Obesity was identified early as a major risk factor for severe coronavirus disease (COVID-19). Patients with obesity have an increased risk of hospitalization, mechanical ventilation, and death compared with nonobese patients (1, 2). Nearly 3 years since severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first identified, it remains largely unclear how much of the increased risk of COVID-19−related disease is because of ventilatory difficulties from decreased lung capacity versus increased obesity-associated inflammation.
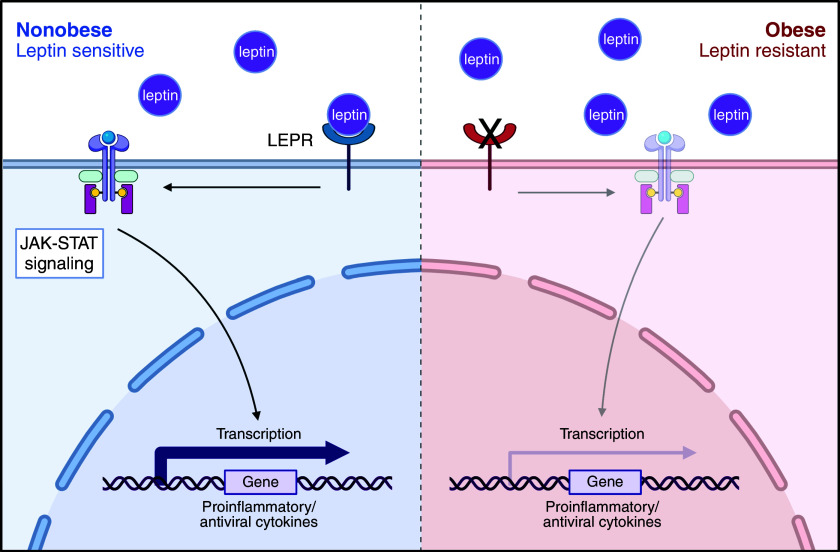
In this issue of the Journal, Guo and colleagues (pp. 566–576) evaluate lung tissue and peripheral blood immune responses with single-cell RNA sequencing (3). They combine their analyses with previously published data from pediatric and adult cohorts, all acquired during the first wave of COVID-19 in China, England, and the United States. Surprisingly, their data suggest that obese subjects exhibit decreased inflammation in early COVID-19 compared with nonobese control subjects. The authors propose a mechanism in which leptin resistance decreases signaling through the Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathway, decreasing type I and II interferons and tumor necrosis factor α signaling in obese patients (Figure 1). These results are similar to previous work that demonstrated through an integrated immune analysis that a deficient type I interferon response was seen in those with severe acute COVID-19 (4).
Figure 1.
Putative mechanism of obesity-related decrease in the inflammatory response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). (Left) In nonobese patients exposed to SARS-CoV-2, leptin rises, binding leptin receptors (LEPRs) on immune cells. This stimulates Janus kinase-signal transducer and activator of transcription (JAK-STAT) signaling and increases the expression of proinflammatory/antiviral cytokines. (Right) In obese patients, there is leptin resistance at baseline. On SARS-CoV-2 exposure, increased leptin fails to stimulate the LEPR, so there is less stimulation of the JAK-STAT pathway and lower expression of proinflammatory/antiviral cytokines. Created with BioRender.com.
These data offer an exciting possible mechanism for obesity conferring an increased risk in patients with COVID-19 that has the potential to translate into therapeutics aimed at increasing interferon signaling. However, there are several limitations to this study. Patient samples were taken during the first wave of COVID-19, and wild-type SARS-CoV-2 was sequenced from 70% of the samples with available sequencing. No subjects were vaccinated, it is unlikely that the subjects had prior exposure to SARS-CoV-2, and less than 20% of their cohort received steroids. Therefore, it is unclear if decreased interferon signaling as a mechanism would hold with current SARS-CoV-2 variants circulating in a largely vaccinated and/or previously infected population in which all hospitalized patients receive steroids. However, obese mice challenged with influenza also exhibit decreased interferon responses (5), and the poor outcomes seen with obesity in COVID-19 mirror that with influenza (6), suggesting that the finding presented herein may broadly apply to other respiratory viruses and the currently circulating SARS-CoV-2 variant. This is important for this pandemic, future pandemics, and seasonal influenza and deserves further evaluation.
We noted two possible confounders in this study. First, the obese subjects were sampled earlier in their ICU course than the nonobese subjects, raising the possibility that interferon responses could rise later in the course and that the differences noted between obese and nonobese subjects were merely a reflection of the difference in sampling times. To address this, the authors graphed interferon responses over time in the ICU (Figure E2E (3) in the online supplement). These graphs suggest that the decreased interferon responses persist over days in the ICU. Second, the racial makeup of the obese and nonobese groups is very different, with Asian Chinese comprising most of the nonobese cohort and white and Black Americans the obese cohort. However, the authors’ finding of differences in interferon signaling persisted in their per-cohort analysis (Figure E2F (3)). In addition, genetic differences between self-identified racial groups are not likely to be substantial (7).
Finally, this manuscript has small numbers and combines unlike groups, including nasal brushings from a pediatric cohort that spans from infants to adolescents (8), BAL samples from mechanically ventilated adults, and peripheral blood mononuclear cells from largely non-ICU inpatient adults (9). The use of body mass index in the pediatric population is imperfect, and its use is not valid for the youngest patients (10), yet it remains widely used. However, the authors examine these data in their individual groups, and this serves to strengthen their findings, suggesting that lower interferon responses in obese patients may persist across a range of ages and with different severity of disease.
Obesity is an established independent risk factor for poor outcomes in acute COVID-19, and the study by Guo and colleagues (3) provides important insights that implicate an impaired antiviral immune response as playing an important role. With obesity such a prevalent problem in the developed world and an emerging issue globally, this is an important area that needs to be better understood.
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.202209-1797ED on September 28, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Kompaniyets L, Goodman AB, Belay B, Freedman DS, Sucosky MS, Lange SJ, et al. Body mass index and risk for COVID-19-related hospitalization, intensive care unit admission, invasive mechanical ventilation, and death—United States, March-December 2020. MMWR Morb Mortal Wkly Rep . 2021;70:355–361. doi: 10.15585/mmwr.mm7010e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature . 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guo SA, Bowyer GS, Ferdinand JR, Maes M, Tuong ZK, Gillman E, et al. Obesity is associated with attenuated tissue immunity in COVID-19. Am J Respir Crit Care Med . 2023;207:566–576. doi: 10.1164/rccm.202204-0751OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science . 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smith AG, Sheridan PA, Tseng RJ, Sheridan JF, Beck MA. Selective impairment in dendritic cell function and altered antigen-specific CD8+ T-cell responses in diet-induced obese mice infected with influenza virus. Immunology . 2009;126:268–279. doi: 10.1111/j.1365-2567.2008.02895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhao X, Gang X, He G, Li Z, Lv Y, Han Q, et al. Obesity increases the severity and mortality of influenza and COVID-19: a systematic review and meta-analysis. Front Endocrinol (Lausanne) . 2020;11:595109. doi: 10.3389/fendo.2020.595109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rosenberg NA, Pritchard JK, Weber JL, Cann HM, Kidd KK, Zhivotovsky LA, et al. Genetic structure of human populations. Science . 2002;298:2381–2385. doi: 10.1126/science.1078311. [DOI] [PubMed] [Google Scholar]
- 8. Yoshida M, Worlock KB, Huang N, Lindeboom RGH, Butler CR, Kumasaka N, et al. NU SCRIPT Study Investigators Local and systemic responses to SARS-CoV-2 infection in children and adults. Nature . 2022;602:321–327. doi: 10.1038/s41586-021-04345-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stephenson E, Reynolds G, Botting RA, Calero-Nieto FJ, Morgan MD, Tuong ZK, et al. Cambridge Institute of Therapeutic Immunology and Infectious Disease-National Institute of Health Research (CITIID-NIHR) COVID-19 BioResource Collaboration Single-cell multi-omics analysis of the immune response in COVID-19. Nat Med . 2021;27:904–916. doi: 10.1038/s41591-021-01329-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vanderwall C, Randall Clark R, Eickhoff J, Carrel AL. BMI is a poor predictor of adiposity in young overweight and obese children. BMC Pediatr . 2017;17:135. doi: 10.1186/s12887-017-0891-z. [DOI] [PMC free article] [PubMed] [Google Scholar]