Abstract
Rationale
Relatives of patients with familial interstitial pneumonia (FIP) are at increased risk for pulmonary fibrosis and develop preclinical pulmonary fibrosis (PrePF).
Objectives
We defined the incidence and progression of new-onset PrePF and its relationship to survival among first-degree relatives of families with FIP.
Methods
This is a cohort study of family members with FIP who were initially screened with a health questionnaire and chest high-resolution computed tomography (HRCT) scan, and approximately 4 years later, the evaluation was repeated. A total of 493 asymptomatic first-degree relatives of patients with FIP were evaluated at baseline, and 296 (60%) of the original subjects participated in the subsequent evaluation.
Measurements and Main Results
The median interval between HRCTs was 3.9 years (interquartile range, 3.5–4.4 yr). A total of 252 subjects who agreed to repeat evaluation were originally determined not to have PrePF at baseline; 16 developed PrePF. A conservative estimate of the annual incidence of PrePF is 1,023 per 100,000 person-years (95% confidence interval, 511–1,831 per 100,000 person-years). Of 44 subjects with PrePF at baseline, 38.4% subjects had worsening dyspnea compared with 15.4% of those without PrePF (P = 0.002). Usual interstitial pneumonia by HRCT (P < 0.0002) and baseline quantitative fibrosis score (P < 0.001) are also associated with worsening dyspnea. PrePF at the initial screen is associated with decreased survival (P < 0.001).
Conclusions
The incidence of PrePF in this at-risk population is at least 100-fold higher than that reported for sporadic idiopathic pulmonary fibrosis (IPF). Although PrePF and IPF represent distinct entities, our study demonstrates that PrePF, like IPF, is progressive and associated with decreased survival.
Keywords: pulmonary fibrosis, idiopathic pulmonary fibrosis, familial idiopathic pulmonary fibrosis
At a Glance Commentary
Scientific Knowledge on the Subject
First-degree relatives of individuals with idiopathic interstitial pneumonia are at risk for asymptomatic pulmonary fibrosis, which we term preclinical pulmonary fibrosis (PrePF).
What this Study Adds to the Field
We define the incidence of fibrotic interstitial lung disease (PrePF) in a familial at-risk cohort. In those with preexisting PrePF, we characterize its progression and demonstrate decreased survival for those having PrePF.
Idiopathic pulmonary fibrosis (IPF) is a poorly understood disease characterized by progressive lung parenchymal scarring, impaired gas exchange, and shortened lifespan. Median survival is approximately 3 years from the time of diagnosis (1), and the clinical course is unpredictable (1). Families with multiple cases of idiopathic interstitial pneumonia (IIP) represent a unique at-risk population to identify early manifestations of IIP (2). In fact, among asymptomatic first-degree relatives (FDRs) from families with two or more cases of IIP (familial interstitial pneumonia, FIP), 14% had interstitial lung abnormalities on high-resolution computed tomography (HRCT) scan, and 35% had an abnormal transbronchial biopsy indicative of interstitial lung disease (3). Among asymptomatic FDRs in FIP families, older age and the gain-of-function MUC5B (mucin 5B) promoter variant (4, 5), the dominant genetic risk variant for IPF (6), are associated with preclinical manifestations of pulmonary fibrosis on HRCT scan (defined by subpleural reticular changes, honeycombing, and/or traction bronchiectasis), and these radiologic lesions appear to be progressive (5). In the Framingham population, the MUC5B promoter variant was predictive of HRCT scan evidence of fibrotic interstitial lung disease (7), and in this population fibrotic interstitial lung disease is associated with radiologic progression, a more rapid decline in lung function, and an increased risk of death, suggesting that early interstitial lung fibrosis is a harbinger of IIP (8). We previously screened a cohort of 493 self-reported asymptomatic family members >40 years of age from families with two or more cases of IIP and found that 15.8% of unaffected subjects had preclinical pulmonary fibrosis (PrePF) defined by visual reads of HRCT (4).
Based on these observations, we hypothesize that this high-risk population (N = 493) derived from FIP families represents an ideal opportunity to define the incidence and progression of PrePF and clinical features and risk factors associated with these outcomes. We obtained survival data on the entire cohort of 493 subjects and performed a repeat health questionnaire and HRCT scan on 296 (60%) of these subjects.
Methods
Overview of the Study
This study was approved by each institutional review board (IRB) (Colorado Multiple IRB #151147; National Jewish Health IFB 1441a; Vanderbilt IRB #020343). A total of 493 non-Hispanic White FDRs (parents, siblings, or offspring) of patients with FIP (see Figure E1 in the online supplement) >40 years of age from across the United States who had not received a clinical diagnosis of IIP were initially screened with a health questionnaire and chest HRCT scan. Preclinical manifestations of pulmonary fibrosis were identified in 15.5% of these at-risk subjects (4). Approximately 4 years after the initial evaluation, all surviving subjects among the 493 participants were recontacted and asked to consent to follow-up. A total of 296 (60%) of the original 493 study subjects participated in the subsequent evaluation. In addition, we report a comprehensive survival analysis on the entire study population (N = 493). A total of 166 of the 493 subjects at baseline and 38 of the 296 follow-up subjects have been previously reported in a study evaluating risk factors for development and progression of familial interstitial lung disease (5). For the purposes of this study, we have limited the definition of PrePF to those with fibrotic interstitial lung disease defined by reticular change with traction bronchiectasis with or without honeycombing (definite PrePF) or by those with reticular change with equivocal traction bronchiectasis and no honeycombing (probable PrePF). Prevalent PrePF is defined as all cases of PrePF identified on the baseline screen. Incident PrePF is defined as a new diagnosis of PrePF upon reimaging approximately 4 years after an initial negative HRCT scan. The progression of prevalent PrePF cases is reported only in those subjects who had PrePF identified on the initial screen.
Health Questionnaire
The health questionnaire included self-reported age, sex, race/ethnicity, smoking status, medical history, or environmental exposures (2) as well as a five-question assessment of dyspnea that was administered at both baseline and follow-up (9). The dyspnea score at each time point is reported as an ordinal variable on a scale of one to five.
Evaluation of HRCT Scans
Visual HRCT review
HRCT scans of the chest were performed at local community centers using the same instructions for image acquisition and reconstruction and were interpreted by study radiologists as previously described (4).
Quantitative HRCT
We used a machine learning data-driven texture analysis (DTA) consisting of a convolutional neural network algorithm trained with image regions of normal lung and lung labeled by a radiologist as having a reticular abnormality, traction bronchiectasis, or honeycombing (4, 10) to provide quantitative assessment of lung fibrosis, expressed as a percentage of lung volume involved. Details of the previously described technique are available in the online supplement.
Radiology consensus
HRCT images were independently read by two radiologists blinded to clinical information; if there was no consensus among the two radiologists, then a third radiologist (D.A.L.) adjudicated the final diagnosis. The radiologists assigned the HRCT scan into one of four diagnostic categories: 1) normal or minor abnormality; 2) nonfibrotic diffuse lung disease; 3) fibrotic interstitial lung disease; or 4) equivocal. The radiology core also assigned a diagnosis of possible, probable, or definite usual interstitial pneumonia (UIP), nonspecific interstitial pneumonia, other IIPs using standard criteria (11).
Multidisciplinary Clinical Consensus Diagnosis
A clinical consensus diagnosis was assigned to each subject in a multistep process: 1) two pulmonologists independently reviewed all data, including demographics, family history, occupation and environmental exposure history, and HRCT images accompanied by the radiology consensus diagnosis to arrive at a final clinical consensus diagnosis; and 2) if the two pulmonologists did not agree, then adjudication was obtained by an interactive consensus meeting with three or more pulmonologists. The clinical core assigned a diagnosis of fibrosis and determined a specific diagnosis, assigning a confidence level of definite, probable, or possible. A specific final diagnosis was then assigned when there was a confidence level of probable or definite. When the clinical consensus assigned the presence of fibrosis but was unable to assign a specific diagnosis, the diagnosis assigned was “uncharacterized fibrosis.” The clinical core assigned a diagnosis of indeterminate when the clinical and HRCT findings were ambiguous.
Blood Processing and Genotyping
DNA was extracted from Paxgene tubes of whole blood (4). The MUC5B promoter variant (rs35705950) was the genotype on all study subjects for whom DNA samples were available (4).
Survival Status
Survival status of 492 of the 493 participants was determined by query of a publicly available source of death records (https://www.obituarydata.com/) after follow-up was completed for all subjects. Participants were identified in the obituary database based on name, date of birth, address, and family member information present in the subject’s obituary then correlated with similar information in our FIP database. Survival follow-up was defined as the time from the first HRCT scan to the date of death or censoring at the date of the database query.
Statistical Analysis
Cohort demographics were summarized using medians and proportions, as appropriate. Change in dyspnea was calculated as the score at follow-up, minus the score at baseline. A Pearson’s chi-square test or t test statistic, as appropriate, was used to test for associations between dyspnea score change (categorized as stable/improved or worsening) and baseline measures of PrePF diagnosis, MUC5B genotype, or quantitative fibrosis score. In sensitivity analyses, we also repeated association testing using a generalized estimating equations approach with robust variance estimation to account for the correlation among outcomes in related individuals. Because none of the conclusions changed, we report P values from standard statistical tests, which were all greater than or equal to those from the generalized estimating equations approach. Kaplan-Meier estimates of survival together with log-rank tests were used to assess differences in survival probabilities between groups. Cox proportional hazards models were used to estimate hazard ratios (HRs) and to test for independent predictors of survival. An additive genetic model was used to assess the effect of MUC5B minor allele on incidence of PrePF because previous studies have observed an additive effect of the variant on risk for IIP (12). All analyses were conducted using R version 3.6.3.
Results
Demographic and Clinical Characteristics of Study Population
Of the initially screened 493 self-reported unaffected FDR family members (baseline), 302 reconsented to additional testing, and 296 (60% of the baseline cohort) subjects completed repeat HRCT screening (Figure E1). Comparing those who chose not to participate in the second screening (n = 197) to those who underwent repeat evaluation (n = 296, follow-up), no significant differences were noted in age, sex, smoking status, or MUC5B genotype (Table 1). The demographics of the follow-up cohort (n = 296) are presented in Table 1; the median interval between HRCTs was 3.9 years (interquartile range, 3.5–4.4 yr). Baseline and follow-up HRCT scans were technically adequate for quantitative DTA HRCT reads for 215 study subjects (Table E1).
Table 1.
Demographics of Cohorts Based on Follow-Up
| Follow-Up | No Follow-Up | P Value | |
|---|---|---|---|
| Number of subjects | 296 | 197 | |
| Male, n (%) | 113 (38) | 75 (38) | 1.00 |
| Age at consent, median (IQR) | 58 (52–66) | 57 (52–64) | 0.64 |
| Non-Hispanic White, n (%) | 290 (98) | 192 (97) | 0.76 |
| Ever-smoker, n (%) | 92 (31) | 61 (33) | 0.70 |
| Time interval between HRCTs, yr, median (IQR) | 3.9 (3.5–4.4) | NA | |
| MUC5B genotype, n (%) | 0.38 | ||
| GG | 170 (57) | 123 (62) | |
| GT | 115 (39) | 70 (35) | |
| TT | 11 (4) | 4 (2) | |
| G alleles | 455 (77) | 316 (80) | |
| T alleles | 137 (23) | 78 (20) | |
| MUC5B promoter variant (rs35705950) MAF | |||
| No PrePF at follow-up | 0.22 | ||
| Incident PrePF | 0.28 | 0.39*† | |
| Prevalent PrePF | 0.28 | 0.18*‡ | |
Definition of abbreviations: HRCT = high-resolution computed tomography; IQR = interquartile range; MAF = minor allele frequency; MUC5B = mucin 5B, oligomeric mucus/gel-forming; PrePF = preclinical pulmonary fibrosis.
Additive genetic model.
Compared with no PrePF at follow-up.
Compared with no PrePF at baseline.
Within the follow-up population (n = 296), 252 had a normal HRCT scan and 44 had PrePF on HRCT at the initial evaluation. The clinical core identified 16 new cases of PrePF (incident cases) among the 252 subjects originally determined not to have PrePF at baseline and 35 cases of persistent PrePF among the 44 subjects originally determined to have PrePF at baseline. Of the 16 subjects with incident PrePF, 1 had chronic hypersensitivity pneumonitis, 9 had uncharacterized fibrosis, and 6 had an IIP (Table E2). Of the 15 subjects who had a diagnosis of indeterminate without fibrosis findings at the initial evaluation, at follow-up, 11 remained indeterminate or normal, and 4 developed PrePF (either IPF or uncharacterized fibrosis; Table E2). This finding suggests that an indeterminate diagnosis by the clinical core frequently represents early PrePF.
Incidence of PrePF
Of 252 subjects with a clinical core consensus diagnosis of no PrePF at baseline, at follow-up, 16 (6.3%) developed PrePF, equating to an annual incidence proportion of PrePF of 1.6% for these at-risk family members. The estimated incidence rate of PrePF in our study population by the clinical core (1,550 per 100,000 person-years; 95% confidence interval [CI], 886–2,517 per 100,000 person-years) is similar to that of our radiology core (1,717 per 100,000 person-years; 95% CI, 1,101–2,714) and far exceeds population-based incidence rates for IPF, which are estimated to be 10 per 100,000 person-years (13). When both the clinical and radiology cores agreed that there was no evidence of PrePF at the initial evaluation (n = 244), 11 subjects were considered by both cores to have developed PrePF during the period of follow-up, resulting in a conservative estimate of the annual incidence of PrePF of 1,023 per 100,000 person-years (95% CI, 511–1,831 per 100,000 person-years). Interestingly, of 23 subjects determined by the radiology core to have nonfibrotic ILD at baseline and who had follow-up HRCTs, 5 developed fibrotic ILD at follow-up (Table E4).
The clinical core determined that nine subjects had PrePF at baseline but were found to be unaffected on follow-up evaluation. Given this unexpected finding, we extensively reviewed these subjects (Table E3 and Figure E2). We found that the clinical and radiology cores did not agree on the assessment of PrePF in six of the nine subjects (Table E3). It is notable that the CT findings of these nine subjects at baseline and follow-up are subtle and, not surprisingly, subject to interobserver variation (Figure E2). Last, quantitative fibrosis scores were available in six of the nine subjects, and two had a decrease in the quantitative fibrosis score, whereas four increased (Table E2).
Symptomatic and Radiographic Progression of PrePF
During the initial evaluation, the clinical core identified 79 cases of PrePF among the 493 asymptomatic FDRs who were screened. Of these 79 baseline prevalent cases of PrePF, 44 (56%) participated in the subsequent evaluation (median follow-up period of 3.9 yr). During the follow-up period, of the 227 subjects with complete dyspnea data, 19.4% had worsening of their dyspnea score (Table E6). Progressive dyspnea was observed in 15 (38.4%) subjects with baseline PrePF compared with 29 (15.4%) without PrePF (P = 0.002; Table 2). Worsening dyspnea during the period of observation was also associated with a UIP pattern on baseline HRCT (Table E7; P < 0.0002) and a higher baseline quantitative fibrosis score (Table 2; P < 0.001). Thus, the presence of PrePF, an HRCT diagnosis of UIP, or a higher quantitative fibrosis score is significantly associated with worsening dyspnea during a brief period of follow-up (median, 3.9 yr). We compared the consensus diagnosis assigned by the clinical core at baseline and at follow-up (Table E2), and we note in subjects with uncharacterized fibrosis at baseline that the follow-up diagnosis remained unchanged in half, whereas others developed a specific IIP or other type of pulmonary fibrosis. There were 23 subjects with nonfibrotic ILD at baseline, and on follow-up 13 were either normal or had minor abnormalities, 5 remained with nonfibrotic ILD, and 5 developed fibrotic ILD (Table E4).
Table 2.
Association between Baseline Diagnosis and Change in Dyspnea
| Dyspnea |
P Value | ||
|---|---|---|---|
| Improved/Stable | Worsening | ||
| Clinical core consensus | |||
| PrePF absent | 159 | 29 | 0.002 |
| PrePF present | 24 | 15 | |
| Quantitative fibrosis score, mean | 1.57 | 4.75 | 0.0005 |
| MUC5B genotype | |||
| GG | 105 | 23 | 0.66 |
| GT/TT | 78 | 21 | |
Definition of abbreviations: MUC5B = mucin 5B, oligomeric mucus/gel-forming; PrePF = preclinical pulmonary fibrosis.
Change in dyspnea is calculated as the score at follow-up minus the score at baseline and collapsed into two categories representing improved/stable dyspnea (score change ≤ 0) and worsening dyspnea (score change > 0). Complete baseline and follow-up questionnaire data available for 227 subjects.
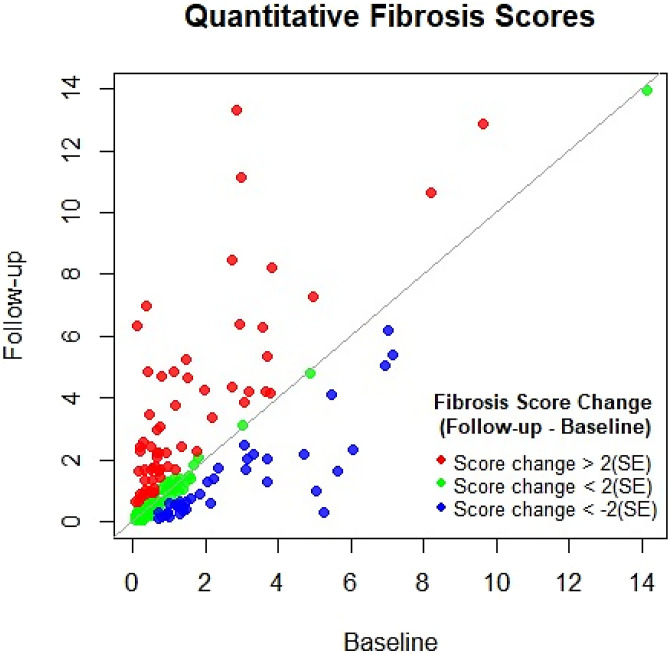
We used machine learning DTA to provide quantitative measure of lung fibrosis to compare baseline and follow-up HRCTs. The scatterplot of the baseline and follow-up DTA quantitative fibrosis score (n = 206; Figure 1) demonstrates an upward shift above the line of identity indicating an increase in the DTA fibrosis score at follow-up (P = 0.003). A total of 41 (20%) subjects had an improvement of their fibrosis score (Figure 1), 98 (47.5%) subjects had no change in their fibrosis score, and 67 (32.5%) subjects had a worsening of their fibrosis score, suggesting that DTA fibrosis score may prove to be a helpful aid in following individuals at risk for lung fibrosis.
Figure 1.
Scatterplot of the quantitative data-driven texture analysis fibrosis score comparing changes in score. Individual subjects are color coded according to fibrosis score change between baseline and follow-up. The fibrosis score changes are coded according to >2 SE change (red), score change < 2 SE (green), and score change < −2 SE (blue). Eight subjects were not scored because of technically limited high-resolution computed tomography scan quality.
Survival
During the follow-up period, there were 18 deaths (Table E9) among the subjects initially screened (N = 493). Among the 79 cases of PrePF diagnosed at baseline, 9 (11.7%) deaths were observed, whereas, among the 416 without PrePF at baseline, 9 subjects (2.2%) died. Among the nine subjects with PrePF at baseline who died during the follow-up period, a review of medical records indicated that three of the deaths were of unknown cause, and six of the deaths were caused by progressive respiratory failure. In contrast, among the nine deaths in subjects without PrePF, no deaths were attributed to respiratory failure.
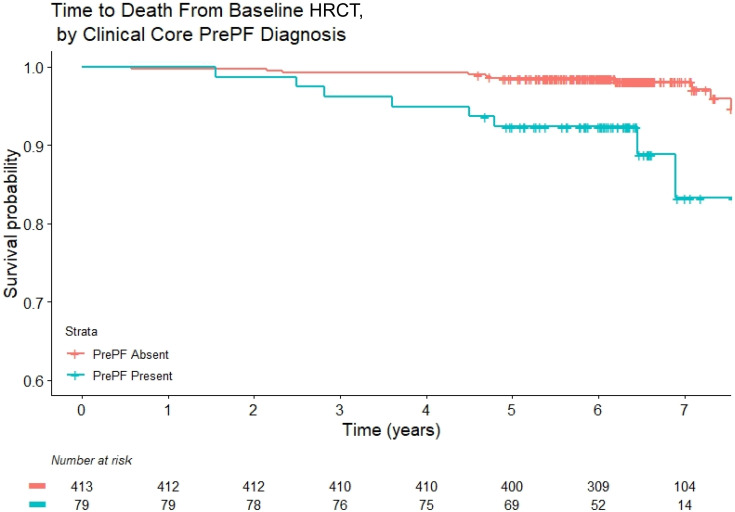
Kaplan-Meier analysis demonstrates that PrePF diagnosed at the initial screen is associated with decreased survival (Figure 2; P < 0.001). By univariate Cox proportional hazards (Table E8), decreased survival is associated with increasing age (HR, 1.13; P < 0.001), PrePF determined by the radiology core (HR, 6.19; P < 0.001), PrePF determined by the clinical core (HR, 4.69; P = 0.001), and quantitative fibrosis score determined by DTA (HR, 1.07; P < 0.001). To identify independent demographic and clinical predictors of survival, we developed a multivariable Cox model. The quantitative DTA fibrosis score is independently associated with decreased survival (HR, 1.06; P = 0.01) after controlling for age and smoking (Table 3).
Figure 2.
Kaplan-Meier plots of survival comparing subjects with preclinical pulmonary fibrosis (PrePF) (cyan) versus no PrePF (pink) as determined by the clinical core. Survival was determined on 492 subjects, because 1 subject had missing identifying information in the database and therefore survival data could not be determined.
Table 3.
Multivariable Cox Proportional Hazards Models of Survival (N = 493)
| Variable | HR (95% CI) | P Value |
|---|---|---|
| Quantitative fibrosis score | 1.06 (1.01–1.10) | 0.013 |
| Age | 1.09 (1.03–1.15) | 0.005 |
| Smoking | ||
| Never | 1 [Ref] | 0.059 |
| Ever | 3.02 (0.96–9.49) |
Definition of abbreviations: CI = confidence interval; HR = hazard ratio; Ref = reference value.
Multivariable Cox model of independent predictors of survival of the 493 asymptomatic at-risk family members screened at baseline.
Effect of MUC5B Genotype on PrePF Disease Progression and PrePF Incidence
We and others (4, 5) previously reported that the minor allele frequency of the gain-of-function MUC5B promoter variant rs35705950 was significantly elevated in the prevalent cases of PrePF compared with those without disease in FDRs in families with FIP. In our follow-up population (n = 296), the minor allele frequency of rs35705950 is 0.28 in the incident and prevalent cases of PrePF compared with 0.22 in subjects without PrePF (Table 1; odds ratio, 1.5; 95% CI, 0.82–2.76). This trend toward being more common in the subjects with PrePF at follow-up compared with unaffected subjects did reach statistical significance (P = 0.042 for an additive genetic model). We also examined the effect of the MUC5B promoter variant on disease progression measured by chest HRCT scan (Table E9), change in dyspnea, and survival (Figure E3) and did not find that the minor allele (T) of MUC5B was associated with disease progression.
Discussion
This is the first study to prospectively determine the incidence of fibrotic lung disease in an at-risk cohort. We found that the annual incidence of developing PrePF in FDRs from FIP families is between 1,000 and 1,500 per 100,000 person-years. The incidence of PrePF in this at-risk population is approximately 100- to 150-fold higher than that reported for sporadic IPF (1, 13–16). Although PrePF and IPF represent distinct entities, our study also demonstrates that PrePF, like IPF, is defined by the same radiographic abnormalities, is progressive, and is associated with decreased survival. In aggregate, these findings should initiate consideration of screening individuals at risk of PrePF and evaluating the potential benefits of early intervention in those with this early presentation of fibrotic lung disease.
Although we found that FDRs arising from families with two or more cases of IIP are at substantial risk of PrePF, there are likely other populations that are at risk of PrePF. For example, the risk among relatives of sporadic cases of IPF appears to be equivalent to that observed for relatives of FIP (17). Because rare (18–23) and common (6, 12, 24–29) genetic variants have been associated with IPF and FIP, it is logical to speculate that gene variants play a role in the risk of developing PrePF and that specific gene variants could be used to trigger screening for interstitial lung disease. In fact, in the Framingham population, we found that the MUC5B promoter variant rs35705950 was predictive of those with fibrotic lung disease (odds ratio, 6.3 per allele; 95% CI, 3.1–12.7) (7). However, it is well established that environmental exposures, such as cigarette smoke (30, 31), asbestos, organic dust, metal and mineral dust (32), wood dust (33–35), and ambient particulate matter (36), place individuals at risk of IIP. Thus, PrePF may develop in nuclear families because of shared genetics and/or a common environment. Although the IPF genetic risk variants establish a vulnerable host, fibrotic lung disease may occur among those who have been sufficiently exposed to an environmental toxin. For example, although the MUC5B promoter variant is the dominant genetic risk factor for IPF and is present in ≈19% of the general population (6, 24), IPF is diagnosed much less frequently (<0.1% [37, 38]), suggesting that IPF is a complex disease that occurs in genetically susceptible hosts with multiple risk factors potentially inclusive of specific environmental exposures. However, our findings also support the possibility that IPF may be underdiagnosed. PrePF is not limited to being an early stage of IPF, because other progressive fibrotic lung diseases are present in the FIP cohort at follow-up, such as chronic hypersensitivity pneumonitis and other IIPs.
The diagnosis of PrePF is challenging. We devised a rigorous phenotyping strategy using a radiology core of multiple readers blinded to clinical data reaching consensus if there was disagreement among the readers. This was followed by a clinical core of multiple independent reviews of all the radiologic and clinical data with an interactive consensus diagnosis if there was disagreement. Even with this rigorous approach, there were nine subjects with PrePF at baseline who were found not to have abnormalities at follow-up. After a detailed evaluation of these nine subjects, we think that the initial radiographic abnormalities were subtle and that quantitative fibrosis scores may be helpful in supplementing visual CT reads of early ILD.
Our modeling of factors determining survival of the cohort identified fibrotic lung disease determined by either clinical consensus, radiology visual HRCT interpretation, or quantitative fibrosis score, as well as age and smoking, to be predictors of survival. However, only quantitative fibrosis score and age remain significant predictors of survival in a multivariable Cox model. These findings lead us to speculate that quantitative measures of lung fibrosis are potentially more specific and could be used as a predictive tool in higher-risk populations. It should be noted that quantitative HRCT is not yet widely available or standardized with respect to methodology. Although we were able to demonstrate a trend toward decreased survival in those with the MUC5B promoter variant, this did not reach statistical significance, likely related to the relatively small sample size. In general, the sample size of our PrePF cohort limits our ability to adequately study the role of genetics, environment, or other risk factors for developing PrePF in FDRs.
There are a number of other limitations to our study. We obtained follow-up in 60% of subjects with PrePF; although we did not detect significant differences in those who followed up compared with those who did not, it is possible bias was introduced based on those who chose to participate in this follow-up study. The FIP cohort is predominately non-Hispanic White, and the generalizability of our findings to PrePF of other ethnicities is uncertain. We do not have surgical lung biopsy in subjects with PrePF. Consequently, those PrePF classified as uncharacterized fibrosis may represent other IIPs, such as nonspecific interstitial pneumonia or other progressive fibrotic ILDs. Finally, vital status might not be perfectly captured using public databases such as ObituaryData.com.
Our findings indicate that the annual incidence of lung fibrosis is high in an at-risk population at 100- to 150-fold higher than the estimated incidence of IPF in the general population and that family members with PrePF having minimal disease burden on HRCT scan and mild dyspnea are at risk of respiratory death. Collectively, these data suggest that unaffected family members >50 years old in families with FIP should be screened for the presence of fibrotic interstitial lung disease, and those with PrePF should be monitored closely, perhaps with periodic HRCT to screen for early pulmonary fibrosis. However, further study is needed to determine the timing, frequency, and type, and radiation dose of HRCT to use. Similarly, it is not clear how other risk factors, such as genetics, environmental exposures, or serum biomarkers, should be incorporated into the decision to screen. These data also raise the interesting question of whether patients with PrePF, once identified, should be considered for treatment approved for established IPF. Based on our findings, we recommend that these topics (screening and earlier treatment) be considered and a randomized prospective clinical treatment trial be conducted among those with early presentation of lung fibrosis.
Footnotes
Supported by NHLBI grants PO1-HL0928701, RO1-HL149836, UH2/3-HL123442, UG3/UH3-HL 151865, and R01-HL158668.
Author Contributions: All authors participated in writing of the manuscript. Data analysis: M.P.S., A.L.P., S.H., and T.E.F. Genotyping: A.D.W. and I.V.Y. Phenotyping: M.P.S., S.K.M., T.J.B., A.O., S.T., G.C., C.S., J.E.L., K.K.B., M.I.S., D.A.L., J.S.L., and D.A.S. Subject recruitment: J.A.K., T.S.B., R.A.W., J.P., C.M., and D.A.S. Funding: D.A.S.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202206-1075OC on September 12, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Ley B, Collard HR. Epidemiology of idiopathic pulmonary fibrosis. Clin Epidemiol . 2013;5:483–492. doi: 10.2147/CLEP.S54815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Steele MP, Speer MC, Loyd JE, Brown KK, Herron A, Slifer SH, et al. Clinical and pathologic features of familial interstitial pneumonia. Am J Respir Crit Care Med . 2005;172:1146–1152. doi: 10.1164/rccm.200408-1104OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kropski JA, Pritchett JM, Zoz DF, Crossno PF, Markin C, Garnett ET, et al. Extensive phenotyping of individuals at risk for familial interstitial pneumonia reveals clues to the pathogenesis of interstitial lung disease. Am J Respir Crit Care Med . 2015;191:417–426. doi: 10.1164/rccm.201406-1162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mathai SK, Humphries S, Kropski JA, Blackwell TS, Powers J, Walts AD, et al. MUC5B variant is associated with visually and quantitatively detected preclinical pulmonary fibrosis. Thorax . 2019;74:1131–1139. doi: 10.1136/thoraxjnl-2018-212430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Salisbury ML, Hewlett JC, Ding G, Markin CR, Douglas K, Mason W, et al. Development and progression of radiologic abnormalities in individuals at risk for familial interstitial lung disease. Am J Respir Crit Care Med . 2020;201:1230–1239. doi: 10.1164/rccm.201909-1834OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moore C, Blumhagen RZ, Yang IV, Walts A, Powers J, Walker T, et al. Resequencing study confirms that host defense and cell senescence gene variants contribute to the risk of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med . 2019;200:199–208. doi: 10.1164/rccm.201810-1891OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hunninghake GM, Hatabu H, Okajima Y, Gao W, Dupuis J, Latourelle JC, et al. MUC5B promoter polymorphism and interstitial lung abnormalities. N Engl J Med . 2013;368:2192–2200. doi: 10.1056/NEJMoa1216076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Putman RK, Gudmundsson G, Axelsson GT, Hida T, Honda O, Araki T, et al. Imaging patterns are associated with interstitial lung abnormality progression and mortality. Am J Respir Crit Care Med . 2019;200:175–183. doi: 10.1164/rccm.201809-1652OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferris BG. Epidemiology standardization project (American Thoracic Society) Am Rev Respir Dis . 1978;118:1–120. [PubMed] [Google Scholar]
- 10. Humphries SM, Swigris JJ, Brown KK, Strand M, Gong Q, Sundy JS, et al. Quantitative high-resolution computed tomography fibrosis score: performance characteristics in idiopathic pulmonary fibrosis. Eur Respir J . 2018;52:1801384. doi: 10.1183/13993003.01384-2018. [DOI] [PubMed] [Google Scholar]
- 11. Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society Diagnosis of idiopathic pulmonary fibrosis: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med . 2018;198:e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 12. Fingerlin TE, Murphy E, Zhang W, Peljto AL, Brown KK, Steele MP, et al. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat Genet . 2013;45:613–620. doi: 10.1038/ng.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nalysnyk L, Cid-Ruzafa J, Rotella P, Esser D. Incidence and prevalence of idiopathic pulmonary fibrosis: review of the literature. Eur Respir Rev . 2012;21:355–361. doi: 10.1183/09059180.00002512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coultas DB, Zumwalt RE, Black WC, Sobonya RE. The epidemiology of interstitial lung diseases. Am J Respir Crit Care Med . 1994;150:967–972. doi: 10.1164/ajrccm.150.4.7921471. [DOI] [PubMed] [Google Scholar]
- 15. Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med . 2006;174:810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 16. Fernández Pérez ER, Daniels CE, Schroeder DR, St Sauver J, Hartman TE, Bartholmai BJ, et al. Incidence, prevalence, and clinical course of idiopathic pulmonary fibrosis: a population-based study. Chest . 2010;137:129–137. doi: 10.1378/chest.09-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hunninghake GM, Quesada-Arias LD, Carmichael NE, Martinez Manzano JM, Poli De Frías S, Baumgartner MA, et al. Interstitial lung disease in relatives of patients with pulmonary fibrosis. Am J Respir Crit Care Med . 2020;201:1240–1248. doi: 10.1164/rccm.201908-1571OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med . 2007;356:1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 19. Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, Weissler JC, et al. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci USA . 2007;104:7552–7557. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thomas AQ, Lane K, Phillips J, III, Prince M, Markin C, Speer M, et al. Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am J Respir Crit Care Med . 2002;165:1322–1328. doi: 10.1164/rccm.200112-123OC. [DOI] [PubMed] [Google Scholar]
- 21. Lawson WE, Grant SW, Ambrosini V, Womble KE, Dawson EP, Lane KB, et al. Genetic mutations in surfactant protein C are a rare cause of sporadic cases of IPF. Thorax . 2004;59:977–980. doi: 10.1136/thx.2004.026336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang Y, Kuan PJ, Xing C, Cronkhite JT, Torres F, Rosenblatt RL, et al. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet . 2009;84:52–59. doi: 10.1016/j.ajhg.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Moorsel CH, van Oosterhout MF, Barlo NP, de Jong PA, van der Vis JJ, Ruven HJ, et al. Surfactant protein C mutations are the basis of a significant portion of adult familial pulmonary fibrosis in a Dutch cohort. Am J Respir Crit Care Med . 2010;182:1419–1425. doi: 10.1164/rccm.200906-0953OC. [DOI] [PubMed] [Google Scholar]
- 24. Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med . 2011;364:1503–1512. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Allen RJ, Porte J, Braybrooke R, Flores C, Fingerlin TE, Oldham JM, et al. Genetic variants associated with susceptibility to idiopathic pulmonary fibrosis in people of European ancestry: a genome-wide association study. Lancet Respir Med . 2017;5:869–880. doi: 10.1016/S2213-2600(17)30387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fingerlin TE, Zhang W, Yang IV, Ainsworth HC, Russell PH, Blumhagen RZ, et al. Genome-wide imputation study identifies novel HLA locus for pulmonary fibrosis and potential role for auto-immunity in fibrotic idiopathic interstitial pneumonia. BMC Genet . 2016;17:74. doi: 10.1186/s12863-016-0377-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mushiroda T, Wattanapokayakit S, Takahashi A, Nukiwa T, Kudoh S, Ogura T, et al. Pirfenidone Clinical Study Group A genome-wide association study identifies an association of a common variant in TERT with susceptibility to idiopathic pulmonary fibrosis. J Med Genet . 2008;45:654–656. doi: 10.1136/jmg.2008.057356. [DOI] [PubMed] [Google Scholar]
- 28. Noth I, Zhang Y, Ma SF, Flores C, Barber M, Huang Y, et al. Genetic variants associated with idiopathic pulmonary fibrosis susceptibility and mortality: a genome-wide association study. Lancet Respir Med . 2013;1:309–317. doi: 10.1016/S2213-2600(13)70045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Allen RJ, Guillen-Guio B, Oldham JM, Ma SF, Dressen A, Paynton ML, et al. Genome-wide association study of susceptibility to idiopathic pulmonary fibrosis. Am J Respir Crit Care Med . 2020;201:564–574. doi: 10.1164/rccm.201905-1017OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baumgartner KB, Samet JM, Stidley CA, Colby TV, Waldron JA. Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med . 1997;155:242–248. doi: 10.1164/ajrccm.155.1.9001319. [DOI] [PubMed] [Google Scholar]
- 31. Washko GR, Hunninghake GM, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, et al. COPDGene Investigators Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med . 2011;364:897–906. doi: 10.1056/NEJMoa1007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fontenot AP, Amicosante M. Metal-induced diffuse lung disease. Semin Respir Crit Care Med . 2008;29:662–669. doi: 10.1055/s-0028-1101276. [DOI] [PubMed] [Google Scholar]
- 33. Hubbard R, Lewis S, Richards K, Johnston I, Britton J. Occupational exposure to metal or wood dust and aetiology of cryptogenic fibrosing alveolitis. Lancet . 1996;347:284–289. doi: 10.1016/s0140-6736(96)90465-1. [DOI] [PubMed] [Google Scholar]
- 34. Riccò M. Lung fibrosis and exposure to wood dusts: two case reports and review of the literature. Med Pr . 2015;66:739–747. doi: 10.13075/mp.5893.00140. [DOI] [PubMed] [Google Scholar]
- 35. Gustafson T, Dahlman-Höglund A, Nilsson K, Ström K, Tornling G, Torén K. Occupational exposure and severe pulmonary fibrosis. Respir Med . 2007;101:2207–2212. doi: 10.1016/j.rmed.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 36. Trethewey SP, Walters GI. The role of occupational and environmental exposures in the pathogenesis of idiopathic pulmonary fibrosis: a narrative literature review. Medicina (Kaunas) . 2018;54:108. doi: 10.3390/medicina54060108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med . 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Olson AL, Swigris JJ, Lezotte DC, Norris JM, Wilson CG, Brown KK. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med . 2007;176:277–284. doi: 10.1164/rccm.200701-044OC. [DOI] [PubMed] [Google Scholar]