To the Editor:
Sepsis is the leading cause of global mortality and is most often attributed to lower respiratory tract infections and subsequent acute respiratory distress syndrome (ARDS) (1). The greatest burden of sepsis rests on sub-Saharan Africa, where lower respiratory tract infections account for approximately 390,000 adult deaths each year (2). However, patients from sub-Saharan Africa are underrepresented in sepsis and ARDS research (3).
ARDS is difficult to diagnose in low-income countries because it requires often unavailable imaging, mechanical ventilation to set positive end-expiratory pressure and deliver a reliable fraction of inspired oxygen, and arterial blood gases to identify hypoxemia (4). To mitigate this gap, the World Health Organization (WHO) pragmatically defined severe respiratory distress without shock (SRD) in adults as oxygen saturation of less than 90% or a respiratory rate of more than 30 breaths per minute, and a systolic blood pressure over 90 mm Hg in the setting of infection and in the absence of clinical cardiac failure (5). The natural history of SRD has not been fully described; accordingly, we aimed to evaluate the prevalence, characteristics, and outcomes of SRD in hospitalized patients in sub-Saharan Africa.
Methods
We conducted a multi-cohort analysis using previously collected deidentified data pooled from 16 hospital-based studies, which were conducted in six countries throughout sub-Saharan Africa from 2009 through 2019, including one previously unpublished dataset (Kitovu Hospital, Masaka, Uganda) (Table 1) (6). Variables in the pooled dataset included: admission age, sex, temperature, heart rate, respiratory rate, systolic blood pressure, diastolic blood pressure, oxygen saturation, Glasgow coma scale (GCS) score, and HIV serostatus. The pooled dataset did not include causes of infection for all participants. We defined SRD according to WHO criteria with the exception of the exclusion of heart failure. We excluded patients younger than 14 years of age. We imputed missing data using multiple imputation with chained equations with 10 iterations (7). We did not impute sex, HIV serostatus, or mortality. We used Stata 13.0 (Stata) and SAS Version 9.4 (SAS Institute) for all analyses.
Table 1.
Data from Hospital-based Cohort Studies Conducted in Six African Countries from 2009 through 2019
| Study | Year | Site | Inclusion Criteria | Total (n) | In-Hospital Mortality (%) | Average Missing Clinical Data per Patient (%)* | Missing Oxygen Saturation per Study (%) | Missing HIV Data per Study (%) |
|---|---|---|---|---|---|---|---|---|
| Adakun | 2013 | Uganda | Meningitis† | 141 | 28 | 12 | 100 | 0 |
| Amir | 2016 | Uganda | Sepsis‡ | 206 | 31 | 15 | 100 | 1 |
| Andrews | 2013 | Zambia | Sepsis‡ | 209 | 41 | 15 | 10 | 2 |
| Andrews | 2014 | Zambia | Sepsis‡ | 109 | 62 | 14 | 30 | 0 |
| Auma | 2013 | Uganda | Sepsis§ | 216 | 19 | 13 | 100 | 22 |
| Huson | 2015 | Gabon | Sepsisǁ | 381 | 4 | 13 | 3 | 0 |
| Jacob | 2009 | Uganda | Sepsis‡ | 381 | 24 | 13 | 100 | 16 |
| Jacob | 2012 | Uganda | Sepsis‡ | 423 | 25 | 4 | 5 | 0 |
| Majwala | 2013 | Uganda | Meningitis† | 145 | 32 | 13 | 100 | 0 |
| Opio | 2013 | Uganda | Hospitalized¶ | 1,664 | 7 | 17 | 23 | 66 |
| Roth | 2015 | Sierra Leone | Fever** | 429 | 19 | 21 | 100 | 94 |
| Rubach | 2015 | Tanzania | Fever†† | 400 | 11 | 1 | 1 | 3 |
| Rylance | 2009 | Tanzania | Hospitalized¶ | 694 | 11 | 6 | 16 | 100 |
| Ssekitoleko | 2011 | Uganda | Sepsis§ | 150 | 30 | 13 | 100 | 13 |
| Wheeler | 2013 | Malawi | Hospitalized¶ | 355 | 23 | 2 | 0 | 19 |
| Unpublished | 2019 | Uganda‡‡ | Hospitalized¶ | 1,482 | 7 | 4 | 1 | 4 |
| Total | — | — | — | 7,385 | 15 | 10 | 39 | 33 |
The data from these hospital-based cohort studies contributed to pooled data for the analysis of the prevalence, characteristics, and outcomes of World Health Organization–defined severe respiratory disease without shock.
Clinical data include temperature, heart rate, respiratory rate, systolic blood pressure, diastolic blood pressure, and Glasgow Coma Scale score.
Admitted with a clinical diagnosis of meningitis.
Admitted with a clinical diagnosis of severe sepsis, defined as systemic inflammatory response syndrome with suspected infection and organ dysfunction.
Admitted with a clinical diagnosis of sepsis defined as systemic inflammatory response syndrome with suspected infection.
Admitted with a temperature ⩾38°C or <36°C and at least one other systemic inflammatory response syndrome criterion.
Admitted with an acute illness to a medical ward with no other specified inclusion criteria.
Admitted with subjective fever or had a documented temperature ⩾38°C within 24 hours of admission.
Admitted with a temperature ⩾38.0°C.
Kitovu Hospital, Masaka, Uganda.
We determined the associations of respiratory rate and oxygen saturation with mortality in separate univariate analyses. We constructed multivariable baseline risk models of in-hospital mortality in all participants and in a subset with infection, using logistic regression models that included 1) age and sex; 2) age, sex, and HIV serostatus; 3) age, sex, and GCS score; and 4) age, sex, HIV serostatus, and GCS score (1). We created separate models for each study with the same variables, but with study-specific coefficients, and then aggregated the models. We included study-specific models with missing baseline variables, such as HIV serostatus, in the aggregate models with the missing variable deleted. We determined the area under the receiver operating characteristic curve (AUC) and absolute risks for mortality for each baseline model, with and without the inclusion of admission SRD as an independent variable.
Results
Of the 7,385 participants, the median age was 37 years (interquartile range [IQR], 27–53), 3,584 (49%) were female, and 2,282 (46%) of the 4,917 participants with a known HIV serostatus were living with HIV. The median respiratory rate was 24 (IQR, 20–30) breaths per minute. Among the 5,121 participants with oxygen saturation recorded, the median value was 96% (IQR, 94–98%).
There were 949 (13%) participants with SRD. Among the 3,575 participants admitted to the hospital with an infection, 578 (16%) had SRD, compared with 371 (10%) of 3,810 participants with undifferentiated causes of hospital admission (odds ratio [OR], 1.78; 95% confidence interval [CI] = 1.55–2.06). Among participants with SRD, the median (and IQR for) oxygen saturation and respiratory rate were 91% (85–97%) and 35 (31–40) breaths per minute, respectively; 235 (25%) met the criterion of oxygen saturation <90% only, 610 (64%) met the criterion of respiratory rate >30 breaths per minute only, and 104 (11%) met both criteria.
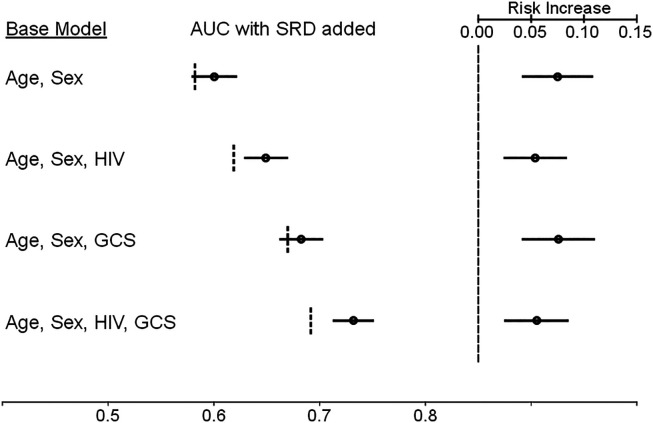
In-hospital mortality occurred in 1,096 (15%) participants. Among participants with SRD, 209 (22%) died in hospital, compared with 887 (14%) of 6,436 participants without SRD (OR, 1.77; 95% CI = 1.49–2.10). The in-hospital case fatality ratio for participants meeting the SRD criteria of respiratory rate only, oxygen saturation only, or both was 111 (18%) of 610, 55 (23%) of 235, and 43 (41%) of 104, respectively (P < 0.001 across groups). In all participants, for every increase of 10 breaths per minute, there was a 75% increase in the odds of mortality (OR, 1.75; 95% CI = 1.64–1.87), and for every 1% increase in oxygen saturation, there was an 8% reduction in the odds of mortality (OR, 0.92; 95% CI = 0.91–0.93). In participants with SRD, there was a nonstatistically significant increase in odds of mortality associated with increased respiratory rate (OR, 1.13; 95% CI = 0.96–1.33), and for every 1% increase in oxygen saturation, there was a 6% reduction in the odds of mortality (OR, 0.94; 95% CI = 0.92–0.96). Using the imputed data, across baseline multivariable models, we found that AUCs ranged from 0.58 to 0.69, which increased, with the addition of SRD, to 0.60 to 0.73. The presence of SRD in the models increased the absolute risk of mortality by 5.4–7.5% over the baseline risk (P < 0.001 for all models) (Figure 1). In participants with infection, the presence of SRD in the models increased the absolute risk for mortality by 2.5–3.7%.
Figure 1.
Comparison of the area under the receiver operating characteristic curve (AUC) for in-hospital mortality models with and without the inclusion of admission severe respiratory disease (SRD), as defined by the World Health Organization, and the estimated mortality risk increases associated with the incorporation of SRD in each model. (Left) Each baseline risk model is provided, followed by the AUC calculated for each model. Vertical dotted lines represent the AUC for each base model. The black dot and horizontal line represent the AUC and 95% confidence interval when SRD is included as an independent variable for each base model. The AUC values are provided according to the scale at the bottom of the figure. (Right) The vertical dashed line at right represents the baseline mortality risk for each model. The black dot and horizontal line represent the estimated absolute mortality risk increase and 95% confidence interval when SRD is present in each base model. The values for the estimated absolute risk increase are provided according to the scale at the top of the figure. GCS = Glasgow Coma Scale score.
Discussion
In the first comprehensive evaluation of the prevalence, characteristics, and outcomes of WHO-defined SRD in hospitalized patients in sub-Saharan Africa, we found that SRD was common with a prevalence that ranged from 10 to 16%, depending on whether the participant was admitted with infection or not. SRD was associated with a high in-hospital case fatality ratio of 22%. Increases in respiratory rate were associated with an increased risk of in-hospital mortality, whereas increases in oxygen saturation were associated with decreased risk of in-hospital mortality. The presence of SRD in each baseline model increased the AUC and the associated absolute risk of mortality. We were limited by missing data but accounted for this by using a robust imputation strategy. We were also unable to rule out heart failure; however, our findings were similar in a sensitivity analysis of participants with infection. We were not able to directly compare SRD to ARDS diagnoses, nor is this likely to be done on a large scale, because of the resource constraints that limit the ability to diagnose ARDS in low-income settings, even when using the less resource-intensive Kigali modification of the Berlin ARDS criteria (8). Nonetheless, our data suggest that SRD identifies patients with acute respiratory disease who are at high risk of death, making SRD a reasonable proxy for ARDS in clinical settings where the components needed for a diagnosis of ARDS are unavailable. Prospective multisite studies could provide further data about the case incidence, etiologies, and outcomes of SRD in Africa.
Acknowledgments
The SRDA investigators are as follows: Ben Andrews, Institute for Global Health, Vanderbilt University, Nashville, Tennessee; Tim Baker, Muhimbili University of Health & Allied Sciences, Dar es Salaam, Tanzania; John A. Crump, Centre for International Health, University of Otago, Dunedin, New Zealand; Martin P. Grobusch, Center of Tropical Medicine and Travel Medicine, Department of Infectious Diseases, Amsterdam University Medical Centers, Amsterdam Infection & Immunity, Amsterdam Public Health, University of Amsterdam, Amsterdam, the Netherlands; Michaela A. M. Huson, Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, the Netherlands; Shevin T. Jacob, Jamie Rylance, and India Wheeler, Department of Clinical Sciences, Liverpool School of Tropical Medicine, Liverpool, United Kingdom; Olamide D. Jarrett, Department of Medicine, University of Illinois at Chicago School of Medicine, Chicago, Illinois; John Kellett, Kitovu Hospital Study Group, Masaka, Uganda; Matthew Rubach, Division of Infectious Diseases and International Health, Duke University Medical Center, Durham, North Carolina; John Schieffelin, Departments of Pediatrics, Section of Infectious Disease, Tulane University, New Orleans, Louisiana.
A complete list of SRDA investigators may be found before the beginning of the References.
Footnotes
Supported by NIH from grant U01AI150508 (C.C.M. and M.C.).
Author and Investigator Contributions: B.H.C., M.C., and C.C.M. contributed to the conception and design of the study and drafted the manuscript. S.A.A., A.A.M., B.A., M.A.A., T.B., P.B., J.A.C., M.P.G., M.A.M.H., S.T.J., O.D.J., J.K., A.M., M.R., J.R., J.S., R.S., and I.W. contributed to data acquisition and edited the manuscript. E.R.M. contributed to data analysis and edited the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.202304-0684LE on July 24, 2023
Author disclosures are available with the text of this letter at www.atsjournals.org.
Contributor Information
on behalf of the Severe Respiratory Distress in Africa (SRDA) Investigators:
Ben Andrews, Tim Baker, John A. Crump, Martin P. Grobusch, Michaela A. M. Huson, Shevin T. Jacob, Jamie Rylance, India Wheeler, Olamide D. Jarrett, John Kellett, Matthew Rubach, and John Schieffelin
References
- 1. Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet . 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. GBD 2016 Lower Respiratory Infections Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis . 2018;18:1191–1210. doi: 10.1016/S1473-3099(18)30310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. LUNG SAFE Investigators; ESICM Trials Group Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA . 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 4. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. ARDS Definition Task Force Acute respiratory distress syndrome: the Berlin definition. JAMA . 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 5.Jacob ST, Lim M, Banura P, Bhagwanjee S, Bion J, Cheng AC, et al. 2013. https://apps.who.int/iris/handle/10665/77751 [DOI] [PMC free article] [PubMed]
- 6. Moore CC, Hazard R, Saulters KJ, Ainsworth J, Adakun SA, Amir A, et al. Derivation and validation of a universal vital assessment (UVA) score: a tool for predicting mortality in adult hospitalised patients in sub-Saharan Africa. BMJ Glob Health . 2017;2:e000344. doi: 10.1136/bmjgh-2017-000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med . 2011;30:377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 8. Riviello ED, Kiviri W, Twagirumugabe T, Mueller A, Banner-Goodspeed VM, Officer L, et al. Hospital incidence and outcomes of the acute respiratory distress syndrome using the Kigali modification of the Berlin definition. Am J Respir Crit Care Med . 2016;193:52–59. doi: 10.1164/rccm.201503-0584OC. [DOI] [PubMed] [Google Scholar]