To the Editor:
Although highly effective cystic fibrosis (CF) transmembrane conductance regulator (CFTR) modulators have improved the lung function and health of individuals with CF, lung infections caused by Pseudomonas aeruginosa and other pathogens often persist, and new infections may develop after treatment begins (1). Robust strategies for diagnosing respiratory pathogens therefore remain essential in CF disease management. Sputum culture is the current standard of care for the detection of infections but has limitations even in subjects not taking modulators. For example, sputum can be difficult to obtain from children, and even induced sputum can yield false-negative results (2). False positives can result from salivary contamination or other sources (3, 4). Treatment with CFTR modulators further limits sputum culture utility by reducing sputum volume and bacterial loads (1).
An emerging analyte in molecular diagnosis is cell-free DNA (cfDNA), nucleic acid released from decomposing cells into patient blood, which can be cataloged using next-generation DNA sequencing (NGS). Although widely applied in neonatal and oncology testing, cfDNA has also been used for infectious disease and can diagnose bacteremia, invasive fungal infection, and acute pneumonia (5). Here, we developed methods for plasma microbial cfDNA sequencing as a noninvasive approach for diagnosing lung infections in people with CF treated with CFTR modulators (Figure 1).
Figure 1.
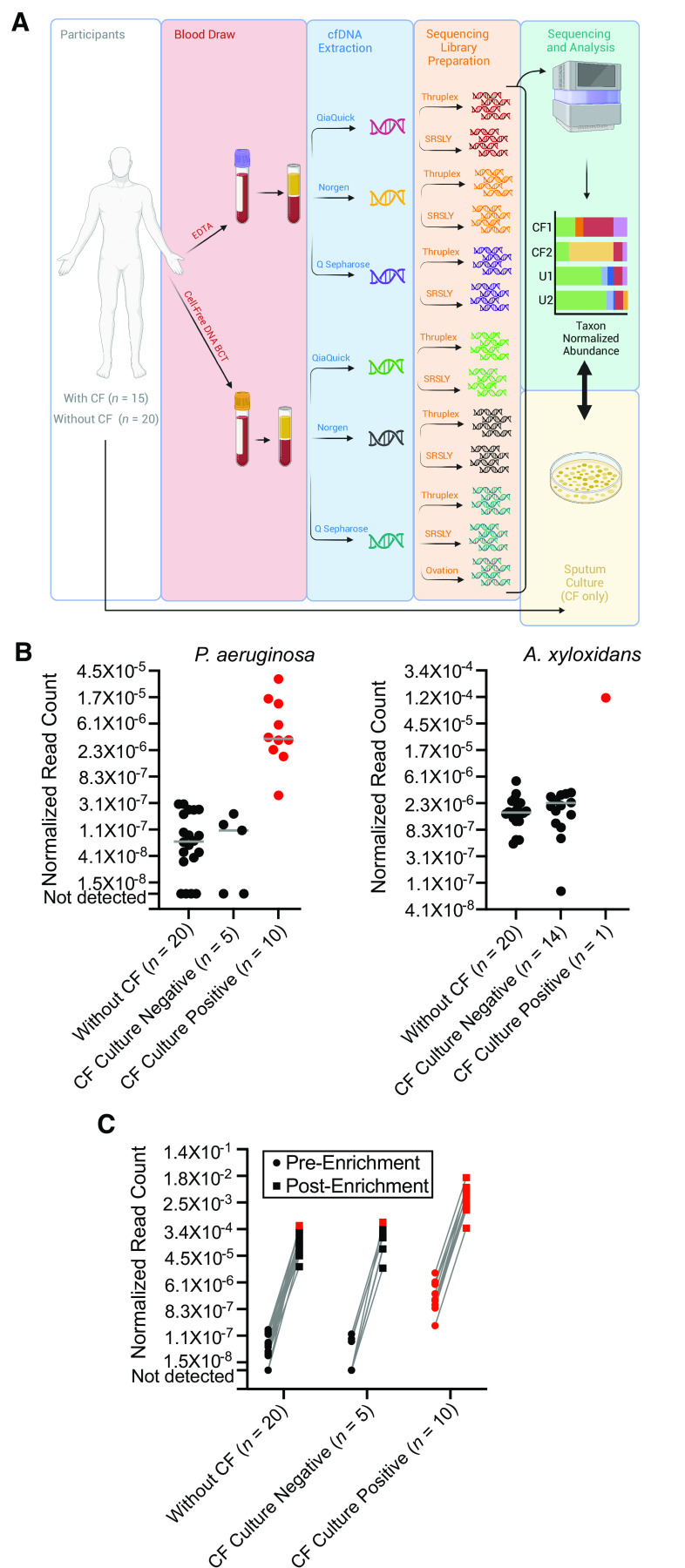
(A) Schematic illustration of the study design. Whole blood was collected from participants with cystic fibrosis (CF) and healthy, unaffected individuals using two different methods (standard ethylenediaminetetraacetic acid collection tubes and Cell-Free DNA BCT tubes), with cell-free DNA (cfDNA) subsequently purified from plasma using each of three different methods (Q Sepharose, Norgen, and QiaQuick). Next-generation DNA sequencing libraries were prepared using different library preparation techniques (SRSLY, Thruplex, and a subset of specimens using Ovation). Sequencing was performed on all libraries, the taxonomic origin of cfDNA fragments was catalogued, and the normalized abundance of species of interest in individuals with CF (CF1 and CF2) was tallied and interpreted relative to background measurements in a population of healthy individuals without CF (U1 and U2). Findings were separately compared against sputum culture results collected from participants with CF. (B) Normalized abundance of Pseudomonas aeruginosa and Achromobacter xylosoxidans cfDNA reads in participants with CF and healthy unaffected individuals. Individuals are stratified by cohort membership (participants with CF or without CF) and microbiological culture results where applicable. Gray solid line indicates the median measurement per group. Significant levels of P. aeruginosa cfDNA (z-score ⩾2, P ⩽ 0.02) and A. xylosoxidans (z-score ⩾5, P ⩽ 0.00001, due to greater population variance) relative to individuals without CF are indicated in red. Note the loge scale on the y-axes. (C) Normalized abundance of P. aeruginosa cfDNA reads shown for standard, unenriched sequencing libraries and the same libraries following whole-genome hybridization enrichment. Individuals are stratified by cohort membership (participants with CF or without CF) and by microbiological culture results where applicable. Significant levels of P. aeruginosa cfDNA (z-score ⩾2, P ⩽ 0.02) relative to individuals without CF are indicated in red. Note the loge scale on the y-axis. EDTA = ethylenediaminetetraacetic acid.
Specimens were prospectively obtained with informed consent at the University of Washington (UW) following approval by the UW Human Subjects Division. Participants with CF (n = 21) were excluded if they were not expectorating at the time of the blood draw but were otherwise not selected for inclusion criteria leaving a final cohort of 15 individuals (Table 1). The FEV1 % predicted of participants with CF was similar between expectorating (average, 66%; SD, 17%) and nonexpectorating (69%; SD, 20%) groups. A total of 13 of 15 participants with CF (87%) were taking CFTR modulator therapy, most with elexacaftor/tezacaftor/ivacaftor. Spontaneously expectorated sputum was collected only from participants with CF at the time of the blood draw and cultured by the UW Clinical Microbiology Laboratory, with pathogen culture positivity defined by this assessment. A convenience sample of unaffected individuals from the general population without acute or chronic respiratory infection (n = 20) approximated the CF cohort’s average age but included more female participants (65%).
Table 1.
Participant Demographic Characteristics
| Characteristic | Without CF (n = 20) | With CF |
|
|---|---|---|---|
| Pseudomonas aeruginosa Culture Positive (n = 10) | Pseudomonas aeruginosa Culture Negative (n = 5) | ||
| Age, yr | 37 ± 12.0 | 36.2 ± 12.9 | 40 ± 13.5 |
| Female sex | 13 (65) | 3 (30) | 1 (20) |
| Race | |||
| White | 21 (100) | 9 (90) | 5 (100) |
| Black | 0 | 1 (10) | 0 |
| FEV1, % predicted | ND | 67.1 ± 8.3 | 58.4 ± 25.0 |
| FVC, % predicted | ND | 81.9 ± 12.1 | 75.6 ± 19.0 |
| FEV1/FVC | ND | 0.677 ± 0.08 | 0.61 ± 0.15 |
| Body mass index, kg/m2 | ND | 25.9 ± 5.2 | 23.7 ± 4.2 |
| CFTR modulator therapy | |||
| Elexacaftor/tezacaftor/ivacaftor | 0 | 8 (80) | 3 (60) |
| Tezacaftor/ivacaftor | 0 | 0 | 1 (5) |
| Ivacaftor | 0 | 0 | 1 (5) |
| None | 0 | 2 (20) | 0 |
Data are presented as mean ± standard deviation where applicable. Values in parentheses are percentages.
Definition of abbreviations: BMI = body mass index; CFTR = cystic fibrosis transmembrane conductance regulator; ND = not determined.
To define diagnostic methods that were optimal in CF, we tested two different formulations of blood collection tubes, three different cfDNA extraction methods, and three different library preparation techniques (13 combinations in all; Figure 1) and identified the combination that best discriminated P. aeruginosa culture-positive from culture-negative cases of CF using unpaired Wilcoxon rank-sum testing. Blood from all participants was collected in Cell-Free DNA BCT (Streck) and EDTA Vacutainer (Becton Dickinson) tubes for processing (6). cfDNA was extracted from 0.5–1 ml of plasma using a QiAmp MinElute ccfDNA kit (Qiagen), a Plasma/Serum Cell-Free Circulating DNA Purification Mini Kit (Norgen Biotek), and Q Sepharose Fast Flow (GE Healthcare) (7). NGS libraries were constructed by SRSLY PicoPlus DNA NGS Library Preparation (Claret Biosciences), ThruPLEX DNA-Seq Library Prep (Takara), and Ovation Ultralow System V2 Library Prep (Tecan). Libraries were sequenced to an average depth of 20.8 million reads using NextSeq2000 (Illumina) with 300 cycle chemistries. Reads were taxonomically classified using Kraken2 (8), with those from cultured pathogens verified by the Basic Local Alignment Search Tool (9) against appropriate reference genomes (accepting E-value ⩽1 e−5), and subsequently normalized to total classified reads. A combination of Cell-Free DNA BCT tubes (specialized for cfDNA stabilization), Q Sepharose cfDNA purification (7), and Thruplex library preparation proved best and was used for subsequent analyses. Notably, Ovation library preparation, a modified version of which is used in commercial microbial plasma cfDNA analysis (“Karius testing” [10]), did not resolve culture-positive from culture-negative participants when used with optimal blood collection and cfDNA purification techniques.
We compared sequencing data generated from the optimal protocols against sputum culture results to evaluate the diagnostic yield of cfDNA for identifying culture-positive respiratory infections in individuals with CF. P. aeruginosa was most commonly cultured (10 participants), followed by methicillin-sensitive Staphylococcus aureus (3 participants) and Candida albicans (3 participants). Other infecting microbes, including Achromobacter xylosoxidans, were identified only in individual participants.
As expected, specimen cfDNA was largely of human origin (6), but participants with CF had greater total microbial cfDNA than participants without CF (1.9% vs. 1.1% nonhuman reads; P = 0.013, two-tailed t test). Although no participants were culture-positive for Burkholderia species, we noted substantial read counts from such organisms in individuals with CF and unaffected participants, suggesting background contamination. The significance of normalized pathogen cfDNA read counts in individuals with CF was assessed relative to the distribution of counts in unaffected individuals using z-scores. For P. aeruginosa and A. xylosoxidans (Figure 1), pathogen plasma cfDNA levels identified culture positivity in corresponding subjects. Significantly increased normalized P. aeruginosa cfDNA levels were observed for all 10 culture-positive participants (z-scores ⩾2.96, P ⩽ 0.0015) but none of the 5 culture-negative individuals with CF, corresponding to a 100% positive predictive value (95% confidence interval, 69.15–100.00%) and a 100.00% negative predictive value (95% confidence interval, 47.82–100.00%). The single individual who was culture-positive for A. xylosoxidans also showed significantly increased levels of A. xylosoxidans cfDNA (z-score 110.89, P ⩽ 0.00001), whereas none of the 14 culture-negative participants did, although this result has limited interpretability because of the low positivity rate. For all participants, culture results obtained at the time of the blood draw were consistent with preceding cultures obtained within 2 years of recruitment. In contrast, normalized cfDNA levels from S. aureus and C. albicans did not differ among culture-positive and culture-negative participants. The finding that cfDNA from infecting P. aeruginosa and A. xylosoxidans was uniquely detectable in this study may reflect the strong inflammatory response precipitated by those pathogens (11, 12), which reportedly correlates with microbial cfDNA plasma levels (13).
We investigated whether methods for P. aeruginosa whole-genome hybridization enrichment (14) could enhance pathogen detection from cfDNA (Figure 1). Enrichment significantly (P = 0.015, two-tailed paired t test) increased normalized read counts from P. aeruginosa while decreasing human cfDNA (P = 6.1 × 10−13, two-tailed paired t test). For the 10 culture-positive participants, P. aeruginosa read counts increased an average of 3,505 fold, indicating that sequencing power could be reduced by the same factor without negatively impacting assay performance. However, relative levels of normalized P. aeruginosa cfDNA remained unchanged when compared among participants (r[35] = 0.947, P < 0.00001, Pearson correlation coefficient). All 10 culture-positive participants continued to show significant levels of P. aeruginosa cfDNA after enrichment relative to unaffected individuals (z-scores ⩾2, P ⩽ 0.02). One culture-negative participant also exhibited significantly increased cfDNA after enrichment; however, an outlier from the non-CF cohort showed similar increases, suggesting that more stringent interpretive thresholds may be required following enrichment procedures.
Finally, because cfDNA isolation and detection is affected by fragment size (7), we examined sequencing data to ascertain cfDNA fragment lengths from host and pathogen genomes. The average size of P. aeruginosa and A. xylosoxidans cfDNA from culture-positive individuals was qualitatively shorter (∼55 bp) than human-derived fragments (∼160 bp). These findings are consistent with previous work (6), highlighting the need for procedures that can identify short DNA.
These results show that plasma cfDNA sequencing can identify P. aeruginosa and A. xylosoxidans respiratory culture positivity in individuals with CF, including those treated with CFTR modulators. Specific methods for the purification and detection of cfDNA optimized diagnostic yields, and whole-genome hybridization enrichment can substantially reduce read-depth requirements for targeted organisms. Our methods are likely feasible for reference laboratories to implement. Future work will expand and validate these methods using larger cohorts, compare results versus those in unaffected individuals with respiratory illness, and assess applicability across a wider range of pathogens.
Acknowledgments
Acknowledgment
The authors thank the individuals who participated in these studies and the University of Washington CF Research Development Program.
Footnotes
Supported by NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases grant K23 AR080209 (to D.R.L.), NIH/National Institute of Diabetes and Digestive and Kidney Diseases grant P30 DK089507 (to S.J.S., C.H.G., and P.K.S.), and Cystic Fibrosis Foundation grants SINGH19R0 (to S.J.S. and P.K.S.) and SALIPA21I0 (to S.J.S.).
Author Contributions: S.J.S. planned the experiments; D.R.L., E.A.H., C.H.G., A.W., and S.J.S. performed the experiments; D.R.L., E.A.H., C.H.G., P.K.S., A.W., and S.J.S. analyzed the data; and D.R.L., E.A.H., C.H.G., P.K.S., A.W., and S.J.S. wrote the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.202305-0844LE on August 4, 2023
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Nichols DP, Morgan SJ, Skalland M, Vo AT, Van Dalfsen JM, Singh SBP, et al. PROMISE-Micro Study Group Pharmacologic improvement of CFTR function rapidly decreases sputum pathogen density, but lung infections generally persist. J Clin Invest . 2023;133:e167957. doi: 10.1172/JCI167957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ronchetti K, Tame JD, Paisey C, Thia LP, Doull I, Howe R, et al. The CF-Sputum Induction Trial (CF-SpIT) to assess lower airway bacterial sampling in young children with cystic fibrosis: a prospective internally controlled interventional trial. Lancet Respir Med . 2018;6:461–471. doi: 10.1016/S2213-2600(18)30171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lu J, Carmody LA, Opron K, Simon RH, Kalikin LM, Caverly LJ, et al. Parallel analysis of cystic fibrosis sputum and saliva reveals overlapping communities and an opportunity for sample decontamination. mSystems . 2020;5:e00296-20. doi: 10.1128/mSystems.00296-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parkins MD, Floto RA. Emerging bacterial pathogens and changing concepts of bacterial pathogenesis in cystic fibrosis. J Cyst Fibros . 2015;14:293–304. doi: 10.1016/j.jcf.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 5. Langelier C, Fung M, Caldera S, Deiss T, Lyden A, Prince BC, et al. Detection of pneumonia pathogens from plasma cell-free DNA. Am J Respir Crit Care Med . 2020;201:491–495. doi: 10.1164/rccm.201904-0905LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barrett SLR, Holmes EA, Long DR, Shean RC, Bautista GE, Ravishankar S, et al. Cell free DNA from respiratory pathogens is detectable in the blood plasma of cystic fibrosis patients. Sci Rep . 2020;10:6903. doi: 10.1038/s41598-020-63970-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oreskovic A, Brault ND, Panpradist N, Lai JJ, Lutz BR. Analytical comparison of methods for extraction of short cell-free DNA from urine. J Mol Diagn . 2019;21:1067–1078. doi: 10.1016/j.jmoldx.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wood DE, Lu J, Langmead B. Improved metagenomic analysis with Kraken 2. Genome Biol . 2019;20:257. doi: 10.1186/s13059-019-1891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol . 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 10. Blauwkamp TA, Thair S, Rosen MJ, Blair L, Lindner MS, Vilfan ID, et al. Analytical and clinical validation of a microbial cell-free DNA sequencing test for infectious disease. Nat Microbiol . 2019;4:663–674. doi: 10.1038/s41564-018-0349-6. [DOI] [PubMed] [Google Scholar]
- 11. Hansen CR, Pressler T, Nielsen KG, Jensen PØ, Bjarnsholt T, Høiby N. Inflammation in Achromobacter xylosoxidans infected cystic fibrosis patients. J Cyst Fibros . 2010;9:51–58. doi: 10.1016/j.jcf.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 12. Pukhalsky AL, Kapranov NI, Kalashnikova EA, Shmarina GV, Shabalova LA, Kokarovtseva SN, et al. Inflammatory markers in cystic fibrosis patients with lung Pseudomonas aeruginosa infection. Mediators Inflamm . 1999;8:159–167. doi: 10.1080/09629359990496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang H, Haidar G, Al-Yousif NS, Zia H, Kotok D, Ahmed AA, et al. Circulating microbial cell-free DNA is associated with inflammatory host-responses in severe pneumonia. Thorax . 2021;76:1231–1235. doi: 10.1136/thoraxjnl-2020-216013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hayden HS, Joshi S, Radey MC, Vo AT, Forsberg C, Morgan SJ, et al. Genome capture sequencing selectively enriches bacterial DNA and enables genome-wide measurement of intrastrain genetic diversity in human infections. MBio . 2022;13:e0142422. doi: 10.1128/mbio.01424-22. [DOI] [PMC free article] [PubMed] [Google Scholar]