Consider two contrasting cases: Ms. A is a 51-year-old woman admitted for community-acquired pneumonia with bilateral opacities on chest imaging. She is started on appropriate antibiotics (cultures eventually grow Streptococcus pneumoniae) and 4 L supplemental oxygen via nasal prongs. She clinically improves and is discharged home on Day 5. Ms. B is a 51-year-old woman with an identical history and pathogen. Despite the timely initiation of antibiotics, she requires high-flow nasal oxygen (HFNO) on Day 2 and has radiographic evidence of worsening lung involvement. On Day 4, she is started on invasive mechanical ventilation (IMV) and prone positioning. An extensive search for other treatable causes is unsuccessful. After a prolonged hospitalization, she is discharged to a rehabilitation facility because of critical illness myopathy.
Intensivists are well acquainted with these scenarios. Ms. B would have been especially familiar to Ashbaugh and colleagues from their 1967 description of what would later be called acute respiratory distress syndrome (ARDS) (1). Since then, intensivists have redefined this syndrome a few times, most recently in the “global definition” of ARDS in this issue of the Journal (2; pp. 37–47). As with other syndromes of critical illness, ARDS is not a disease. No diagnostic gold standard exists to test for the presence of inflammatory lung injury with capillary leak, the construct ARDS seeks to capture, and we have only a handful of treatments that affect outcomes. The clinical status quo might argue that (re)defining ARDS is pointless. Some might even suggest that a bedside diagnosis of ARDS is clinically irrelevant and that such syndromic definitions benefit only researchers. So why might we need another definition?
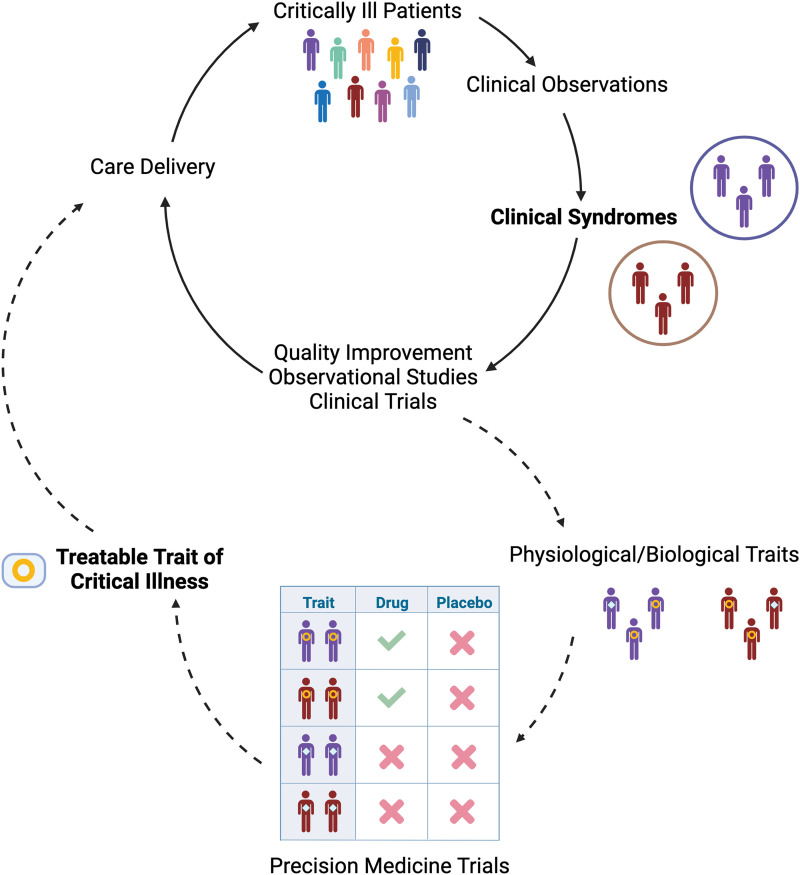
Since Avicenna introduced the concept in the Canon of Medicine, the medical field has relied on syndromic definitions and their periodic revisions to advance care. From rheumatologic to psychiatric and paraneoplastic, syndromic definitions aim to identify groups of patients with shared signs and symptoms in an objective and reproducible manner. These patients can then be included in studies to advance prognostic capability and identify potential therapies (3). Thus, clinical care and research progress are closely intertwined. Prognostically, clinicians expect very different trajectories and outcomes when caring for patients with noncomplicated pneumonia versus ARDS. Therapeutically, the construct of ARDS led to studies that revealed the lifesaving benefits of lung-protective ventilation and prone positioning. Decades of clinical observations led to the creation of syndromic definitions; these definitions have helped advance scientific knowledge that enhances care delivery, providing the opportunity for new clinical observations and ideas to improve their constructs. Without this feedback loop (Figure 1), there would be little hope for progress in medical care. Syndromic definitions are meant to evolve.
Figure 1.
Feedback loop of clinical and research progress in critical care medicine. Solid arrows indicate the existing paradigm. Dashed arrows indicate a potential future direction of the field guided by advances in precision medicine.
The global definition builds on the Berlin definition of ARDS in several ways (4). Some changes aim to make diagnosing ARDS more feasible, especially in resource-constrained locations. For instance, the new definition recognizes that clinicians detect bilateral lung infiltrates using different imaging modalities, that the ratio of oxygen saturation as measured by pulse oximetry to FiO2 can supplant the ratio of PaO2 to FiO2 (with some caveats) (5), and that it is time to include the Kigali modification to the Berlin criteria in resource-constrained settings, because a lack of access to positive pressure ventilation does not prevent ARDS (6). Recent guidelines put forth by the European Society of Intensive Care Medicine similarly call for an updated definition of ARDS, cognizant of these shifts in clinical practice and the diversity of locations in which care is delivered (7).
Perhaps the most fundamental change in the proposed global definition of ARDS comes from allowing patients on HFNO of ⩾30 L/min to now be eligible for the diagnosis. Some say that such broadening of the definition will threaten the validity of this already heterogeneous syndrome. After all, FiO2 delivered with HFNO is imprecise, and positive end-expiratory pressure is unknown. Yet clinically, Ms. B’s incipient lung injury likely was recognized when her oxygen requirements began to escalate; intubation was simply a process of care in a trajectory of progressive respiratory failure. One clinician might have chosen to initiate IMV on Day 2, reasoning that her rapid decline suggested that she would not sustain respiration on HFNO for long, whereas another chose to wait until Day 4. Should this difference in practice alter the time of her ARDS diagnosis by 2 days? Did the biological process of increased alveolar–capillary permeability, resulting in noncardiogenic pulmonary edema, begin only when IMV was initiated? If not, might earlier treatment be more effective than waiting until the horse has left the barn? Before the increase in the use of HFNO spurred by the FLORALI (Clinical Effect of the Association of Noninvasive Ventilation and High Flow Nasal Oxygen Therapy in Resuscitation of Patients With Acute Lung Injury: A Randomised Study) trial (8) and then the coronavirus disease (COVID-19) pandemic, Ms. B would have been just as likely to receive noninvasive ventilation and qualify for ARDS under the Berlin criteria on the basis of geographical practice preferences. The pandemic also showed that the mortality of patients on HFNO who would have otherwise met the Berlin criteria for ARDS is similar to that of patients with Berlin-defined ARDS on noninvasive ventilation (9). Thus, although the global definition’s inclusion of HFNO may broaden the population with diagnoses of ARDS to some extent, it is largely catching up to clinical practice by acknowledging that typical care has fundamentally changed since the Berlin criteria were introduced in 2012.
If some of the changes proposed by the global definition do increase the heterogeneity of patients labeled as having ARDS, will this be problematic? After all, the syndrome’s notorious heterogeneity has already made it difficult to find effective therapies in clinical trials. However, most landmark trials have used stringent clinical criteria to test interventions only on subsets of patients with ARDS (enrichment). Even in patients on IMV, there is evidence of a heterogeneous inflammatory response associated with different mortality outcomes and treatment effects (10). As such, some upcoming trials of ARDS are designed around biological phenotypes of ARDS, aiming to recruit those most likely to benefit from specific interventions (predictive enrichment). Perhaps recruitment of patients with nonintubated ARDS will enable interventional studies that affect progression to IMV, a key outcome especially where resources are constrained.
Will this global definition be the final ARDS definition? Likely not. Rigorous studies testing the implementation, reliability, and validity of the global definition versus the Berlin criteria are needed to inform intensivists and inspire future modifications (3). More fundamentally, in a decade or two, we may have moved beyond syndromic concepts in favor of treatable traits—that is, specific patterns of biological derangements that appear across syndromic definitions and have discrete responses to therapeutic interventions (Figure 1). Critical care is on the cusp of an era of precision medicine, resulting from advances in omics science, data science, and machine learning, which have led to a new understanding of shared molecular host response signatures across a variety of critical illness syndromes (11). But although we hold such a future in sight, it remains aspirational. For now, the updated ARDS definition better reflects the current care of patients with acute lung injury in diverse settings and should enable progress toward better outcomes for our patients around the world.
Footnotes
Supported by NHLBI grant R35HL140026 and grant F32HL165828.
Originally Published in Press as DOI: 10.1164/rccm.202308-1441VP on October 10, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults Lancet 1967. 2 319 323 4143721 [Google Scholar]
- 2.Matthay MA, Arabi Y, Arroliga AC, Bernard G, Bersten AD, Brochard LJ, et al. A new global definition of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2024;209:37–47. doi: 10.1164/rccm.202303-0558WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ranieri VM, Rubenfeld G, Slutsky AS. Rethinking acute respiratory distress syndrome after COVID-19: if a “better” definition is the answer, what is the question? Am J Respir Crit Care Med . 2023;207:255–260. doi: 10.1164/rccm.202206-1048CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. ARDS Definition Task Force Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 5. Chen W, Janz DR, Shaver CM, Bernard GR, Bastarache JA, Ware LB. Clinical characteristics and outcomes are similar in ARDS diagnosed by oxygen saturation/FiO2 ratio compared with PaO2/FiO2 ratio. Chest . 2015;148:1477–1483. doi: 10.1378/chest.15-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Riviello ED, Kiviri W, Twagirumugabe T, Mueller A, Banner-Goodspeed VM, Officer L, et al. Hospital incidence and outcomes of the acute respiratory distress syndrome using the Kigali modification of the Berlin definition. Am J Respir Crit Care Med . 2016;193:52–59. doi: 10.1164/rccm.201503-0584OC. [DOI] [PubMed] [Google Scholar]
- 7. Grasselli G, Calfee CS, Camporota L, Poole D, Amato MBP, Antonelli M, et al. European Society of Intensive Care Medicine Taskforce on ARDS ESICM guidelines on acute respiratory distress syndrome: definition, phenotyping and respiratory support strategies. Intensive Care Med . 2023;49:727–759. doi: 10.1007/s00134-023-07050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frat JP, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, et al. FLORALI Study Group REVA Network. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med . 2015;372:2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 9. Ranieri VM, Tonetti T, Navalesi P, Nava S, Antonelli M, Pesenti A, et al. High-flow nasal oxygen for severe hypoxemia: oxygenation response and outcome in patients with COVID-19. Am J Respir Crit Care Med . 2022;205:431–439. doi: 10.1164/rccm.202109-2163OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Calfee CS, Delucchi KL, Sinha P, Matthay MA, Hackett J, Shankar-Hari M, et al. Irish Critical Care Trials Group Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir Med . 2018;6:691–698. doi: 10.1016/S2213-2600(18)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maslove DM, Tang B, Shankar-Hari M, Lawler PR, Angus DC, Baillie JK, et al. Redefining critical illness. Nat Med . 2022;28:1141–1148. doi: 10.1038/s41591-022-01843-x. [DOI] [PubMed] [Google Scholar]