Cystic fibrosis (CF) transmembrane conductance regulator protein modulator (CFTRm) drugs have transformed the prognosis and quality of life of 90% of people with CF (pwCF) in high-income countries (1). However, they are not accessible in low- and middle-income countries (LMICs) because of a lack of registration and the prohibitively high price set by Vertex Pharmaceuticals, which has a global monopoly on these life-saving drugs. Vertex has protected itself from competition by filing restrictive patents across the world, effectively blocking the importation of generic drugs in almost all LMICs, many of which are still awaiting registration of the original drugs (2). The prohibitive cost of CFTRm drugs may also endanger access for pwCF in the United States who face the threat of unaffordable out-of-pocket copayments (3).
Vertex was not granted patents for CFTRm drugs in Argentina. Generic formulations of highly effective CFTRm drugs, including elexacaftor/tezacaftor/ivacaftor (ETI), can therefore be manufactured locally at a significantly lower retail price ($4,000 U.S. per month) than the U.S. listed price ($27,000 per month) (4). While awaiting the outcome of a legal challenge in South Africa (SA) to import generic CFTRm drugs through a compulsory license, some pwCF in SA have started traveling to Argentina to personally collect and pay out-of-pocket costs for generic ETI, thereby avoiding the risk of infringing on patent regulations (5).
Clarithromycin is a strong Cytochrome P450 3A (CYP3A) inhibitor that delays liver metabolism of ETI when administered alongside ETI. The recommended dose adjustment is to reduce ETI to twice weekly when coadministered with a strong CYP3A inhibitor (6). Left with little choice to save costs, some pwCF in SA opted to exploit this drug interaction on a long-term basis under the supervision of their medical practitioner.
We documented the early real-world outcomes and safety profile in pwCF in SA initiating treatment with generic ETI from December 2021 to March 2023 in standard (daily) or off-label “modulator-sparing” (twice weekly) dosing in combination with a strong CYP3A inhibitor (clarithromycin 250 mg or 500 mg twice daily). Changes in FEV1% predicted and body mass index for different dosing schedules were recorded at follow-up encounters at 1, 3, and 6 months. Sweat chloride tests were performed at baseline, 1 month after ETI was initiated, and again after 1 month if the ETI dose was changed. Repeated-measures analyses compared outcomes stratified in three ETI dosing strategies: standard, modulator-sparing, and changed from standard to modulator-sparing. Informed consent was obtained from participants, and the study was approved by the University of Cape Town Research Ethics Committee (approval HREC 087/2023).
Of the total 523 people in the SA CF registry in 2021 (69% White, 19% mixed ancestry, 10% Black African), 43 (8.2%) aged between 8 and 52 years initiated generic ETI with the standard dose (n = 31) or the modulator-sparing dose (n = 12) (7). Ten people (23.3%) changed from the standard to the modulator-sparing dose during the follow-up period. Thirty-one (72%) and 10 (23%) were homozygous and heterozygous for F508del, respectively. One pair of siblings were heterozygous with A455E and Y563N mutations.
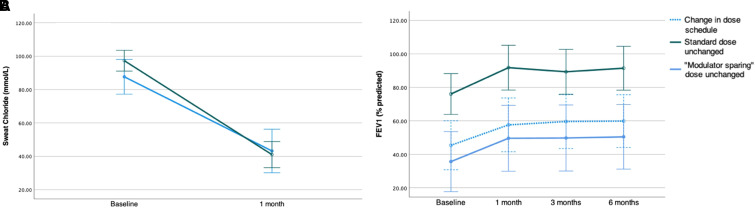
There were significant decreases in sweat chloride in those receiving the standard dose (mean ± SD, −56 ± 18 mmol/L) and the modulator-sparing dose (−44 ± 21 mmol/L), with no statistical difference between dose groups (P = 0.1) (Figure 1A). The mean sweat chloride decreased further by 4 ± 12 mmol/L in seven people who initiated treatment at the standard dose but changed to the modulator-sparing dose between 3 and 10 months after starting treatment. There were overall improvements from baseline in FEV1% predicted (15.1; P < 0.01) after 1 month, which were sustained throughout follow-up, with no difference in the change over time between dosing groups (P > 0.1) (Figure 1B). Similar improvements in body mass index were observed after 1 month (1.0 kg/m2; P < 0.001), with continued improvement over time. Adverse events reported included acne (n = 4), distal intestinal obstruction syndrome (n = 3), dizziness (n = 3), and mood or sleep disorders (n = 4). No hepatoxicity requiring adjustment of ETI treatment was observed. There was one death in an adult with established advanced lung disease that was thought to be unrelated to ETI. Our study is limited by its retrospective design, small sample size (reflecting the limited access to even generic CFTRm drugs in SA), and incomplete follow-up data. In addition, therapeutic drug monitoring of ETI in those receiving the modulator-sparing dose was not done because this is unavailable in SA.
Figure 1.
Baseline and change in sweat chloride (A) and FEV1% predicted (B) in people with cystic fibrosis who initiated treatment with generic elexacaftor/tezacaftor/ivacaftor on a standard, modulator-sparing, or a combination of dosing strategies. Markers indicate mean values and vertical bars 95% confidence intervals.
Our experience with generic ETI is comparable to experience using the original product in terms of efficacy and safety (1, 8). Furthermore, there was no clinically significant difference in outcomes between the modulator-sparing and standard dosing approaches. We propose that modulator-sparing strategies with a strong CYP3A inhibitor may, in extreme circumstances, be an acceptable, effective, and cost-saving interim measure to increase access to lifesaving CFTRm drugs in LMICs. Pharmacokinetic studies and continued monitoring are needed to optimize efficacy and safety, including adverse effects of clarithromycin such as gastrointestinal symptoms and prolongation of the QT interval corrected for heart rate.
The clinical benefits of generic ETI experienced by a fortunate small minority of mostly White and wealthier pwCF in SA highlights the gaping global disparity in CF care between rich and poor in this new era of CF treatment. The unjustified high cost of CFTRm drugs and accompanying restrictive patent conditions have effectively left further behind thousands of pwCF living in LMICs, where CF diagnosis and care is often suboptimal (2, 4). Initiatives such as the United Nations–backed Medicines Patent Pool have been successful vehicles to expand affordable access to other life-altering medicines in LMICs, but it requires a willingness by pharmaceutical industry stakeholders to enter into such licensing agreements (9). Vertex is at the forefront of novel therapy development pipelines for sickle-cell disease, which disproportionately affects people in LMICs, especially children in sub-Saharan Africa (10). Although these advances are applauded, governments and the pharmaceutical industry need to reflect on their responsibility to provide affordable access to life-altering novel drug therapies to everyone who is eligible (10). Achieving a balance between rewarding innovation and the right to health care is a moral obligation. If the current status of global inequalities in access to CFTRm drugs is anything to go by, we are very far away from achieving this balance.
Acknowledgments
Acknowledgment
The authors thank their collaborators, Tony Biebuyck, Carla Els, Paul Gebers, and Dave Richards; and Hanri Truter and Marina Coetzee for assisting with data collection; and the families in South Africa for allowing us to share these data and for the extraordinary sacrifices they make for their loved ones.
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.202307-1200VP on August 7, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. King JA, Nichols AL, Bentley S, Carr SB, Davies JC. An update on CFTR modulators as new therapies for cystic fibrosis. Paediatr Drugs . 2022;24:321–333. doi: 10.1007/s40272-022-00509-y. [DOI] [PubMed] [Google Scholar]
- 2. Zampoli M, Morrow BM, Paul G. Real-world disparities and ethical considerations with access to CFTR modulator drugs: mind the gap! Front Pharmacol . 2023;14:1163391. doi: 10.3389/fphar.2023.1163391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McGarry ME, Gibb ER, Laguna TA, O’Sullivan BP, Sawicki GS, Zobell JT. How many billions is enough? Prioritizing profits over patients with cystic fibrosis. Pediatr Pulmonol . 2023;58:1595–1597. doi: 10.1002/ppul.26335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guo J, Wang J, Zhang J, Fortunak J, Hill A. Current prices versus minimum costs of production for CFTR modulators. J Cyst Fibros . 2022;21:866–872. doi: 10.1016/j.jcf.2022.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Robbins SNR.Miracle cystic fibrosis drug kept out of reach in developing countries. New York Times . 2023.
- 6.Food and Drug Administration. 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2021/212273s008lbl.pdf
- 7.Zampoli M, Verstraete J, Fraeundorf M, CF Steering Committee 2021. https://sacfa.org.za/registry-annual-report-2021/
- 8. Bower JK, Volkova N, Ahluwalia N, Sahota G, Xuan F, Chin A, et al. Real-world safety and effectiveness of elexacaftor/tezacaftor/ivacaftor in people with cystic fibrosis: interim results of a long-term registry-based study. J Cyst Fibros . 2023 doi: 10.1016/j.jcf.2023.03.002. [DOI] [PubMed] [Google Scholar]
- 9. Shadlen KC. Accelerating pooled licensing of medicines to enhance global production and equitable access. Lancet . 2022;400:632–634. doi: 10.1016/S0140-6736(22)01013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iyer JK.Sickle cell cures are coming. African children can’t be left behind. StatNews . 2023. https://www.statnews.com/2023/07/12/sickle-cell-gene-therapy-cures-price-africa-access/