Abstract
Rationale
Immature control of breathing is associated with apnea, periodic breathing, intermittent hypoxemia, and bradycardia in extremely preterm infants. However, it is not clear if such events independently predict worse respiratory outcome.
Objectives
To determine if analysis of cardiorespiratory monitoring data can predict unfavorable respiratory outcomes at 40 weeks postmenstrual age (PMA) and other outcomes, such as bronchopulmonary dysplasia at 36 weeks PMA.
Methods
The Prematurity-related Ventilatory Control (Pre-Vent) study was an observational multicenter prospective cohort study including infants born at <29 weeks of gestation with continuous cardiorespiratory monitoring. The primary outcome was either “favorable” (alive and previously discharged or inpatient and off respiratory medications/O2/support at 40 wk PMA) or “unfavorable” (either deceased or inpatient/previously discharged on respiratory medications/O2/support at 40 wk PMA).
Measurements and Main Results
A total of 717 infants were evaluated (median birth weight, 850 g; gestation, 26.4 wk), 53.7% of whom had a favorable outcome and 46.3% of whom had an unfavorable outcome. Physiologic data predicted unfavorable outcome, with accuracy improving with advancing age (area under the curve, 0.79 at Day 7, 0.85 at Day 28 and 32 wk PMA). The physiologic variable that contributed most to prediction was intermittent hypoxemia with oxygen saturation as measured by pulse oximetry <90%. Models with clinical data alone or combining physiologic and clinical data also had good accuracy, with areas under the curve of 0.84–0.85 at Days 7 and 14 and 0.86–0.88 at Day 28 and 32 weeks PMA. Intermittent hypoxemia with oxygen saturation as measured by pulse oximetry <80% was the major physiologic predictor of severe bronchopulmonary dysplasia and death or mechanical ventilation at 40 weeks PMA.
Conclusions
Physiologic data are independently associated with unfavorable respiratory outcome in extremely preterm infants.
Keywords: extremely premature infant, bronchopulmonary dysplasia, apnea, intermittent hypoxemia, predictive value of tests, heart rate
At a Glance Commentary
Scientific Knowledge on the Subject
Extremely preterm infants have immature control of breathing, which is often associated with apnea, periodic breathing, intermittent hypoxemia, and bradycardia. Such abnormalities in control of breathing can be detected and quantitated using mathematical analysis of high-resolution cardiorespiratory monitoring data. Extremely preterm infants also have poor respiratory outcomes, including the need for prolonged respiratory support, but the contribution of abnormal control of breathing to respiratory outcomes has not been determined.
What This Study Adds to the Field
Analysis of high-resolution cardiorespiratory data from extremely preterm infants provides important prognostic information, and physiologic data can predict unfavorable respiratory outcomes, with prediction accuracy increasing with advancing postnatal age. The physiologic variable that contributes most to prediction of unfavorable outcome is intermittent hypoxemia with oxygen saturation as measured by pulse oximetry <90%, and the physiologic variable that contributes most to prediction of highly unfavorable outcome or severe bronchopulmonary dysplasia is intermittent hypoxemia with oxygen saturation as measured by pulse oximetry <80%.
Preterm birth is a major cause of infant mortality and morbidity worldwide (1, 2) and a leading contributor to disability (3). Abnormal control of breathing is common in preterm infants and usually manifests as apnea, intermittent hypoxemia (IH), bradycardia, and periodic breathing (PB). Apnea generally resolves by term-corrected age (4), although some infants have delayed resolution of apnea and/or bradycardia (5, 6). Extremely preterm infants may also have impaired gas exchange caused by respiratory distress syndrome and subsequent bronchopulmonary dysplasia (BPD). The combination of apnea and lung disease often leads to more prolonged episodes of IH (5). Such chronic IH is associated with worse short-term outcomes (e.g., increased respiratory support, retinopathy of prematurity) (7) and neurodevelopmental impairment (8) that are difficult to predict in the neonatal ICU (9).
Because extreme prematurity predisposes to both abnormalities of control of breathing (apnea, IH, bradycardia, and PB) and worse respiratory outcomes, the association between abnormal control of breathing and worse outcomes may be due to the common antecedent of more extreme immaturity. Respiratory outcomes such as BPD are operational definitions based on the magnitude of respiratory support and do not indicate the contribution of impaired control of breathing. Caffeine is known to decrease apnea and reduces BPD (10), suggesting that repeated apneic episodes may contribute to (and are not just associated with) pulmonary outcomes. However, although caffeine reduces apnea, hypoxemic episodes persist (11), and higher doses or extended treatment may be needed (12, 13). Importantly, preterm infants experience PB, very brief bradycardic episodes, and IH not identified by clinical cardiorespiratory monitors. The importance and relationship of these “non–clinically evident” events to outcomes is not established.
The Pre-Vent (Prematurity-related Ventilatory Control; ClinicalTrials.gov identifier NCT03174301) study was an NIH-funded (NHLBI) cooperative agreement among six academic centers (five clinical sites and one data coordinating center) with the primary goal of elucidating the role of ventilatory control as a determinant of respiratory dysfunction in preterm infants (14). The hypothesis of the Pre-Vent multicenter protocol was that features of ventilatory control instability using clinical neonatal ICU cardiorespiratory monitoring data can predict unfavorable respiratory outcomes at 40 weeks postmenstrual age (PMA), as well as other respiratory outcomes such as BPD.
Methods
Additional details regarding the methods are provided in the online supplement.
Study Population
This was a multicenter prospective cohort study including infants <29 weeks gestational age (GA) with continuously archived cardiorespiratory monitoring data (Figure 1) (14). Institutional review board approval was obtained at all sites, with waiver of consent at three clinical centers and informed consent required at two clinical centers. Oversight was provided by an observational safety monitoring board appointed by NHLBI.
Figure 1.
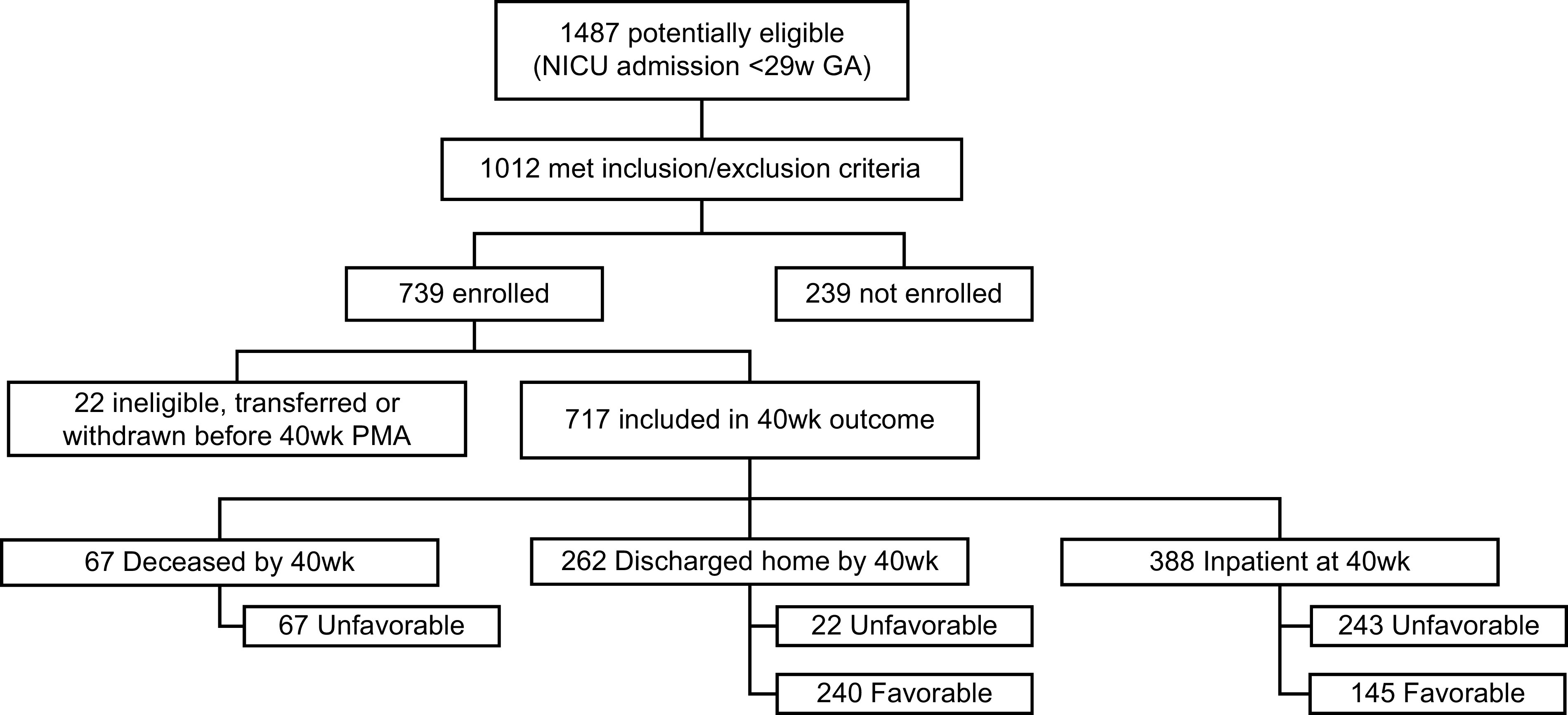
Flow diagram of infants studied in Pre-Vent. GA = gestational age; NICU = neonatal ICU; PMA = postmenstrual age.
Outcome
Primary outcome
Primary outcome was defined as follows:
-
•
Favorable: Either 1) an inpatient at 40 weeks PMA and not on oxygen, not on other flow/pressure respiratory support, and not on inhaled/oral/intravenous respiratory medications, or 2) discharged home before 40 weeks PMA and not on respiratory medications, oxygen, or other respiratory support.
-
•
Unfavorable: Either 1) deceased at or before 40 weeks, 2) inpatient on medications or O2 or other respiratory support at 40 weeks PMA, or 3) discharged before 40 weeks on medications or O2 or other respiratory support.
Unfavorable outcome was further categorized as highly unfavorable (death or invasive mechanical ventilation [IMV] at 40 wk PMA), moderately unfavorable (noninvasive mechanical ventilation/continuous positive airway pressure [CPAP] at 40 wk PMA), or mildly unfavorable (O2 or only medications at 40 wk PMA or discharged home on medications or O2 at <40 wk PMA).
Secondary outcomes
Prespecified secondary outcomes included the following:
Data
Clinical data consisted of baseline clinical characteristics and variables of neonatal illness severity, medications, outcomes, neonatal comorbidities, and respiratory support. Physiologic data consisted of features extracted from cardiorespiratory monitoring data (defined in the online supplement). These features were quantified as the count of events per day, total daily duration of all events, and average duration per event (dpe) for each of the five control of breathing variables:
-
1.
Apnea (⩾20 s [17])
-
2.
PB (wavelet transform using a five-breath template [18])
-
3.
IH events with oxygen saturation as measured by pulse oximetry <80% (IH80) (for 10–300 s [19])
-
4.
IH events with oxygen saturation as measured by pulse oximetry <90% (IH90) (for 10–300 s [19])
-
5.
Bradycardia (<80 beats/min for 5 s or longer)
Analysis
Prediction models were developed (see online supplement) at four time points of Day 7, Day 14, Day 28, and 32 weeks PMA using 24 hours of data as a cross-sectional snapshot. At each time point, models were developed using 1) physiologic data variables alone, 2) clinical variables alone, and 3) combined physiologic + clinical variables. Additional prespecified analyses were done using the longitudinal burden of data up to the time point in each infant to determine if this improved predictive ability.
The primary outcome (favorable vs. unfavorable) was compared using multivariable logistic regression. The cross-validated area under the curve (cvAUC) using 10-fold cross-validation was used as a primary performance measure. Penalized logistic regression (least absolute shrinkage and selection operator) selected model variables, with the final model chosen to optimize cvAUC. Goodness of fit was assessed by McFadden’s pseudo R2 (20). Additional leave-one-site-out analyses were done for validation.
Results
Patient Characteristics
Overall, 717 infants were evaluated (mean ± SD; median: birth weight [BW] 871 ± 259 g; 850 g; GA, 26.4 ± 1.7 wk; 26.4 wk) (Table 1, Figure 1). A total of 385 infants (53.7%) had a favorable outcome (BW, 989 ± 226 g; 961 g; GA, 27.2 ± 1.3 wk; 27.4 wk), whereas 332 (46.3%) had an unfavorable outcome (BW, 735 ± 226 g; 692.5 g; GA, 25.6 ± 1.7 wk; 25.4 wk).
Table 1.
Perinatal and Demographic Information and Illness Severity by Outcome
| Variable | Combined Population | Favorable Outcome | Unfavorable Outcome |
|---|---|---|---|
| Baseline information | |||
| Birth weight, g, mean ± SD; median; 25th–75th, n | 871.3 ± 259; 850; 676–1,050; n = 717 | 988.7 ± 226; 961; 830–1,140; n = 385 | 735.2 ± 226; 693; 579–848; n = 332 |
| <10% percentile weight (Fenton), % (n/N) | 11.3 (81/717) | 3.1% (12/385) | 20.8% (69/332) |
| Birth weight percentile (Fenton), mean ± SD; median; 25th–75th, n | 51 ± 28; 53; 26–75; n = 715 | 57 ± 26; 61; 38–77, n = 385 | 43 ± 30; 44; 16–70; n = 330 |
| Birth length, cm, mean ± SD; median; 25th–75th, n | 33.6 ± 3.5; 33.5; 31–36; n = 697 | 35.1 ± 2.8; 35; 33–37, n = 376 | 31.8 ± 3.2; 31; 29.5–33.6; n = 321 |
| Birth head circumference, cm, mean ± SD; median; 25th–75th, n | 23.7 ± 2.5; 23.5; 22–25.5; n = 685 | 24.7 ± 2.1; 24.5; 23.5–26.0; n = 371 | 22.4 ± 2.3; 22; 21–23.5; n = 314 |
| GA, wk, mean ± SD; median; 25th–75th, n | 26.43 ± 1.71; 26.4; 25.14–28.0; n = 717 | 27.18 ± 1.34; 27.4 ; 26.1–28.3; n = 385 | 25.6 ± 1.7; 25.4; 24.3–26.9; n = 332 |
| Sex (M), % (n/N) | 51% (366/717) | 47.5% (183/385) | 55.1% (183/332) |
| Multiple gestation, % (n/N) | 23% (165/717) | 23.6% (91/385) | 22.3% (74/332) |
| Born outside study center, % (n/N) | 8.9% (64/717) | 8.1% (31/385) | 9.9% (33/332) |
| Clinical chorioamnionitis, % (n/N) | 2.2% (13/591) | 2.5% (8/316) | 1.8% (5/275) |
| Histological chorioamnionitis, % (n/N) | 35.9% (212/591) | 35.8% (113/316) | 36% (99/275) |
| Rupture of membranes before delivery, % (n/N) | 40.7% (277/680) | 43.8% (161/368) | 37.2% (116/312) |
| Rupture of membranes for >18 h, % (n/N) | 68.3% (181/265) | 63% (97/154) | 75.7% (84/111) |
| Rupture of membranes >7 d, % (n/N) Denominator N = all babies who answered yes to the above variable |
32.1% (85/265) | 29.9% (46/154) | 35.1% (39/111) |
| Antenatal steroids: none, % (n/N) | 8.5% (59/696) | 8.0% (30/375) | 9.0% (29/321) |
| Antenatal steroids: number of complete courses per infant, mean ± SD; median, 25th–75th; n | 0.76 ± 0.58; 1; 0–1; n = 694 | 0.80 ± 0.59; 1.0; 0–1.0; n = 374 | 0.72 ± 0.55; 1.0; 0–1.0; n = 320 |
| Maternal antibiotics, % (n/N) | 77.1% (n = 516/669) | 77.1% (n = 280/363) | 77.1% (n = 236/306) |
| Presentation, vertex, % (n/N) | 52.7% (n = 320/607) | 54.1% (180/333) | 51.1% (140/274) |
| Mode of birth, cesarean section, % (n/N) | 67.8% (n = 486/717) | 68.3% (263/385) | 67.2% (223/332) |
| Maternal information | |||
| Maternal race, Black, % (n/N) | 51% (n = 346/678) | 53.3% (195/366) | 48.4% (151/312) |
| Maternal ethnicity, Hispanic, % (n/N) | 16.1% (n = 112/696) | 15.7% (59/376) | 16.6% (53/320) |
| Maternal age, yr, mean ± SD, median, 25th–75th; n | 29.36 ± 6.1; 29; 25–34; n = 717 | 29.47 ± 5.7; 29; 25–34; n = 385 | 29.22 ± 6.5; 30; 24–34; N = 332 |
| Baby’s health insurance, Medicaid/public, % (n/N) | 63.2% (450/712) | 63.4% (241/380) | 63.0% (209/332) |
| Baby’s health insurance, private, % (n/N) | 33.4% (238/712) | 34.5% (131/380) | 32.2% (107/332) |
| Maternal hypertension, % (n/N) | 40.2% (250/622) | 40.2% (135/336) | 40.2% (115/286) |
| Maternal hypertension before pregnancy, % (n/N) | 59.1% (139/235) | 60.6% (77/127) | 57.4% (62/108) |
| Maternal gestational diabetes, % (n/N) | 5.3% (32/603) | 5.9% (19/322) | 4.6% (13/281) |
| Maternal diabetes mellitus before pregnancy, % (n/N) | 6.0% (36/599) | 6.6% (21/317) | 5.3% (15/282) |
| Maternal asthma during pregnancy in medical record, % (n/N) | 10.4% (n = 62/597) | 12.5% (40/321) | 8.0% (22/276) |
| Antepartum hemorrhage, % (n/N) | 16.1% (96/597) | 16.3% (52/320) | 15.9% (44/277) |
| Medications to prolong pregnancy, % (n/N) | 35.9% (243/677) | 34.6% (126/364) | 37.4% (117/313) |
| Maternal tobacco smoking, % (n/N) | 11.9% (73/611) | 11.5% (38/331) | 12.5% (35/280) |
| Maternal opiates (oral/intravenous) or sedatives or other illicit drugs, % (n/N) | 12.6% (77/613) | 13.3% (44/330) | 11.7% (33/283) |
| Illness severity | |||
| Apgar score at 1 min, median; 25th–75th, n | 4; 2–6; n = 704 | 5; 3–7, n = 377 | 3; 2–5; n = 327 |
| Apgar score at 5 min, median; 25th–75th, n | 7; 5.5–8; n = 704 | 7; 6–8; n = 377 | 6; 4–7; n = 327 |
| Resuscitation at birth: O2, % (n/N) | 89.6% (638/712) | 86.3% (328/380) | 93.4% (310/332) |
| Resuscitation at birth: CPAP, % (n/N) | 58.6% (417/712) | 67.1% (255/380) | 48.8% (162/332) |
| Resuscitation at birth: bag and mask, % (n/N) | 77.0% (548/712) | 72.6% (276/380) | 81.9% (272/332) |
| Resuscitation at birth: intubation, % (n/N) | 53.9% (384/712) | 40.0% (152/380) | 69.9% (232/332) |
| Resuscitation at birth, chest compression, % (n/N) | 2.0% (14/712) | 1.1% (4/380) | 3.0% (10/332) |
| Resuscitation at birth epinephrine, % (n/N) | 0.6% (4/712) | 0.5% (2/380) | 0.6% (2/332) |
| Admission temperature to NICU, °C, mean ± SD, n | 36.8 ± 0.8; n = 717 | 36.9 ± 0.7; n = 385 | 36.7 ± 0.8; n = 332 |
| Prophylactic indomethacin, ibuprofen, acetaminophen, % (n/N) | 37.7% (n = 268/710) | 33.1% (n = 126/381) | 43.2% (n = 142/329) |
Definition of abbreviations: CPAP = continuous positive airway pressure; GA = gestational age; NICU = neonatal ICU.
Outcomes and neonatal comorbidities are listed in Table 2, respiratory support in Table 3, and medication use in Table E1 in the online supplement. Of the 332 infants with unfavorable outcomes, 113 (34%) had a highly unfavorable outcome (16% of total) because of either IMV at 40 weeks PMA or death before or at 40 weeks PMA (20% of unfavorable; 9% of total) (Table E2). Forty-three (13% of unfavorable; 6% of total) had a moderately unfavorable outcome (nasal IMV or CPAP at 40 wk PMA), whereas 176 (53% of unfavorable; 25% of total) had a mildly unfavorable outcome.
Table 2.
Outcomes and Other Neonatal Morbidities in Survivors Only
| Variable | Combined Population | Primary Favorable Outcome | Primary Unfavorable Outcome |
|---|---|---|---|
| Favorable primary outcome | |||
| Only on respiratory medications at discharge <40 wk PMA, % (n/N) | 1.5% (11/717) | 0% (0/385) | 3.3% (11/332) |
| Only on respiratory support at discharge <40 wk PMA, % (n/N) | 0.7% (5/717) | 0% (0/385) | 1.5% (5/332) |
| On respiratory medications and respiratory support at discharge <40 wk PMA, % (n/N) | 0.8% (6/717) | 0% (0/385) | 1.8% (6/332) |
| Only on respiratory medications as inpatient at 40 wk PMA, % (n/N) | 3.1% (22/717) | 0% (0/385) | 6.6% (22/332) |
| Only on respiratory support as inpatient at 40 wk PMA, % (n/N) | 11.6% (83/717) | 0% (0/385) | 25.0% (83/332) |
| On respiratory medications and respiratory support as inpatient at 40 wk PMA, % (n/N) | 19.2% (138/717) | 0% (0/385) | 41.6% (138/332) |
| Death ⩽40 wk PMA, % (n/N) | 9.3% (67/717) | 0% (0/385) | 20.2% (67/332) |
| Discharged alive on no respiratory medications or respiratory support <40 wk PMA, % (n/N) | 33.5% (240/717) | 62.3% (240/385) | 0% (0/332) |
| Alive on no respiratory medications or respiratory support as inpatient at 40 wk PMA, % (n/N) | 20.2% (145/717) | 37.7% (145/385) | 0% (0/332) |
| Secondary outcomes | |||
| Moderate BPD (NICHD definition, % (n/N) Need for any oxygen at 36 wk PMA or discharge, whichever comes first; 36 wk survivors only |
44% (288/654) | 17.4% (67/385) | 82.2% (221/269) |
| Severe BPD (NICHD definition), % (n/N) Need for ⩾30% oxygen and/or positive pressure (PPV or NCPAP) at 36 wk PMA or discharge, whichever comes first; infants alive at 36 wk PMA |
31.2% (204/654) | 7.0% (27/385) | 65.8% (177/269) |
| Severe BPD, % (n/N) On invasive mechanical ventilation at 36 wk PMA; infants alive at 36 wk PMA |
9.9% (65/654) | 0.5% (2/385) | 23.4% (63/269) |
| Highly unfavorable outcome: death or IMV at 40 wk PMA, % (n/N) | 15.8% (113/717) | 0% (0/385) | 34.0% (113/332) |
| Moderately unfavorable outcome: noninvasive positive pressure at 40 wk PMA, % (n/N) | 6.0% (43/717) | 0% (0/385) | 13.0% (43/332) |
| Mildly unfavorable outcome: nasal cannula or only medications at 40 wk PMA or discharged home on medications or oxygen before 40 wk, % (n/N) | 24.5% (176/717) | 0% (0/385) | 53.0% (176/332) |
| Duration of respiratory support (IMV or CPAP or O2), d, mean ± SD; median, 25th–75th, n through discharge, up to 40 wk (infants alive at 40 wk PMA) | 57.79 ± 33.21; 59, 28.25–88; n = 651 | 38.88 ± 25.53; 36; 16–58; n = 385 | 85.16 ± 22.12; 90; 75–101; n = 266 |
| Duration of mechanical ventilation, d, mean ± SD; median, 25th–75th, n through discharge, up to 40 wk; 40 wk survivors only | 21.57 ± 28.01; 7; 1–35.75, n = 651 | 7.94 ± 13.2; 2; 0–9.25; n = 385 | 41.28 ± 31.83; 38; 10–65; n = 266 |
| Pulmonary hypertension diagnosis >34 wk (echocardiography proven), % (n/N) through discharge up to 40 wk; 40 wk survivors only | 9.4% (61/650) | 0.5% (2/385) | 22.3% (59/265) |
| Other morbidity in 40 wk survivors | |||
| Any intraventricular hemorrhage (grades 1–4) through exit or 36 wk PMA, % (n/N) | 30.3% (197/651) | 22.9% (88/385) | 41% (109/266) |
| Severe intraventricular hemorrhage (grade 3 or 4) through exit or 36 wk PMA, % (n/N) | 10.0% (65/651) | 5.7% (22/385) | 16.2% (43/266) |
| Periventricular leukomalacia through exit or 36 wk PMA, % (n/N) | 4.1% (27/651) | 2.3% (9/385) | 6.8% (18/266) |
| Posthemorrhagic hydrocephalus through exit or 36 wk PMA, % (n/N) | 9.4% (61/651) | 4.7% (18/385) | 16.2% (43/266) |
| Early-onset septicemia (blood culture positive before 72 h of age), % (n/N) | 2.51% (18/717) | 0.52% (2/385) | 4.82% (16/332) |
| Late-onset septicemia (blood culture positive beyond 3 d of age) through exit up to 40 wk, % (n/N) | 20.39% (145/711) | 10.9% (42/385) | 31.60% (103/326) |
| Antibiotic courses of ⩾5 d duration without positive cultures through exit up to 40 wk, % (n/N) | 0.14%(1/717) | 0% (0/385) | 0.30% (1/332) |
| Retinopathy of prematurity (ROP): any stage/zone through exit up to 52 wk (of infants who received an ROP exam), % (n/N) |
57.1% (358/627) | 41.4% (154/372) | 80.0% (204/255) |
| Retinopathy of prematurity (ROP): severe (needing laser/bevacizumab/surgery) through exit up to 40 wk, % (n/N) | 7.9% (50/634) | 1.6% (6/372) | 16.8% (44/262) |
| Patent ductus arteriosus: any (diagnosed on echocardiography) through exit up to 40 wk, % (n/N) | 40.5% (263/650) | 31.2% (120/385) | 54.0% (143/265) |
| Patent ductus arteriosus: diagnosed through exit up to 40w and medically treated with indomethacin/ibuprofen/acetaminophen through exit up to 52 wk, % (n/N) | 26.9% (175/650) | 19.0 (73/385) | 38.5% (102/265) |
| Patent ductus arteriosus: surgically ligated or cardiac catheter closure through exit up to 40 wk, % (n/N) | 4.6% (30/650) | 1.0% (4/385) | 9.8% (26/265) |
| Necrotizing enterocolitis: medical (stage II or III) through exit up to 40 wk, % (n/N) | 4.8% (31/650) | 3.6% (14/385) | 6.4% (17/265) |
| Necrotizing enterocolitis: surgical (stage II or III) through exit up to 40 wk, % (n/N) | 3.4% (22/650) | 1.0% (4/385) | 6.8% (18/265) |
| Spontaneous intestinal perforation through exit up to 40 wk, % (n/N) | 3.1% (20/650) | 1.3% (5/385) | 5.7% (15/265) |
| Discharge weight, g, at exit up to 52 wk, mean ± SD; median; 25th–75th, n | 3,506.03 ± 1,162; 3,230; 2,611–4,125; n = 649 | 2,946.26 ± 706; 2,855; 2,390–351; n = 385 | 4,322.36 ± 1,213; 4,177.5; 3,347.5–5,120; n = 364 |
| Discharge weight percentile for PMA at exit up to 52 wk, mean ± SD; median; 25th–75th, n | 50.5 ± 28.6; 50.4; 25.5–75.2; n = 649 | 36.6 ± 23.5; 34.4; 16.4–55.2; n = 385 | 70.6 ± 22.9; 76.6; 55.2–89.2; n = 264 |
| Discharge length, cm, mean ± SD; median; range, n at exit up to 52 wk | 48.77 ± 4.67; 48; 45.5–51.50; n = 649 | 47.07 ± 3.63; 46.5; 44.5–49.50, n = 385 | 51.26 ± 4.9; 51; 48.0–54.5; n = 264 |
| Discharge length percentile for PMA, mean ± SD; median; 25th–75th, n at exit up to 52 wk | 50.6 ± 28.6; 48; 25.8– 75.2; n = 649 | 39.9 ± 25.2; 35.8; 17.3–59.9; n = 385 | 66.2 ± 26.0; 71.6; 48.0–88.3; n = 264 |
| Discharge head circumference, cm, at exit up to 52 wk, mean ± SD; median; 25th–75th, n | 34.57 ± 2.99; 34.5; 32.94–36.13; n = 649 | 33.35 ± 2.34; 33.5; 32–34.5; n = 385 | 36.34 ± 2.94; 36.0; 34.5–38.0; n = 264 |
| Discharge head circumference percentile for PMA at exit up to 52 wk, mean ± SD; median; 25th–75th, n | 50.4 ± 28.6; 53.5; 28.6–73.7; n = 649 | 38.6 ± 24.7; 37.0; 19.1–53.5; n = 385 | 67.7 ± 24.8; 72.5; 53.5–88.0, n = 264 |
| Hospital length of stay, d, mean ± SD; median; 25th–75th, n | 111 ± 44;199; 76–138; n = 649 | 86 ± 26; 83; 67–100; n = 385 | 148 ± 40; 145; 119–182; n = 264 |
Definition of abbreviations: BPD = bronchopulmonary dysplasia; CPAP = continuous positive airway pressure; IMV = invasive mechanical ventilation; NCPAP = nasal continuous positive airway pressure; NICHD = Eunice Kennedy Shriver National Institute of Child Health and Human Development; PMA = postmenstrual age; PPV = positive pressure ventilation.
Table 3.
Clinical Respiratory Data
| Variable | Cumulative from Day 1 to 32 Wk PMA* | Day 1 | Day 7 | Day 14 | Day 28 | 32 Wk PMA |
|---|---|---|---|---|---|---|
| Respiratory support (any) (%; n/N) | 90.01% (n = 714/717) | 98.74% (n = 707/716) | 91.32% (n = 642/703) | 88.76% (n = 608/ 685) | 84.43% (564/668) | 81.85% (n = 541/661) |
| Any invasive IMV (%; n/N) | 78.52% (n = 563/717) | 68.01% (n = 487/716) | 43.1% (n = 303/703) |
39.12% (n = 268/685) |
31.7% (n = 212/668) | 19.51% (n = 129/661) |
| Mean airway pressure on IMV (mean ± SD; median, 25th–75th, n | 10.0 ± 2.8; 9.5; 8–11.1; n = 563 | 8.0 ± 2.2; 8; 6.8–9; n = 484 | 9.2 ± 2.2; 8.9; 7.7–10; n = 303 | 10.2 ± 2.7; 9.9; 8.4–12; n = 268 | 10.4 ± 2.5; 10; 8.8–12; n = 209 | 11.0 ± 2.9; 10; 9–13; n = 128 |
| On CPAP, % (n/N) | 23% (547/717) | 20.3% (145/716) | 26.5% (186/703) | 23.2% (159/685) | 21.1% (141/668) | 25.6% (169/661) |
| On supplemental oxygen, yes/no, % | 75.9% (706/717) | 79.9% (572/716) | 68.9% (484/703) | 73.9% (506/685) | 69.3% (463/668) | 65.4% (432/661) |
| FiO2 (closest to noon), mean ± SD; median, 25th–75th, n | 0.33 ± 0.18; 0.26; 0.21–0.37; n = 717 | 0.28 ± 0.12; 0.24; 0.21–0.30; n = 716 | 0.30 ± 0.15; 0.24; 0.21–0.32; n = 703 | 0.34 ± 0.19; 0.26; 0.21–0.36; n = 685 | 0.32 ± 0.17; 0.25; 0.21–0.36; n = 668 | 0.30 ± 0.16; 0.23; 0.21–0.32; n = 661 |
| FiO2 closest to noon is >30% = yes, % (n/N) | 34.6% (571/717) | 23.5% (168/716) | 26.0% (183/703) | 34.7% (238/685) | 32.6% (218/668) | 25.9% (171/661) |
| Maximum PaCO2, %, mean ± SD; median, 25th–75th, n | 52.2 ± 13; 40; 44–59; n = 575 | 47.9 ± 11.5; 46; 40–54; n = 418 | 55.3 ± 11.3; 54; 48–64, n = 93 | 59.4 ± 32; 50; 43–58.5; n = 15 | 58.6 ± 17.9; 53.5; 43.5–73; n = 8 | 51.0 ± 4.6; 51.5; 48–54.1; n = 4 |
| Max capillary or venous PCO2, %, mean ± SD; median, 25th–75th, n | 53.0 ± 10.5; 52; 46–59; n = 693 | 46.6 ± 11; 45; 40–51; n = 250 | 50.8 ± 10.7; 49; 44–57; n = 425 | 54.1 ± 10.4; 53; 47–60; n = 685 | 53.2 ± 10.4; 52; 46–59; n = 322 | 53.7 ± 10.2; 52; 47–60; n = 221 |
| Longitudinal burden of respiratory support: number of days alive and off ventilation, mean ± SD; median, 25th–75th, n | N/A | N/A | 3.8 ± 3.4; 4; 0–7; n = 703 | 8.1 ± 6.4; 11; 0–14; n = 685 | 17.2 ± 12; 23; 2–28; n = 668 | 22.8 ± 12.9; 24; 14–31; n = 661 |
| Number of days alive and off ventilation in past 2 wk, mean ± SD; median, 25th–75th, n | N/A | N/A | N/A | N/A | 9 ± 6.2; 14; 0–14; n = 668 | 10.8 ± 5.4; 14; 9–14; n = 661 |
Definition of abbreviations: IMV = invasive mechanical ventilation; PMA = postmenstrual age.
For the cumulative data column, % is the percentage of days until 32 weeks PMA; n/N represents infants on any day until 32 weeks PMA.
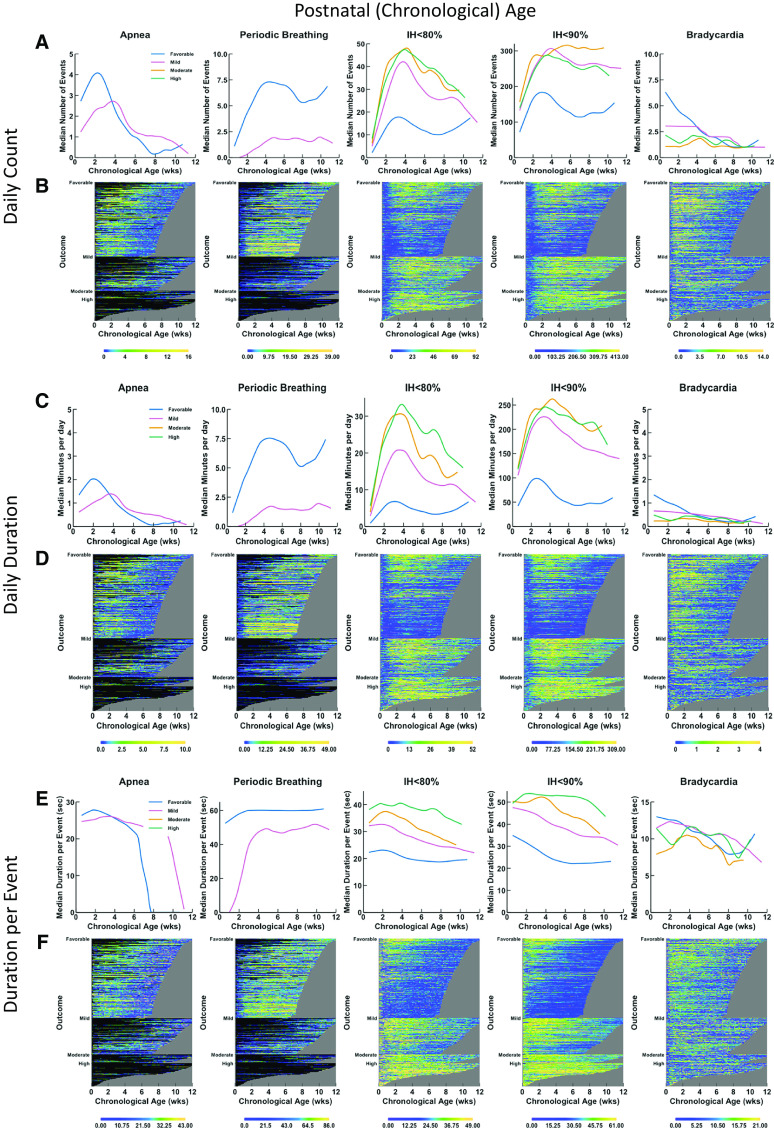
Physiologic Data over Time
Physiologic data were graphed over chronological age (Figure 2, left panels) and PMA (Figure 2, right panels) with number of events per day (Figure 2, top panels), minutes per day (Figure 2, middle panels), and dpe (Figure 2, bottom panels) shown as trajectories over time in terms of outcome (favorable, mildly unfavorable, moderately or highly unfavorable) and as heatmaps representing individual infants. Because apnea and PB were not evaluable when infants were intubated and on mechanical ventilation, the data are shown only for times of spontaneous breathing (including noninvasive ventilation) without invasive respiratory support. These features are summarized in Table E3.
Figure 2.
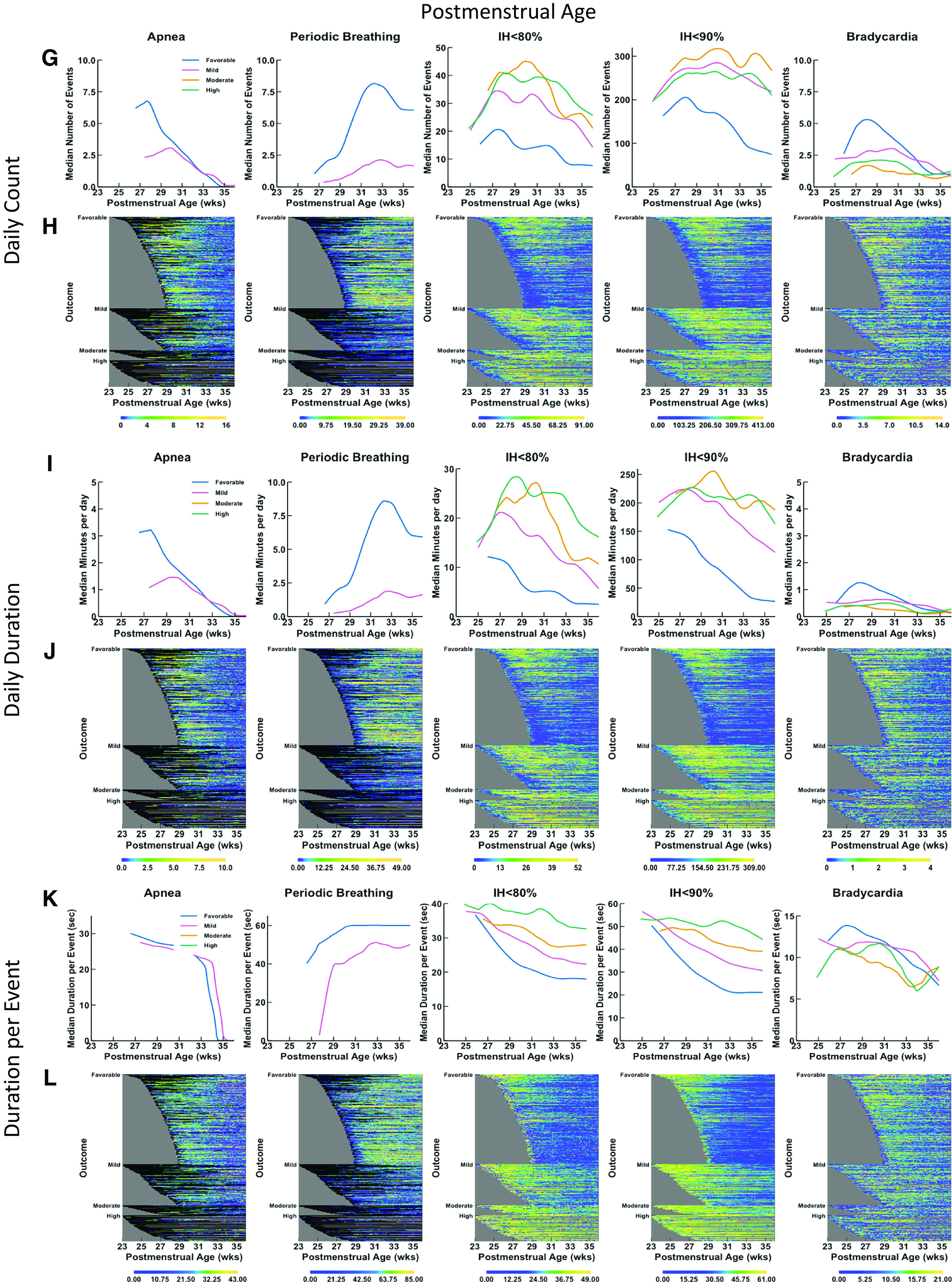
Trajectories and heat maps for daily counts, daily duration, and median duration per event of apnea, periodic breathing, intermittent hypoxemia (IH) ⩽80%, IH ⩽90%, and bradycardia for infants enrolled in Pre-Vent. Chronologic (postnatal) age is shown on the x-axis in the left panels (A–F) and postmenstrual age is shown on the x-axis in the right panels (G–L). Data are shown from 3 days of age, and a minimum number of 30 infants was required to calculate a trajectory curve. Only infant days that have at least 12 hours of signal available are included. Top row of graphs (A, G) and heatmaps (B, H) show data for daily counts, whereas the middle row of graphs (C, I) and heatmaps (D, J) show data for daily duration of events, and bottom row of graphs (E, K) and heatmaps (F, L) show data for median duration per event. The top row of graphs (A, G) shows trajectories of daily count of event by outcome (color-coded lines), with the number of events on the y-axis and age on the x-axis. The second row of panels shows heatmaps (B, H) for daily counts of events. The heat map shows data for all infants, with each infant per horizontal line, grouped by outcome as favorable (at top), mildly unfavorable (in middle), and moderately or highly unfavorable (at bottom). Within each category of outcome, infants are grouped by gestational age with oldest infants at the bottom and youngest infants at the top, with the data plotted horizontally by chronological age on the x-axis. Color coding for number of events per day is shown with yellow indicating more events and deepening shades of blue indicating fewer events. Black indicates no data, such as for apnea or periodic breathing when the infant is on a ventilator. The gray area represents data truncation when the infant has reached 36 weeks, 6 days postmenstrual age, the predetermined endpoint for the Pre-Vent physiologic outcomes analysis. The middle row of graphs (C, I) shows trajectories of daily duration of event by outcome (color-coded lines), with the number of events on the y-axis and the age on the x-axis. The second row of panels shows heatmaps (D, J) for daily duration of the events. The lower row of graphs (E, F) shows trajectories of median duration per event by outcome (color-coded lines), with the number of events on the y-axis and the chronologic age on the x-axis. The bottom row of panels shows heatmaps (F, L) for median duration per event.
Apnea
Infants with moderately or highly unfavorable outcomes could not be analyzed for association with apnea (or PB), because there were few infants with such outcomes not intubated at the four time points. Excluding days on IMV when apnea could not be evaluated, infants with favorable outcomes initially had more apneic events and of longer daily duration in the first 4 weeks (until 33 wk PMA) than did those with mildly unfavorable outcomes but subsequently less apnea thereafter. Because infants may be intubated due to apnea, these results may not reflect the relationship between apnea and outcome. Apnea cessation (median) was generally by 8 weeks chronologic age in infants with a favorable outcome, by 12 weeks in those with a mildly unfavorable outcome, and by 35–36 weeks PMA in both groups because infants with unfavorable outcomes were more premature than those with favorable outcomes.
PB
PB, similar to apnea, was also increased in both number and duration among infants with a favorable outcome as compared with those with a mildly unfavorable outcome (as with apnea, many infants with unfavorable outcomes were intubated on IMV and were not evaluable for PB). PB persisted until 40 weeks PMA in infants with favorable and mildly unfavorable outcomes.
IH
Data are shown for IH80 and IH90. IH75 and IH85 were also investigated but did not improve prediction (data not shown). Infants with unfavorable outcomes had increased IH80 and IH90 counts and daily duration. IH90 dpe and IH80 dpe discriminated best among the different categories of outcome, with different trajectories over time between infants with favorable, mildly unfavorable, moderately unfavorable, and highly unfavorable outcomes (Figures 2E and 2F). The median daily counts, daily duration, and dpe for the infants with favorable and unfavorable outcomes diverged from the earliest time point (25 wk PMA), with dpe again illustrating maximal separation among different outcomes (Figures 2K and 2L).
Bradycardia
Bradycardia trajectories of infants with favorable outcomes and different categories of unfavorable outcomes had overlap, with convergence by ∼14 weeks chronologic age or 33 weeks PMA for counts and daily duration.
Primary outcome
The primary outcome was measured using unfavorable versus favorable outcome models with 24-hour (snapshot) data.
Physiologic Models
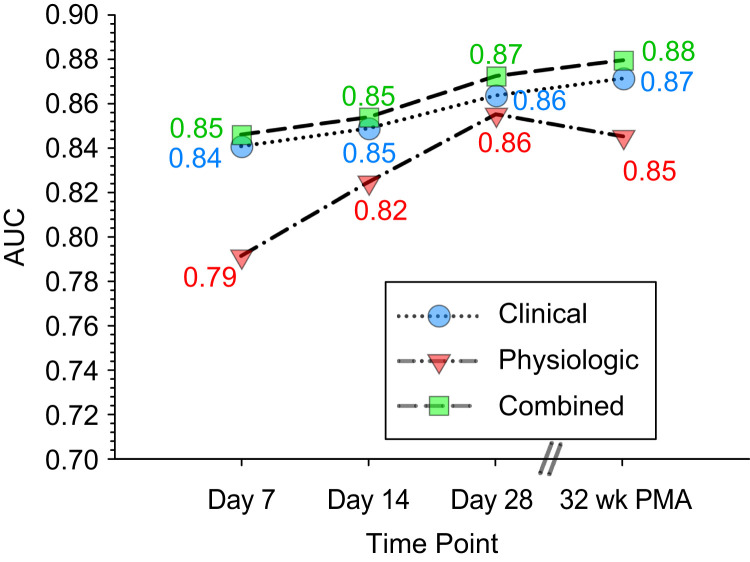
Physiologic data predicted unfavorable outcomes, with the accuracy of prediction increasing with advancing postnatal age (Tables 4–7, Figures 3 and 4). The AUC of the receiver operating characteristic curve (ROC) of models improved from 0.792 at Postnatal Day 7 to 0.825 at Day 14, 0.855 at Day 28, and 0.845 at 32 weeks PMA. The primary variables contributing the most overall to predictive accuracy were IH <90% (IH90) duration per event (IH90dpe), followed by IH90 total duration per day and IH90 count per day. In contrast, longer PB and apnea events (dpe) were associated with favorable outcomes, but these variables could be evaluated only in infants not on a ventilator, and many infants with unfavorable outcome were mechanically ventilated at earlier time points.
Table 4.
Model at 7 Days of Age, Including Survivors to Day 7 (Limited to Importance 0.0005 or More)
| Variable (Selected by LASSO) | AUC C-Statistic | Importance of Variable | OR (95% CI) or Similar | P Value |
|---|---|---|---|---|
| Clinical variables alone model | 0.841 | |||
| Gestational age | 0.0117 | 0.51 (0.39–0.68) | <0.00001 | |
| SGA | 0.0070 | 4.73 (2.04–10.97) | 0.0003 | |
| FiO2 >0.3* | 0.0050 | 2.28 (1.35–3.83) | 0.002 | |
| IMV | 0.0036 | 2.53 (1.27–5.05) | 0.008 | |
| Positive pressure | 0.0035 | 1.95 (1.1–3.47) | 0.022 | |
| Black race | 0.0028 | 0.57 (0.37–0.89) | 0.014 | |
| Supplemental oxygen >12 h/24 h | 0.0026 | 1.76 (1.11–2.78) | 0.016 | |
| Growth percentile | 0.0024 | 0.74 (0.58–0.93) | 0.010 | |
| Apgar 1-min score | 0.0023 | 0.79 (0.65–0.96) | 0.016 | |
| Sedatives | 0.0014 | 2.42 (1.12–5.21) | 0.024 | |
| Male | 0.0012 | 1.40 (0.96–2.04) | 0.084 | |
| Physiologic variables alone model | 0.792 | |||
| PB dpe | 0.0179 | 0.56 (0.42–0.73) | 0.00004 | |
| IH90 daily duration | 0.0099 | 1.66 (1.2–2.32) | 0.003 | |
| IH90 dpe | 0.0006 | 1.15 (0.87–1.53) | 0.321 | |
| Combined model (physiologic model + clinical model) | 0.846 | |||
| SGA | 0.0233 | 9.57 (4.18–21.91) | <0.00001 | |
| Gestational age | 0.0095 | 0.53 (0.39–0.71) | 0.00002 | |
| PB dpe | 0.0064 | 0.60 (0.45–0.81) | 0.0009 | |
| FiO2 >0.3* | 0.0046 | 2.04 (1.16–3.58) | 0.013 | |
| Black race | 0.0038 | 0.61 (0.39–0.97) | 0.035 | |
| IH90 daily duration | 0.0038 | 1.45 (1.01–2.09) | 0.045 | |
| Positive pressure | 0.0027 | 2.04 (1.04–4.01) | 0.037 | |
| Apgar 1-min score | 0.0025 | 0.77 (0.63–0.95) | 0.017 | |
| Caffeine | 0.0006 | 0.63 (0.27–1.49) | 0.295 | |
| IMV | 0.0006 | 1.72 (0.74–4.0) | 0.208 |
Definition of abbreviations: AUC = area under the curve; CI = confidence interval; dpe = duration per event; IH90 = intermittent hypoxemia events with oxygen saturation as measured by pulse oximetry <90%; IMV = invasive mechanical ventilation; LASSO = least absolute shrinkage and selection operator; OR = odds ratio; PB = periodic breathing; SGA = small for gestational age.
When FiO2 is listed as a variable, it is the continuous variable of FiO2 value closest to noon. If FiO2 >0.3 is listed, it is the binary variable FiO2 >0.3.
Table 7.
Model at 32 Weeks Postmenstrual Age, Including Survivors to 32 Weeks Postmenstrual Age (Limited to Importance 0.0005 or More)
| Variable (Selected by LASSO) | AUC C-Statistic | Importance of Variable | OR (95% CI) | P Value |
|---|---|---|---|---|
| Clinical variables alone model | 0.871 | |||
| SGA | 0.0142 | 6.76 (2.92–15.64) | 0.00001 | |
| IMV | 0.0136 | 6.86 (3.43–13.71) | 0.00000 | |
| Gestational age | 0.0105 | 0.56 (0.43–0.72) | 0.00001 | |
| Positive pressure | 0.0079 | 2.51 (1.57–4.01) | 0.0001 | |
| FiO2* | 0.0050 | 2.17 (1.36–3.46) | 0.001 | |
| Apgar 1-min score | 0.0034 | 0.77 (0.62–0.95) | 0.015 | |
| Diuretic | 0.0033 | 2.96 (1.42–6.15) | 0.004 | |
| Supplemental oxygen >12 h/24 h | 0.0022 | 2.09 (1.27–3.44) | 0.004 | |
| Black race | 0.0011 | 0.66 (0.41–1.08) | 0.098 | |
| Physiologic variables alone model | 0.845 | |||
| IH90 dpe | 0.0146 | 2.57 (1.74–3.81) | <0.00001 | |
| PB count | 0.0084 | 0.53 (0.33–0.87) | 0.012 | |
| IH90 count | 0.0067 | 1.54 (1.23–1.93) | 0.0002 | |
| Apnea dpe | 0.0028 | 0.80 (0.62–1.02) | 0.07 | |
| Combined model (physiologic model + clinical model) | 0.879 | |||
| SGA | 0.0101 | 5.33 (2.13–13.32) | 0.0003 | |
| Gestational age | 0.0052 | 0.58 (0.43–0.79) | 0.0005 | |
| PB count | 0.0037 | 0.63 (0.39–1.01) | 0.054 | |
| Positive pressure | 0.0025 | 1.94 (1.12–3.33) | 0.017 | |
| IH90 count | 0.0020 | 1.33 (1.03–1.70) | 0.027 | |
| IMV | 0.0018 | 2.63 (0.99–6.99) | 0.053 | |
| Diuretic | 0.0018 | 2.75 (1.24–6.08) | 0.013 | |
| IH90 dpe | 0.0016 | 1.62 (1.14–2.29) | 0.007 | |
| Apgar 1-min score | 0.0014 | 0.81 (0.64–1.03) | 0.080 | |
| FiO2 >0.3* | 0.0014 | 2.32 (1.25–4.31) | 0.008 | |
| Apnea dpe | 0.0012 | 0.82 (0.63–1.07) | 0.144 | |
| Black race | 0.0011 | 0.65 (0.41–1.05) | 0.075 |
Definition of abbreviations: AUC = area under the curve; CI = confidence interval; dpe = duration per event; IH90 = intermittent hypoxemia events with oxygen saturation as measured by pulse oximetry <90%; IMV = invasive mechanical ventilation; LASSO = least absolute shrinkage and selection operator; OR = odds ratio; PB = periodic breathing; SGA = small for gestational age.
When FiO2 is listed as a variable, it is the continuous variable of FiO2 value closest to noon. If FiO2 >0.3 is listed, it is the binary variable FiO2 >0.3.
Figure 3.
Graph of predictive accuracy of models using clinical, physiologic, and combined (clinical and physiological) variables at different postnatal time points (Postnatal Days 7, 14, and 28 and 32 wk PMA). PMA = postmenstrual age.
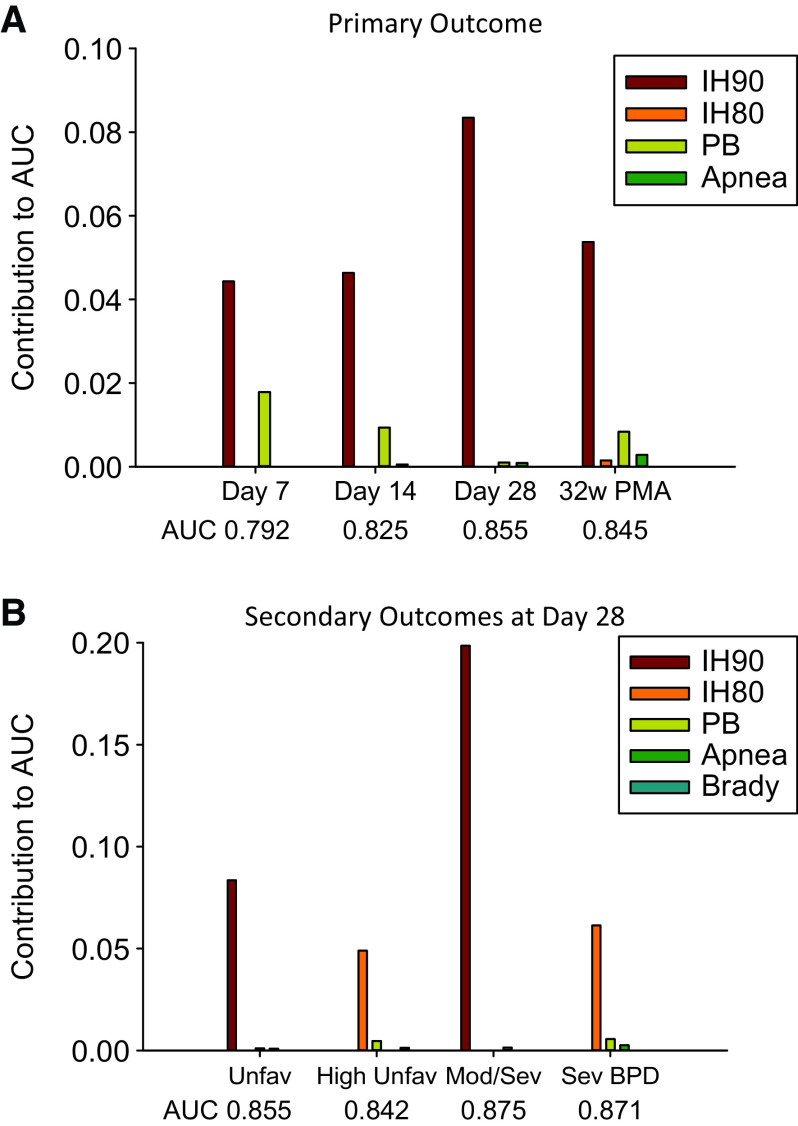
Figure 4.
Graph showing contributions of different physiologic variables (each variable incorporating different features: duration per event, daily duration, and count) to outcome. (A) Unfavorable versus favorable outcome at different time points. (B) Primary and secondary outcomes at Postnatal Day 28. AUC = area under the curve; BPD = bronchopulmonary dysplasia; IH80 = intermittent hypoxemia events with oxygen saturation as measured by pulse oximetry <80%; IH90 = intermittent hypoxemia events with oxygen saturation as measured by pulse oximetry <90%; PB = periodic breathing; PMA = postmenstrual age.
Table 5.
Model at 14 Days of Age, Including Survivors to Day 14 (Limited to Importance 0.0005 or More)
| Variable (Selected by LASSO) | AUC C-Statistic | Importance of Variable | OR (95% CI) or Similar | P Value |
|---|---|---|---|---|
| Clinical variables alone model | 0.849 | |||
| IMV | 0.0170 | 6.70 (3.2–14) | <0.00001 | |
| SGA | 0.0153 | 7.69 (3.57–16.56) | <0.00001 | |
| Positive pressure | 0.0117 | 3.47 (1.9–6.32) | 0.0001 | |
| FiO2* | 0.0069 | 1.66 (1.15–2.38) | 0.006 | |
| Black race | 0.0022 | 0.61 (0.39–0.94) | 0.027 | |
| Gestational age | 0.0021 | 0.64 (0.49–0.83) | 0.0007 | |
| Apgar 1-min score | 0.0014 | 0.81 (0.67–0.99) | 0.035 | |
| Temperature at admission | 0.0013 | 0.84 (0.68–1.05) | 0.121 | |
| Hispanic ethnicity | 0.0006 | 0.60 (0.33–1.07) | 0.085 | |
| Sedatives | 0.0005 | 2.30 (0.96–5.50) | 0.062 | |
| Physiologic variables alone model | 0.825 | |||
| IH90 dpe | 0.0156 | 1.71 (1.29–2.27) | 0.0002 | |
| PB dpe | 0.0094 | 0.57 (0.42–0.77) | 0.0002 | |
| IH90 count | 0.0038 | 1.30 (1.05–1.61) | 0.014 | |
| Apnea dpe | 0.0005 | 0.83 (0.62–1.10) | 0.184 | |
| Combined model (physiologic model + clinical model) | 0.854 | |||
| SGA | 0.0083 | 6.00 (2.65–13.59) | 0.00002 | |
| Positive pressure | 0.0047 | 2.84 (1.46–5.56) | 0.002 | |
| PB dpe | 0.0046 | 0.58 (0.42–0.81) | 0.001 | |
| Gestational age | 0.0041 | 0.62 (0.47–0.82) | 0.0009 | |
| FiO2* | 0.0016 | 1.38 (0.96–1.97) | 0.082 | |
| IH90 dpe | 0.0016 | 1.25 (0.91–1.72) | 0.162 | |
| Male | 0.0014 | 1.36 (0.89–2.09) | 0.153 | |
| Apnea dpe | 0.0010 | 0.83 (0.60–1.15) | 0.267 | |
| Apgar 1-min score | 0.0010 | 0.82 (0.67–1.01) | 0.062 | |
| Sedatives | 0.0008 | 2.09 (0.87–4.99) | 0.097 |
Definition of abbreviations: AUC = area under the curve; CI = confidence interval; dpe = duration per event; IH90 = intermittent hypoxemia events with oxygen saturation as measured by pulse oximetry <90%; IMV = invasive mechanical ventilation; LASSO = least absolute shrinkage and selection operator; OR = odds ratio; PB = periodic breathing; SGA = small for gestational age.
When FiO2 is listed as a variable, it is the continuous variable of FiO2 value closest to noon. If FiO2 >0.3 is listed, it is the binary variable FiO2 >0.3.
Table 6.
Model at 28 Days of Age, Including Survivors to Day 28 (Limited to Importance 0.0005 or More)
| Variable (Selected by LASSO) | AUC C-Statistic | Importance of Variable | OR (95% CI) | P Value |
|---|---|---|---|---|
| Clinical variables alone model | 0.864 | |||
| IMV | 0.0169 | 6.86 (3.64–12.92) | <0.00001 | |
| FiO2* | 0.0085 | 1.82 (1.24–2.69) | 0.002 | |
| Positive pressure | 0.0053 | 2.51 (1.48–4.27) | 0.001 | |
| SGA | 0.0039 | 3.55 (1.53–8.21) | 0.003 | |
| Apgar 1-min score | 0.0026 | 0.76 (0.62–0.93) | 0.007 | |
| Supplemental oxygen >12 h/24 h | 0.0026 | 1.97 (1.15–3.39) | 0.014 | |
| Birth weight | 0.0020 | 0.73 (0.53–1.00) | 0.050 | |
| Diuretics | 0.0008 | 2.37 (0.80–7.0) | 0.118 | |
| Male | 0.0008 | 1.34 (0.89–2.01) | 0.156 | |
| Physiologic variables alone model | 0.855 | |||
| IH90 dpe | 0.0246 | 2.92 (2.04–4.18) | <0.00001 | |
| IH90 count | 0.0042 | 1.48 (1.21–1.81) | 0.0002 | |
| PB duration | 0.0010 | 0.69 (0.44–1.08) | 0.103 | |
| Apnea dpe | 0.0009 | 0.83 (0.64–1.06) | 0.142 | |
| Combined model (physiologic model + clinical model) | 0.872 | |||
| FiO2* | 0.0092 | 1.89 (1.28–2.79) | 0.0014 | |
| IH90 count | 0.0067 | 1.49 (1.20–1.84) | 0.0003 | |
| SGA | 0.0054 | 4.11 (1.69–9.97) | 0.002 | |
| Positive pressure | 0.0041 | 3.00 (1.59–5.65) | 0.0007 | |
| IMV | 0.0024 | 3.70 (1.56–8.74) | 0.003 | |
| Apnea dpe | 0.0019 | 0.77 (0.58–1.02) | 0.068 | |
| Hydrocortisone | 0.0019 | 2.91 (0.92–9.19) | 0.069 | |
| Multiple gestation | 0.0011 | 0.75 (0.45–1.26) | 0.277 | |
| IH90 dpe | 0.0008 | 1.62 (1.11–2.38) | 0.013 |
Definition of abbreviations: AUC = area under the curve; CI = confidence interval; dpe = duration per event; IH90 = intermittent hypoxemia events with oxygen saturation as measured by pulse oximetry <90%; IMV = invasive mechanical ventilation; LASSO = least absolute shrinkage and selection operator; OR = odds ratio; PB = periodic breathing; SGA = small for gestational age.
When FiO2 is listed as a variable, it is the continuous variable of FiO2 value closest to noon. If FiO2 >0.3 is listed, it is the binary variable FiO2 >0.3.
Clinical Models
Clinical data models predicted unfavorable outcomes with good accuracy at Day 7 (AUC of ROC, 0.841), which remained relatively stable over time (0.849 on Day 14, 0.864 on Day 28, and 0.871 at 32 wk PMA) (Tables 4–7, Figure 3). At Day 7, lower GA, being small for gestational age (SGA), on high (>0.3) FiO2, and on IMV or positive pressure (including CPAP or nasal IMV) were the major contributors to unfavorable outcomes. These variables were also important at Days 14 and 28, although the relative importance was different because GA (most important on Day 7) was replaced by IMV on Day 14 and Day 28 as the most contributory variable. Black infants had lower odds of unfavorable outcome at Days 7 and 14, although this was no longer statistically significant at Day 28 or 32 weeks PMA. At 32 weeks PMA, being SGA, on IMV, and of lower GA were the major contributors.
Physiologic and Clinical Models
Models combining physiologic and clinical data had good accuracy, with AUC of ROC of 0.846 at Day 7, 0.854 on Day 14, 0.872 on Day 28, and 0.879 at 32 weeks PMA (Tables 4–7, Figure 3). This accuracy was comparable to that with clinical data alone (less than 0.01 increase in AUC). The major clinical variables included in this model were SGA and GA on Day 7, SGA and positive pressure on Day 14, FiO2 and SGA on Day 28, and SGA and GA at 32 weeks PMA. The major physiologic variables were those related to IH90 and PB (absence of PB in nonventilated infants and duration of PB if present).
Secondary outcomes evaluated using 24-hour data at 28 days of age were as outlined below.
-
1.
Highly unfavorable outcome (Death or IMV at 40w PMA) (Table 8; Figure 4). The physiologic model had overall model accuracy of 0.842 (cvAUC), with the important variables in order of contribution to AUC being higher IH80 dpe, followed by lower PB count, and increased bradycardia daily duration. The clinical model had accuracy of 0.862 with the important variables being use of mechanical ventilation, lower GA, SGA, higher FiO2, being male, lower 1 minute Apgar, and non-Hispanic ethnicity. The combined model had accuracy of 0.872, with major variables being SGA, lower GA, use of sedatives, higher IH80 dpe, non-Hispanic ethnicity, and IH90 count and IH90 dpe.
-
2.
BPD at 36 weeks PMA (NICHD definition) (Table 9; Figure 4). The physiologic model had an accuracy of 0.875, with major variables being higher IH90 dpe and IH90 daily duration, and lower Apnea dpe. The clinical model had an accuracy was 0.883, with important contributors being use of IMV, higher FiO2, use of positive pressure (CPAP, non-invasive or invasive ventilation), prolonged supplemental oxygen (>12 h/24 h), SGA, lower 5 minute Apgar, and lack of caffeine use. The combined model had an accuracy of 0.894, with major contributors being higher FiO2, use of positive pressure, non-Black race, higher IH90 daily duration, SGA, lower Apnea dpe, use of IMV, use of supplemental oxygen, non-Hispanic ethnicity, and lower 5 minute Apgar.
-
3.
Grade 3 BPD (Jensen and colleagues [16]. definition) (Table 10; Figure 4). The physiologic model had an accuracy of 0.871, with major contributors being higher IH80 dpe, and lower PB daily duration and Apnea daily duration. The clinical model had an accuracy of 0.887, with major contributors being use of IMV, sedation, SGA, lower 1 minute Apgar, lower GA, black race, and high FiO2 (>0.3). The combined model had an accuracy of 0.900, with major contributors being use of sedatives, SGA, IH80 dpe, lower GA, less PB daily duration, less apnea daily duration, and lower 1 minute Apgar.
Table 8.
Secondary Outcomes: Highly Unfavorable Outcome at 40 Weeks Postmenstrual Age (Limited to Importance 0.0005 or More)
| Variable (selected by LASSO) | AUC C-Statistic | Importance of Variable | OR (95% CI) | P Value |
|---|---|---|---|---|
| Clinical variables alone model | 0.862 | |||
| IMV | 0.0156 | 3.92 (1.65–9.32) | 0.002 | |
| Gestational age | 0.0135 | 0.52 (0.33–0.80) | 0.003 | |
| SGA | 0.0133 | 4.77 (2.11–10.76) | 0.0002 | |
| FiO2* | 0.0024 | 1.28 (0.95–1.72) | 0.101 | |
| Male | 0.0018 | 1.80 (0.96–3.39) | 0.068 | |
| Apgar 1-min score | 0.0016 | 0.79 (0.55–1.12) | 0.183 | |
| Hispanic ethnicity | 0.0005 | 0.49 (0.20–1.20) | 0.120 | |
| Physiologic variables alone model | 0.842 | |||
| IH80 dpe | 0.0490 | 1.86 (1.27–2.71) | 0.001 | |
| PB count | 0.0047 | 0.13 (0.01–2.37) | 0.170 | |
| Bradycardia daily duration | 0.0013 | 1.32 (1.02–1.71) | 0.038 | |
| Combined model (physiologic model + clinical model) | 0.872 | |||
| SGA | 0.0134 | 4.73 (1.98–11.31) | 0.0005 | |
| Gestational age | 0.0112 | 0.50 (0.33–0.76) | 0.001 | |
| Sedatives | 0.0065 | 3.10 (1.26–7.65) | 0.014 | |
| IH80 dpe | 0.0056 | 1.69 (1.03–2.78) | 0.038 | |
| Hispanic ethnicity | 0.0025 | 0.45 (0.19–1.09) | 0.078 | |
| IH90 count | 0.0013 | 1.24 (0.90–1.71) | 0.192 | |
| IH90 dpe | 0.0013 | 1.50 (0.93–2.41) | 0.097 |
Definition of abbreviations: AUC = area under the curve; CI = confidence interval; dpe = duration per event; IH80 = intermittent hypoxemia events with oxygen saturation as measured by pulse oximetry <80%; IH90 = intermittent hypoxemia events with oxygen saturation as measured by pulse oximetry <90%; IMV = invasive mechanical ventilation; LASSO = least absolute shrinkage and selection operator; OR = odds ratio; PB = periodic breathing; SGA = small for gestational age.
When FiO2 is listed as a variable, it is the continuous variable of FiO2 value closest to noon. If FiO2 >0.3 is listed, it is the binary variable FiO2 >0.3.
Table 9.
Secondary Outcomes: Moderate-Severe Bronchopulmonary Dysplasia (Eunice Kennedy Shriver National Institute of Child Health and Human Development) Outcome at 36 Weeks Postmenstrual Age (Limited to Importance 0.0005 or More)
| Variable (Selected by LASSO) | AUC C-Statistic | Importance of Variable | OR (95% CI) | P Value |
|---|---|---|---|---|
| Clinical variables alone model | 0.883 | |||
| IMV | 0.0182 | 9.47 (4.87–18.43) | <0.00001 | |
| FiO2* | 0.0104 | 1.87 (1.23–2.84) | 0.0035 | |
| Positive pressure | 0.0094 | 4.10 (2.37–7.08) | <0.00001 | |
| Supplemental oxygen >12 h/24 h | 0.0072 | 3.94 (2.26–6.86) | <0.00001 | |
| SGA | 0.0051 | 4.22 (2.06–8.67) | <0.0001 | |
| Apgar 5-min score | 0.0014 | 0.77 (0.62–0.96) | 0.0213 | |
| Caffeine | 0.0007 | 0.66 (0.26–1.71) | 0.397 | |
| Physiologic variables alone model | 0.875 | |||
| IH90 dpe | 0.0122 | 2.78 (1.83–4.20) | <0.00001 | |
| IH90 daily duration | 0.0084 | 1.95 (1.46–2.61) | 0.00001 | |
| Apnea dpe | 0.0014 | 0.82 (0.64–1.06) | 0.128 | |
| Combined model (physiologic model + clinical model) | 0.894 | |||
| FiO2* | 0.0102 | 1.84 (1.22–2.79) | 0.0038 | |
| Positive pressure | 0.0059 | 3.53 (1.82–6.84) | 0.0002 | |
| Black race | 0.0059 | 0.50 (0.29–0.86) | 0.012 | |
| IH90 daily duration | 0.0057 | 1.62 (1.20–2.19) | 0.002 | |
| SGA | 0.0035 | 4.12 (1.80–9.43) | 0.001 | |
| Apnea dpe | 0.0032 | 0.73 (0.54–1.0) | 0.047 | |
| IMV | 0.0026 | 3.85 (1.62–9.15) | 0.002 | |
| Supplemental oxygen | 0.0023 | 2.78 (1.19–6.51) | 0.019 | |
| Hispanic ethnicity | 0.0021 | 0.58 (0.27–1.25) | 0.165 | |
| Apgar 5 min | 0.0020 | 0.71 (0.55–0.92) | 0.009 |
Definition of abbreviations: AUC = area under the curve; CI = confidence interval; dpe = duration per event; IH90 = intermittent hypoxemia events with oxygen saturation as measured by pulse oximetry <90%; IMV = invasive mechanical ventilation; LASSO = least absolute shrinkage and selection operator; OR = odds ratio; PB = periodic breathing; SGA = small for gestational age.
When FiO2 is listed as a variable, it is the continuous variable of FiO2 value closest to noon. If FiO2 >0.3 is listed, it is the binary variable FiO2 >0.3.
Table 10.
Secondary Outcomes: Grade 3 Bronchopulmonary Dysplasia (Invasive Mechanical Ventilation; Jensen and Colleagues) Outcome at 36 Weeks Postmenstrual Age (Limited to Importance 0.0005 or More)
| Variable (Selected by LASSO) | AUC C-Statistic | Importance of Variable | OR (95% CI) | P Value |
|---|---|---|---|---|
| Clinical variables alone model | 0.887 | |||
| IMV | 0.0207 | 6.96 (3.11–15.56) | <0.00001 | |
| Sedatives | 0.0125 | 4.44 (1.97–10) | 0.0003 | |
| SGA | 0.0067 | 3.75 (1.70–8.25) | 0.001 | |
| Apgar 1-min score | 0.0063 | 0.72 (0.53–1.0) | 0.0465 | |
| Gestational age | 0.0027 | 0.68 (0.47–1.0) | 0.050 | |
| Black race | 0.0020 | 1.79 (1.03–3.13) | 0.0396 | |
| FiO2 high | 0.0006 | 2.25 (1.15–4.43) | 0.0185 | |
| Physiologic variables alone model | 0.871 | |||
| IH80 dpe | 0.0614 | 1.70 (1.18–2.45) | 0.0047 | |
| PB daily duration | 0.0056 | 0.02 (0.01–0.50) | 0.0183 | |
| Apnea daily duration | 0.0025 | 0.60 (0.31–1.18) | 0.138 | |
| Combined model (physiologic model + clinical model) | 0.900 | |||
| Sedatives | 0.0164 | 4.78 (1.65–13.86) | 0.004 | |
| SGA | 0.0092 | 4.01 (1.66–9.71) | 0.002 | |
| IH80 dpe | 0.0087 | 1.60 (1.02–2.53) | 0.042 | |
| Gestational age | 0.0087 | 0.57 (0.38–0.87) | 0.009 | |
| PB daily duration | 0.0042 | 0.05 (0.01–0.72) | 0.027 | |
| Apnea daily duration | 0.0025 | 0.50 (0.23–1.08) | 0.079 | |
| Apgar 1-min score | 0.0009 | 0.83 (0.51–1.34) | 0.442 | |
| Steroids | 0.0008 | 0.51 (0.22–1.18) | 0.118 | |
| Apgar 5-min score | 0.0008 | 0.82 (0.54–1.25) | 0.361 | |
| Hispanic ethnicity | 0.0006 | 0.70 (0.32–1.53) | 0.369 | |
| IMV | 0.0005 | 2.80 (1.04–7.51) | 0.041 |
Definition of abbreviations: AUC = area under the curve; CI = confidence interval; dpe = duration per event; IH80 = intermittent hypoxemia events with oxygen saturation as measured by pulse oximetry <80%; IMV = invasive mechanical ventilation; LASSO = least absolute shrinkage and selection operator; OR = odds ratio; PB = periodic breathing; SGA = small for gestational age.
Snapshot (24 h) versus longitudinal models for outcome
Compared with the snapshot (24 h) model at Day 28, the aggregate model (summary data up to 28 d) and mixed effects model (longitudinal model using all daily data to 28 d) for unfavorable, highly unfavorable, moderate/severe BPD (NICHD [15]), and grade 3 BPD (Jensen and colleagues [16]) had an accuracy about 0.01 AUC higher (Table E4).
Validation of models
In addition to 10-fold cross-validation, leave-one-site-out analysis was done for internal validation. Although there was some variation in performance across sites as expected, out-of-sample AUCs remained high for all models (Table E5). Understanding the subtlety of site differences remains a topic for further research.
Discussion
The major findings of our study were that mathematical models based on cardiorespiratory bedside monitoring can identify alterations in physiologic measures that are independently associated with unfavorable respiratory outcomes in extremely preterm infants. We defined the association of specific parameters of abnormal physiologic data (apnea, PB, IH, and bradycardia) with respiratory outcome at 40 weeks PMA. Both physiologic and clinical variables contributed to good prediction models, with the contribution by physiologic variables increasing with postnatal age. The major physiologic variable associated with unfavorable respiratory outcome was IH90, with the best predictor being the IH90dpe.
Although apnea and bradycardia are traditional measures of abnormal control of breathing, our study suggests that IH90 and IH80 may be valuable biomarkers for poor respiratory outcomes. Our observation of IH90 and IH80 as predictors is consistent with recent publications on the association between IH80 daily duration and prolonged IH80 events with BPD (21, 22) and prolonged IH80 with pulmonary hypertension and death in infants with BPD (23). Because IH90 events were much more frequent than IH80 in our study, and because they were closer to the usual oxygen saturation targets (91–95%) (24–26), this suggests that inclusion of periods of “milder” hypoxemia creates a more sensitive biomarker of worse outcome than IH80. An explanation for how IH may be mechanistically associated with worse outcome requires further study. It is possible that it is not just brief hypoxemia but the associated reoxygenation and oxidative stress that contribute to morbidity (8). It is not clear if avoidance of or shortening the duration of IH events, such as with automated control of inspired oxygen concentration (“closed-loop automated oxygen control”) (27, 28), would reduce unfavorable outcomes or whether IH90 is simply a marker of illness severity. It is possible that the risk factors identified in the prediction models lie on causal pathways, but our intent was not causal analysis. For example, IH90 and IH80 were associated with worse outcome, and it needs to be determined if IH is in the causal pathway. If IH contributes to lung damage, reducing IH would increase favorable outcome, but if reoxygenation or clinical therapy of IH causes unfavorable outcome, perhaps increasing ventilator settings/FiO2 to reduce IH would increase lung injury and make outcomes worse.
We also found that baseline clinical variables such as GA and SGA were strongly associated with unfavorable outcome. Respiratory outcomes such as BPD are strongly dependent on the magnitude of immaturity (29, 30). Models using BW and GA in combination with respiratory support variables such as FiO2 have good predictive accuracy (30). Our models confirm that GA or BW is an important variable soon after birth, whereas the magnitude of respiratory support becomes increasingly important with advancing postnatal age (31). SGA preterm infants are known to be at higher risk of BPD (32–34), probably because of impairment of lung development at critical developmental stages.
Models combining physiologic and clinical data did not substantially improve overall predictive performance compared with models with only clinical variables or models with only physiologic variables at later time points, even though the contributing variables were different. It is possible that predictive accuracies higher than 0.90 may not be achievable, because accuracies above 0.85 are already excellent, and some infants develop severe illness later (e.g., necrotizing enterocolitis, sepsis) with respiratory deterioration. Future studies may address discordance in prediction to identify unidentified or unmeasured risk factors. Some changes in clinical data (e.g., respiratory support) may be in response to changes in physiologic measures (e.g., increases in FiO2 secondary to frequent IH or provision of CPAP in response to frequent apnea), and some changes in physiologic data may be secondary to clinical therapies (e.g., CPAP or caffeine). Hence, it is necessary to consider the interdependence of the physiologic and clinical data variables in their contribution to outcomes.
It is important to compare and contrast the physiologic variables associated with the secondary outcomes versus the primary outcome. IH80 dpe was a major physiologic predictor of the “highly unfavorable” outcome and grade 3 BPD (16) outcome, whereas IH90 dpe and IH90 daily duration were predictors of the moderate/severe BPD (15) outcome. The deeper desaturations (IH80) are associated with increased severity of unfavorable outcome or BPD, whereas desaturations closer to target range (IH90) are associated with less unfavorable outcomes. SGA and use of sedatives were also stronger contributors to the highly unfavorable and severe BPD outcomes, whereas variables such as FiO2 and use of positive pressure were associated with moderate-severe BPD. The use of sedatives is common in the neonatal ICU, especially in younger gestation and SGA infants who remain intubated at older postnatal ages (35) and hence may be a marker for severe lung disease, with the need for sedation reflecting infant agitation in the setting of prolonged mechanical ventilation.
Major strengths of this study are the analysis of a large multicenter prospective cohort with high-resolution cardiorespiratory data. Events such as apnea, PB, IH, and bradycardia were measured using sophisticated algorithms. However, some limitations need to be acknowledged. One limitation is that the outcomes are short term by 40 weeks PMA/discharge, and longer-term respiratory outcomes are pending follow-up evaluation. Short-term outcomes also introduce time-related bias because prediction at later time points is closer to the outcome. Also, apnea or PB could not be detected during IMV because of the intervention of mechanical breaths. Thus, apnea and PB could not be adequately characterized in infants with the anticipated highest likelihood of worse outcomes.
It is essential to consider the relevance of our results for the field of neonatology. Despite advances in neonatal care and reductions in neonatal mortality and many neonatal morbidities, the rate of BPD in extremely preterm infants has increased in recent years (36), indicating that additional research on the causes of respiratory morbidity is essential. Our data demonstrate that although clinical variables such as BW, GA, and magnitude of respiratory support are strong predictors of respiratory outcome, physiologic data that reflect abnormalities of control of breathing are also associated with poor outcome. Although there is only incremental improvement in prediction accuracy in combining physiologic and clinical variables, physiologic data alone are independent predictors of unfavorable respiratory outcome. Indeed, many clinical parameters such as respiratory support may be responses to physiologic instability. It is probable that better and clinically accessible quantitative measurements of IH90 and IH80 would advance clinical care. It is possible that IH90 is a more sensitive marker of worse outcome than apnea or bradycardia, which are less frequent and associated with more severe or prolonged IH. IH may also be secondary to agitation and other behavioral changes in addition to abnormal control of breathing and hence may have additional predictive value. Early prediction of outcomes by IH estimation, perhaps in combination with clinical models (30) and other measures such as lung ultrasound (37, 38), may enable selective use of potential therapies, reducing adverse effects of such therapies in infants at lower risk and targeting therapies to those at higher risk (39). Future clinical studies may identify mechanisms regulating IH (e.g., by alterations in peripheral chemosensitivity [40]), determine optimal interventions to reduce IH (e.g., by servo-controlled oxygen supplementation, increasing caffeine dose or duration, prolonged CPAP), and then test in randomized trials if such interventions improve outcomes in preterm infants.
Acknowledgments
Acknowledgment
Participating sites collected and stored the data, and the University of Virginia, the lead data coordinating center (LDCC), analyzed the data. The co–principal investigators (co-PIs) at each site had full access to individual site data and take responsibility for the integrity of the raw waveforms, and Drs. Randall Moorman (LDCC co-PI) and Douglas Lake (LDCC co-PI) take responsibility for the integrity of the data and accuracy of the data analysis. We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. The following individuals, in addition to those listed as authors, participated in this study:
Pre-Vent Investigators: Karen D. Fairchild and Amanda M. Zimmet, University of Virginia Center for Advanced Medical Analytics, Charlottesville, VA; Erin K Lonergan, Casey M. Rand: Ann & Robert H. Lurie Children’s Hospital of Chicago, and Pediatric Autonomic Medicine, Stanley Manne Children’s Research Institute, Chicago, IL; Arlene Zadell, Department of Pediatrics, University Hospitals Cleveland Medical Center, Rainbow Babies and Children’s Hospital, Cleveland, OH; Arie Nakhmani, Department of Electrical and Computer Engineering, University of Alabama at Birmingham, Birmingham, AL; Waldemar A. Carlo, Deborah Laney, and Colm P. Travers, Division of Neonatology, Department of Pediatrics, University of Alabama at Birmingham School of Medicine, Birmingham, AL; Ana Cecilia Aguilar and Alini Schott, Division of Neonatology, Department of Pediatrics, University of Miami Miller School of Medicine, Holtz Children’s Hospital – University of Miami/Jackson Memorial Medical Center, Miami, FL; and Julie Hoffmann, Washington University School of Medicine in St. Louis, St. Louis, MO.
NIH/NHLBI:
Neil Aggarwal, M.D.: Division of Lung Diseases, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD
Lawrence Baizer, Ph.D.: Division of Lung Diseases, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD
Peyvand Ghofrani, M.D.E.: Division of Lung Diseases, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD
Aaron D. Laposky, Ph.D.: Division of Lung Diseases, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD
Aruna Natarajan M.D., Ph.D.: Division of Lung Diseases, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD
Barry Schmetter, B.S.: Division of Lung Diseases, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD
Observational Study Monitoring Board (OSMB):
Estelle B. Gauda, M.D. (Chair): Division of Neonatology, University of Toronto Hospital for Sick Children, Toronto, ON, Canada
Jonathan M. Davis, M.D.: Division of Newborn Medicine, Tufts Clinical and Translational Science Institute, Boston, MA
Roberta L. Keller, M.D.: Department of Pediatrics, University of California, San Francisco School of Medicine, San Francisco CA
Robinder G. Khemani, M.D.: Department of Anesthesiology and Critical Care Medicine, Children’s Hospital Los Angeles, Los Angeles, CA
Renee H. Moore, Ph.D.: Department of Epidemiology and Biostatistics, Drexel University, Philadelphia, PA
Elliott M. Weiss, M.D., M.S.M.E.: Department of Pediatrics, University of Washington School of Medicine, Seattle, WA; Treuman Katz Center for Pediatric Bioethics, Seattle Children’s Research Institute, Seattle, WA
University of Virginia:
Amy K. Camblos, B.S.: Clinical Trials Office, University of Virginia School of Medicine, Charlottesville, VA
Gina M. Duda, B.S.: Clinical Trials Office, University of Virginia School of Medicine, Charlottesville, VA
Abigail A. Flower, Ph.D.: Data Science Institute, University of Virginia, Charlottesville, VA
Steven A. Fowler, B.S.: Clinical Trials Office, University of Virginia School of Medicine, Charlottesville, VA
Patcharin Pramoonjago, Ph.D.: Biorepository and Tissue Research Facility, University of Virginia School of Medicine, Charlottesville, VA
Craig A. Rumpel, M.S.: Biorepository and Tissue Research Facility, University of Virginia School of Medicine, Charlottesville, VA
Northwestern University:
Allaa Fadl-Alla, B.S.: Ann & Robert H. Lurie Children’s Hospital of Chicago, and Pediatric Autonomic Medicine, Stanley Manne Children’s Research Institute, Chicago, IL
Lynn D. Boswell, P.T., M.S.: Ann & Robert H. Lurie Children’s Hospital of Chicago, and Pediatric Autonomic Medicine, Stanley Manne Children’s Research Institute, Chicago, IL
Michael S. Carroll, Ph.D.: Ann & Robert H. Lurie Children’s Hospital of Chicago, and Data Analytics and Reporting, Stanley Manne Children’s Research Institute, Chicago, IL
Raye-Ann DeRegnier, M.D.: Northwestern University Feinberg School of Medicine, Ann & Robert H. Lurie Children’s Hospital of Chicago, and Neonatology, Stanley Manne Children’s Research Institute, Chicago, IL
Bradley S. Hopkins, R.R.T.: Ann & Robert H. Lurie Children’s Hospital of Chicago, and Pediatric Autonomic Medicine, Stanley Manne Children’s Research Institute, Chicago, IL
Narayanan Krishnamurthi, Ph.D.: Ann & Robert H. Lurie Children’s Hospital of Chicago, and Pediatric Autonomic Medicine, Stanley Manne Children’s Research Institute, Chicago, IL
University of Alabama at Birmingham:
David Paydarfar, M.D.: Department of Neurology at Dell Medical School, University of Texas at Austin, Austin, TX
Elisabeth Salisbury, Ph.D.: Department of Pediatrics and Neurology, University of Massachusetts Medical School, Worcester, MA
Bradley Troxler, M.D.: Division of Pulmonary and Sleep Medicine, Department of Pediatrics, University of Alabama at Birmingham School of Medicine, Birmingham, AL
Washington University:
Ryan Colvin, M.S.: Division of General Medicine, Washington University School of Medicine in St. Louis, St. Louis, MO
Joey Egan, R.R.T.: Respiratory Care, St. Louis Children’s Hospital, St. Louis, MO
Elise Eiden, M.S.: Institute for Informatics, Washington University School of Medicine in St. Louis, St. Louis, MO
Jeffery Hoover, M.D.: Division of Newborn Medicine, Washington University School of Medicine in St. Louis, St. Louis, MO
Laura Linneman, R.N.: Washington University School of Medicine in St. Louis, St. Louis, MO
Daniel Mammel, M.D.: Division of Newborn Medicine, Washington University School of Medicine in St. Louis, St. Louis, MO
Michael McLeland, Ph.D.: Sleep Laboratory, St. Louis Children’s Hospital, St. Louis, MO
Harley Pyles, R.R.T.: Respiratory Care, St. Louis Children’s Hospital, St. Louis, MO
Barbara Warner, M.D.: Division of Newborn Medicine, Washington University School of Medicine in St. Louis, St. Louis, MO
Footnotes
A complete list of Prematurity-related Ventilatory Control (Pre-Vent) Investigators Study Group members may be found before the beginning of the References.
Supported by NHLBI, NIH, grants U01 HL133708, U01 HL133643, U01 HL133704, U01 HL133536, U01 HL133689, and U01 HL133700. The NIH and the NHLBI provided grant support through cooperative agreements. This research was supported in part by the Intramural Research Program of the NIH and NHLBI. Although NHLBI staff did have input into the study design, conduct, analysis, and manuscript drafting, the content and views expressed in this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or the U.S. Department of Health and Human Services.
Author Contributions: Substantial contributions to the conception or design of the work or the acquisition, analysis, or interpretation of data for the work: all authors, including all Pre-Vent Investigators. Drafting the work: N.A. Revising the work critically for important intellectual content: all authors. Final approval of the version to be published: all authors, including all Pre-Vent Investigators. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: all authors, including all Pre-Vent Investigators.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202210-1971OC on May 23, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Prematurity-related Ventilatory Control (Pre-Vent) Investigators:
Karen D. Fairchild, Amanda M. Zimmet, Erin K Lonergan, Casey M. Rand, Arlene Zadell, Arie Nakhmani, Waldemar A. Carlo, Deborah Laney, Colm P. Travers, Ana Cecilia Aguilar, Alini Schott, Julie Hoffmann, Neil Aggarwal, Lawrence Baizer, Peyvand Ghofrani, Aaron D. Laposky, Aruna Natarajan, Barry Schmetter, Estelle B. Gauda, Jonathan M. Davis, Roberta L. Keller, Robinder G. Khemani, Renee H. Moore, Elliott M. Weiss, Amy K. Camblos, Gina M. Duda, Abigail A. Flower, Steven A. Fowler, Patcharin Pramoonjago, Craig A. Rumpel, Allaa Fadl-Alla, Lynn D. Boswell, Michael S. Carroll, Raye-Ann DeRegnier, Bradley S. Hopkins, Narayanan Krishnamurthi, David Paydarfar, Elisabeth Salisbury, Bradley Troxler, Ryan Colvin, Joey Egan, Elise Eiden, Jeffery Hoover, Laura Linneman, Daniel Mammel, Michael McLeland, Harley Pyles, and Barbara Warner
References
- 1. Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet . 2015;385:430–440. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 2. Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet . 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 3. Mwaniki MK, Atieno M, Lawn JE, Newton CR. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet . 2012;379:445–452. doi: 10.1016/S0140-6736(11)61577-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Henderson-Smart DJ. The effect of gestational age on the incidence and duration of recurrent apnoea in newborn babies. Aust Paediatr J . 1981;17:273–276. doi: 10.1111/j.1440-1754.1981.tb01957.x. [DOI] [PubMed] [Google Scholar]
- 5. Di Fiore JM, Martin RJ, Gauda EB. Apnea of prematurity—perfect storm. Respir Physiol Neurobiol . 2013;189:213–222. doi: 10.1016/j.resp.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 6. Hofstetter AO, Legnevall L, Herlenius E, Katz-Salamon M. Cardiorespiratory development in extremely preterm infants: vulnerability to infection and persistence of events beyond term-equivalent age. Acta Paediatr . 2008;97:285–292. doi: 10.1111/j.1651-2227.2007.00618.x. [DOI] [PubMed] [Google Scholar]
- 7. Di Fiore JM, Bloom JN, Orge F, Schutt A, Schluchter M, Cheruvu VK, et al. A higher incidence of intermittent hypoxemic episodes is associated with severe retinopathy of prematurity. J Pediatr . 2010;157:69–73. doi: 10.1016/j.jpeds.2010.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martin RJ, Wang K, Köroğlu O, Di Fiore J, Kc P. Intermittent hypoxic episodes in preterm infants: do they matter? Neonatology . 2011;100:303–310. doi: 10.1159/000329922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Travers CP, Carlo WA, Ambalavanan N. The future of outcome prediction for preterm infants in the neonatal ICU. Am J Respir Crit Care Med . 2022;205:6–8. doi: 10.1164/rccm.202109-2188ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al. Caffeine for Apnea of Prematurity Trial Group Caffeine therapy for apnea of prematurity. N Engl J Med . 2006;354:2112–2121. doi: 10.1056/NEJMoa054065. [DOI] [PubMed] [Google Scholar]
- 11. Bucher HU, Duc G. Does caffeine prevent hypoxaemic episodes in premature infants? A randomized controlled trial. Eur J Pediatr . 1988;147:288–291. doi: 10.1007/BF00442697. [DOI] [PubMed] [Google Scholar]
- 12. Dobson NR, Rhein LM, Darnall RA, Corwin MJ, Heeren TC, Eichenwald E, et al. Caffeine Study Group Caffeine decreases intermittent hypoxia in preterm infants nearing term-equivalent age. J Perinatol . 2017;37:1135–1140. doi: 10.1038/jp.2017.82. [DOI] [PubMed] [Google Scholar]
- 13. Rhein LM, Dobson NR, Darnall RA, Corwin MJ, Heeren TC, Poets CF, et al. Caffeine Pilot Study Group Effects of caffeine on intermittent hypoxia in infants born prematurely: a randomized clinical trial. JAMA Pediatr . 2014;168:250–257. doi: 10.1001/jamapediatrics.2013.4371. [DOI] [PubMed] [Google Scholar]
- 14. Dennery PA, Di Fiore JM, Ambalavanan N, Bancalari E, Carroll JL, Claure N, et al. Pre-Vent: the prematurity-related ventilatory control study. Pediatr Res . 2019;85:769–776. doi: 10.1038/s41390-019-0317-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med . 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 16. Jensen EA, Dysart K, Gantz MG, McDonald S, Bamat NA, Keszler M, et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants. An evidence-based approach. Am J Respir Crit Care Med . 2019;200:751–759. doi: 10.1164/rccm.201812-2348OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee H, Rusin CG, Lake DE, Clark MT, Guin L, Smoot TJ, et al. A new algorithm for detecting central apnea in neonates. Physiol Meas . 2012;33:1–17. doi: 10.1088/0967-3334/33/1/1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mohr MA, Fairchild KD, Patel M, Sinkin RA, Clark MT, Moorman JR, et al. Quantification of periodic breathing in premature infants. Physiol Meas . 2015;36:1415–1427. doi: 10.1088/0967-3334/36/7/1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Di Fiore JM, Kaffashi F, Loparo K, Sattar A, Schluchter M, Foglyano R, et al. The relationship between patterns of intermittent hypoxia and retinopathy of prematurity in preterm infants. Pediatr Res . 2012;72:606–612. doi: 10.1038/pr.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McFadden D. In: Frontiers in econometrics. Zarembka P, editor. New York: Academic Press; 1973. Conditional logit analysis of qualitative choice behavior; pp. 105–142. [Google Scholar]
- 21. Jensen EA, Whyte RK, Schmidt B, Bassler D, Vain NE, Roberts RS, Canadian Oxygen Trial Investigators Association between intermittent hypoxemia and severe bronchopulmonary dysplasia in preterm infants. Am J Respir Crit Care Med . 2021;204:1192–1199. doi: 10.1164/rccm.202105-1150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Raffay TM, Dylag AM, Sattar A, Abu Jawdeh EG, Cao S, Pax BM, et al. Neonatal intermittent hypoxemia events are associated with diagnosis of bronchopulmonary dysplasia at 36 weeks postmenstrual age. Pediatr Res . 2019;85:318–323. doi: 10.1038/s41390-018-0253-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gentle SJ, Travers CP, Nakhmani A, Indic P, Carlo WA, Ambalavanan N. Intermittent hypoxemia and bronchopulmonary dysplasia with pulmonary hypertension in preterm infants. Am J Respir Crit Care Med . 2023;207:899–907. doi: 10.1164/rccm.202203-0580OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carlo WA, Finer NN, Walsh MC, Rich W, Gantz MG, Laptook AR, et al. SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med . 2010;362:1959–1969. doi: 10.1056/NEJMoa0911781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Askie LM, Darlow BA, Finer N, Schmidt B, Stenson B, Tarnow-Mordi W, et al. Neonatal Oxygenation Prospective Meta-analysis (NeOProM) Collaboration Association between oxygen saturation targeting and death or disability in extremely preterm infants in the neonatal oxygenation prospective meta-analysis collaboration. JAMA . 2018;319:2190–2201. doi: 10.1001/jama.2018.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schmidt B, Whyte RK, Asztalos EV, Moddemann D, Poets C, Rabi Y, et al. Canadian Oxygen Trial (COT) Group Effects of targeting higher vs lower arterial oxygen saturations on death or disability in extremely preterm infants: a randomized clinical trial. JAMA . 2013;309:2111–2120. doi: 10.1001/jama.2013.5555. [DOI] [PubMed] [Google Scholar]
- 27. Claure N, Bancalari E. New modes of respiratory support for the premature infant: automated control of inspired oxygen concentration. Clin Perinatol . 2021;48:843–853. doi: 10.1016/j.clp.2021.08.002. [DOI] [PubMed] [Google Scholar]
- 28. Bachman TE, Onland W, van Kaam AH, Roubik K, Hummler HD, Lal M, et al. Frequency and duration of extreme hypoxemic and hyperoxemic episodes during manual and automatic oxygen control in preterm infants: a retrospective cohort analysis from randomized studies. BMC Pediatr . 2022;22:350. doi: 10.1186/s12887-022-03407-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Onland W, Debray TP, Laughon MM, Miedema M, Cools F, Askie LM, et al. Clinical prediction models for bronchopulmonary dysplasia: a systematic review and external validation study. BMC Pediatr . 2013;13:207. doi: 10.1186/1471-2431-13-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Laughon MM, Langer JC, Bose CL, Smith PB, Ambalavanan N, Kennedy KA, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med . 2011;183:1715–1722. doi: 10.1164/rccm.201101-0055OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Greenberg RG, McDonald SA, Laughon MM, Tanaka D, Jensen E, Van Meurs K, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network Online clinical tool to estimate risk of bronchopulmonary dysplasia in extremely preterm infants. Arch Dis Child Fetal Neonatal Ed . 2022;107:638–643. doi: 10.1136/archdischild-2021-323573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jensen EA, Foglia EE, Dysart KC, Simmons RA, Aghai ZH, Cook A, et al. Adverse effects of small for gestational age differ by gestational week among very preterm infants. Arch Dis Child Fetal Neonatal Ed . 2019;104:F192–F198. doi: 10.1136/archdischild-2017-314171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Regev RH, Lusky A, Dolfin T, Litmanovitz I, Arnon S, Reichman B, Israel Neonatal Network Excess mortality and morbidity among small-for-gestational-age premature infants: a population-based study. J Pediatr . 2003;143:186–191. doi: 10.1067/S0022-3476(03)00181-1. [DOI] [PubMed] [Google Scholar]
- 34. Reiss I, Landmann E, Heckmann M, Misselwitz B, Gortner L. Increased risk of bronchopulmonary dysplasia and increased mortality in very preterm infants being small for gestational age. Arch Gynecol Obstet . 2003;269:40–44. doi: 10.1007/s00404-003-0486-9. [DOI] [PubMed] [Google Scholar]
- 35. Zimmerman KO, Smith PB, Benjamin DK, Laughon M, Clark R, Traube C, et al. Sedation, analgesia, and paralysis during mechanical ventilation of premature infants. J Pediatr . 2017;180:99–104.e1. doi: 10.1016/j.jpeds.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA . 2015;314:1039–1051. doi: 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Loi B, Vigo G, Baraldi E, Raimondi F, Carnielli VP, Mosca F, et al. Lung ultrasound to monitor extremely preterm infants and predict bronchopulmonary dysplasia. A multicenter longitudinal cohort study. Am J Respir Crit Care Med . 2021;203:1398–1409. doi: 10.1164/rccm.202008-3131OC. [DOI] [PubMed] [Google Scholar]
- 38. Pezza L, Alonso-Ojembarrena A, Elsayed Y, Yousef N, Vedovelli L, Raimondi F, et al. Meta-analysis of lung ultrasound scores for early prediction of bronchopulmonary dysplasia. Ann Am Thorac Soc . 2022;19:659–667. doi: 10.1513/AnnalsATS.202107-822OC. [DOI] [PubMed] [Google Scholar]
- 39. Steinhorn R, Davis JM, Göpel W, Jobe A, Abman S, Laughon M, et al. International Neonatal Consortium Chronic pulmonary insufficiency of prematurity: developing optimal endpoints for drug development. J Pediatr . 2017;191:15–21.e1. doi: 10.1016/j.jpeds.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 40. Martin RJ, Di Fiore JM, Macfarlane PM, Wilson CG. Physiologic basis for intermittent hypoxic episodes in preterm infants. Adv Exp Med Biol . 2012;758:351–358. doi: 10.1007/978-94-007-4584-1_47. [DOI] [PubMed] [Google Scholar]