and characterization of genes involved in the resistance of B. bronchiseptica to cationic peptides.
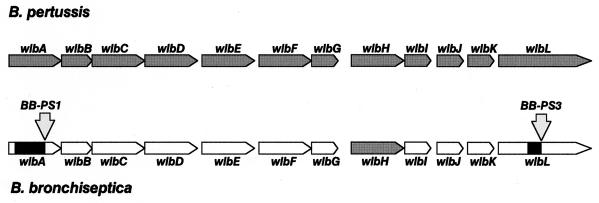
To understand the molecular basis of peptide resistance, we attempted to clone the gene loci inactivated by Tn5 insertions. In the case of the two mutants BB-PS1 and BB-PS3, an inverse PCR strategy allowed the amplification of DNA sequences containing the transposon ends and the flanking DNA regions. DNA sequencing revealed that the transposons were integrated in the B. bronchiseptica counterparts of two genes recently implicated in the biosynthesis of LPS in B. pertussis (1), wlbA in the case of strain BB-PS1 and wlbL in the case of BB-PS3. Partial DNA sequences of both genes were determined and found to be identical to those of the corresponding B. pertussis genes (data not shown). This suggests that the wlb locus is highly conserved between B. pertussis and B. bronchiseptica (Fig. 3), confirming recent data obtained by a comparison of several restriction digest patterns of the cloned B. pertussis and B. bronchiseptica wlb loci (2). The wlbA gene was recently proposed to code for a dehydrogenase which is involved in the biosynthesis of 2,3-dideoxy-2,3-di-N-acetylmannosaminuronic acid (2,3-diNAcManA), a constituent of the so-called band A trisaccharide of B. pertussis LPS (see below). The product of the wlbL gene shows significant homologies with proteins of various bacteria involved in modification of nucleotide sugars and may be required for biosynthesis of the 2,6-dideoxy-galactose derivative of N-acetyl-N-methylfucosamine (FucNAcMe), which is also a constituent of the band A trisaccharide (1). Unfortunately, so far we have not been able to identify the transposon integration site in the third mutant, BB-PS2.
FIG. 3.
Structures of the wlb locus in B. pertussis (top) and in B. bronchiseptica (bottom) (1, 2). Grey arrows identify genes for which the DNA sequence is available; e.g., in the case of B. bronchiseptica, only the wlbH gene has been sequenced so far (1, 2). The integration sites of Tn5 in the wlbA and wlbL genes of B. bronchiseptica BB-PS1 and BB-PS3, respectively, are indicated by downward arrows. The black boxes in the wlbA and wlbL structures of B. bronchiseptica indicate those parts of the two genes sequenced during this study.
Changes in the LPS of mutated peptide-sensitive B. bronchiseptica strains.
The identification of mutations in LPS biosynthesis genes in two of the peptide-sensitive strains suggested alterations in their LPS structure. The LPS of B. pertussis does not contain extended O-specific side chains common to many enteric bacteria. In LPS preparations of B. pertussis, typically two bands are visible in silver-stained acrylamide gels (9, 35). The slower-migrating band A corresponds to a charged trisaccharide containing N-acetylglucosamine (GlcNAC), 2,3-diNAcManA, and FucNAcMe linked to the LPS core region. The faster-migrating band B corresponds to the core region lacking the trisaccharide (3). In contrast, B. bronchiseptica strains were shown to contain a smooth LPS form with O-specific side chains linked to the trisaccharide and consisting of linear unbranched polymers of 1,4-linked 2,3-diacetamido-2,3-dideoxy-α-l-galactopyranosyluronic acid residues (12). Some strain-dependent polymorphism of these structures regarding the presence or absence of the O-specific side chains, but also concerning variations in other parts of the LPS molecules, has been reported (25). Whereas the wlb loci of B. pertussis and B. bronchiseptica containing genes involved in the biosynthesis of the trisaccharide have been characterized (1, 2), the genes required for the biosynthesis of the O-specific side chains in B. bronchiseptica have not yet been identified.
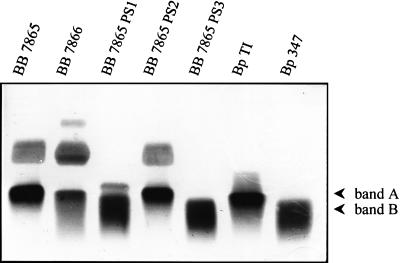
The LPSs of B. bronchiseptica BB7865, BB7866, BB-PS1, BB-PS2, and BB-PS3, B. pertussis Tohama I, and B. pertussis BP347 were isolated and separated on polyacrylamide gels. After silver staining, in the case of the B. pertussis Tohama I, a single band which corresponds to the previously described LPS band A could be detected. The Tohama I-derived bvg mutant BP347 mainly expressed band B. A bvg-dependent switch from band A to band B has already been described for various B. pertussis strains (37). In the case of the B. bronchiseptica strain BB7865, a diffuse smear of higher-molecular-weight bands, which represents O-specific side chains, could be seen in addition to band A. Similarly, in the case of the phase variant BB7866, O-specific side chains appear to be present, although there are some differences in the pattern of LPS-derived bands as compared to that of the wild-type strain, suggesting that as in the case of B. pertussis, phase variation affects LPS structure in B. bronchiseptica (Fig. 4).
FIG. 4.
Silver-stained polyacrylamide gels with LPS preparations of various Bordetella strains. The positions of band A and band B are indicated. O-specific side chains in the BB7865, BB7866, and BB-PS2 strains are visible as a diffuse cloud above band A. Abbreviations: BB 7865 PS1, PS2, and PS3, mutants BB-PS1, BB-PS2, and BB-PS3, respectively; Bp TI, B. pertussis Tohama I; Bp 347, B. pertussis BP347.
As suggested by the Tn5 insertions into the wlbA and wlbL genes of the peptide-sensitive BB-PS1 and BB-PS3 mutants, respectively, these mutants showed major changes in the LPS profile as compared to that of their parent strain BB7865. In agreement with the assumed function of the wlbA and wlbL gene products in the biosynthesis of the trisaccharide linked to the LPS core (1, 2), band A was replaced by band B in the two mutants. In line with the absence of band A, the O-specific side chains linked to the trisaccharide disappeared in BB-PS1 and in BB-PS3. In the case of BB-PS1, a new band of unknown composition appeared above band B, which had a slightly higher molecular weight than band A. Therefore, the Tn5 insertions in BB-PS1 and BB-PS3, which rendered them highly susceptible to cationic peptides, caused alterations in the LPS structure resulting in a change from a smooth to a rough phenotype. The fact that phase variation also influences the LPS structure in B. bronchiseptica may explain the increased susceptibility of strain BB7866 to the various peptides, at least in part.
Interestingly, the third transposon mutant, BB-PS2, did not reveal any obvious change in its LPS profile (Fig. 4). As already mentioned, so far we have not been able to identify the gene locus inactivated by the transposon in this mutant. However, since no changes in the LPS profile could be detected, it is likely that as-yet-unknown LPS-independent mechanisms account for the observed increase in the susceptibility of this mutant to the cationic peptides. Alternative resistance strategies may involve efflux pumps such as the recently identified mtr system of Neisseria gonorrhoeae (43). Additional mechanisms may account for the still very significant difference between rough B. bronchiseptica mutants and the “naturally” rough B. pertussis strains in their susceptibility to cationic peptides.
The identification of mutations in LPS biosynthesis genes after screening for peptide-sensitive B. bronchiseptica strains confirms previous studies which indicated that factors implicated in the transport of peptides across the outer membrane are important for peptide resistance in gram-negative bacteria. These factors include the charge of the LPS molecules, the LPS concentration, the presence or absence of the O-antigen side chains, and their length (36, 51). Since the LPS of B. bronchiseptica is highly charged due to the presence of uronic acids in the O-specific side chains, they may shield the negative charges present on the membranes and thereby prevent an efficient membrane attack by the peptides. Surface charges also seem to be involved in the susceptibility of B. pertussis to various antibiotics, including tetracycline and novobiocin, as transposon-induced LPS mutants were recently shown to have altered susceptibilities to these drugs (47).
ACKNOWLEDGMENTS
We thank Michael Kuhn and Hans-Dieter Zucht for many helpful discussions, Dagmar Beier for providing us with the transposon delivery vector, Gaby Gerlach for help with the radial diffusion assays, and Dagmar Beier, Justin Daniels, and Michael Kuhn for critical reading of the manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft (SFB479/A2) and the Fonds der Chemischen Industrie.
REFERENCES
- 1.Allen A G, Maskell D J. The identification, cloning and mutagenesis of a genetic locus required for lipopolysaccharide biosynthesis in Bordetella pertussis. Mol Microbiol. 1996;19:37–52. doi: 10.1046/j.1365-2958.1996.354877.x. [DOI] [PubMed] [Google Scholar]
- 2.Allen A G, Thomas R M, Cadisch J T, Maskell D J. Molecular and functional analysis of the lipopolysaccharide biosynthesis locus wlb from Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Mol Microbiol. 1998;29:27–38. doi: 10.1046/j.1365-2958.1998.00878.x. [DOI] [PubMed] [Google Scholar]
- 3.Amano K-I, Fukushi K, Watanabe M. Biochemical and immunological comparison of lipopolysaccharides from Bordetella species. J Gen Microbiol. 1990;136:481–487. doi: 10.1099/00221287-136-3-481. [DOI] [PubMed] [Google Scholar]
- 4.Aspedon A, Groisman E A. The antibacterial action of protamine: evidence for disruption of cytoplasmic membrane energization in Salmonella typhimurium. Microbiology. 1996;142:3389–3397. doi: 10.1099/13500872-142-12-3389. [DOI] [PubMed] [Google Scholar]
- 5.Banemann A, Gross R. Phase variation affects long-term survival of Bordetella bronchiseptica in professional phagocytes. Infect Immun. 1997;65:3469–3473. doi: 10.1128/iai.65.8.3469-3473.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beier D, Schwarz B, Fuchs T M, Gross R. In vivo characterization of the unorthodox BvgS two-component sensor protein of Bordetella pertussis. J Mol Biol. 1995;248:596–610. doi: 10.1006/jmbi.1995.0245. [DOI] [PubMed] [Google Scholar]
- 7.Bensch K W, Raida M, Magert H J, Schulz-Knappe P, Forssmann W G. hBD-1: a novel β-defensin from human plasma. FEBS Lett. 1995;368:331–335. doi: 10.1016/0014-5793(95)00687-5. [DOI] [PubMed] [Google Scholar]
- 8.Bordet J, Gengou O. L’endotoxine coquechuleuse. Ann Inst Pasteur (Paris) 1909;23:415–419. [Google Scholar]
- 9.Chaby R, Caroff M. Lipopolysaccharides of Bordetella pertussis endotoxin. In: Wardlaw A C, Parton R, editors. Pathogenesis and immunity in pertussis. Chichester, United Kingdom: John Wiley; 1988. pp. 247–271. [Google Scholar]
- 10.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diamond G, Zasloff M, Eck H, Brasseur M, Maloy W L, Bevin C L. Tracheal antimicrobial peptide, a cysteine-rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of cDNA. Proc Natl Acad Sci USA. 1991;88:3952–3956. doi: 10.1073/pnas.88.9.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Fabio J L, Caroff M, Karibian D, Richards J C, Perry M B. Characterization of the common antigenic lipopolysaccharide O-chains produced by Bordetella bronchiseptica and Bordetella parapertussis. FEMS Microbiol Lett. 1992;97:275–282. doi: 10.1016/0378-1097(92)90348-r. [DOI] [PubMed] [Google Scholar]
- 13.Ewanowich C A, Melton A R, Weiss A A, Sherburne R K, Peppler M S. Invasion of HeLa 229 cells by virulent Bordetella pertussis. Infect Immun. 1989;57:2698–2704. doi: 10.1128/iai.57.9.2698-2704.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falkow S, Isberg R R, Portnoy D A. The interaction of bacteria with mammalian cells. Annu Rev Cell Biol. 1992;8:333–363. doi: 10.1146/annurev.cb.08.110192.002001. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez R C, Weiss A A. Susceptibilities of Bordetella pertussis strains to antimicrobial peptides. Antimicrob Agents Chemother. 1996;40:1041–1043. doi: 10.1128/aac.40.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finn T M, Stevens L A. Tracheal colonization factor: a Bordetella pertussis secreted virulence determinant. Mol Microbiol. 1995;16:625–634. doi: 10.1111/j.1365-2958.1995.tb02425.x. [DOI] [PubMed] [Google Scholar]
- 17.Goldman M J, Anderson G M, Stolzenberg E D, Kari U P, Zasloff M, Wilson J M. Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell. 1997;88:553–560. doi: 10.1016/s0092-8674(00)81895-4. [DOI] [PubMed] [Google Scholar]
- 18.Graeff-Wohlleben H, Killat S, Banemann A, Guiso N, Gross R. Cloning and characterization of a manganese-containing superoxide dismutase from Bordetella pertussis. J Bacteriol. 1997;179:2194–2201. doi: 10.1128/jb.179.7.2194-2201.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groisman E A. Bacterial responses to host-defense peptides. Trends Microbiol. 1996;4:127–128. doi: 10.1016/0966-842x(96)30013-9. [DOI] [PubMed] [Google Scholar]
- 20.Gross R, Rappuoli R. Positive regulation of pertussis toxin expression. Proc Natl Acad Sci USA. 1988;85:3913–3917. doi: 10.1073/pnas.85.11.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gueirard P, Weber C, Le Coustumier A, Guiso N. Human Bordetella bronchiseptica infection related to contact with infected animals: persistence of bacteria in host. J Clin Microbiol. 1995;33:2002–2006. doi: 10.1128/jcm.33.8.2002-2006.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guzman C A, Rohde M, Bock M, Timmis K N. Invasion and intracellular survival of Bordetella bronchiseptica in mouse dendritic cells. Infect Immun. 1994;62:5528–5537. doi: 10.1128/iai.62.12.5528-5537.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harder J, Bartels J, Christophers E, Schröder J M. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 24.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Le Blay K, Gueirard P, Guiso N, Chaby R. Antigenic polymorphism of the lipopolysaccharides from human and animal isolates of Bordetella bronchiseptica. Microbiology. 1997;143:1433–1441. doi: 10.1099/00221287-143-4-1433. [DOI] [PubMed] [Google Scholar]
- 26.Lee C K, Roberts A L, Finn T M, Knapp S, Mekalanos J J. A new assay for invasion of HeLa229 cells by Bordetella pertussis: effect of inhibitors, phenotypic modulation, and genetic alterations. Infect Immun. 1990;58:2516–2522. doi: 10.1128/iai.58.8.2516-2522.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lehrer R I, Roseman M, Harwig S, Jackson R, Eisenhauer P. Ultrasensitive assays for endogenous antimicrobial polypeptides. J Immunol Methods. 1991;137:167–173. doi: 10.1016/0022-1759(91)90021-7. [DOI] [PubMed] [Google Scholar]
- 28.Lehrer R I, Selsted M E, Szklarek D, Fleischmann J. Antibacterial activity of microbicidal cationic proteins 1 and 2, natural peptide antibiotics of rabbit lung macrophages. Infect Immun. 1983;42:10–14. doi: 10.1128/iai.42.1.10-14.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leininger E, Roberts M, Kenimer J G, Charles I G, Fairweather N, Novotny P, Brennan M J. Comparative roles of the Arg-Gly-Asp sequence present in the Bordetella pertussis adhesins pertactin and filamentous hemagglutinin. Infect Immun. 1992;60:2380–2385. doi: 10.1128/iai.60.6.2380-2385.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maloy W L, Kari U P. Structure-activity studies on magainin and other host defense peptides. Biopolymers. 1995;37:105–122. doi: 10.1002/bip.360370206. [DOI] [PubMed] [Google Scholar]
- 31.McCray, P. B., Jr., and L. Bentley. Human airway epithelia express a β-defensin. Am. J. Respir. Cell. Mol. Biol. 16:343–349. [DOI] [PubMed]
- 32.Monack D, Arico B, Rappuoli R, Falkow S. Phase variants of Bordetella bronchiseptica arise by spontaneous deletions in the vir locus. Mol Microbiol. 1989;3:1719–1728. doi: 10.1111/j.1365-2958.1989.tb00157.x. [DOI] [PubMed] [Google Scholar]
- 33.Nicolas P, Mor A. Peptides as weapons against microorganisms in the chemical defense system of vertebrates. Annu Rev Microbiol. 1995;49:277–304. doi: 10.1146/annurev.mi.49.100195.001425. [DOI] [PubMed] [Google Scholar]
- 34.Parra-Lopes C, Baer M T, Groisman E A. Molecular genetic analysis of a locus required for resistance to antimicrobial peptides in Salmonella typhimurium. EMBO J. 1993;12:4053–4062. doi: 10.1002/j.1460-2075.1993.tb06089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peppler M S. Two physically and serologically distinct lipopolysaccharide profiles in strains of Bordetella pertussis and their phenotype variants. Infect Immun. 1984;43:224–232. doi: 10.1128/iai.43.1.224-232.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rana F R, Macias E A, Sultany C M, Modzrakowsky M C, Blazyk J. Interactions between magainin 2 and Salmonella typhimurium outer membranes: effect of lipopolysaccharide structure. Biochemistry. 1991;30:5858–5866. doi: 10.1021/bi00238a008. [DOI] [PubMed] [Google Scholar]
- 37.Ray A, Redhead K, Selkirk S, Poole S. Variability in LPS composition, antigenicity and reactogenicity of phase variants of Bordetella pertussis. FEMS Microbiol Lett. 1991;79:221–218. doi: 10.1016/0378-1097(91)90088-r. [DOI] [PubMed] [Google Scholar]
- 38.Relman D A, Tuomanen E, Falkow S, Golenbock D T, Saukkonen K, Wright S D. Recognition of a bacterial adhesin by an integrin: macrophage CR3 (αMβ2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell. 1990;61:1375–1382. doi: 10.1016/0092-8674(90)90701-f. [DOI] [PubMed] [Google Scholar]
- 39.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 40.Savelkoul P H M, Kremer B, Kusters J G, van der Zeijst B A M, Gaastra W. Invasion of HeLa cells by Bordetella bronchiseptica. Microb Pathog. 1993;14:161–168. doi: 10.1006/mpat.1993.1016. [DOI] [PubMed] [Google Scholar]
- 41.Schipper H, Krohne G, Gross R. Epithelial cell invasion and survival of Bordetella bronchiseptica. Infect Immun. 1994;62:3008–3011. doi: 10.1128/iai.62.7.3008-3011.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Selsted M E, Szklarek D, Lehrer R I. Purification and antibacterial activity of antimicrobial peptides of rabbit granulocytes. Infect Immun. 1984;45:150–154. doi: 10.1128/iai.45.1.150-154.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shafer W M, Qu X, Waring A J, Lehrer R I. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc Natl Acad Sci USA. 1998;95:1829–1833. doi: 10.1073/pnas.95.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stainer D W, Scholte M J. A simple chemically defined medium for production of phase I Bordetella pertussis. J Gen Microbiol. 1970;63:211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- 45.Stibitz S, Yang M S. Subcellular location and immunological detection of proteins encoded by the vir locus of Bordetella pertussis. J Bacteriol. 1991;173:4288–4296. doi: 10.1128/jb.173.14.4288-4296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsai C M, Frasch C E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 47.Turcotte M L, Martin D, Brodeur B R, Peppler M S. Tn5-induced lipopolysaccharide mutations in Bordetella pertussis that affect outer membrane function. Microbiology. 1997;143:2381–2394. doi: 10.1099/00221287-143-7-2381. [DOI] [PubMed] [Google Scholar]
- 48.Uhl M A, Miller J F. Integration of multiple domains in a two-component sensor protein: the Bordetella pertussis BvgAS phosphorelay. EMBO J. 1996;15:1028–1036. [PMC free article] [PubMed] [Google Scholar]
- 49.Weiss A A, Falkow S. Genetic analysis of phase change in Bordetella pertussis. Infect Immun. 1984;43:263–269. doi: 10.1128/iai.43.1.263-269.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weiss A A, Hewlett E L. Virulence factors of Bordetella pertussis. Annu Rev Microbiol. 1986;40:661–686. doi: 10.1146/annurev.mi.40.100186.003305. [DOI] [PubMed] [Google Scholar]
- 51.Weiss J, Beckerdite-Quagliata S, Elsbach P. Resistance of gram-negative bacteria to purified bactericidal leukocyte proteins: relation to binding and bacterial lipopolysaccharide structure. J Clin Invest. 1980;65:619–628. doi: 10.1172/JCI109707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woolfrey F B, Moody J A. Human infections associated with Bordetella bronchiseptica. Clin Microbiol Rev. 1991;4:234–255. doi: 10.1128/cmr.4.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao C, Wang I, Lehrer R I. Widespread expression of β-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett. 1996;396:319–322. doi: 10.1016/0014-5793(96)01123-4. [DOI] [PubMed] [Google Scholar]