Abstract
Background:
Episodic high-altitude exposure leads to optic disc edema and retinopathy. It is uncertain whether high-altitude exposure is a risk factor for nonarteritic anterior ischemic optic neuropathy (NAION).
Methods:
We performed a single-center, retrospective, cross-sectional case study of 5 patients with high-altitude–associated NAION (HA-NAION) from April 2014 to April 2019. Main study parameters included known vascular risk factors for NAION, evolution of visual acuity, visual field, optic disc, and macula measurements.
Results:
We studied 5 eyes of 5 patients with HA-NAION that occurred at 7,000–9,000 ft above sea level, 28 patients with classic NAION that developed at sea level (normal altitude NAION or NA-NAION), and 40 controls. All 5 patients with HA-NAION had clinically confirmed NAION by a neuro-ophthalmologist within 3–21 days of onset and comprehensive follow-up evaluations (average follow-up of 23 months). Other than high-altitude exposure, 4 of 5 patients had undiagnosed obstructive sleep apnea (OSA, apnea–hypopnea index 5.4–22.2) and 1 had systemic vascular risk factors. All patients had disc-at-risk in the contralateral eye. The best-corrected distance visual acuity was 20/20 to 20/70 (median logMAR 0) at presentation and 20/70 to counting finger (median logMAR 0) at ≥6 months. Automated static perimetry revealed average mean deviation of −18.6 dB at presentation and −22.1 dB at ≥6 months. The average retinal nerve fiber layer was 244 μm (80–348 μm) at onset and 59 μm (55–80 μm) at ≥6 months. The average ganglion cell complex thickness was 50 μm (43–54 μm) at onset and 52 μm (50–55 μm) at ≥6 months. The patients with OSA were started on home continuous positive airway pressure treatment. Visual outcomes were similar in patients with HA-NAION and NA-NAION. - After addressing all NAION risk factors, no new events occurred in the HA-NAION group within 2–8 years with or without repeat high-altitude exposure.
Conclusions:
NAION can occur under high-altitude conditions. HA-NAION is associated with relatively younger age at onset, disc-at-risk, and OSA. These patients exhibit a relatively progressive course of vision loss after initial onset and severe thinning of optic nerves on optical coherence tomography. Treatment for OSA is recommended, especially with repeated high-altitude exposure.
Acute mountain sickness can manifest with visual disturbances, which have been associated with evidence of high-altitude cerebral edema. The incidence of high-altitude retinopathy and optic neuropathy ranges from 3.8% to 90.5% (1). Optic disc swelling happens in mountaineers at high altitudes with an incidence of 59%–79% (2,3), depending on the altitude above the sea level and the speed of ascent (4), and may be associated with younger age (5). Most of them experience complete resolution on descent. At 1 extreme high-altitude expedition (11,500 to 18,000 feet or 5,486.4 m above the sea level), 79% of all climbers exhibited retinal hemorrhages (6). Tian et al studied 91 healthy Chinese military members assigned to Tibet and found significant increase in optical coherence tomography (OCT) retinal nerve fiber layer (RNFL) and macula thickness (7).
Symptoms of acute mountain sickness are related to hypobaric hypoxia, and the symptoms typically increase as the altitude increases. Although ambient air contains 20.9% oxygen (O2) at all altitudes, lower barometric pressure at higher altitudes causes hypobaric hypoxia, which leads to effectively lower percentage of O2. At Sierra Nevada (8,000 ft above sea level), the effective O2 concentration of 15.4% means each breath contains 26% less oxygen than at sea level. At Mount Kilimanjaro (19,344 ft above sea level), the effective O2 concentration of 10% means each breath contains 51% less oxygen than at level. At highest peak of Mount Everest (29,000 ft above sea level), the effective O2 concentration of 6.9% means each breath contains 67% less oxygen than at the sea level.
Nonarteritic anterior ischemic optic neuropathy (NAION) is the most common acute optic neuropathy in those older than 50 years (8). It typically presents with acute unilateral painless vision loss accompanied by sectoral or diffuse optic nerve edema. After onset, vision loss can progress over weeks. In NAION, ischemia of the posterior ciliary artery territory (posterior to lamina cribrosa) (9) leads to altitudinal visual field loss and subsequent chronic optic atrophy. Common risk factors include an underlying small/crowded or anomalous optic disc (92%) and isolated disc anomalies (26%) such as optic disc drusen, cardiovascular risk factor (74%), hypercoagulable states, and vasculitis (8%) (10).
There have been reported cases of NAION at extremely high altitude. One case described a 41-year-old man US Air Force pilot who developed superior paracentral visual field loss in the left eye with associated optic disc blurred margins (11). Another case occurred in a 33-year-old man who spent 3 months at 17,953 ft above sea level was attributed to hypoxia-induced vasospasm and impaired autoregulation (12). NAION as part of acute mountain sickness at high altitude has not previously been reported. In this study, we report a cohort of 5 patients who developed NAION after high-altitude exposure (HA-NAION) and compared their clinical characteristics, visual function, optical coherence tomography measurements with that of normal altitude (sea level) NAION (NA-NAION), and controls.
METHODS
We performed a retrospective, cross-sectional study at the Byers Eye Institute, Department of Ophthalmology, Stanford University School of Medicine. Control eye data were collected through convenience sampling in patients with normal examinations. This study protocol was approved by the Stanford University Institutional Review Board in accordance with the Declaration of Helsinki, and the study procedures were performed according to Health Insurance Portability and Accountability Act.
Participants
The timeline from high-altitude exposure to visual disturbance onset was recorded as part of the history of present illness. Altitude at the locations of exposure was recorded by patient report with verification from the treating physicians. Sleep apnea was evaluated by a sleep medicine–trained physician using polysomnography. We performed a retrospective study of 5 eyes from 5 patients with HA-NAION, 40 eyes of 28 patients with NA-AION, and 40 control eyes. The average follow-up duration was 30 months (11–56 months). Inclusion criteria for those with HA-NAION: age older than 18 years who presented with vision loss within 2 weeks after exposure to high altitude (>4,000 feet), without major vascular risk factors, such as uncontrolled diabetes, hyperlipidemia, hypertension, prior ischemic or hemorrhagic strokes, transient ischemic attacks, or heart attacks. NA-NAION patient data were collected from patients who had a confirmed diagnosis of NAION without high-altitude exposure. Controls were obtained from patients without major eye diseases and without NAION. The best-corrected distance visual acuity better than or equal to 20/30 on the Snellen eye chart and automated visual field (HVF) median deviation better than or equal to −2 dB. Exclusion criteria: Flight-related NAION cases were excluded given variabilities in presentation and possibly different mechanisms of action from that of HA-NAION. All patients underwent neuro-ophthalmologic examination within 3–21 days after developing vision loss. OCT of the RNFL and ganglion cell complex (GCC) thickness, as well as HVF, was performed at minimum during initial and the most recent clinic visit.
Statistical Analysis
The Mann-Whitney U test (Graph Pad Prism) was used to determine the difference in mean and SD between control and exposure groups. A P value of <0.05 was considered to be statistically significant.
RESULTS
Clinical Presentation of High-altitude Nonarteritic Anterior Ischemic Optic Neuropathy
Patient 1 is a typical example of HA-NAION. He was a 49-year-old man with no past medical history who was hiking rigorously in Sierra Nevada at 8,000 feet above sea level for 3 days when he noted left eye blurriness. He descended but continued white water rafting for another day. Neuro-ophthalmology evaluation occurred 8 days after symptom onset. He had visual acuity of 20/20 in both eyes, normal color vision, normal intraocular pressures, a left relative afferent pupillary defect, disc at risk in the right optic nerve, and a diffusely swollen left optic disc (Fig. 1). Static perimetry showed an inferior altitudinal visual field defect in the left eye and a normal visual field in the right eye. The OCT showed thick RNFL of 266 μm and thin GCC of 54 μm. His visual acuity declined to 20/150 6 weeks after onset, and his inferior altitudinal visual field defect at onset progressed to generalized severe constriction by next HVF 3 months later. The disc edema evolved to optic atrophy. Home polysomnography performed a few weeks after initial diagnosis of NAION showed mild OSA (AHI 5.4, multiple episodes of flow limitation not accounted for by AHI, history of snoring, narrow oropharynx), and he elected to be treated with continuous positive airway pressure (CPAP) during sleep when at high altitude. With this regimen, he had no further NAION events despite multiple high-altitude exposures, all while using CPAP at night.
FIG. 1.
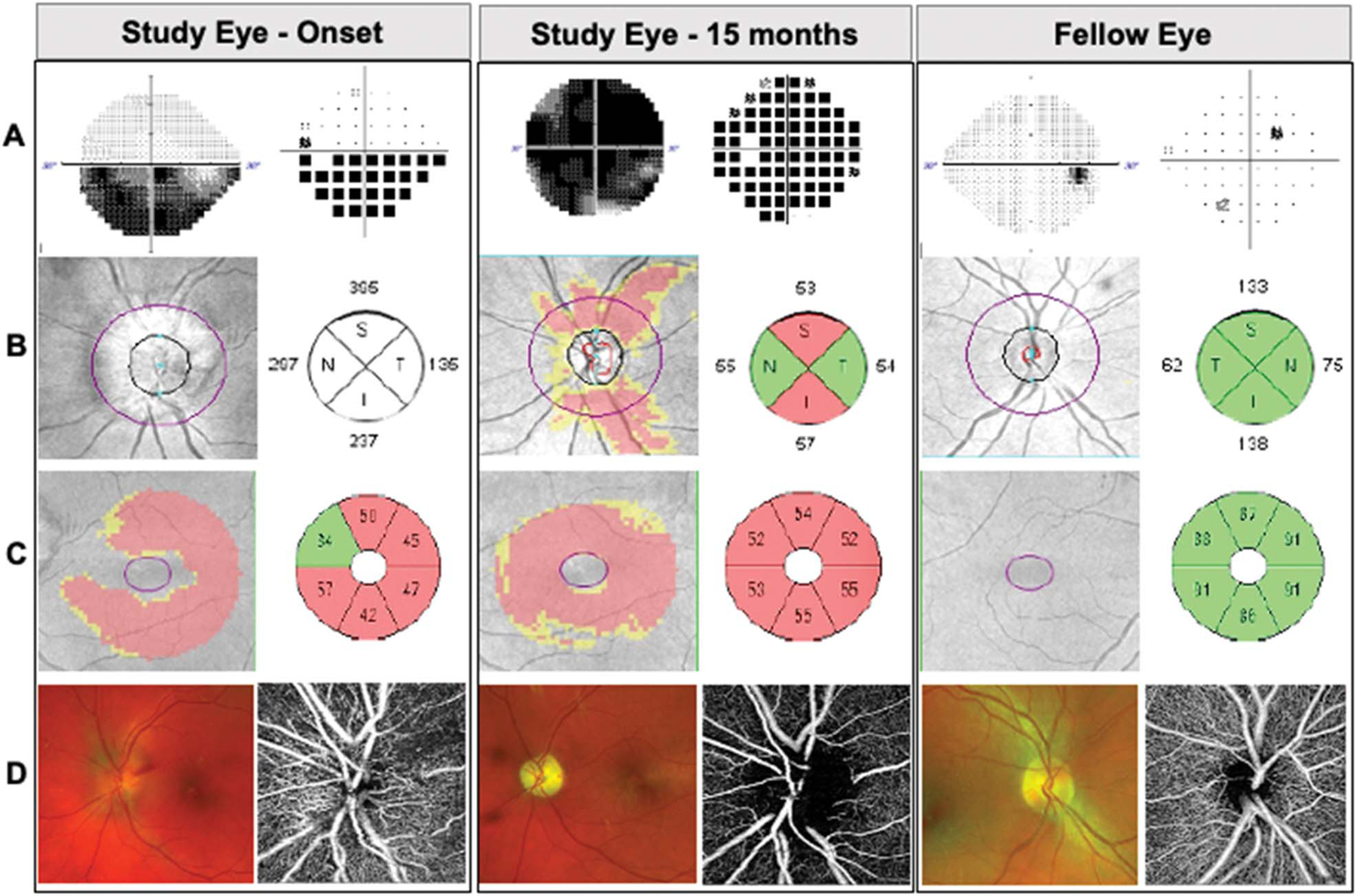
Representative patient with high-altitude NAION in left eye. Images demonstrating study eye at onset and, at 15-month follow-up, and fellow eye. A. Static perimetry revealed inferior altitudinal visual field defect that worsened over time. Gray scale (left) and pattern deviation (right). B. Spectral-domain optical coherence tomography (OCT) and peripapillary retinal nerve fiber layer thickness deviation map and quantification by quadrants (in microns). C. OCT macular ganglion cell complex thickness deviation map and quantification by sectors (in microns). D. Scanning laser ophthalmoscopy images of the optic disc (Optos, left) and OCT angiography of the optic disc (Zeiss AngioPlex, right).
Patient 2 was a 74-year-old woman with multiple past history, including Sjogren’s syndrome (biopsy proven), hypertension, hyperlipidemia, microvascular right cranial nerve 3 paresis occurred at high altitude 1 year prior to HA-NAION (temporal artery biopsy negative, normal ESR, CRP), and incidentally noted and right upper lobe lung adenocarcinoma (stage IB, T2 N0 M0 resected 2 years prior to HA-NAION). She had sudden onset vision loss of the right eye while camping in Sierra Nevada at 7,000 feet. She was seen immediately on returning to the sea level by general ophthalmologist and ruled out retinal pathology. Six weeks later, she was diagnosed with NAION by neuro-ophthalmologist following negative ESR, CRP, and temporal artery biopsy, and at that time, her visual acuity was 20/60 in the right eye, and she had optic disc edema. Static perimetry revealed diffuse visual field constriction in right eye. There was thick RNFL of 348 μm on OCT. Despite systemic high dose corticosteroid treatment, her visual acuity subsequently declined to counting fingers at 2 feet within 2 weeks and remained the same for 7 years of follow-up, and her OCT had severe generalized RNFL and GCC thinning. Her polysomnography showed moderate OSA, and she was treated with CPAP but had difficulty tolerating it and stopped after 2 years. She had no new events for 7 years.
Patient 3 was a 49-year-old man with history of well-controlled hyperlipidemia who was fly fishing at Yellowstone State Park at 8,000 feet when he noted left eye inferior visual field loss. He reported poor sleep 2 nights before symptoms onset, which he attributed to allergies at high altitude. His initial left eye visual acuity was 20/20 with unilateral optic disc edema. The OCT measurement showed RNFL of 328 μm and static perimetry showed severe visual field constriction in the left eye. The most recent follow-up was at 35 months. His visual acuity remained at 20/20, and his static perimetry improved had persistent inferonasal defect. He had symptoms of sleep apnea for years. The polysomnography showed moderate OSA (AHI 22.2), and he was treated with CPAP. He had no further NAION events during 8 years of followup despite high-altitude exposure.
Patient 4 was a professional high-altitude climber with frequent high-altitude exposure in the 8,000–9,000 feet range. Approximately 10 days before onset, he noted 20%–30% vision loss in the left eye. We did not have any information of his right eye because he suffered from trauma in his youth and underwent enucleation. His initial visual acuity was 20/20 with optic disc edema, and static perimetry showed a mildly enlarged blind spot. However, over the next 3 months, there was progression of optic disc edema and vision loss that evolved into an inferior altitudinal defect. At the 13-month follow-up, his static perimetry showed stable inferior altitudinal field defect, while his visual acuity remained 20/20. He declined polysomnography.
Patient 5 noted inferior visual field defect in the right eye at the sea level. Within the same week, he flew to Bryce Canyon at 8,000 feet for 4 days where his vision worsened. On his return to the sea level, ophthalmic examination showed a visual acuity of 20/70 in the right eye and right optic disc edema. He also used sildenafil before onset. Static perimetry showed right inferonasal defect. At the 80-month follow-up, his visual acuity was 20/80 and static perimetry showed dense inferior loss, patch superior loss nasally more than temporally. His polysomnography showed very mild OSA (AHI 8.7), but he did not initiate CPAP as per sleep medicine’s recommendation. He had no further NAION events.
Comparison of High-altitude Nonarteritic Anterior Ischemic Optic Neuropathy and Normal Altitude Nonarteritic Anterior Ischemic Optic Neuropathy
We compared 5 eyes of 5 patients with high-altitude NAION with 28 normal-altitude NAION eyes and 40 healthy control eyes. The onset of HA-NAION was during high-altitude exposure at 7,000–9,000 ft in patients 1–4 and before high-altitude exposure in patient 5. All 5 patients had clinically confirmed isolated NAION by a neuro-ophthalmologist within 3–21 days of onset and comprehensive workup. The average age of HA-NAION group was 56 years (range 48–74 years) (Table 1), which was the same compared with that of the healthy control group 51 ± 1.7 years (P = 0.58) but younger than that of the NA-NAION group 63 years (P = 0.06). None of the 5 HA-NAION patients developed fellow eye involvement up to 8 years after onset (range 11–56 months). In addition, none of the 5 patients developed typical symptoms of mountain sickness or other signs of high-altitude retinopathy.
TABLE 1.
Clinical characteristics of patients with high-altitude NAION
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Summary (n = 5) | |
|---|---|---|---|---|---|---|
| Sex | M | F | M | M | M | M:F = 4:1 |
| Age (yrs) | 49 | 74 | 48 | 55 | 56 | 56 |
| Risk factors | None | multiple | Hyperlipidemia | None | Prior sildenafil use | None |
| Altitude exposure before onset (feet) | 8,000 | 7,000 | 8,000 | 5,000–9,000 | 9,000 | 8,000 |
| Sleep apnea | Mild | Moderate | Moderate | Deferred | Mild | Present |
| Subsequent CPAP treatment | Yes | Yes | Yes | NA | No | |
| AHI | 5.4 | 18.2 | 22.2 | NA | 8.7 | 5.4–22.2 |
| Nadir O2 saturation | 90% | 87% | 87% | NA | 97% | 87%–97% |
| ODI | 4.8 | 3.6 | NA | NA | NA | 3.6–4.8 |
| RDI | - | NA | NA | NA | NA |
AHI, apnea–hypopnea index; CPAP, continuous positive airway pressure; NA, not available.
On neuro-ophthalmic examination at presentation, the visual acuity ranged from 20/20 to 20/70 (median logMAR 0) (Table 2). Three of 5 patients presented with inferior altitudinal visual field loss, and 2 of 5 patients presented with cloudy/blurry vision at onset. On peripapillary OCT analysis, the average RNFL thickness was 244 μm (range 80–348 μm), and GCC was 48 μm (43–54 μm). Static perimetry measured the mean deviation of −18.61 dB (range −13.45 to −22.20 dB). These were not significantly different from patients with NA-NAION. At the most recent follow-up, the visual acuity ranged from 20/70 to counting finger (mean logMAR 0). Three of 5 patients had worse mean deviation on static perimetry from the initial visual field at presentation, 1 patient improved, and 1 remained stable. HA-NAION eyes had significantly worse median deviation (P < 0.001), RNFL thickness (P = 0.00), and GCC thickness (P = 0.001) compared with controls. However, RNFL and GCC measurements in chronic HA-NAION eyes were not different compared with that of the chronic NA-NAION eyes. In the fellow eyes, when compared with normal controls, patients with HA-NAION showed smaller cup-to-disc ratio (P = 0.02, Table 2), smaller cup volume (P = 0.01), and increased macular cube average thickness (P = 0.03).
TABLE 2.
Comparison of visual function and OCT measurements of controls, high-altitude and normal altitude NAION eyes, and their unaffected fellow eyes. Note all NA-NAION and HA-NAION eyes are ≥ 6 months after onset.
| Controls (n = 40) | NA-NAION (n = 28) | NA-NAION (n = 8) (fellow eye) |
HA-NAION (n = 5) | HA-NAION (n = 4) (fellow eye) |
Ctrl vs HA-NAION P |
HA-NAION vs NA-NAION P |
Ctrl vs HA-NAION fellow eyes P |
HA-NAION vs NA-NAION fellow eyes P |
|
|---|---|---|---|---|---|---|---|---|---|
| Visual function | |||||||||
| LogMAR | 0.02 ± 0.01 (0.00–0.50) | 0.59 ± 0.12 (0.00–1.88) | −0.03 ± 0.09 (20.20–0.10) | 0.58 ± 0.40 (0.00–2.00) | 0.03 ± 0.05 (0–0.10) | 0.11 | 0.57 | 0.87 | 0.04 |
| Mean deviation (dB) | −1.28 ± 0.26* (−5.75 to 4.56) | −15.8 ± 1.7 (−0.71 to 30) | −1.69±1.86 (−4.6 to 0.38) | −22.14 ± 3.99 (−11.67 to 29.10) | −1.26±1.81 (−0.01 to 3.87) | <0.0001 | 0.15 | 0.15 | 0.72 |
| OCT, disc | |||||||||
| RNFL (μm) | 91 ± 1 (71–110) | 65 ± 2 (44–99) | 97 ± 10 (81–114) | 59 ± 6 (50–80) | 92 ± 8 (84–102) | 0.00 | 0.14 | 0.90 | 0.35 |
| Disc area (mm2) | 1.84 ± 0.06† (71–105) | 1.80 ± 0.06 (1.33–2.86) | 1.60 ± 0.20 (1.29–1.89) | 1.59 ± 0.10 (0.36–0.75) | 1.74 ± 0.28 (1.36–2.02) | 0.18 | 0.16 | 0.64 | 0.14 |
| Rim area (mm2) | 1.41 ± 0.03 (0.97–1.93) | 1.26 ± 0.06 (0.73–0.85) | 1.41 ± 0.20 (1.15–1.76) | 1.16 ± 0.11 (0.84–1.33) | 1.59 ± 0.17 (1.36–1.77) | 0.07 | 0.61 | 0.05 | 0.41 |
| Mean C/D ratio | 0.40 ± 0.020 (0.07–0.68) | 0.49 ± 0.036 (0.09–0.81) | 0.29 ± 0.17 (0.07–0.53) | 0.46 ± 0.13 (0.14–0.64) | 0.24 ± 0.15 (0.08–0.44) | 0.42 | 0.97 | 0.02 | 0.62 |
| Vertical C/D ratio | 0.38 ± 0.019 (0.05–0.64) | 0.50 ± 0.038 (0.09–0.86) | 0.26 ± 0.15 (0.07–0.5) | 0.51 ± 0.25 (0.14–0.71) | 0.22 ± 0.17 (0.07–0.46) | 0.08 | 0.99 | 0.07 | 0.72 |
| Cup volume (mm2) | 0.10 ± 0.01 (0.00–0.50) | 0.12 ± 0.03 (0–0.55) | 0.03 ± 0.04 (0–0.12) | 0.07 ± 0.04 (0–0.165) | 0.02 ± 0.04 (0–0.07) | 0.69 | 0.72 | 0.01 | 0.66 |
| OCT, macula | |||||||||
| GCC (μm) | 80 ± 1 (67–93) | 60 ± 2 (45–76) | 82 ± 6 (70–90) | 58 ± 5 (50–78) | 85 ± 8 (76–94) | 0.001 | 0.57 | 0.78 | 0.57 |
| Central subfield thickness (μm) | 259 ± 1 (241–324) | 257 ± 5.7 (203–306) | 285 ± 20 (255–312) | 255 ± 15 (237–274) | 271 ± 25 (245–294) | 0.33 | 0.93 | 0.35 | 0.44 |
| Cube volume (mm3) | 9.9 ± 0.1 (8.8–10.5) | 9.6 ± 0.2 (8.2–11.9) | 10.7 ± 0.7 (9.7–11.9) | 9.7 ± 0.8 (9–10.9) | 10.8 ± 0.6 (10.3–11.4) | 0.16 | 0.91 | 0.05 | 0.68 |
| Cube average thickness (μm) | 281 ± 2 (244–320) | 265 ± 5 (227–329) | 297 ± 17 (270–329) | 270 ± 23 (249–302) | 301 ± 16 (286–17) | 0.18 | 0.91 | 0.03 | 0.69 |
Mann–Whitney U test.
Calculated automatically by the OCT analysis and typically larger C/D ratios than what’s qualitatively determined on color fundus photography.
GCC, ganglion cell complex; HA, high altitude; NA, normal altitude; NAION, nonarteritic anterior ischemic optic neuropathy; OCT, optical coherence tomography; RNFL, retinal nerve fiber layer; VA, visual acuity.
CONCLUSIONS
We report 5 patients with HA-NAION occurring during or immediately after high-altitude exposure. The patients with HA-NAION were relatively younger at onset of vision loss compared with NA-NAION. They had disc at risk (except for 1 monocular patient), progressive vision loss, severe visual field defects, and subsequent severe RNFL and GCC thinning after resolution of optic disc edema. However, there was not significantly different between the HA-NAION and NA-NAION groups. Those with AION in 1 eye have approximately 15% chance of developing AION in the contralateral eye, which raises questions regarding the risk of subsequent high-altitude exposure and possible need for lifestyle modification. So far, none of our patients with HA-NAION have developed new events with up to 8 years of follow-up (range 2–8 years) by avoiding high-altitude exposure or using CPAP at high altitude.
HA-NAION is rare and may be related to adaptive changes in retinal autoregulation in response to hypobaric hypoxia (13). In preclinical studies, exposure to systemic hypoxia leads to endoplasmic reticulum stress, glial cell reactivity and loss, retinal thickness changes, and upregulation of inflammatory pathways (14,15). This is consistent with increased inflammatory biomarkers in the blood of patients with NAION (16). Because hypoxia worsens with increased altitude, arterial oxygen saturation and ocular perfusion pressure decrease, retinal venous pressure increases, and intraocular pressure remains stable (17). These 5 cases with HA-NAION raised important high-altitude–related factors that need further investigation. Possible factors contributing to NAION at high altitude include (1) hypoxia-induced cerebral edema and elevated intracranial pressure, (2) vasogenic edema causing breakdowns in the blood–brain barrier, (3) increased cerebral blood flow resulting from altered autoregulation leading to capillary overperfusion and vasogenic cerebral edema, (4) contribution of vigorous physical exercise during high-altitude exposure, and (5) sleep disturbances and hypoxia during sleep. Decreased oxygen concentration and worsened sleep apnea may contribute to lower oxygen supplies of optic nerve head at high altitude. A meta-analysis found that patients with OSA had a more than 6-fold increase of NAION when compared with patients without OSA (18). When patients with OSA stay at high altitudes, hypobaric hypoxia may promote central sleep apnea, resulting in combined hypoxia (19). Other risk factors for HA-NAION may include altitude on starting of the ascent, higher and faster ascent, longer duration at high altitude, lower hematocrit, increased strenuous activities, and high baseline intraocular pressure.
There is currently no effective treatment for HA-NAION. Treatment to prevent or reduce symptoms of high-altitude sickness includes oxygen, acetazolamide, and immediate descent. However, in previous studies, the use of acetazolamide was not associated with less optic disc edema or less central nervous system symptoms, although when combined with steroids, some showed encouraging results by improving perfusion pressure in the vessels of the optic nerve head and alleviating the imbalance between the intraocular pressure and perfusion pressure (20). Hypobaric hypoxia has not been proven to worsen OSA in patients with NAION. However, given the known risk for recurrent NAION and the known effectiveness of CPAP for OSA, we recommend that all patients with clinically confirmed OSA are treated, especially during repeated high-altitude exposure.
Limitations of this study include its retrospective nature and small sample size. Because there was no examination just before high-altitude exposure, we cannot rule out that these patients may have had presymptomatic swelling of the optic nerve head and then manifest with symptoms during high-altitude exposure. Although none of the patients in our cohort developed NAION during air travel, the potential high-altitude exposure during long trans-Atlantic flights has becoming an increasingly concerning risk factor because commercial aircraft cabin pressurization is maintained at 4,000–8,000 ft. Further studies are necessary for the diagnosis and management of air travel–associated NAION.
NAION can occur with high-altitude exposure resulting in severe irreversible vision loss. The visual outcome is similar to NAION that occurs in normal altitude. Hypobaric hypoxia and sleep apnea may be contributing factors. A prospective study is needed to further clarify the association between high-altitude exposure and NAION. Understanding the physiologic changes in patients at high risk of NAION before and after high-altitude exposure can more clearly identify changes that contribute to developing HA-NAION.
ACKNOWLEDGMENTS
The authors thank Dr. Yan Yan from Shanghai Jiaotong University School of Medicine and Dr. Robert O’Donnell for their critical review of this manuscript.
Supported by unrestricted grant from Research to Prevent Blindness, Inc. and the National Eye Institute P30-EY026877. Y. J. Liao and H. E. Moss are both supported by NANOS Pilot Grant. L. A. Mesentier-Louro is supported by the Stanford School of Medicine TRAM grant. H. E. Moss is supported by an NIH K23-EY024345 unrestricted grant. Y. A. Liu is supported in part by the UC Davis Paul Calabresi Career Development Award for Clinical Oncology as funded by the National Cancer Institute/National Institutes of Health through Grant #2K12CA138464-11.
Footnotes
STATEMENT OF AUTHORSHIP
Conception and design: Y. A. Liu, H. E. Moss, S. J. Beres, Y. J. Liao; Acquisition of data: Y. A. Liu, M. A. Shariati, H. E. Moss, S. J. Beres, Y. J. Liao; Analysis and interpretation of data: Y. A. Liu, L. A. Mesentier-Louro, M. A. Shariati, H. E. Moss, S. J. Beres, Y. J. Liao. Drafting the manuscript: Y. A. Liu, Y. J. Liao; Revising the manuscript for intellectual content: Y. A. Liu, L. A. Mesentier-Louro, H. E. Moss, S. J. Beres, Y. J. Liao. Final approval of the completed manuscript: Y. A. Liu, L. A. Mesentier-Louro, M. A. Shariati, H. E. Moss, S. J. Beres, Y. J. Liao.
The authors report no conflicts of interest.
Contributor Information
Yin A. Liu, Department of Ophthalmology, Stanford University School of Medicine, Stanford, California; Departments of Ophthalmology & Vision Science, Neurology, and Neurological Surgery, University of California, Davis, Sacramento, California.
Louise A. Mesentier-Louro, Department of Ophthalmology, Stanford University School of Medicine, Stanford, California.
Mohammad A. Shariati, Department of Ophthalmology, Stanford University School of Medicine, Stanford, California.
Heather E. Moss, Department of Ophthalmology, Stanford University School of Medicine, Stanford, California; Department of Neurology & Neurological Sciences, Stanford University School of Medicine, Stanford, California..
Shannon J. Beres, Department of Ophthalmology, Stanford University School of Medicine, Stanford, California; Department of Neurology & Neurological Sciences, Stanford University School of Medicine, Stanford, California..
Yaping Joyce Liao, Department of Ophthalmology, Stanford University School of Medicine, Stanford, California; Department of Neurology & Neurological Sciences, Stanford University School of Medicine, Stanford, California..
REFERENCES
- 1.Wiedman M, Tabin GC. High-altitude retinopathy and altitude illness. Ophthalmology 1999;106:1924–1927. discussion 1927. [DOI] [PubMed] [Google Scholar]
- 2.Willmann G, Fischer MD, Schatz A, Kai S, Andre M, Eberhart Z, Bartz-Schmidt KU, Gekeler F. Quantification of optic disc edema during exposure to high altitude shows no correlation to acute mountain sickness. PLoS One 2011;6:e27022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willmann G, Gekeler F, Schommer K, Bartsch P. Update on high altitude cerebral edema including recent work on the eye. High Alt Med Biol 2014;15:112–122. [DOI] [PubMed] [Google Scholar]
- 4.Willmann G, Fischer MD, Schatz A, Schommer K, Messias A, Zrenner E, Bartz-Schmidt KU, Gekeler F. Quantification of optic disc edema during exposure to high altitude shows no correlation to acute mountain sickness. PLoS One 2011;6:ARTN e27022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosch MM, Barthelmes D, Merz TM, Bloch KE, Turk AJ, Hefti U, Sutter FKP, Maggiorini M, Wirth MG, Schoch OD, Landau K. High incidence of optic disc swelling at very high altitudes. Arch Ophthalmol 2008;126:644–650. [DOI] [PubMed] [Google Scholar]
- 6.Barthelmes D, Bosch MM, Merz TM, Barthelmes D, Bosch MM, Merz TM, Petrig BL, Truffer F, Bloch KE, Holmes TA, Cattin P, Hefti U, Sellner M, Sutter FKP, Maggiorini M, Landau K. Delayed appearance of high altitude retinal hemorrhages. PLoS One 2011;6:e11532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian X, Zhang B, Jia Y, Wang C, Li Q. Retinal changes following rapid ascent to a high-altitude environment. Eye (Lond) 2018;32:370–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berry S, Lin WV, Sadaka A, Lee AG. Nonarteritic anterior ischemic optic neuropathy: cause, effect, and management. Eye Brain 2017;9:23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayreh SS. Ischemic optic neuropathy. Prog Retin Eye Res 2009;28:34–62. [DOI] [PubMed] [Google Scholar]
- 10.Preechawat P, Bruce BB, Newman NJ, Biousse V. Anterior ischemic optic neuropathy in patients younger than 50 years. Am J Ophthalmol 2007;144:953–960. [DOI] [PubMed] [Google Scholar]
- 11.Distefano AG, Lam BL. Non-arteritic anterior ischemic optic neuropathy in pilots. Aerosp Med Hum Perform 2018;89:1005–1007. [DOI] [PubMed] [Google Scholar]
- 12.Bandyopadhyay S, Singh R, Gupta V, Gupta A. Anterior ischaemic optic neuropathy at high altitude. Indian J Ophthalmol 2002;50:324–325. [PubMed] [Google Scholar]
- 13.Newman WD, Dorrell ED. Anterior ischemic optic neuropathy associated with disc drusen. J Neuroophthalmol 1996;16:7–8. [PubMed] [Google Scholar]
- 14.Mesentier-Louro LA, Shariati MA, Dalal R, Camargo A, Kumar V, Shamskhou EA, de Jesus Perez V, Liao YJ. Systemic hypoxia led to little retinal neuronal loss and dramatic optic nerve glial response. Exp Eye Res 2020;193:107957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mesentier-Louro LA, Rangel B, Stell L, Shariati MA, Dalal R, Nathan A, Yuan K, de Jesus Perez V, Liao YJ. Hypoxia-induced inflammation: profiling the first 24-hour posthypoxic plasma and central nervous system changes. PLoS One 2021;16:e0246681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mesentier-Louro LA, Stell L, Yan Y, Montague AA, de Jesus Perez V, Liao YJ. Immunoprofiling of nonarteritic anterior ischemic optic neuropathy. Transl Vis Sci Technol 2021;10:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baertschi M, Dayhaw-Barker P, Flammer J. The effect of hypoxia on intra-ocular, mean arterial, retinal venous and ocular perfusion pressures. Clin Hemorheol Microcirc 2016;63:293–303. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y, Zhou LM, Lou H, Cheng JW, Wei RL. The association between obstructive sleep apnea and nonarteritic anterior ischemic optic neuropathy: a systematic review and meta-analysis. Curr Eye Res 2016;41:987–992. [DOI] [PubMed] [Google Scholar]
- 19.Burgess KR, Ainslie PN. Central sleep apnea at high altitude. Adv Exp Med Biol 2016;903:275–283. [DOI] [PubMed] [Google Scholar]
- 20.Hayreh SS, Zimmerman MB. Optic disc edema in non-arteritic anterior ischemic optic neuropathy. Graefes Arch Clin Exp Ophthalmol 2007;245:1107–1121. [DOI] [PubMed] [Google Scholar]


