Abstract
Rationale
The identification of early chronic obstructive pulmonary disease (COPD) is essential to appropriately counsel patients regarding smoking cessation, provide symptomatic treatment, and eventually develop disease-modifying treatments. Disease severity in COPD is defined using race-specific spirometry equations. These may disadvantage non-White individuals in diagnosis and care.
Objectives
Determine the impact of race-specific equations on African American (AA) versus non-Hispanic White individuals.
Methods
Cross-sectional analyses of the COPDGene (Genetic Epidemiology of Chronic Obstructive Pulmonary Disease) cohort were conducted, comparing non-Hispanic White (n = 6,766) and AA (n = 3,366) participants for COPD manifestations.
Measurements and Main Results
Spirometric classifications using race-specific, multiethnic, and “race-reversed” prediction equations (NHANES [National Health and Nutrition Examination Survey] and Global Lung Function Initiative “Other” and “Global”) were compared, as were respiratory symptoms, 6-minute-walk distance, computed tomography imaging, respiratory exacerbations, and St. George’s Respiratory Questionnaire. Application of different prediction equations to the cohort resulted in different classifications by stage, with NHANES and Global Lung Function Initiative race-specific equations being minimally different, but race-reversed equations moving AA participants to more severe stages and especially between the Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage 0 and preserved ratio impaired spirometry groups. Classification using the established NHANES race-specific equations demonstrated that for each of GOLD stages 1–4, AA participants were younger, had fewer pack-years and more current smoking, but had more exacerbations, shorter 6-minute-walk distance, greater dyspnea, and worse BODE (body mass index, airway obstruction, dyspnea, and exercise capacity) scores and St. George’s Respiratory Questionnaire scores. Differences were greatest in GOLD stages 1 and 2. Race-reversed equations reclassified 774 AA participants (43%) from GOLD stage 0 to preserved ratio impaired spirometry.
Conclusions
Race-specific equations underestimated disease severity among AA participants. These effects were particularly evident in early disease and may result in late detection of COPD.
Keywords: COPD, early disease, race-specific spirometry prediction equations, dyspnea, health inequities
At a Glance Commentary
Scientific Knowledge on the Subject
Race-specific prediction equations are commonly used to interpret spirometry results. For non-White populations, these equations tend to normalize the lower mean values observed in marginalized populations that may have experienced deprivation-associated exposures. As a result, their percentage predicted values are inflated. The impact on the classification of chronic obstructive pulmonary disease by race-specific equations has not been comprehensively studied in African American (AA) relative to non-Hispanic White (NHW) smokers.
What This Study Adds to the Field
Race-specific prediction equations established for AA individuals classified AA participants in COPDGene (Genetic Epidemiology of Chronic Obstructive Pulmonary Disease) into lower Global Initiative for Chronic Obstructive Lung Disease (GOLD) stages (healthier) than if NHW or multiethnic equations were used. These participants were younger but had more exacerbations, dyspnea, and worse St. George’s Respiratory Questionnaire total scores than the NHW participants in each stage. Their raw spirometry values were lower on average in each stage. For smoking-exposed participants not meeting GOLD criteria for chronic obstructive pulmonary disease, the use of NHW equations reclassified AA participants into preserved ratio impaired spirometry from the “normal spirometry” group and was associated with more respiratory symptoms. The impact of these misclassifications is important for non-White smokers because it may affect their access to care and treatment.
Chronic obstructive pulmonary disease (COPD) is a worldwide health problem encompassing diverse populations, with more than 392 million prevalent cases and 3.3 million deaths (1–3). Race-specific spirometry prediction equations were established to improve precision in assessing lung health, including in patients with COPD. The use of prediction equations defined by race has come into question as concerns about the concept of race as a social construct rather than a biologic factor have increased (4). Efforts to interpret raw spirometry volumes to percentage predicted values that define expected values for healthy lung function have resulted in a proliferation of prediction equations in non-White populations around the world (5), but the utility of these prediction equations for predicting outcomes has been questioned (5–7). The Global Lung Function Initiative (GLI) has taken a lead in developing race-specific and multiethnic (8) equations, with a recent effort at a globalized equation (9). However, previous studies have shown that race-specific equations in population-based cohorts did not predict exacerbations or mortality for African American (AA) individuals as well as raw spirometry values or equations developed among non-Hispanic White (NHW) individuals (6, 7, 10).
COPD is uniquely affected by race-specific equations because its diagnosis and severity classification rely on spirometric measures, specifically Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria (11, 12). Although the criteria of FEV1:FVC < 0.7 for diagnosis of COPD and percentage predicted values for severity stages are unchanged, there has been recognition of respiratory symptoms as an important aspect of the disease. Prediction equations have typically been defined in reference to White populations, and the major effect has been to normalize lower non-White group values (13). That process increases the resultant percentage predicted values for these non-White individuals. The question of whether spirometry is useful for COPD diagnosis across all populations is important for non-White people around the world (14–17). The history of spirometry in race-based evaluations of non-White populations was described in detail by Braun (18, 19), and the potential social and clinical consequences of “adjustment” by race linked with recognition of socioeconomic and environmental exposures have been discussed more recently (4). However, there has not been a detailed assessment of the impact of race-specific equations on COPD and related illness. Baugh and colleagues looked at a group of 530 AA participants from the SPIROMICS (Study of COPD Subgroups and Biomarkers) study but did not distinguish groups by severity (20). We recently reported on underdiagnosis among AA individuals using FEV1:FVC (fixed ratio) as the criterion for COPD diagnosis (21). But in the present analysis, we focus on the impact of race-based prediction equations on severity classification rather than diagnosis. The role of spirometry in both diagnosis and disease severity in COPD are linked and important to properly counsel patients and initiate the available therapies. We evaluated the impact of these equations on participants with definite COPD by GOLD classification and those undiagnosed but with potential disease from smoking exposure.
The COPDGene (Genetic Epidemiology of Chronic Obstructive Pulmonary Disease) study enrolled more than 3,366 AA participants (33% of the cohort) who have been highly characterized for respiratory symptoms, function, and lung structure. In the present work, we hypothesized that race-specific prediction equations might underestimate disease severity in AA participants. Race-specific equations tend to normalize lower values in non-White groups, leading to inflated predicted spirometry values (13). We studied the COPDGene cohort and evaluated participants who smoked, comparing race-specific, multiethnic, and “race-reversed” prediction equations from NHANES (National Health and Nutrition Examination Survey) and GLI for classification and assessed clinical differences by self-reported race within the classified groups.
Methods
Ever-Smoker Cohort
COPDGene (ClinicalTrials.gov identifier NCT 00608764) has been previously described (22). Briefly, 10,198 people who had ever smoked were enrolled between 2007 and 2012 (phase 1) at 21 clinical centers across the United States, and 10,132 participants had complete spirometry data for this analysis. Enrollment included general community-based recruitment and enhanced recruitment of participants with known COPD and planned recruitment of one-third non-Hispanic AA participants. Participants were enrolled, within self-reported racial categories on the basis of a history of ⩾10 pack-years of smoking and absence of chronic lung disease, excepting COPD or a history of asthma. The research protocol was approved by the institutional review board at each of the 21 clinical centers, and all participants provided written informed consent. Subjects returned at five-year intervals for additional study visits, with phase 2 occurring between 2012 and 2017. Participants completed standardized pre- and postbronchodilator spirometry, a 6-minute-walk test, and standardized questionnaires of respiratory symptoms, including St. George’s Respiratory Questionnaire (SGRQ), the modified Medical Research Council (mMRC) dyspnea scale, and an inventory of comorbid diseases. A comorbidity score was developed as a summed value for each participant of all the comorbid diseases that were reported from the queried diseases in the phase 1 data collection. One point is scored for each self-reported physician diagnosis of any cancer, diabetes, congestive heart failure, coronary artery disease, high blood pressure, stroke, blood clots, pneumothorax, gastroesophageal reflux, stomach ulcers, and osteoporosis. The BODE (body mass index, airway obstruction, dyspnea, and exercise capacity) score was calculated from body mass index, spirometry, mMRC dyspnea scale score, and 6-minute-walk distance (6MWD).
COPD Classification
We classified participants on the basis of postbronchodilator spirometry values using NHANES race-specific equations and the 2023 GOLD criteria (12). Because these criteria address only participants with FEV1:FVC ratios <0.7, we extended the classification to include those with FEV1:FVC ratios ⩾0.7 and FEV1% predicted ⩾ 80% as GOLD stage 0 (23) and those with FEV1:FVC ratios ⩾0.7 and FEV1% predicted < 80% as preserved ratio impaired spirometry (PRISm) (24, 25). Values for FEV1:FVC ratio were calculated to two significant figures for classification. Although neither PRISm nor GOLD stage 0 is currently recognized as defining COPD, evidence of symptoms and differential progression to COPD from the two groups make identification of these categories and distinctions between them important (26–28).
Comparison of Spirometry Prediction Equations
We looked at different race-specific prediction equations and reversed (NHW to AA and AA to NHW) them to apply to the opposite racial group to determine the maximal difference for classification of NHW and AA participants. The underlying assumption was that reversed equations (NHW and AA) provided the polar opposites for classification. For the smoker cohort with complete spirometry results (n = 10,132), we applied eight different sets of prediction equations to the postbronchodilator raw spirometry results: 1) NHANES race-specific equations stratified by self-reported race (race-specific equations); 2) NHANES NHW equations applied to AA participants (race-reversed equations); 3) NHANES AA equations applied to NHW participants (race-reversed equations); 4) race-specific GLI equations (8) stratified by self-reported race (race-specific equations); 5) GLI AA equations applied to NHW (race-reversed equations) and GLI NHW equations applied to AA participants (race-reversed equations); 6) GLI “Other” equations applied to all participants (multiethnic equations); 7) GLI “Global” equations applied to all participants (multiethnic equations) (9); and 8) (FEV1/height2) × 100 applied to all participants (race-free) (29).
COPD Disease Score
To characterize participants in COPDGene without relying solely on spirometry and identify the heterogeneity in COPD, we implemented a COPD disease score, derived from our published work, which also includes structural changes, symptoms, and functional limitation (30). This score included spirometry ([FEV1(milliliters)/height (meters)2] × 100 < 80%), as described by Checkley and colleagues (29). We quantified three additional measures of disease severity: 1) symptoms (report of any of mMRC dyspnea scale score ⩾ 2, chronic bronchitis, or history of severe exacerbation in the prior year); 2) structural changes on computed tomography scan (emphysema [⩾5% low-attenuation area < −950 Hounsfield units], gas trapping [⩾20% low-attenuation area at −856 Hounsfield units], and average airway wall thickness normalized to a theoretical airway lumen of 10-mm inner perimeter > 2.2 units, a marker of airway disease [>95th percentile for never smoker group]); and 3) reduced function (6MWD < 1,150 ft). For each criterion, we scored 1 point for having the disease finding and then summed values to obtain an individual’s disease score.
GOLD Stage 0 and PRISm Reclassification with Race-reversed Prediction Equations
We evaluated the effect of NHANES race-specific equations when assigning GOLD stage 0 (23) and PRISm groups, by using reversed prediction equations (NHANES NHW-specific equations on AA participants and AA-specific equations on NHW participants). This process identified participants who changed classification from GOLD stage 0 to PRISm on the basis of an FEV1 percentage predicted cutoff of 80%. We compared the characteristics of the reclassified participants with those in their original groups.
Statistical Analysis
Participant characteristics are summarized as mean and SD for continuous variables and as count and percentage for categorical variables. Differences between groups were evaluated using a two-tailed t test for continuous variables and the chi-square test for categorical variables. Ordinal or nonnormally distributed variables were evaluated using the Wilcoxon rank sum test. Significant differences were identified with a P value <0.05. Data were analyzed using JMP 16 (SAS Institute) and R Statistical Software (v4.2.2.) (https://www.R-project.org/).
Results
Cohort Characteristics by Self-described Race
At enrollment, among all ever-smoking participants with complete spirometry (n = 10,132), the proportions of NHW (n = 6,766) and AA (n = 3,366) participants differed across the extended GOLD stages (Tables 1 and 2); percentages AA were as follows: GOLD stage 1, 23%; GOLD stage 2, 25%; GOLD stage 3, 21%; GOLD stage 4, 19%; PRISm, 43%; and GOLD stage 0, 41%. In each GOLD stage, the AA participants were younger; were more likely to be current smokers; reported more severe exacerbations and more dyspnea; and had shorter 6MWDs, worse SGRQ total scores, higher BODE scores, higher COPD disease scores, and lower FEV1 and FVC. Pack-years were lower in AA participants, as were chronic bronchitis and the overall comorbidity score.
Table 1.
Baseline Characteristics of COPDGene Participants with COPD Diagnosis Using Race-Specific Prediction Equations, by Self-described Race and GOLD Stage
| GOLD Stage 1 |
GOLD Stage 2 |
GOLD Stage 3 |
GOLD Stage 4 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| African American | Non-Hispanic White | P Value | African American | Non-Hispanic White | P Value | African American | Non-Hispanic White | P Value | African American | Non-Hispanic White | P Value | |
| Number | 178 | 609 | — | 482 | 1,444 | — | 244 | 920 | — | 114 | 492 | — |
| Age, yr | 56.9 (8.2) | 63.2 (8.8) | <0.0001 | 57.7 (8.0) | 64.1 (8.6) | <0.0001 | 60.8 (8.1) | 65.4 (8.1) | <0.0001 | 60.5 (7.2) | 64.7 (7.2) | <0.0001 |
| Gender (% female) | 46% | 41% | NS | 47% | 46% | NS | 45% | 42% | NS | 39% | 42% | NS |
| Current smoking | 88% | 46% | <0.0001 | 73% | 42% | <0.0001 | 52% | 48% | <0.0001 | 23% | 22% | NS |
| Pack-years | 41.2 (25.6) | 46.0 (24.2) | 0.02 | 41.2 (22.8) | 53.9 (27.4) | <0.0001 | 44.5 (22.5) | 57.4 (28.0) | <0.0001 | 42.5 (25.6) | 60.0 (29.2) | <0.0001 |
| Chronic bronchitis | 15% | 16% | NS | 23% | 28% | 0.03 | 23% | 32% | 0.007 | 16% | 30% | 0.002 |
| Severe exacerbations | 6% | 4% | NS | 23% | 13% | <0.0001 | 39% | 23% | <0.0001 | 58% | 33% | <0.0001 |
| Comorbidity score | 1.1 (1.3) | 1.2 (1.2) | NS | 1.3 (1.3) | 1.7 (1.4) | <0.0001 | 1.6 (1.3) | 1.8 (1.5) | 0.05 | 1.5 (1.3) | 1.6 (1.3) | NS |
| Diabetes | 8% | 8% | NS | 15% | 12% | NS | 21% | 14% | 0.006 | 14% | 9% | NS |
| CAD | 10% | 13% | NS | 10% | 17% | 0.0002 | 11% | 20% | 0.002 | 13% | 17% | NS |
| 6MWD, ft | 1,368 (379) | 1,542 (321) | <0.0001 | 1,151 (376) | 1,356 (349) | <0.0001 | 960 (376) | 1,143 (354) | <0.0001 | 796 (360) | 888 (348) | 0.01 |
| mMRC dyspnea scale score | 1.3 (1.4) | 0.7 (1.1) | 0.0002 | 1.9 (1.5) | 1.5 (1.4) | <0.0001 | 2.6 (1.3) | 2.4 (1.2) | 0.03 | 3.1 (0.1) | 3.2 (1.1) | NS |
| mMRC dyspnea scale score ⩾ 2 | 33% | 19% | <0.0001 | 61% | 47% | <0.0001 | 82% | 78% | NS | 90% | 95% | NS |
| BODE score | 1.04 (1.3) | 0.5 (0.9) | <0.0001 | 2.5 (1.8) | 1.7 (1.6) | <0.0001 | 5.2 (1.9) | 4.6 (1.7) | <0.0001 | 7.2 (1.8) | 6.7 (1.5) | 0.003 |
| COPD disease score | 2.26 (1.54) | 1.8 (1.28) | 0.001 | 4.1 (1.7) | 3.8 (1.6) | 0.0006 | 5.6 (1.4) | 5.4 (1.2) | NS | 6.3 (1.0) | 6.2 (0.9) | NS |
| SGRQ total score | 21.9 (19.6) | 17.0 (16.9) | 0.0013 | 37.6 (23.3) | 31.5 (20.8) | <0.0001 | 49.3 (20.9) | 45.0 (18.6) | 0.002 | 59.4 (17.7) | 55.4 (15.8) | 0.02 |
| FEV1, L | 2.40 (0.60) | 2.7 (0.7) | <0.0001 | 1.76 (0.49) | 1.92 (0.51) | <0.0001 | 1.06 (0.29) | 1.16 (0.29) | <0.0001 | 0.60 (0.20) | 0.66 (0.19 | 0.002 |
| FVC, L | 3.71 (0.87) | 4.23 (1.01) | <0.0001 | 2.96 (0.78) | 3.33 (0.87) | <0.0001 | 2.31 (0.68) | 2.77 (0.77) | <0.0001 | 1.8 (0.67) | 2.2 (0.67) | <0.0001 |
| FEV1:FVC | 0.65 (0.04) | 0.65 (0.04) | NS | 0.60 (0.07) | 0.58 (0.08) | <0.0001 | 0.47 (0.09) | 0.43 (0.09) | <0.0001 | 0.34 (0.1) | 0.31 (0.1) | <0.0001 |
| FEV1% predicted | 91.0% | 90.6% | NS | 65.4% | 64.9% | NS | 40.8% | 40.1% | NS | 21.9% | 22.7% | NS |
| FVC% predicted | 110.2% | 106.6% | 0.0005 | 86.9% | 85.6% | NS | 69.4% | 71.8% | 0.01 | 51.5% | 56.6% | 0.0003 |
Definition of abbreviations: 6MWD = 6-minute-walk distance; BODE = body mass index, airway obstruction, dyspnea, and exercise capacity; CAD = coronary artery disease; COPD = chronic obstructive pulmonary disease; COPDGene = Genetic Epidemiology of Chronic Obstructive Pulmonary Disease; GOLD = Global Initiative for Chronic Obstructive Lung Disease; mMRC = modified Medical Research Council; NS = nonsignificant; SGRQ = St. George’s Respiratory Questionnaire.
Data are expressed as mean (SD) or percentage.
Table 2.
Baseline Characteristics of COPDGene Participants Not Classified as Having COPD Using Race-Specific Prediction Equations
| GOLD Stage 0 |
PRISm |
Reclassified |
|||||
|---|---|---|---|---|---|---|---|
| African American | NHW | P Value | African American | NHW | P Value | AA Reclassified to PRISm from GOLD Stage 0 by NHW NHANES Equations* | |
| Number | 1,807 | 2,580 | 541 | 721 | 774 (43% of GOLD stage 0 AA) | ||
| Age, yr | 52.7 (6.0) | 59.3 (8.7) | <0.0001 | 53.5 (6.0) | 60.0 (8.6) | <0.0001 | 52.6 (5.8), P = 0.65 |
| 55 yr or younger (%) | 72% | 36% | <0.0001 | 67% | 31% | <0.0001 | 75%, P = 0.67 |
| Gender (% male) | 57% | 50% | <0.0001 | 57% | 50% | <0.0001 | 50%, P = 0.003 |
| Current smoking | 87% | 40% | <0.0001 | 83% | 49% | <0.0001 | 87%, P = 0.67 |
| Height, cm | 171.2 (9.4) | 169.7 (9.4) | <0.0001 | 171.7 (10.3) | 169.4 (9.4) | <0.0001 | 170.4 (9.7), P = 0.05 |
| BMI, kg/m2 | 29.0 (6.1) | 28.8 (5.6) | 0.37 | 31.9 (7.8) | 31.9 (7.0) | NS | 30.1 (6.6), P = 0.0001 |
| Pack-years | 37.8 (21.6) | 46.2 (25.3) | <0.0001 | 36.1 (20.0) | 37.8 (20.3) | <0.0001 | 36.2 (20.6), P = 0.95 |
| Chronic bronchitis | 12% | 13% | NS | 16% | 19% | NS | 14%, P = 0.25 |
| Severe exacerbations | 6% | 3% | <0.0001 | 11% | 11% | NS | 8%, P = 0.09 |
| Comorbidity score | 1.1 (1.2) | 1.4 (1.34) | <0.0001 | 1.28 (1.3) | 1.62 (1.5) | <0.0001 | 0.95 (1.2), P = 0.02 |
| 6MWD, ft | 1,373 (351) | 1,576 (325) | <0.0001 | 1,190 (366) | 1,322 (366) | <0.0001 | 1,331 (347), P = 0.005 |
| mMRC dyspnea scale score | 1.1 (1.4) | 0.56 (0.89) | <0.0001 | 1.72 (1.5) | 1.36 (1.4) | <0.0001 | 1.32 (1.44), P = 0.0002 |
| mMRC dyspnea scale score ⩾ 2 | 35% | 16% | <0.0001 | 52% | 43% | 0.003 | 42%, P = 0.0003 |
| Current respiratory medications | 16% | 12% | <0.0001 | 34% | 32% | <0.0001 | 20%, P = 0.01 |
| SGRQ total score | 21.5 (20.0) | 13.8 (15.6) | <0.0001 | 32.9 (23.6) | 27.5 (22.5) | <0.0001 | 24.7 (21.4), P = 0.0004 |
| FEV1, L | 2.79 (0.65) | 2.96 (0.69) | 0.0005 | 1.96 (0.5) | 2.13 (0.5) | 0.0005 | 2.44 (0.51), P < 0.0001 |
| FVC, L | 3.51 (0.84) | 3.82 (0.90) | <0.0001 | 2.53 (0.66) | 2.82 (0.67) | <0.0001 | 3.09 (0.68), P < 0.0001 |
| FEV1:FVC | 0.80 (0.05) | 0.78 (0.05) | <0.0001 | 0.78 (0.05) | 0.76 (0.05) | <0.0001 | 0.7995 (0.051), P = 0.03 |
| FEV1% predicted† | 98.4 (12.1) | 96.7 (10.9) | <0.0001 | 69.4 (8.9) | 70.8 (8.0) | 0.004 | 87.9 (4.3), P < 0.0001 |
| FVC% predicted† | 98.2 (12.7) | 95.4 (11.1) | <0.0001 | 71.3 (9.7) | 71.7 (8.8) | NS | 88.4 (6.65), P < 0.0001 |
| COPD disease score | 1.4 (1.2) | 0.9 (1.1) | <0.0001 | 2.9 (1.4) | 2.4 (1.5) | <0.0001 | 1.85 (1.34), P = 0.0006 |
| BODE score | 1.01 (1.27) | 0.41 (0.85) | <0.0001 | 2.03 (1.75) | 1.44 (1.7) | <0.0001 | 1.21 (1.37), P = 0.0003 |
Definition of abbreviations: 6MWD = 6-minute-walk distance; AA = African American; BMI = body mass index; BODE = body mass index, airway obstruction, dyspnea, and exercise capacity; COPD = chronic obstructive pulmonary disease; COPDGene = Genetic Epidemiology of Chronic Obstructive Pulmonary Disease; GOLD = Global Initiative for Chronic Obstructive Lung Disease; mMRC = modified Medical Research Council; NHANES = National Health and Nutrition Examination Survey; NHW = non-Hispanic White; NS = nonsignificant; PRISm = preserved ratio impaired spirometry; SGRQ = St. George’s Respiratory Questionnaire.
Data are expressed as mean (SD) or percentage.
Significance values are reported for the comparison of the reclassified group compared with the original GOLD stage 0 AA group.
NHANES race-specific equations.
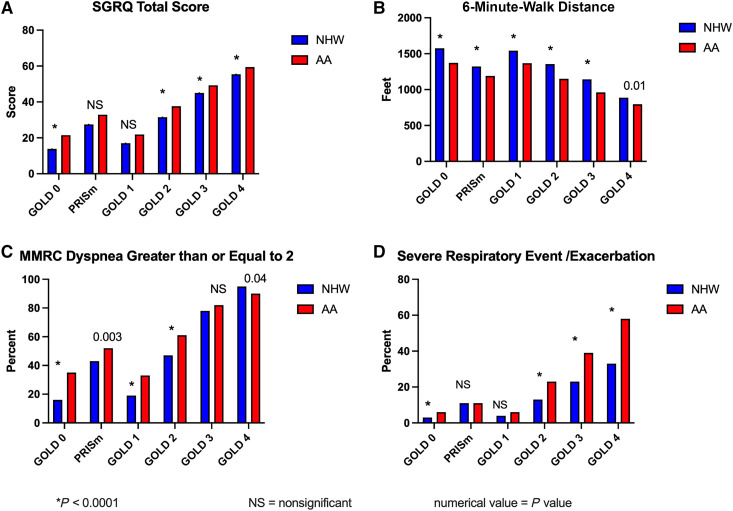
When stratified by GOLD stage, respiratory symptoms and exercise capacity differed by race (Figure 1), with consistently worse results among AA participants for SGRQ total score (higher scores are worse), 6MWD, mMRC dyspnea scale score ⩾ 2, and the percentage of participants who reported severe exacerbations in the previous year. PRISm, as a category of spirometry that includes a disproportionate number of AA individuals, also stands out as demonstrating worse quality of life, shorter walk distance, greater dyspnea, and more exacerbations than GOLD stage 1.
Figure 1.
Disease characteristics of smokers in COPDGene phase 1 by self-reported race and disease severity derived from race-specific prediction equations. In each category of disease severity (using a modified Global Initiative for Chronic Obstructive Lung Disease [GOLD] stage classification that adds GOLD stage 0 and preserved ratio impaired spirometry [PRISm]), African American (AA) participants have worse symptoms, function, and quality of life. The effects are more pronounced in the early disease groups but persist across all stages. Spirometry prediction equations for classification in this figure are the NHANES race-specific equations. PRISm generally shows worse characteristics in each measure than GOLD stage 0 or GOLD stage 1 and is more similar to GOLD stage 2 for St. George’s Respiratory Questionnaire (SGRQ) score, modified Medical Research Council (MMRC) dyspnea scale score, and 6MWD. (A) SGRQ total score, a disease-specific quality-of-life measure. SGRQ score increases across GOLD stages 1–4 but also demonstrates that there are measurable effects in the GOLD and PRISm groups and differences between AA and NHW participants across each group. (B) Reduced physical function on the 6-minute-walk test with advancing GOLD stage and significant reductions in each group for AA participants. (C) Significantly greater reports of dyspnea as defined by the MMRC dyspnea scale score ⩾ 2 in AA participants except in GOLD stage 4. This is particularly notable in the GOLD stage 0 group. (D) Although severe respiratory events or exacerbations are reported across all groups, they are more common in advanced disease and significantly increased in AA participants in all GOLD stages except PRISm and GOLD stage 1. 6MWD = 6-minute-walk distance; COPDGene = Genetic Epidemiology of Chronic Obstructive Pulmonary Disease; NHANES = National Health and Nutrition Examination Survey; NHW = non-Hispanic White. *P < 0.0001.
Classification of COPD Severity by Various Prediction Equations
The impact of different prediction equations on diagnostic classification is shown in Table 3 and Figures 2 and 3. There were consistent shifts of AA participants from less severe to more severe stages using prediction equations that were not race specific. In GOLD stages 1 to 4, AA participants moved into higher (worse) GOLD stages with GLI Other, GLI Global, and race-reversed NHW equations. The largest number of reclassifications occurred in the race-reversed comparison. For the COPD-undiagnosed AA participants, large shifts were seen from GOLD stage 0 to PRISm classification. Applying the NHANES AA equations or the GLI AA equations to NHW participants had the opposite effect on the classification of COPD, moving NHW participants to less severe categories, but fewer participants were affected (see Figures E2 and E3 in the online supplement; Table 3). GLI Global equations (9) had a greater effect than the GLI Other equations but did not show as large an impact as the race-reversed NHANES equations.
Table 3.
Effect of Different Prediction Equations on Disease Severity Classification in COPDGene Participants
| Race | GOLD Stage 0 | PRISm | GOLD Stage 1 | GOLD Stage 2 | GOLD Stage 3 | GOLD Stage 4 | |
|---|---|---|---|---|---|---|---|
| Multiethnic prediction equations | |||||||
| 1.NHANES race-specific (reference) | NHW | 2,515 | 695 | 674 | 1,468 | 922 | 492 |
| 2.NHANES AA applied to NHW | NHW | 3,023 | 187 | 1,289 | 1,194 | 743 | 330 |
| 3.GLI race-specific (NHW) | NHW | 2,571 | 639 | 698 | 1,454 | 912 | 492 |
| 4.GLI AA applied to NHW | NHW | 3,023 | 187 | 1,267 | 1,221 | 743 | 325 |
| 5.GLI Other applied | NHW | 2,843 | 367 | 955 | 1,359 | 824 | 418 |
| 6.GLI Global applied | NHW | 2,774 | 436 | 859 | 1,405 | 855 | 437 |
| 1.NHANES race-specific (reference) | AA | 1,791 | 525 | 194 | 495 | 245 | 116 |
| 2.NHANES NHW applied to AA | AA | 1,017 | 1,299 | 63 | 485 | 331 | 171 |
| 3.GLI race-specific (AA) | AA | 1,747 | 569 | 185 | 492 | 258 | 115 |
| 4.GLI NHW applied to AA | AA | 1,046 | 1,270 | 64 | 490 | 328 | 168 |
| 5.GLI Other applied | AA | 1,416 | 900 | 105 | 519 | 286 | 140 |
| 6.GLI Global applied | AA | 1,319 | 997 | 93 | 515 | 291 | 151 |
| Race-free (neutral) prediction equations | |||||||
| % Predicted by (FEV1/height2) × 100 | NHW | 2,515 | 695 | 757 | 1,311 | 960 | 528 |
| % Predicted by (FEV1/height2) × 100 | AA | 1,518 | 798 | 145 | 436 | 301 | 168 |
Definition of abbreviations: AA = African American; COPDGene = Genetic Epidemiology of Chronic Obstructive Pulmonary Disease; GLI = Global Lung Function Initiative; GOLD = Global Initiative for Chronic Obstructive Lung Disease; NHANES = National Health and Nutrition Examination Survey; NHW = non-Hispanic white; PRISm = preserved ratio impaired spirometry.
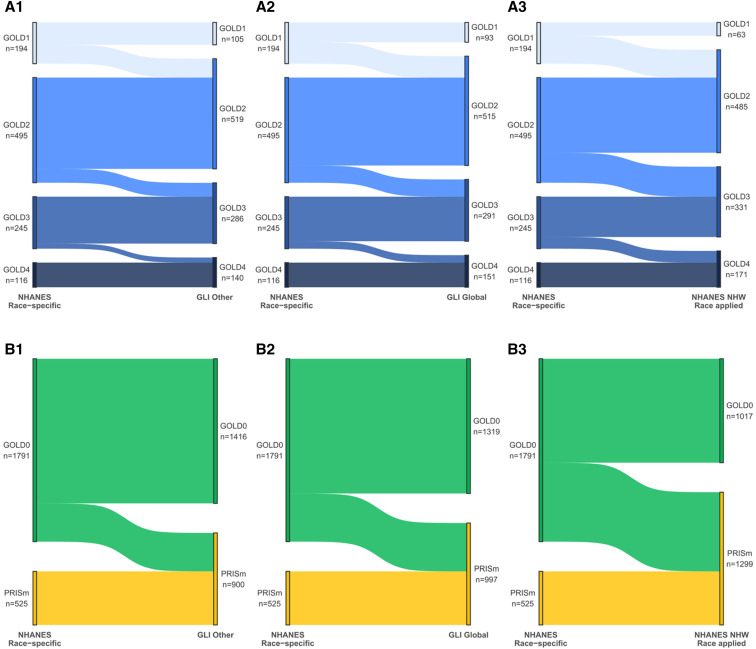
Figure 2.
Reclassification of African American (AA) participants with chronic obstructive pulmonary disease (COPD) and undiagnosed participants using different prediction equations. (A1–A3) Each panel demonstrates the shifts of participants from the reference NHANES (National Health and Nutrition Examination Survey) AA race-specific classification to an alternative prediction equation (Global Lung Function Initiative [GLI] Other, GLI Global, and the NHANES non-Hispanic White (NHW) race-specific “race-reversed”). The overall pattern shows that the AA race-specific equations classify individuals as less severely diseased compared with other equations. GLI Other and GLI Global are similar, but there were slightly more reclassifications under GLI Global (198 vs. 210 out of a total of 1,050 AA participants classified as having COPD [approximately 20%]). (A1) GLI Other classifications, whereby 89 AA participants were reclassified in Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage 2. Similar reclassification occurred at each GOLD stage, and in all cases, participants were reclassified to worse disease stages. (A2) GLI Global equations and their impact on classification, with 101 participants reclassified from GOLD stage 1 to GOLD stage 2. (A3) The greatest impact was noted using race-reversed equations and applying the NHW NHANES equations to AA participants. In this scenario, 131 of the initial 191 GOLD stage 1 participants were reclassified to GOLD stage 2. The trend continued with a large number of AA participants reclassified from GOLD stage 2 to GOLD stage 3, while the reclassifications to more advanced disease from GOLD stage 3 to GOLD stage 4 were fewer. (B1–B3). The proportion of AA participants (70%) who did not meet the fixed-ratio criteria (FEV1:FVC < 0.7) for COPD was greater than that of NHW (49%) in the COPDGene cohort and was consequent to the effect of lower FVC values in a portion of the AA group, as previously reported (21). Thus, these participants could not be reclassified into any of the GOLD stages on the basis of different race-specific equations and could only move between preserved ratio impaired spirometry (PRISm) and GOLD stage 0 groups, as described in our extended classification. As for the COPD-diagnosed participants, the different prediction equations resulted in large numbers of participants being reclassified as sicker (moving to PRISm), with the GLI Other equations (B1) having the fewest (n = 375) reclassified to PRISm; GLI Global equations had 472 participants reclassified to PRISm (B2), and race-reversed NHANES equations had 774 participants (43% of the GOLD stage 0 AA participants) reclassified to PRISm. COPDGene = Genetic Epidemiology of Chronic Obstructive Pulmonary Disease.
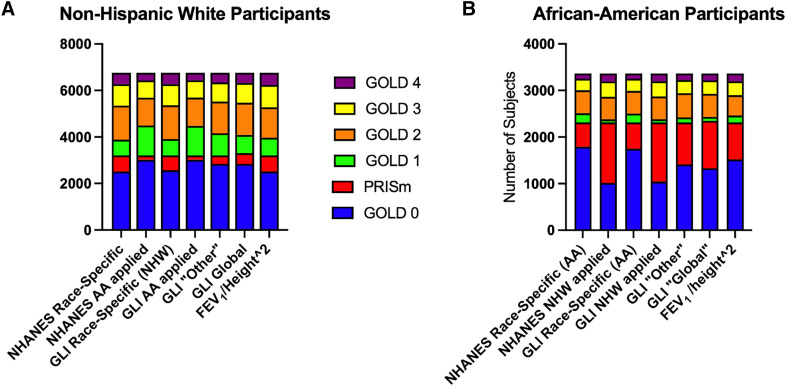
Figure 3.
Extended Global Initiative for Chronic Obstructive Lung Disease (GOLD) classifications for non-Hispanic White (NHW) and African American (AA) participants in COPDGene using alternative prediction equations. This provides a visual comparison of differences between AA and NHW participants using different prediction equations and emphasizes the potential impact of the fixed-ratio discrepancy for AA individuals with an inability to move into GOLD stages, as noted in the legend for Figure 2. This is characterized by the large number of PRISm-classified AA participants and fewer GOLD stage 1 participants. GLI = Global Lung Function Initiative; NHANES = National Health and Nutrition Examination Survey; PRISm = preserved ratio impaired spirometry.
Figure 3 graphically illustrates the shifting seen with NHW prediction equations (Figure 3A) compared with AA equations (Figure 3B) and also illustrates an apparent impact of the problematic fixed-ratio criteria limiting the movement of AA participants above and below the FEV1:FVC < 0.7 line (21). The figure demonstrates larger proportions of AA participants in PRISm who might have a similar disease status to those in GOLD stage 2 or 3 and fewer AA participants in GOLD stage 1, because of limited movement across the fixed ratio. Conversely, application of the AA equations to NHW participants shifts the population strongly to the right (less severe disease) for both groups (Figure 3).
Early COPD-like Disease: GOLD Stage 0 and PRISm Reclassification with Race-reversed Equations
The use of race-reversed prediction equations resulted in reclassification of 774 (43%) AA participants from GOLD stage 0 to PRISm (reclassified percentage predicted FEV1 less than 80%). The results of the baseline GOLD stage 0 and PRISm groups by race are summarized in Table 2. The last column in Table 2 shows the characteristics of the reclassified participants from GOLD stage 0 to PRISm and identifies significant differences between the reclassified AA participants and the original group of AA participants in GOLD stage 0. The COPD disease scores (initial GOLD stage 0 = 1.13 vs. reclassified to PRISm participants = 1.85; P < 0.001), and BODE scores (initial GOLD stage 0 = 0.66 vs. reclassified to PRISm = 1.21; P < 0.0001) of these reclassified AA participants were significantly higher (indicating worse disease) than the original GOLD stage 0 group means. Other key findings in the reclassified group included 20% using respiratory medications (compared with 14% in the original GOLD stage 0 group), increased mean values for mMRC dyspnea scale score, and shorter 6MWD. The younger mean age of 52.6 years for these AA participants suggests that there could be an impact of misclassification on early disease diagnosis.
Discussion
Our analysis of COPDGene, a diverse national cohort of people who have smoked, showed that current race-specific spirometry equations underestimate COPD severity among AA individuals by multiple metrics. NHANES and GLI race-specific equations for NHW and AA participants resulted in higher percentage predicted spirometry values and less severe group classifications of AA participants across the GOLD groups compared with race-free, multiethnic, and race-reversed equations. We demonstrate that within each GOLD stage defined by race-based equations, AA participants clinically appeared to be worse, with shorter 6MWD, more dyspnea, and worse quality of life. By contrast, applying NHW equations to the AA participants, or the use of either GLI Other or “Global” prediction equations, reclassified many GOLD stages 1 and 2 AA participants into GOLD stage 3 or 4. The effect was strongest with the use of the race-reversed NHW equations, followed by the GLI Global and Other equations. Because such “down-staging” of individuals with COPD can affect determinations of disability and access to lung volume reduction procedures or lung transplantation, the current use of race-based spirometric equations likely maintains a serious health inequity.
However, the numerically greater effect of race-based spirometric equations may be on AA individuals in early COPD-like disease (GOLD stage 0 and PRISm groups), a large and relatively young group of people who have smoked and are at risk for progressive lung disease. We show that the application of NHW prediction equations to AA participants resulted in a striking reclassification of many AA participants from GOLD stage 0 to PRISm. Misclassifying appropriate severity for AA individuals represents a lost opportunity to identify and inform individuals of the consequences of their smoking.
Neither the GOLD stage 0 nor the PRISm group is currently designated as having COPD, but in the COPDGene study, both groups have sizable numbers of participants with symptoms and structural changes that imply actual lung disease. They also have been shown to have some progression of lung disease over time, with segments of the GOLD stage 0 group typically progressing to PRISm, GOLD stage 1, or GOLD stage 2 over five years and PRISm participants progressing to higher GOLD stages (28). Thus, these categories contain crucial individuals to identify for preventive strategies and early interventions. Importantly, using reversed race-specific equations, we demonstrated that AA GOLD stage 0 subjects who were reclassified into the PRISm group were indeed sicker than the GOLD stage 0 group as a whole. Race-specific equations for these participants with early-stage disease minimized the severity of illness and hindered the recognition of early disease.
Our findings raise questions about the use of spirometry alone for diagnosis of COPD and reliance on race-specific equations for defining severity of disease. The long history of identifying lower mean values for spirometry measures in perceived non-White “racial” groups led to an assumption that those individuals could not be compared against an NHW population (despite extensive overlap in measurement distributions) and that their spirometric values needed to be adjusted for group or race. These adjustments are based on measuring mean values for the selected non-White population and then “normalizing” the lower mean for that subgroup. This allows a lower value to be “normal” for the subgroup member and gives a higher percentage predicted value than the same measurement would provide for an NHW participant. This creates a different standard of disease, suggesting better lung health at a given measured volume for the non-White individuals. Burney and Hooper (31) and others (6, 7, 32) found that raw FVC was a better predictor of mortality and recommended that ethnic adjustments not be used for prognosis.
An important consideration is that spirometry has substantial variance within an individual beyond the basic categories of age, sex, and height, which makes it more difficult to reliably define clinical disease at a single time point. The extensive within-group variability for spirometry further affects its reproducibility. This leads to questions about the necessity of separating race as a “distinct” group needing specific equations (31, 33). At the same time, perhaps in part because of the historical role of spirometry in medical racism (19), there has been a long-standing awareness that AA and other populations had lower measured values for spirometry, and yet the clinical implications of those measured differences were not established in terms of health or clinical disease.
This is an important area for additional study given that occupational health metrics founded on spirometry would lead to a conclusion of disease, if individuals manifesting lower values of FEV1 and FVC were measured against only NHW population values (32, 34). Better information is needed on all sides of this issue to decide whether percentage predicted values from spirometry provide an adequate assessment of lung health or disease across subpopulations. With substantial and growing admixture in the world, as well as understanding that race is a social construct without a precise biologic meaning (16), race-specific equations will be difficult to implement accurately, lead to underclassification of disease in vulnerable populations, and overstate the impact of lower spirometry values in healthy individuals.
Strengths of this study include a large AA population with a broad spectrum of disease and smoking exposure and very detailed characterization of symptoms and function. Limitations include the cohort structure of an exposure and disease cohort, unlike balanced recruitment in a randomized trial, which may result in confounding. The use of reversed race-specific equations is provided to demonstrate the maximum effect of differential race-specific equations on classification. It is also possible that the reclassification of GOLD stage 0 participants to PRISm represents overdiagnosis of disease, although the symptom burden in this population suggests real disease. One study looked at symptomatic versus asymptomatic tobacco-exposed people, finding no difference in progression between the two, so symptoms without other metrics may not be adequate to define the risk of progression (35). Our previous work suggested that risk of progression in patients with established COPD is multifactorial (36). Further studies are needed to address the issue of disease progression in these individuals. Early disease identification is a key goal for managing the worldwide problem of COPD (37). An important limitation in our analysis is the current diagnostic structure for COPD, which is linked to the fixed FEV1:FVC ratio of 0.7 for COPD diagnosis and affects AA as well as race-specific equations, so we cannot fully define the effect of spirometry on diagnosis and early disease. There are currently no disease-modifying treatments for COPD. The tissue loss driving structural changes of emphysema and pruning of small airways and vasculature (38) make it is unlikely that tissue restoration treatments can be identified and trialed in more advanced disease. To effectively test disease-modifying treatments, there needs to be consensus on the characteristics of very early disease and a search for treatments that interrupt the pathologic tissue destruction. Limiting the description of COPD to spirometric criteria may hamper forward progress in treatment.
Conclusions
Our data indicate that assigning COPD severity solely on the basis of race-specific prediction equations fails to accurately reflect disease symptoms and may not provide correct representation of disease in AA individuals. The impacts of these prediction equations go beyond the United States to worldwide populations and to the issue of health versus disease within variable human morphology. We question the assumption that spirometry can function as the sole predictor of clinical lung disease and suggest that consideration should be given to incorporating broader metrics of disease for COPD and developing criteria for early disease to facilitate testing disease-modifying treatments. We advocate developing usable criteria for early disease across all subgroups to facilitate testing disease-modifying treatments. Symptoms and structural changes may be equally as important as spirometry in assessing COPD. In the future, criteria for early disease, and COPD diagnosis, especially if they include spirometry, should be carefully evaluated in all populations before implementation.
A recent American Thoracic Society statement on race and pulmonary function testing has identified concerns about the use of race, and we concur with those concerns (39). Solutions are more difficult. Previous responses to these issues were first to eliminate “standard adjustments” for AA spirometry in favor of stratified race-specific equations. Now, these stratifications are appearing inadequate and unfair. We believe that we should be meticulous in removing medical practices that harm individuals or groups in our society. This holds especially true for those who are already affected by health disparities. Although additional study is needed to improve understanding of COPD initiation, manifestations, and progression, the use of race-specific equations should be eliminated and race-free or multiethnic alternatives substituted while better methodology for COPD diagnosis is developed.
Footnotes
Supported by NHLBI awards U01 HL089897, U01 HL089856, R01 HL159805, and R01 HL157879. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI or the NIH. COPDGene is also supported by the COPD Foundation through contributions made to an industry advisory board that has included AstraZeneca, Bayer Pharmaceuticals, Boehringer Ingelheim, Genentech, GlaxoSmithKline, Novartis, Pfizer, and Sunovion.
Author Contributions: Conception and design: E.A.R., M.E.L., J.K.O., A.K.B., J.D.C., and B.J.M. Analysis and interpretation: E.A.R., M.E.L., R.W.G., A.A.D., S.P.B., A.K.B., T.H.B., J.D.C., E.K.S., N.R.M., R.A.W., and R.C. Drafting the manuscript for important intellectual content: E.A.R., M.E.L., R.C., J.L. Curtis, G.R.O., D.J.C., A.N., J.K., R.A.W., and R.C. Critical review and revision of the manuscript: all authors. Statistical analysis and data management: E.A.R., M.E.L., J.L. Crooks, Q.G.C., and C.W.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202303-0444OC on August 23, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Quaderi SA, Hurst JR. The unmet global burden of COPD. Glob Health Epidemiol Genom . 2018;3:e4. doi: 10.1017/gheg.2018.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Safiri S, Carson-Chahhoud K, Noori M, Nejadghaderi SA, Sullman MJM, Ahmadian Heris J, et al. Burden of chronic obstructive pulmonary disease and its attributable risk factors in 204 countries and territories, 1990–2019: results from the Global Burden of Disease Study 2019. BMJ . 2022;378:e069679. doi: 10.1136/bmj-2021-069679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adeloye D, Song P, Zhu Y, Campbell H, Sheikh A, Rudan I, NIHR RESPIRE Global Respiratory Health Unit Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. Lancet Respir Med . 2022;10:447–458. doi: 10.1016/S2213-2600(21)00511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schluger NW, Dozor AJ, Jung YEG. Rethinking the race adjustment in pulmonary function testing. Ann Am Thorac Soc . 2022;19:353–356. doi: 10.1513/AnnalsATS.202107-890PS. [DOI] [PubMed] [Google Scholar]
- 5. Cooper BG, Stocks J, Hall GL, Culver B, Steenbruggen I, Carter KW, et al. The Global Lung Function Initiative (GLI) Network: bringing the world’s respiratory reference values together. Breathe (Sheff) . 2017;13:e56–e64. doi: 10.1183/20734735.012717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McCormack MC, Balasubramanian A, Matsui EC, Peng R, Wise RA, Keet CA. Race, lung function and long-term mortality in the National Health and Examination Survey III. Am J Respir Crit Care Med . 2022;205:723–724. doi: 10.1164/rccm.202104-0822LE. [DOI] [PubMed] [Google Scholar]
- 7. Elmaleh-Sachs A, Balte P, Oelsner EC, Allen NB, Baugh AD, Bertoni AG, et al. Race/ethnicity, spirometry reference equations and prediction of incident clinical events: the Multi-Ethnic Study of Atherosclerosis (MESA) lung study. Am J Respir Crit Care Med . 2022;205:700–710. doi: 10.1164/rccm.202107-1612OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. ERS Global Lung Function Initiative Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J . 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bowerman C, Bhatka NR, Brazzale D, Cooper BR, Cooper J, Gochicoa-Rangel L, et al. A race-neutral approach to the interpretation of lung function measurements. Am J Respir Crit Care Med . 2023;207:768–774. doi: 10.1164/rccm.202205-0963OC. [DOI] [PubMed] [Google Scholar]
- 10. Burney PG, Hooper R. Forced vital capacity, airway obstruction and survival in a general population sample from the USA. Thorax . 2011;66:49–54. doi: 10.1136/thx.2010.147041. [DOI] [PubMed] [Google Scholar]
- 11.Global Initiative for Chronic Obstructive Lung Disease. 2021. https://staging.goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf
- 12.Global Initiative for Chronic Obstructive Lung Disease. 2023. https://goldcopd.org/wp-content/uploads/2022/12/GOLD-2023-ver-1.1-2Dec2022_WMV.pdf
- 13. Brems JH, Ferryman K, McCormack MC, Sugarman J. Ethical considerations regarding the use of race in pulmonary function testing. Chest . 2022;162:878–881. doi: 10.1016/j.chest.2022.05.006. [DOI] [PubMed] [Google Scholar]
- 14. Bhakta NR, Kaminsky DA, Bime C, Thakur N, Hall GL, McCormack MC, et al. Addressing race in pulmonary function testing by aligning intent and evidence with practice and perception. Chest . 2022;161:288–297. doi: 10.1016/j.chest.2021.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Witzig R. The medicalization of race: scientific legitimization of a flawed social construct. Ann Intern Med . 1996;125:675–679. doi: 10.7326/0003-4819-125-8-199610150-00008. [DOI] [PubMed] [Google Scholar]
- 16. Sirugo G, Tishkoff SA, Williams SM. The quagmire of race, genetic ancestry, and health disparities. J Clin Invest . 2021;131:e150255. doi: 10.1172/JCI150255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Flanagin A, Frey T, Christiansen SL, AMA Manual of Style Committee Updated guidance on the reporting of race and ethnicity in medical and science journals. JAMA . 2021;326:621–627. doi: 10.1001/jama.2021.13304. [DOI] [PubMed] [Google Scholar]
- 18. Braun L. Race, ethnicity and lung function: a brief history. Can J Respir Ther . 2015;51:99–101. [PMC free article] [PubMed] [Google Scholar]
- 19. Braun L. Race correction and spirometry: why history matters. Chest . 2021;159:1670–1675. doi: 10.1016/j.chest.2020.10.046. [DOI] [PubMed] [Google Scholar]
- 20. Baugh AD, Shiboski S, Hansel NN, Ortega V, Barjaktarevic I, Barr RG, et al. Reconsidering the utility of race-specific lung function prediction equations. Am J Respir Crit Care Med . 2022;205:819–829. doi: 10.1164/rccm.202105-1246OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Regan EA, Lowe ME, Make BJ, Curtis JL, Chen QG, Cho MH, et al. Use of the spirometric “fixed-ratio” underdiagnoses COPD in African-Americans in a longitudinal cohort study. J Gen Intern Med . 2023 doi: 10.1007/s11606-023-08185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, et al. Genetic Epidemiology of COPD (COPDGene) study design. COPD . 2010;7:32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Regan EA, Lynch DA, Curran-Everett D, Curtis JL, Austin JH, Grenier PA, et al. Genetic Epidemiology of COPD Investigators Clinical and radiologic disease in smokers with normal spirometry. JAMA Intern Med . 2015;175:1539–1549. doi: 10.1001/jamainternmed.2015.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wan ES, Hokanson JE, Murphy JR, Regan EA, Make BJ, Lynch DA, et al. COPDGene Investigators Clinical and radiographic predictors of GOLD-unclassified smokers in the COPDGene study. Am J Respir Crit Care Med . 2011;184:57–63. doi: 10.1164/rccm.201101-0021OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wan ES, Castaldi PJ, Cho MH, Hokanson JE, Regan EA, Make BJ, et al. COPDGene Investigators Epidemiology, genetics, and subtyping of preserved ratio impaired spirometry (PRISm) in COPDGene. Respir Res . 2014;15:89. doi: 10.1186/s12931-014-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wan ES, Hokanson JE, Regan EA, Young KA, Make BJ, DeMeo DL, et al. Significant spirometric transitions and preserved ratio impaired spirometry among ever smokers. Chest . 2022;161:651–661. doi: 10.1016/j.chest.2021.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Young AL, Bragman FJS, Rangelov B, Han MK, Galbán CJ, Lynch DA, et al. COPDGene Investigators disease progression modeling in chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2020;201:294–302. doi: 10.1164/rccm.201908-1600OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Young KA, Strand M, Ragland MF, Kinney GL, Austin EE, Regan EA, et al. COPDGene® Investigators Pulmonary subtypes exhibit differential Global Initiative for Chronic Obstructive Lung Disease spirometry stage progression: the COPDGene® study. Chronic Obstr Pulm Dis (Miami) . 2019;6:414–429. doi: 10.15326/jcopdf.6.5.2019.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Checkley W, Foreman MG, Bhatt SP, Dransfield MT, Han M, Hanania NA, et al. COPDGene Study Investigators Differences between absolute and predicted values of forced expiratory volumes to classify ventilatory impairment in chronic obstructive pulmonary disease. Respir Med . 2016;111:30–38. doi: 10.1016/j.rmed.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowe KE, Regan EA, Anzueto A, Austin E, Austin JHM, Beaty TH, et al. COPDGene® 2019: redefining the diagnosis of chronic obstructive pulmonary disease Chronic Obstr Pulm Dis (Miami) 20196 384–399.31710793 [Google Scholar]
- 31. Burney PG, Hooper RL. The use of ethnically specific norms for ventilatory function in African-American and White populations. Int J Epidemiol . 2012;41:782–790. doi: 10.1093/ije/dys011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gaffney AW, McCormick D, Woolhandler S, Christiani DC, Himmelstein DU. Prognostic implications of differences in forced vital capacity in Black and White US adults: findings from NHANES III with long-term mortality follow-up. EClinicalMedicine . 2021;39:101073. doi: 10.1016/j.eclinm.2021.101073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Myers JE. Differential ethnic standards for lung functions, or one standard for all? S Afr Med J . 1984;65:768–772. [PubMed] [Google Scholar]
- 34.Townsend MC, Cowl CT. U.S. occupational historical perspective on race and lung function. Am J Respir Crit Care Med. 2022;206:789–790. doi: 10.1164/rccm.202203-0565LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McKleroy W, Shing T, Anderson WH, Arjomandi M, Awan HA, Barjaktarevic I, et al. Longitudinal follow-up of participants with tobacco exposure and preserved spirometry. JAMA . 2023;330:442–453. doi: 10.1001/jama.2023.11676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Strand M, Khatiwada A, Baraghoshi D, Lynch D, Silverman EK, Bhatt SP, et al. Predicting COPD progression in current and former smokers using a joint model for forced expiratory volume in 1 second and forced expiratory volume in 1 second to forced vital capacity ratio. Chronic Obstr Pulm Dis (Miami) . 2022;9:439–453. doi: 10.15326/jcopdf.2022.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martinez FJ, Han MK, Allinson JP, Barr RG, Boucher RC, Calverley PMA, et al. At the root: defining and halting progression of early chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2018;197:1540–1551. doi: 10.1164/rccm.201710-2028PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Estépar RS, Kinney GL, Black-Shinn JL, Bowler RP, Kindlmann GL, Ross JC, et al. COPDGene Study Computed tomographic measures of pulmonary vascular morphology in smokers and their clinical implications. Am J Respir Crit Care Med . 2013;188:231–239. doi: 10.1164/rccm.201301-0162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bhakta NR, Bime C, Kaminsky DA, McCormack MC, Thakur N, Stanojevic S, et al. Race and ethnicity in pulmonary function test interpretation: an official American Thoracic Society statement. Am J Respir Crit Care Med . 2023;207:978–995. doi: 10.1164/rccm.202302-0310ST. [DOI] [PMC free article] [PubMed] [Google Scholar]