Abstract
Background:
Animal models of optic nerve injury are often used to study central nervous system (CNS) degeneration and regeneration, and targeting the optic nerve is a powerful approach for axon-protective or remyelination therapy. However, the experimental delivery of drugs or cells to the optic nerve is rarely performed because injections into this structure are difficult in small animals, especially in mice.
New method:
We investigated and developed methods to deliver drugs or cells to the mouse optic nerve through 3 different routes: a) intraorbital, b) through the optic foramen and c) transcranial.
Results:
The methods targeted different parts of the mouse optic nerve: intraorbital proximal (intraorbital), intracranial middle (optic-foramen) or intracranial distal (transcranial) portion.
Comparison with existing methods:
Most existing methods target the optic nerve indirectly. For instance, intravitreally delivered cells often cannot cross the inner limiting membrane to reach retinal neurons and optic nerve axons. Systemic delivery, eye drops and intraventricular injections do not always successfully target the optic nerve. Intraorbital and transcranial injections into the optic nerve or chiasm have been performed but these methods have not been well described. We approached the optic nerve with more selective and precise targeting than existing methods.
Conclusions:
We successfully targeted the murine optic nerve intraorbitally, through the optic foramen, and transcranially. Of all methods, the injection through the optic foramen is likely the most innovative and fastest. These methods offer additional approaches for therapeutic intervention to be used by those studying white matter damage and axonal regeneration in the CNS.
Keywords: Optic nerve injections, Intraorbital, Optic foramen, Transcranial injection
1. Introduction
The optic nerve is a central nervous system (CNS) white matter tract that conveys the information from the retina to the brain. The human optic nerve is composed of 1.2 million axons of the retinal ganglion cells (RGCs) (Vrabec and Levin, 2007). The soma of the RGCs reside in the inner layer of the retina, while the axons travel through the optic nerve head, exits the globe, become myelinated by optic nerve oligodendrocytes, and synapse onto the brainstem and other nuclei (Benowitz et al., 2017; Calkins et al., 2017). This anatomical compartmentalization is advantageous for diverse investigative approaches. Indeed, the optic nerve has been widely used as a model to study CNS degeneration and regeneration (Benowitz and Yin, 2008). In addition, the optic nerve is a therapeutic target for a wide group of disorders named optic neuropathies, such as glaucoma and ischemic optic neuropathies, which may cause progressive or sudden vision loss (Hayreh, 2009; Bessero and Clarke, 2010; Sun and Liao, 2017).
The optic nerve can be experimentally approached indirectly through different routes (Fig. 1). For instance, eye drops containing nerve growth factor (NGF) can reach the retina and optic nerve (Lambiase et al., 2005), but many drugs cannot be delivered in eye drops because of poor penetration, short half-life, systemic toxicity, or other factors (Patel et al., 2013; Alvarez-Trabado et al., 2017). Systemic administration through oral or intravenous routes are the most common ways to deliver drugs to the body, but the need to cross the blood-brain barrier (or blood-retina and blood-optic nerve barriers) limits delivery of effective therapies and may increase side-effects due to the large doses required for penetrating these barriers (Choonara et al., 2010). Intravitreal injection is the most common route of delivery for stem cells or drugs to treat visual loss (Johnson and Martin, 2013; Mead et al., 2017). However, intravitreal injection is limited to delivery of treatment to the retina, not the optic nerve, and the inner limiting membrane is a barrier that limits penetration of the drug to reach retinal neurons and optic nerve axons (Johnson et al., 2010). Intraorbital delivery to the anterior optic nerve (Asavapanumas et al., 2014) has been performed previously but to our knowledge this method has not been described in detail. Intraventricular delivery is a common, invasive way to directly deliver cells or drugs to the brain (DeVos and Miller, 2013; Cohen-Pfeffer et al., 2017), but it does not specifically target the optic nerve, which may reduce efficacy and increase the risk of CNS side effects. Intracranial delivery to the optic chiasm has been described (Dehghan et al., 2016), but not targeting the optic nerve before it reaches the chiasm.
Fig. 1.
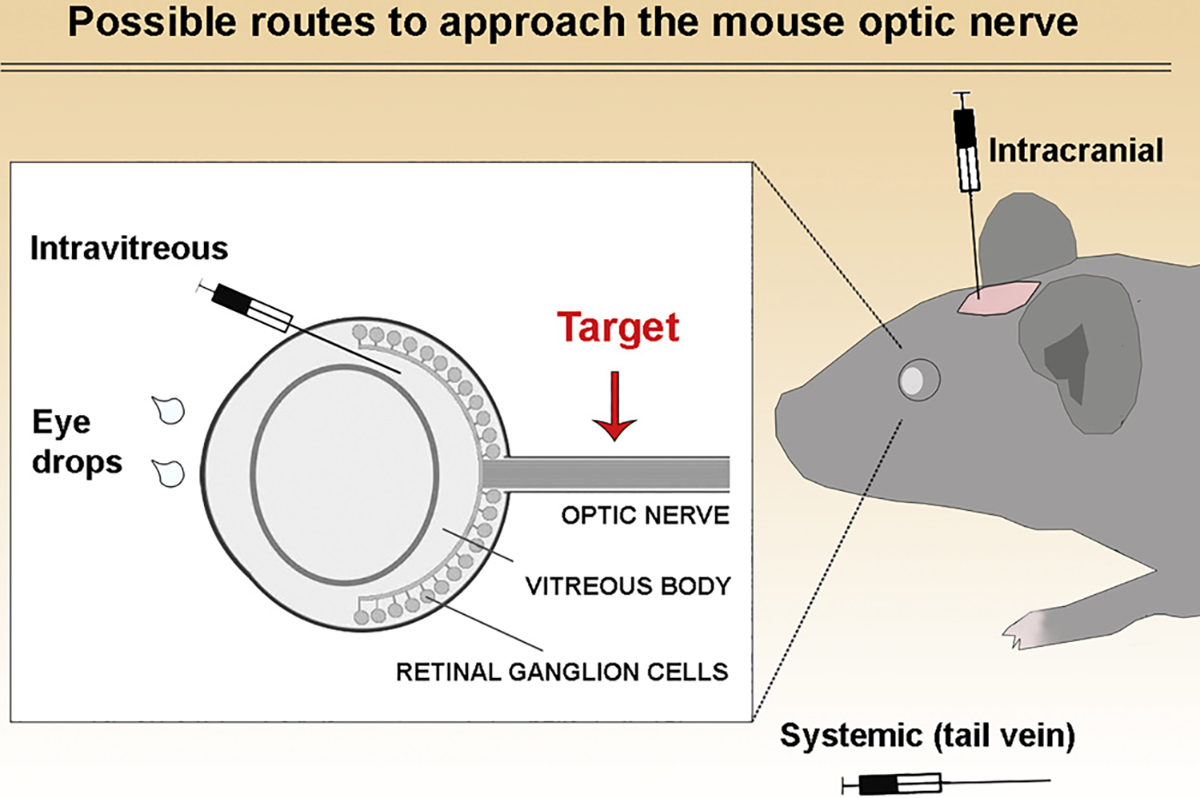
Possible routes to approach the optic nerve. Drugs or cells have been delivered in different ways to target the visual system. Eye drops and intravitreal injections containing growth factors have efficiently targeted the retina and optic nerve, but this may not be feasible for all drugs given the transport is by unclear mechanisms (Lambiase et al., 2005 IOVS). Direct application of cells to the optic nerve sheath have been performed in adult rats but not in mice (Guo et al., 2014 Cell Death and Dis.). Other potential approaches to target the optic nerve include intracranial and systemic injections. Intracranial injections can target the optic nerve indirectly or directly depending on where the needle is inserted. Indirect methods listed above have variable levels of success in targeting the optic nerve. In this study, we propose direct injections into the optic nerve to approach this structure in mice.
Although intra-optic nerve injections have been performed in humans in clinical trials (Weiss et al., 2016), most preclinical studies targeting the optic nerve deliver treatment through intravitreal injections (Mesentier-Louro et al., 2016). Although RGC cell soma can likely be targeted through intravitreal injections, the optic nerve axons may need direct delivery of axon-protective therapy. In addition, the optic nerve is a white matter tract that can be used to study transplantation of oligodendroglia, which holds promise as a treatment to central white matter disease. There are a few studies that were successful in targeting the optic nerve of adult rats (Guo et al., 2014; Raykova et al., 2015), but, to our knowledge, there are no well described methods of direct delivery to the mouse optic nerve. Experimental assessment of the mouse optic nerve for drug or cell delivery is particularly important in preclinical studies because the mouse is the preferred species for most preclinical in vivo studies due to easy manipulation, availability of transgenic animals and well-characterized injury models, while the optic nerve is an excellent model to study CNS degeneration and regeneration.
In this study, we delivered dyes or cells by targeting the mouse optic nerve through 3 different routes: a) intraorbital, b) through the optic foramen and c) transcranial.
2. Materials and methods
2.1. Animals
All animal care and experiments were carried out in accordance with the Association for Research in Vision and Ophthalmology statement for the Use of Animals in Ophthalmic and Vision Research and with approval from the Stanford University Administrative Panel on Laboratory Animal Care (SU-APLAC 15886). Adult wild-type C57BL/6 mice (Charles River Laboratories, Inc., Wilmington, MA, USA) were housed in cages at constant temperature, with a 12:12-hour light/dark cycle, with food and water available ad libitum. All efforts were made to minimize animal suffering, including the use of discarded animal carcasses for explorative procedures. Animals were kept under supervision and warmed by a heat pad until they recovered from the anesthesia.
2.2. Dyes used for injection
There are several dyes that can be used to assess whether injections were successful. We chose to work with Toluidine blue (saturated solution in water) to visualize the nerve after dissection.
2.3. Intraorbital injection
This method requires the exposure of the intraorbital portion of the optic nerve, utilizing a similar approach as described for optic nerve crush (Meyer and Miotke, 1990; Moore et al., 2009; Tang et al., 2011). We performed this procedure in 16 optic nerves to establish this method.
Mice were anesthetized by intraperitoneal injection of ketamine (80–100 mg/kg) and xylazine (5–10 mg/kg) and one drop of 0.5% proparacaine hydrochloride ophthalmic solution USP was applied on the eye. A subcutaneous injection of carprofen (5 mg/kg) was given for analgesia.
The mouse was placed in right lateral decubitus to expose the left optic nerve.
We used fine forceps to hold the conjunctiva and dissected a small window with Vannas scissors along the globe half way between the limbus and the back of the eye (Video 1). This allowed visualization of the retro-orbital sinus (Fig. 2B, arrowhead). Avoiding damage to the vessels, two fine forceps were used to expose the optic nerve (Fig. 2B, arrow), by inserting the forceps in between the two branches of the vessels.
Fig. 2.
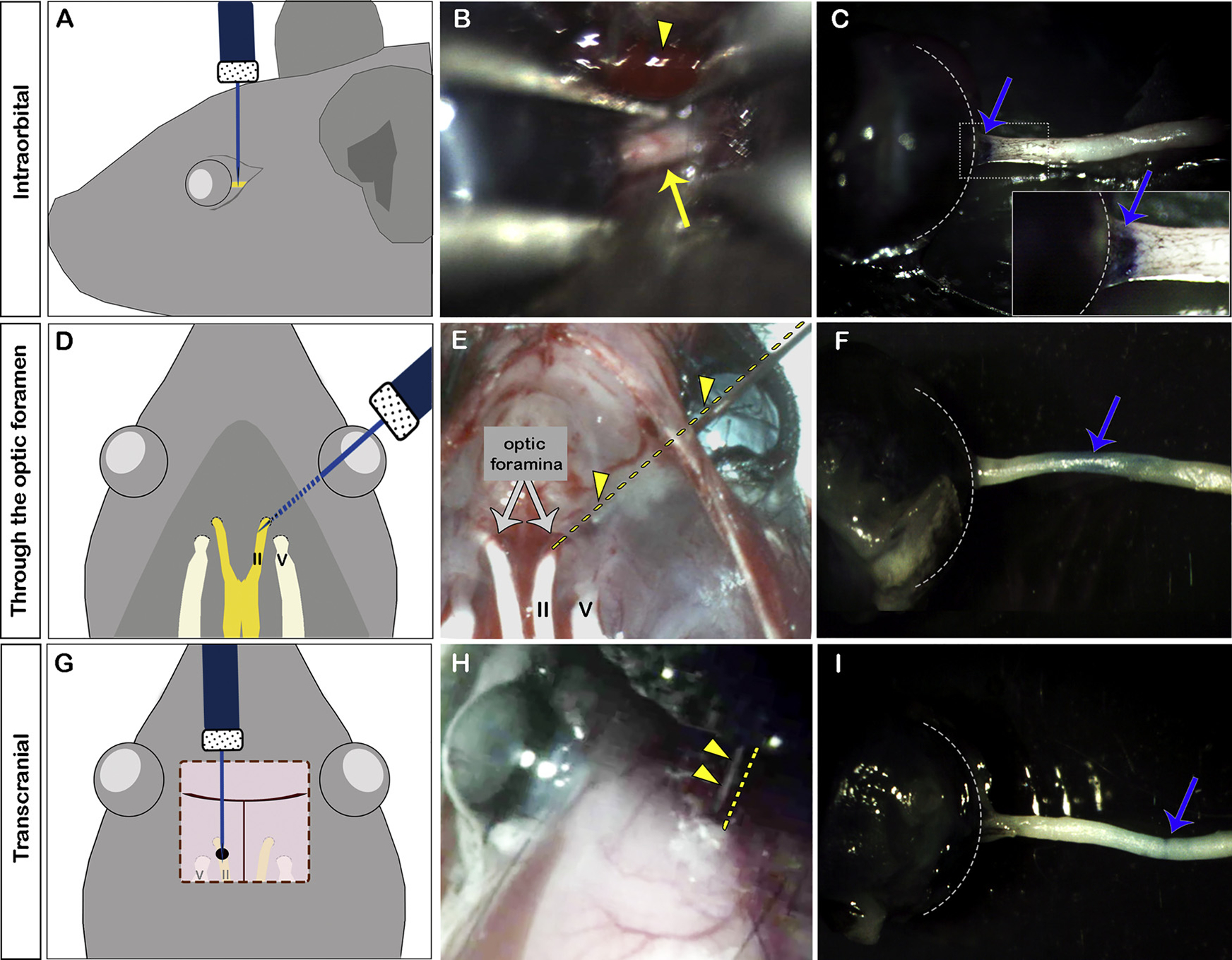
Three different routes to target the optic nerve. Injection of Toluidine blue into the optic nerve intraorbitally (A–C), through the optic foramen (D–F), or transcranially (G–I). A, D, G: diagrams or each approach. B, E, H: color photograph illustrating each approach. Arrowhead in B indicates the retro-orbital sinus; arrow in B indicates the optic nerve. E and H are photographs taken of mice carcasses partially dissected for better visualization of the structures. The two optic foramina are indicated by gray arrows in E. Arrowheads and dashed lines in E and H indicate the needle. C, F, I: color photograph of dissected optic nerve showing Toluidine blue staining (blue arrows) of the optic nerve after injection via each approach. Inset in C shows higher magnification of the area inside the dashed rectangle. Dashed lines in C, F and I delineate the globe. V: cranial nerve V (trigeminal nerve); II: cranial nerve II (optic nerve).
Once the optic nerve was exposed, the eye was kept in a steady position with one of the forceps in the left hand of the surgeon, avoiding excessive stretching that could cause damage to the optic nerve. By holding the syringe (Hamilton precision syringe, 5–10 μL, Hamilton Company) with the right hand, the needle (33 g, sharp, Hamilton Company) was inserted into the optic nerve (Video 1). We preferred to hold the syringe with one hand and release the retraction on the globe in order to free up the other hand to carefully push the piston of the syringe to deliver its contents into the nerve. By using this one-person only method, the injection is not visualized but this does not affect the delivery. If direct visualization is preferred, especially in the beginning of trying this approach, the injection can be done by an assistant or a with a controlled delivery system. After the procedure is done, the conjunctiva can be sutured or not depending on the size of the incision.
For the first three days after the procedure, a subcutaneous injection of carprofen (5 mg/kg) was given once a day for analgesia and Bacitracin ointment (500 units/gm) was applied over the eye twice a day to prevent infections.
2.4. Injection through the optic foramen
A better way to target the posterior intraorbital or the intracranial optic nerve without performing craniotomy is through the optic foramen. Although very simple to perform, this method has not been published to our knowledge. We performed this approach in 29 optic nerves to establish this method. In mice, the intraorbital optic nerve exits the orbit through the optic foramen, which is ~0.6 mm in diameter in adult mice and is located at the skull base, half way between the midline and the lateral end of the wing and rostral and caudal borders of the wing (Bab, 2007).
Video 2 shows how the injection through the optic foramen without direct visualization is performed in the live animal, and Video 3 illustrates the same injection in a mouse carcass with an open skull, showing how a 33-gauge sharp needle (~0.2 mm in outer diameter) introduced through the conjunctiva reaches the optic nerve (II cranial nerve, Fig. 2D) by sliding in parallel to the orbital bone. Because this is a blind injection, we recommend practicing with partially dissected (brain removed) murine carcasses first.
Mice were anesthetized by intraperitoneal injection of ketamine (80–100 mg/kg) and xylazine (5–10 mg/kg) and one drop of 0.5% proparacaine hydrochloride ophthalmic solution USP was applied on the eye. A subcutaneous injection of carprofen (5mg/kg) was given for analgesia.
The anesthetized mouse was placed in ventral decubitus. We gently fixed the head of the animal using one hand (the head can also be fixed using a stereotaxic frame if desired) and used forceps in the other hand to feel the superior rim of the orbital bone to estimate the entry point of the needle. Once this has been established, the needle was inserted just superior to the globe and slided through the conjunctiva in a 45° trajectory relative to the midline (Videos 2 and 3). The surgeon should feel the needle sliding along the edge of the orbital bone and allow it to penetrate approximately 4 mm to correctly target the intracranial portion of the optic nerve just after it exits the optic foramen. Injection into the posterior intraorbital optic nerve can also be performed by penetrating between 3–4 mm in the same angle. Prior to injection, the surgeon should feel the resistance of the optic nerve dural sheath and then deliver the content of the syringe into the optic nerve.
2.5. Transcranial injection
To target the intracranial optic nerve, traditional transcranial injections can be done. We targeted the intracranial portion of the optic nerve located at 0.5 mm lateral and 1.5 mm anterior of bregma, which is ~1.0 mm anterior to the optic chiasm. For comparison, previous publication showed that injections into the nearby optic chiasm can be performed by changing the coordinates more medially and posteriorly to 0.0 mm lateral and 0.5 mm anterior of bregma (Dehghan et al., 2016).
Since transcranial injections have been well described using stereotaxic frame (Cetin et al., 2006), we only performed this method in 3 mouse carcasses in order to compare the different methods performed in this study (Fig. 2). Briefly, the mouse carcass was placed in a stereotaxic frame, and the scalp cleaned. An incision was made on the scalp along the midline using a scalpel. Using a drill to perforate the skull, a small hole was made on the located site of injection and the needle was inserted until it touched the cranial base to deliver the syringe contents. To perform this technique in live animals, the animals should be anesthetized with ketamine (80–100 mg/kg) and xylazine (5–10 mg/kg), and lubricant eye drops should be applied to the eyes.
3. Results and discussion
3.1. Comparison of different optic nerve injection methods
The easiest way to learn these different injection techniques is to practice injecting a dye such as Toluidine blue. In Fig. 2, intraorbital optic nerve injection led to blue staining of the most proximal optic nerve just posterior to the globe (arrow in Fig. 2C). Injection through the optic foramen led to blue staining of the middle portion of the optic nerve (arrow in Fig. 2F). Finally, transcranial injection led to blue staining of distal portion of the optic nerve (arrow in Fig. 2I).
Of the 3 methods, injection through the optic foramen is probably the fastest and does not require extensive dissection or suturing. However, it is an injection without direct visualization, so this technique may be harder to learn initially. This technique should be practiced with a partially dissected skull before performing the technique on a live animal. In the live animal, this injection technique can be consistently performed by feeling the rigid needle against the edge of the orbital bone and penetrating the optic nerve sheath.
The intraorbital method is relatively easy to those who already have experience with optic nerve crush. For novice, this method is challenging and requires greater surgical dexterity because there is limited space to work and any accidental disruption of the retro-orbital sinus will lead to bleeding. The transcranial technique requires craniotomy and takes the most time to perform but has lower risk of bleeding and incorrect targeting.
3.2. Potential ways to minimize damage to the optic nerve
When injecting into the optic nerve, axonal damage may occur, but for some approaches such as stem cell transplantation it may be necessary to deliver cells locally. Instead of injecting through the nerve sheath, which increases risk of damage to the optic nerve axons, the same approach can be used to deliver drug or cells coated in some type of biomaterials like hydrogels, particles and fibrous materials (Forbes and Andrews, 2017; Ziemba and Gilbert, 2017). However, the sheath may be a barrier, so sometimes a small cut on the nerve sheath needs to be made (Guo et al., 2014).
We used a needle gauge that could be suitable either for drug or cell delivery (33 gauge), but we anticipate that, if no cells need to be injected, a higher gauge or glass micropipettes may be used, improving the precision of injection, particularly after exposure of the nerve. That would minimize damage to the optic nerve when inserting the needle into the nerve. In addition, although we used an injection volume of 2 μl to allow troubleshooting of the methods with abundant dye suspension, we recommend injecting smaller volumes to minimize damage and avoid leakage.
3.3. In vivo visualization after injection
Although fluorescent dyes like CellTracker or CellTrace (Thermo Fisher Scientific, MA, USA) are not currently easily used to track cells injected anywhere other than the eye, labeled cells can be injected into the optic nerve and then the location of the cells can be assessed using traditional histology and microscopy in multiple time points. A better way to track cells is to do some type of in vivo imaging. However, because visualization of the injected cells is not always possible in live animals, we recommend in vivo tracking of the drug or cells injected whenever possible. There are different ways of labeling cells and then tracking them. This depends on the cell type used, availability of equipment, and duration of follow-up. In vivo bioluminescence imaging is a useful technique to track previously labeled cells (e.g. transduced with viral vectors to express luciferase). Because the signal is only present after the expressed luciferase protein catalyzes a reaction with intraperitoneally injected luciferin to produce light, this method only detects signal from cells that are metabolically active and not dead cells or non-cell components. However, this method is limited because it has limited spatial resolution capacity (2–3 mm) and therefore depth penetration (1cm) (Arbab et al., 2009). In a small number of animals, we injected luciferase-expressing fibroblasts using the intraorbital and through the optic foramen approaches and used the bioluminescence imaging of the whole animal 24 h after injection to determine the approximate location of the cells (Mesentier-Louro et al., unpublished data). Another method of cell tracking that has better spatial resolution is to label cells with superparamagnetic iron oxide nanoparticles and then image with magnetic resonance imaging (MRI) machine. This method can localize the cells within the optic nerve and can be used for serial, long-term tracking in vivo for weeks to months (Mesentier-Louro et al., 2014). Another method is to use radioisotopes to label cells prior to injection, since the radioactive cells can be tracked by scintigraphy over days to weeks, depending on the half-life of the radioisotope, using a SPECT/CT camera (Zaverucha-do-Valle et al., 2014).
4. Conclusions
Treatments that improve optic nerve health and function may be most appropriately delivered by targeting the optic nerve instead of intravitreal, intraventricular, or systemic delivery. However, targeted delivery of drugs or cells to the optic nerve is difficult in the mouse, the most common small animal used in preclinical studies. In this study, we developed and compared 3 different methods to target different portions of the mouse optic nerve using injections intraorbitally, through the optic foramen, and transcranially. The best method can be chosen depending on the location of the injury and the preferred part of the optic nerve to target. Of all methods, the injection through the optic foramen is likely the most innovative, fastest and does not require extensive equipment or dexterity of the surgeon. These methods offer additional approaches for therapeutic intervention to be used by those studying white matter damage and axonal regeneration in the CNS.
Supplementary Material
Acknowledgments
We thank Roopa Dalal for tissue preparation and sectioning and Joana Galvao for help with surgical methods.
Funding
This work was supported by the North American Neuro-Ophthalmology Society Pilot Grant, Bonderman grant and grants from the National Eye Institute (NEI P30-EY026877) and Research to Prevent Blindness, Inc, all from United States of America.
Footnotes
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jneumeth.2018.10.038.
References
- Alvarez-Trabado J, Diebold Y, Sanchez A, 2017. Designing lipid nanoparticles for topical ocular drug delivery. Int. J. Pharm. 532, 204–217. [DOI] [PubMed] [Google Scholar]
- Arbab AS, Janic B, Haller J, Pawelczyk E, Liu W, Frank JA, 2009. In vivo cellular imaging for translational medical research. Curr. Med. Imaging Rev. 5, 19–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asavapanumas N, Ratelade J, Papadopoulos MC, Bennett JL, Levin MH, Verkman AS, 2014. Experimental mouse model of optic neuritis with inflammatory demyelination produced by passive transfer of neuromyelitis optica-immunoglobulin G. J. Neuroinflamm. 11, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bab I, 2007. Micro-tomographic Atlas of the Mouse Skeleton. Springer, New York. [Google Scholar]
- Benowitz L, Yin Y, 2008. Rewiring the injured CNS: lessons from the optic nerve. Exp. Neurol. 209, 389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz LI, He Z, Goldberg JL, 2017. Reaching the brain: advances in optic nerve regeneration. Exp. Neurol. 287, 365–373. [DOI] [PubMed] [Google Scholar]
- Bessero AC, Clarke PG, 2010. Neuroprotection for optic nerve disorders. Curr. Opin. Neurol. 23, 10–15. [DOI] [PubMed] [Google Scholar]
- Calkins DJ, Pekny M, Cooper ML, Benowitz L, IIoA Lasker, Neurodegeneration Glaucomatous, 2017. The challenge of regenerative therapies for the optic nerve in glaucoma. Exp. Eye Res. 157, 28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin A, Komai S, Eliava M, Seeburg PH, Osten P, 2006. Stereotaxic gene delivery in the rodent brain. Nat. Protoc. 1, 3166–3173. [DOI] [PubMed] [Google Scholar]
- Choonara YE, Pillay V, Danckwerts MP, Carmichael TR, du Toit LC, 2010. A review of implantable intravitreal drug delivery technologies for the treatment of posterior segment eye diseases. J. Pharm. Sci. 99, 2219–2239. [DOI] [PubMed] [Google Scholar]
- Cohen-Pfeffer JL, Gururangan S, Lester T, Lim DA, Shaywitz AJ, Westphal M, Slavc I, 2017. Intracerebroventricular delivery as a safe, long-term route of drug administration. Pediatr. Neurol. 67, 23–35. [DOI] [PubMed] [Google Scholar]
- Dehghan S, Hesaraki M, Soleimani M, Mirnajafi-Zadeh J, Fathollahi Y, Javan M, 2016. Oct4 transcription factor in conjunction with valproic acid accelerates myelin repair in demyelinated optic chiasm in mice. Neuroscience 318, 178–189. [DOI] [PubMed] [Google Scholar]
- DeVos SL, Miller TM, 2013. Direct intraventricular delivery of drugs to the rodent central nervous system. J. Vis. Exp. 75, e50326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes LH, Andrews MR, 2017. Restoring axonal localization and transport of transmembrane receptors to promote repair within the injured CNS: a critical step in CNS regeneration. Neural Regen. Res. 12, 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Davis B, Nizari S, Normando EM, Shi H, Galvao J, Turner L, Shi J, Clements M, Parrinello S, Cordeiro MF, 2014. Direct optic nerve sheath (DONS) application of Schwann cells prolongs retinal ganglion cell survival in vivo. Cell Death Dis. 5, e1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayreh SS, 2009. Ischemic optic neuropathy. Progr. Retin Eye Res. 28, 34–62. [DOI] [PubMed] [Google Scholar]
- Johnson TV, Martin KR, 2013. Cell transplantation approaches to retinal ganglion cell neuroprotection in glaucoma. Curr. Opin. Pharmacol. 13, 78–82. [DOI] [PubMed] [Google Scholar]
- Johnson TV, Bull ND, Martin KR, 2010. Identification of barriers to retinal engraftment of transplanted stem cells. Invest. Ophthalmol. Vis. Sci. 51, 960–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambiase A, Tirassa P, Micera A, Aloe L, Bonini S, 2005. Pharmacokinetics of conjunctivally applied nerve growth factor in the retina and optic nerve of adult rats. Invest. Ophthalmol. Vis. Sci. 46, 3800–3806. [DOI] [PubMed] [Google Scholar]
- Mead B, Logan A, Berry M, Leadbeater W, Scheven BA, 2017. Concise review: dental pulp stem cells: a novel cell therapy for retinal and central nervous system repair. Stem Cells 35 (1), 61–67. [DOI] [PubMed] [Google Scholar]
- Mesentier-Louro LA, Zaverucha-do-Valle C, Rosado-de-Castro PH, Silva-Junior AJ, Pimentel-Coelho PM, Mendez-Otero R, Santiago MF, 2016. Bone Marrow-Derived Cells as a Therapeutic Approach to Optic Nerve Diseases. Stem Cells Int. 2016, 5078619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesentier-Louro LA, Zaverucha-do-Valle C, da Silva-Junior AJ, Nascimento-Dos-Santos G, Gubert F, de Figueiredo AB, Torres AL, Paredes BD, Teixeira C, Tovar-Moll F, Mendez-Otero R, Santiago MF, 2014. Distribution of mesenchymal stem cells and effects on neuronal survival and axon regeneration after optic nerve crush and cell therapy. PLoS One 9, e110722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RL, Miotke J, 1990. Rapid initiation of neurite outgrowth onto laminin from explants of adult mouse retina induced by optic nerve crush. Exp. Neurol. 107, 214–221. [DOI] [PubMed] [Google Scholar]
- Moore DL, Blackmore MG, Hu Y, Kaestner KH, Bixby JL, Lemmon VP, Goldberg JL, 2009. KLF family members regulate intrinsic axon regeneration ability. Science 326, 298–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Cholkar K, Agrahari V, Mitra AK, 2013. Ocular drug delivery systems: an overview. World J. Pharmacol. 2, 47–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raykova K, Jones MV, Huang H, Hoffman PF, Levy M, 2015. Minimally-invasive technique for injection into rat optic nerve. J. Vis. Exp. 99, 52249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun MH, Liao YJ, 2017. Structure-Function Analysis of Nonarteritic Anterior Ischemic Optic Neuropathy and Age-Related Differences in Outcome. J. Neuroophthalmol. 37, 258–264. [DOI] [PubMed] [Google Scholar]
- Tang Z, Zhang S, Lee C, Kumar A, Arjunan P, Li Y, Zhang F, Li X, 2011. An optic nerve crush injury murine model to study retinal ganglion cell survival. J. Vis. Exp. 50, 2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrabec JP, Levin LA, 2007. The neurobiology of cell death in glaucoma. Eye 21 (Suppl 1), S11–14. [DOI] [PubMed] [Google Scholar]
- Weiss JN, Levy S, Benes SC, 2016. Stem Cell Ophthalmology Treatment Study (SCOTS): bone marrow-derived stem cells in the treatment of Leber’s hereditary optic neuropathy. Neural Regen. Res. 11, 1685–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaverucha-do-Valle C, Mesentier-Louro L, Gubert F, Mortari N, Padilha AB, Mencalha A, Abdelhay E, Teixeira C, Tovar-Moll F, Lopes de Souza SA, Gutfilen B, Mendez-Otero R, 2014. Sustained effect of bone marrow mononuclear cell therapy in axonal regeneration in a model of optic nerve crush. Brain Res. 1587, 54–68. [DOI] [PubMed] [Google Scholar]
- Ziemba AM, Gilbert RJ, 2017. Biomaterials for local, controlled drug delivery to the injured spinal cord. Front. Pharmacol. 8, 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


