Abstract
Background:
Cortisol response to stressors (hypothalamic–pituitary–adrenal axis, autonomic nervous system, and immune system) plays a vital role in maintaining stable metabolic homeostasis. This study was done to assess the prevalence of hypocortisolemia in patients presenting to ED with sepsis and/or septic shock.
Methods:
This prospective observational study was done from July 2020 to April 2021. Serum cortisol levels were measured in patients with sepsis and septic shock, and their clinical and laboratory profile was categorized, coded, and analyzed.
Results:
Ninety-eight patients were included, of which serum Cortisol <10 μg/dl was noted in 7 (7.2%) patients. The cohort’s mean age was 52.9 (SD: 15.3) years with a male predominance (n-61; 62.2%). Most common presenting complaint was fever (n-52; 53.1%), followed by abdominal pain (n-24; 24.5%), and breathing difficulty (n-14; 14.3%). Systolic blood pressure <90 mmHg and tachycardia were seen in 63 patients (64.3%). Assessment of diet and native medication use did not demonstrate a predisposition to hypocortisolemia. The median (IQR) arterial lactate values were lower in the hypocortisolemic group: 2.2 (1.2–2.5) as compared to the non-hypocortisolemic group: 3.7 (2.2–8.0). Patients with septic shock without hypocortisolemia were noted to have a higher mean lactate level (2.6 ± 1.3 Vs 5.4 ± 3.9) and lower platelet counts compared to those with low cortisol levels. Patients with normal cortisol levels (n-38; 38.8%) still had low ACTH values.
Conclusion:
The prevalence of hypocortisolemia was lower when compared to other Indian studies. Diet and native medication use do not predispose Indians to hypocortisolemia.
Keywords: ACTH, cortisol levels, hypocortisolemia, sepsis, septic shock
INTRODUCTION
An individual’s response to stress relies on three major systems, i.e., the hypothalamic-pituitary-adrenal (HPA) axis, the autonomic nervous system, and the immune system. A correct balance between the activation of these systems allows for controlling infection while maintaining metabolic homeostasis and cardiovascular stability.[1,2,3] There has been interest in investigating the role of glucocorticoids in the pathophysiology of critical illness for many years, as it has been observed that the adrenal glands are crucial to surviving under conditions of physiologic stress.
As per the National Advisory General Medical Sciences Council (NAGMSC) working group report, sepsis is defined as an acute serious organ dysfunction caused by a dysregulated host response to infection, whereas septic shock is defined as sepsis that has circulatory, cellular, and metabolic abnormalities that are associated with a greater risk of mortality than sepsis alone.[4,5] It includes patients who fulfil the criteria for sepsis and whose lactate levels are rising above 2 mmol/liter without volume loss and the requirement of inotropes to keep a mean arterial pressure of above 65 mmHg.[4] Hemodynamic instability in patients with sepsis or septic shock is mostly linked with inhibition of the HPA axis.[1,2] Dysfunction of the HPA axis in the setting of sepsis or septic shock is referred to as critical illness-related corticosteroid insufficiency (CIRCI), which is defined as inadequate cellular corticosteroid activity for the severity of the illness.[6,7,8] Recent guidelines by the Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) suggested that adrenal insufficiency (AI) in critically ill patients is best made by a delta total serum cortisol of <9 microg/dl after adrenocorticotrophic hormone (250 microg) administration or random total cortisol of <10 μg/dl.[6,9] The neuroendocrine response in shock and acute or critical illness is to activate the anterior pituitary function and inhibit peripheral anabolic pathways.[2,10] Hence there is increased adrenocorticotropic hormone (ACTH) production and a resultant increase in cortisol levels.[7,10] Unfortunately, there is no study done in an Indian Emergency Department (ED) set up to support this. The response among Indian patients has not been studied adequately earlier and we postulate a possible difference in the neuroendocrine response, as the general population is exposed to multiple forms of steroids in indigenous native medications and food. This is important because critical illnesses activating the HPA axis may vary according to the type of infection, time of presentation to ED from the disease onset, chronicity of the illness, and immunity of the individual.
METHODS
Study design: This was a prospective observational cohort study assessing the prevalence of hypocortisolemia in patients with sepsis or septic shock.
Setting: This study was conducted in a tertiary referral health care center. Our ED is a 50-bedded department and caters to the need of about 75,000 patients per year, including medical, surgical, and trauma patients. Six priority I beds are dedicated exclusively to patients with hemodynamic compromise.
Study period: This study was conducted over a period of 9 months (July 2020 to April 2021).
Participants: Adult patients (age ≥18 years) who presented to us with a history of fever and/or clinical features of infection with quick Sequential Organ Failure Assessment (qSOFA) ≥ 2 were included in the study after obtaining informed written consent from the patient or the closest relative. The following were excluded: patients who were already on steroids in the past 30 days (oral, inhalational, and parenteral), previously known disease of the HPA axis (itself causing adrenal insufficiency), COVID-19 positive or suspects, and those who did not give consent for the study.
Methodology: An initial random serum cortisol sample and iced ACTH sample were collected in blood collection tubes with a clot activator from each participant. In order to separate and freeze samples for ACTH right away, they were sent to the clinical biochemistry laboratory in an ice box. This was done by the phlebotomist involved in each patient’s care, without any delay. Our laboratory is accessible on foot in 3 minutes or less from the ED and works round the clock. Patients were categorized into hypocortisolemic and non-hypocortisolemic using a cortisol cut-off value of 10 μg/dl. ACTH levels as per our lab assay were normal if above 46 pg/ml.
Variables: The patients and relatives were interviewed and the relevant details of history, clinical findings, laboratory investigations, and qSOFA score were documented in the study form. Triage priority categorization was as per hospital protocol, and priority 1 and 2 patients were included in the study.[11] Triage criteria stress tremendously on vital parameters that are also a part of our inclusion criteria. It helps understand the severity of the illness in those who have been included.
Priority 1: Patients with severely compromised vital signs, whose clinical status necessitates immediate life-saving interventions, which often include resuscitation and advanced life support.
Priority 2: Patients with complaints perceived as urgent, and with the possibility of deterioration, but with no immediate threat to life.
Outcome Variable: The prevalence of hypocortisolemia was determined and correlated with factors like Ayurveda drug use, food habits, ACTH levels, ED, and hospital outcome.
Laboratory test: Besides routine laboratory investigations and relevant radiological tests, random serum cortisol levels and ACTH levels were sent at the presentation from ED. Random serum cortisol level and ACTH level are quantitative variables processed by a Chemiluminescence Immunoassay (CLIA). Based on random serum cortisol levels, we segregated patients into two groups for statistical analysis.
Bias: The primary or co-investigators collected the data, and not the treating physician. To eliminate interviewer bias, a standard set of questions were asked based on a pre-made clinical questionnaire.
Sample size: The sample size was calculated based on a study by Suresh R et al.,[12] with a 48.3% prevalence of hypocortisolemia in patients with severe sepsis or shock. With a precision of 10% and a confidence interval of 95%, the sample size was calculated to be 96.
Data source and Statistical analysis: Clinical data of the patient was entered in a standard datasheet from the questionnaire. Laboratory investigations and outcomes were extracted from our hospital’s electronic database. The data were analyzed using Statistical Package for Social Science (SPSS Inc. Released 2018, version 25.0.0.0 Armonk, NY, USA) software. Quantitative variables data were summarized by using mean and standard deviation (SD) or median with Inter Quartile Range (IQR), and qualitative variables, such as gender, presence, or absence of hypocortisolemia, and native drug usage, were reported using frequency and percentage. Arterial blood gas (ABG) and baseline laboratory investigations at the presentation were analyzed using the Kruskal-Wallis H test. A Violin graph was plotted using R (Version 4.1.2) software, based on random cortisol values in patients presenting in shock versus the normotensive patients. A univariate logistic regression analysis was done, and the P value was calculated with significance defined as less than 0.05.
Ethical considerations: The Institutional Review Board (IRB) and ethical committee clearance were obtained before the commencement of the study (IRB Min no: 12809 dated January 05, 2020). Unique identity documents and password-protected data entry software with limited users were used to guarantee patient confidentiality.
RESULTS
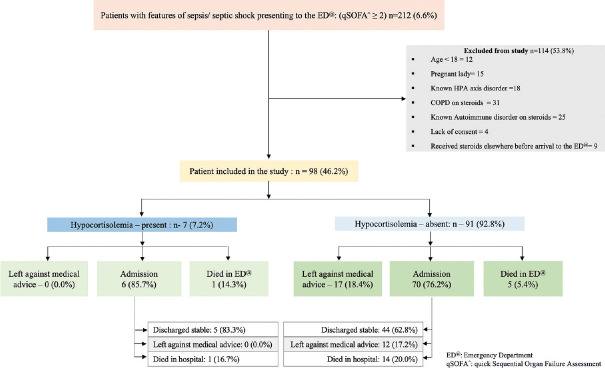
Participants: This study included a total of 98 patients fulfilling inclusion criteria, of which the prevalence of hypocortisolemia was 7.2% (n-7). The age of the study cohort ranged from 18–87 years with a mean of 52.9 (SD: 15.3) years. There was a male predominance (n-61; 62.2%) [Table 1]. According to the physiological status at arrival, most of these patients were triaged as a priority I (n-92; 93.8%).
Table 1.
Baseline characteristics and presenting complaints at presentation to the Emergency Department
| Variables | Overall n-98 (%) | Hypocortisolemic group n-7 (7.2%) | Non-hypocortisolemic group n-91 (92.8%) | P-value |
|---|---|---|---|---|
| Mean age (SD*) years | 52.9±15.3 | 58.7±9.3 | 52.5±15.6 | 0.334 |
| Sex – Males | 61 (62.2) | 4 (5.7) | 57 (62.6) | 0.408 |
| Use of native medications | 11 (11.2) | 2 (33.3) | 9 (9.8) | 0.176 |
| Dietary habits (Nonvegetarian) | 92 (93.9) | 6 (85.7) | 86 (94.5) | 0.367 |
|
| ||||
| Presenting complaints | ||||
|
| ||||
| Fever | 52 (53.1) | 5 (71.4) | 47 (52.2) | 0.442 |
| Abdominal pain | 24 (24.5) | 1 (14.2) | 23 (25.2) | 1.000 |
| Breathing difficulty | 14 (14.3) | 1 (14.2) | 13 (14.9) | 1.000 |
| Vomiting | 10 (10.2) | 0 (0) | 10 (10.9) | 1.000 |
| Seizures/altered sensorium | 7 (7.1) | 0 (0) | 7 (7.7) | 1.000 |
| Cough | 6 (6.1) | 1 (14.2) | 5 (5.4) | 0.367 |
| Dysuria | 5 (5.1) | 0 (0) | 5 (5.4) | 0.724 |
| Loose stools | 5 (5.1) | 0 (0) | 5 (5.4) | 1.000 |
|
| ||||
| Comorbidities | ||||
|
| ||||
| Diabetes Mellitus | 43 (43.9) | 4 (57.1) | 39 (42.8) | 0.696 |
| Hypertension | 24 (24.5) | 1 (14.2) | 23 (25.2) | 1.000 |
| Chronic Liver disease | 9 (9.1) | 0 (0) | 9 (9.8) | 1.000 |
| Chronic Kidney disease | 7 (7.1) | 0 (0) | 7 (7.7) | 1.000 |
SD* – Standard Deviation
Presenting complaints, comorbidities, and vital signs at presentation: Most common presenting complaint was fever (n-52; 53.1%), followed by abdominal pain (n-24; 24.5%), breathing difficulty (n-14; 14.3%), and vomiting (n-10;10.2%) [Table 1]. Taking the cut-off value of random cortisol as <10 μg/dl, hypocortisolemia was seen in 7 (7.2%) patients. [Figure 1] We found no specific presenting complaints specific to the hypocortisolemic group [Table 1]. Comorbidities included diabetes mellitus (n-43;43.9%), hypertension (n-24; 24.5%), chronic liver disease (n-9; 9.1%), and chronic kidney disease (n-7; 7.1%). [Table 1] Approximately two-thirds (n-63; 64.3%) of the patients presented in shock (systolic blood pressure <90 mmHg) and with tachycardia. Details of the vital signs at the time of presentation are given in Table 2.
Figure 1.
Strengthening the Reporting of Observational studies in Epidemiology (STROBE) diagram
Table 2.
Vital signs at presentation to the Emergency Department
| Variables | Overall n-98 (%) | Hypocortisolemic group n-7 (7.2%) | Non-hypocortisolemic group n-91 (92.8%) | P-value |
|---|---|---|---|---|
| Shock (Systolic BP <90 mmHg) | 63 (64.3) | 4 (57.1) | 59 (64.8) | 0.698 |
| Tachycardia (Heart rate >100) | 67 (68.4) | 4 (57.1) | 63 (69.2) | 0.675 |
| Tachypnea (Respiratory rate >22) | 82 (83.7) | 4 (57.1) | 78 (85.7) | 0.084 |
| Altered sensorium | 46 (46.9) | 4 (57.1) | 42 (46.1) | 0.703 |
| Hypoxia (SpO2 <94% at room air) | 25 (25.5) | 1 (14.2) | 24 (26.3) | 0.674 |
Arterial blood gas (ABG) and baseline laboratory investigations: We found no statistically significant difference in baseline characteristics in both groups [Table 1]. Diet and utilization of native medications didn’t predispose patients to hypocortisolemia [Table 1]. ABG lactate values were noted to be lower in the hypocortisolemic group as compared to the non-hypocortisolemic group. [Table 3] Sub-analysis of the baseline characteristics and laboratory findings of patients in shock is given in Table 4. The patients with septic shock and no hypocortisolemia had a higher lactate level and creatinine and lower platelet counts in comparison with those who had low cortisol levels. Low ACTH values were seen in 38 (38.8%) patients. The profile of these patients is elaborated in Table 5. Figure 2 demonstrates that the probability distribution of cortisol values was similar in the group of patients who were in shock versus the normotensive patients. The medians also lie close to each other with an almost similar interquartile range. This is supported by an insignificant P value (0.698).
Table 3.
Arterial blood gas and baseline laboratory investigations at presentation
| Variables | Frequency (Overall) n-98 (%) | Hypocortisolemic group S. Cortisol <10 µg/dl n-7 (7.2%) | Non-hypocortisolemic group S. Cortisol: 10–25 n-16 (16.3%) | Non-hypocortisolemic group S. Cortisol >25 n- 75 (76.5%) | P-value |
|---|---|---|---|---|---|
| pH (SD!) | 7.29 (0.2) | 7.37 (0.1) | 7.33 (0.1) | 7.28 (0.2) | 0.388 |
| Lactates (Median – IQR) – mmol/l | 3.25 (2.0–7.2) | 2.20 (1.2–2.5) | 2.9 (1.5–5.5) | 3.7 (2.2–8.0) | 0.047 |
| PF ratio (Median – IQR) | 380 (295–400) | 350 (350–400) | 395 (300–400) | 350 (280–400) | 0.807 |
| Total Counts (Median – IQR)/cumm | 13200 (7950–20850) | 19950 (16625–25475) | 12950 (6575–22475) | 11500 (7600–19800) | 0.109 |
| Neutrophil (Median – IQR@)/cumm | 85 (76.8–90.0) | 86 (78–87.0) | 81.0 (74.0–89.0) | 86.0 (76.0–91.0) | 0.529 |
| Lymphocyte (Median – IQR@)/cumm | 8 (4–13.3) | 7 (2.00–10.0) | 8.50 (3.3–16.3) | 8 (4–13) | 0.501 |
| Platelets (Median – IQR@)/cumm | 138000 (67000–274000) | 296000 (240500–505500) | 128500 (63750–226500) | 121000 (60000–262500) | 0.044 |
| Creatinine (Median – IQR@) mg% | 1.88 (1.2–3.3) | 1.73 (1.1–3.7) | 1.67 (1.2–3.4) | 1.89 (1.3–3.2) | 0.830 |
| Total bilirubin (Median – IQR@) mg/dl | 1.29 (0.6–3.6) | 0.58 (0.5–1.2) | 1.42 (0.6–4.8) | 1.34 (0.6–3.9) | 0.298 |
| ACTH# (Median – IQR@) | 58.00 (21.3–142.5) | 25.95 (19.5–131.7) | 67.00 (24.1–208.0) | 67 (24.1–174) | 0.046 |
| ACTH# <46 | 43 (43.8%) | 5 (5.1%) | 11 (11.2%) | 27 (27.5%) | 0.018 |
SD!: Standard Deviation, IQR@: Interquartile range, ACTH#: Adrenocorticotropic hormone
Table 4.
Baseline characteristics, arterial blood gas, and laboratory investigations in patients presenting with septic shock
| Septic shock n – 63 (64.3%) | Hypocortisolemic group n-4 | Non-Hypocortisolemic group n-59 | P-value |
|---|---|---|---|
| Age* | 59.2±12.2 | 51.5±16.3 | 0.324 |
| Sex – Male | 2 (50%) | 36 (61.7%) | 1.000 |
| Use of native medications | 1 (25%) | 6 (10.2%) | 0.383 |
| Dietary habits (Nonvegetarian) | 1 (25%) | 57 (96.6%) | 0.181 |
| ABG – PF ratio* | 375.0±28.0 | 337.9±101.8 | 0.646 |
| ABG – Lactates (mmol/l)* | 2.6±1.3 | 5.35±3.86 | 0.171 |
| Creatinine (mg%)* | 2.04±1.25 | 2.26±1.56 | 0.778 |
| Platelet count (/cumm)* | 413000±221975 | 144709±126266 | 0.024 |
| Total counts (/cumm)* | 22666±5400 | 17262±22662 | 0.113 |
| Total bilirubin (mg/dl)* | 0.63±0.25 | 3.32±5.30 | 0.093 |
ABG - PF: Mean and standard deviation*
Table 5.
Characteristics of patients with adequate cortisol response but low adrenocorticotropic hormone
| Variables | Frequency: n-38 (38.8%) |
|---|---|
| Age (years)# | 50.9±17.1 |
| Sex (Males) | 21 (55.3%) |
| Use of native medications | 5 (13.2) |
| Dietary habits (Nonvegetarian) | 37 (97.4%) |
| Shock (Systolic BP <90 mmHg) | 26 (68.4%) |
| Arterial Blood Gas – pH# | 7.34±0.16 |
| Arterial lactates (mmol/l)* | 2.80 (1.75–6.15) |
| PF ratio* | 400 (333–400) |
| Total leukocyte counts (/cumm)* | 10100 (5950–19200) |
| Neutrophils (/cumm)* | 82.5 (75.7–87.2) |
| Platelets (/cumm)* | 1,51,500 (51,500–2,93,750) |
| Creatinine – (mg%)* | 1.71 (1.15–2.64) |
| Total bilirubin (mg/dl)* | 0.86 (0.52–2.62) |
#Mean and standard deviation, *Median, and IQR
Figure 2.
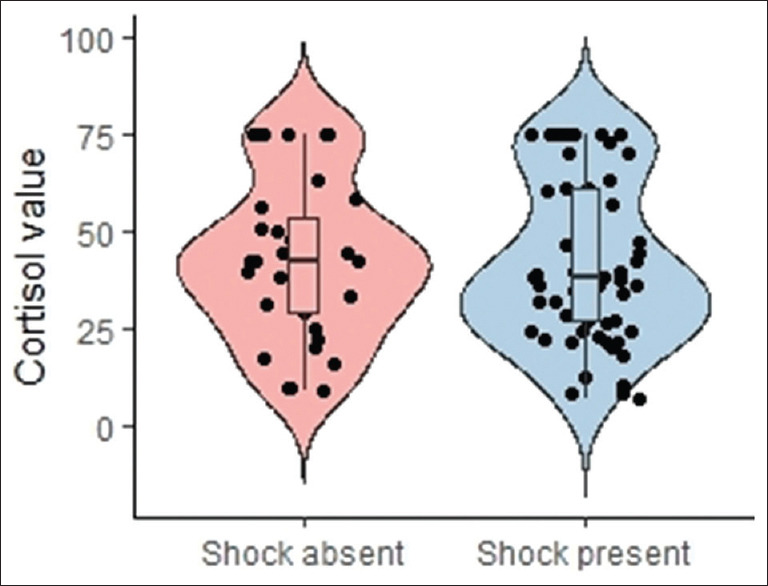
Distribution of cortisol values in patients in shock versus the normotensive patients
Clinical diagnosis in ED and outcome: Initial diagnosis based on the clinical features in ED were respiratory tract infection (n-21; 21.4%), acute febrile illnesses (n-9; 9.2%), acute gastroenteritis (n-5; 5.1%), urosepsis (n-14; 14.3%), acute abdomen (perforation/gastrointestinal bleeding: n-8; 8.2%), cholangitis (n-10; 10.2%), soft tissue infection (necrotizing/non-necrotizing: n-16; 16.3), undifferentiated illness (n-5; 5.1%), and previous surgical site infection (n-2; 2.1%). The ED team was involved in the resuscitation and management of all the patients following which patients were handed over to the respective medical or surgical units. ED disposition was as follows: 77.6% (n = 76) of patients warranted an admission, which included almost all who had hypocortisolemia (n-6; 85.7%). Despite the need for admission, 17 (17.3%) patients were discharged against medical advice. Out of those admitted, the majority (n-49; 64.5%) were discharged in a stable hemodynamic condition. The ED mortality rate was 6.1% (n = 6), while the in-hospital mortality rate was 15.3% (n = 15). [Figure 1] We have included the hospital outcome, however, follow-up and assessment of duration of ICU/hospital stay were not a part of the study objectives.
DISCUSSION
This study describes the prevalence of hypocortisolemia in patients with sepsis and septic shock, in a South Indian tertiary care ED setup and its correlation with practices of food and lifestyle that are specific to our population. There are many uncertainties as the cortisol cut-off values to diagnose adrenal insufficiency are still debated. The patients we studied had a qSOFA of more than 2 and were primarily triaged to priority 1. The large majority were nonvegetarians who helped assess its relevance in hypocortisolemia. However, we noted no association between them. This was studied because the use of anabolic steroids in the rearing of animals has been postulated to be one of the reasons for relative adrenal insufficiency in the Indian population. A subset of the study population had a history of native medication intake. The relevance of this information lies in the fact that there is a debate and evaluation of these medicines for containing steroids with documentation of steroid-induced Cushing’s after usage of the same.[13,14] However, given the small sample size and limitations of this study, it is not possible to affirmatively conclude that using alternative medicines does not predispose to a relative cortisol insufficiency. Studies have shown evidence of the mixing of steroids with indigenous medicines as well.[15] Chang SS et al.[16] showed that hypocortisolemic patients were often herbal medicine users, however, a similar finding was not seen in our study. Ragavendra Suresh et al.[12] demonstrated a hypocortisolemic prevalence in critically ill patients of 48.3%, considering the cut-off value of random basal cortisol as ≤24 μg/dl. In our study, we took the random cortisol cut-off value as <10 μg/dl following the SCCM and ESICM 2017 criteria.[6] On sub-analysis with a cut-off of ≤24 μg/dl, the percentage of patients with hypocortisolemia was only 22.4% (n-22). Contrary to a few other Indian studies, the prevalence of hypocortisolemia was found to be low in our study.[12] The evaluated difference may be due to the cut-off value taken into consideration and the fact that most other studies had very low sample sizes. There may be a general gross overestimation of the prevalence of hypocortisolemia in sepsis in the Indian setting. All patients with hypocortisolemia were admitted and most were discharged stable. A minority of individuals within our study cohort chose to be discharged against medical advice, primarily due to factors such as inadequate family support, unfavorable prognosis, financial limitations, and a preference for treatment at a particular hospital. Patients who were discharged stable had a much lesser serum cortisol value at presentation than those who died in the hospital. The association between high cortisol and high mortality is seen here just as it was in studies done by Rocío De Castro et al.[17] and Susan Sam et al.[18]
To identify the nature of HPA axis suppression in sepsis, ACTH values were simultaneously analyzed. ACTH values were below normal in most of the patients with low cortisol levels, implying a significant secondary adrenal insufficiency. Hence the HPA axis suppression in sepsis-induced adrenal insufficiency is central, which is consistent with what has been studied.[7,19] Hélène Prigent et al.[20] have described that the multifactorial nature of the insults in sepsis may cause both primary and secondary adrenal insufficiency.
There was a subgroup of patients who have mounted an adequate cortisol response despite a low ACTH value. This is unusual considering the postulated physiology of sepsis. Our hypothesis in this situation is of a local irritant or inflammatory response from the structures around the adrenal gland that are causing adrenal stimulation and hormone secretion. One more finding was that the mean creatinine among these patients was not normal: 1.71 mg%, which may be in keeping with the postulation. This is a hypothesis that would need further evaluation and assessment. Doing an ACTH stimulation test would have been ideal but is tedious, especially amidst treating patients in septic shock in ED, hence was not included in the study protocol.
This study throws light on the probable overestimation of hypocortisolemia in sepsis, in Indian patients and that there isn’t much difference because of our culture and food habits.
Limitation: A larger sample size would give a better comparable statistical outcome. A multivariate logistic regression analysis was not performed since no variable had a significant P value in the univariate analysis, which may have been related to the limited sample size in this study. A good number of the recruited patients who left against medical advice could not be included in the final analysis. It is a single-center study, and more studies will be required in determining and understanding the response of cortisol to stressors in the general Indian population.
Highlights: This study has prospectively assessed the prevalence of hypocortisolemia in sepsis and septic shock in a tertiary care center in India. It might negate the theory stating increased prevalence of the same, secondary to alternative medicine use and steroids in poultry. Adrenal insufficiency was seen in 7.2% of the study population, most of which was secondary as ACTH was also measured to be low. High cortisol and lactates predisposed to higher mortality.
CONCLUSION
The prevalence of hypocortisolemia was lower as compared to other Indian studies.[3,12] Cultural aspects, diet, and medication use do not predispose Indians to hypocortisolemia. High cortisol and lactate values predispose to higher mortality. The adrenal insufficiency was secondary, and there was also an unusual subset with normal cortisol but low ACTH levels.
Research quality and ethics statement
The authors of this publication declare that this scientific work follows the EQUATOR Network’s reporting quality, formatting, and reproducibility requirements. The authors further state that this clinical study was initiated after approval from the Institutional Evaluation Board/Ethics Committee review and that the protocol/approval number is (IRB Min no: 12809 dated January May, 2020). We also certify that the contents of this submission have not been plagiarized and that we have conducted a plagiarism check.
Financial support and sponsorship
Fluid research grant of Christian Medical College, Vellore.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Silverman MN, Pearce BD, Biron CA, Miller AH. Immune modulation of the hypothalamic-pituitary-adrenal (HPA) axis during viral infection. Viral Immunol. 2005;18:41–78. doi: 10.1089/vim.2005.18.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsigos C, Kyrou I, Kassi E, Chrousos GP. Stress: Endocrine physiology and pathophysiology. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, et al., editors. Endotext. South Dartmouth (MA): MDText.com, Inc; 2020. [Google Scholar]
- 3.Maqbool M, Shah ZA, Wani FA, Wahid A, Parveen S, Nazir A. Prevalence of occult adrenal insufficiency and the prognostic value of a short corticotropin stimulation test in patients with septic shock. Indian J Crit Care Med. 2009;13:85–91. doi: 10.4103/0972-5229.56054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NAGMSC Working Group on Sepsis: Final Report. In: National Institute of General Medical Sciences NIH, editor. Available from: https://www.nigms.nih.gov/News/reports/Documents/nagmsc-working-group-onsepsis-final-report.pdf. NIH; 2019 .
- 5.Chatterjee S, Bhattacharya M, Todi SK. Epidemiology of adult-population sepsis in India: A single center 5 year experience. Indian J Crit Care Med. 2017;21:573–7. doi: 10.4103/ijccm.IJCCM_240_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Annane D, Pastores SM, Rochwerg B, Arlt W, Balk RA, Beishuizen A, et al. Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (Part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Crit Care Med. 2017;45:2078–88. doi: 10.1097/CCM.0000000000002737. [DOI] [PubMed] [Google Scholar]
- 7.Marino LO, Souza HP. Dysfunction of the hypothalamic-pituitary-adrenal axis in critical illness: A narrative review for emergency physicians. Eur J Emerg Med. 2020;27:406–13. doi: 10.1097/MEJ.0000000000000693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouachour G, Tirot P, Gouello JP, Mathieu E, Vincent JF, Alquier P. Adrenocortical function during septic shock. Intensive Care Med. 1995;21:57–62. doi: 10.1007/BF02425155. [DOI] [PubMed] [Google Scholar]
- 9.Marik PE, Pastores SM, Annane D, Meduri GU, Sprung CL, Arlt W, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients:consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med. 2008;36:1937–49. doi: 10.1097/CCM.0b013e31817603ba. [DOI] [PubMed] [Google Scholar]
- 10.Téblick A, Langouche L, Van den Berghe G. Anterior pituitary function in critical illness. Endocr Connect. 2019;8:R131–43. doi: 10.1530/EC-19-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George AS, Ganesan P, Christopher J, Paul S, Abhilash KP. A review of triage practices and evolution of Christian Medical College, Vellore triage system (CMCTS) during the COVID-19 pandemic. Curr Med Issues. 2021;19:292–9. [Google Scholar]
- 12.Suresh R, Wig N, Panda PK, Jyotsna VP, Chaturvedi PK, Pandey RM. Serum cortisol level in Indian patients with severe sepsis/septic shock. J Emerg Trauma Shock. 2017;10:194–8. doi: 10.4103/JETS.JETS_123_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hendarto H. Atlantis Press; 2017. [[Last accessed on 2022 Mar 31]]. Iatrogenic Cushing's syndrome caused by treatment with traditional herbal medicine, a case report. Available from: https://www.atlantis-press.com/proceedings/ichlas-17/25886992 . [Google Scholar]
- 14.Sazlina S, Zaiton A. Cushing's syndrome secondary to adulterated complementary and alternative medicine. Malays Fam Physician Off J Acad Fam Physicians Malays. 2009;4:94–7. [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou T, Zhou P, Hua H, Liu X. Beneficial effects and safety of corticosteroids combined with traditional Chinese medicine for pemphigus: A systematic review. Evid Based Complement Alternat Med. 2015;2015:e815358. doi: 10.1155/2015/815358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang S-S, Liaw S-J, Bullard MJ, Chiu T-F, Chen J-C, Liao H-C. Adrenal insufficiency in critically ill emergency department patients: A Taiwan preliminary study. Acad Emerg Med. 2001;8:761–4. doi: 10.1111/j.1553-2712.2001.tb00202.x. [DOI] [PubMed] [Google Scholar]
- 17.Castro RD, Ruiz D, Lavín B-A, Lamsfus JÁ, Vázquez L, Montalban C, et al. Cortisol and adrenal androgens as independent predictors of mortality in septic patients. PLoS One. 2019;14:e0214312. doi: 10.1371/journal.pone.0214312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sam S, Corbridge TC, Mokhlesi B, Comellas AP, Molitch ME. Cortisol levels and mortality in severe sepsis. Clin Endocrinol (Oxf) 2004;60:29–35. doi: 10.1111/j.1365-2265.2004.01923.x. [DOI] [PubMed] [Google Scholar]
- 19.Arafah BM. Hypothalamic pituitary adrenal function during critical illness: Limitations of current assessment methods. J Clin Endocrinol Metab. 2006;91:3725–45. doi: 10.1210/jc.2006-0674. [DOI] [PubMed] [Google Scholar]
- 20.Prigent H, Maxime V, Annane D. Science review: Mechanisms of impaired adrenal function in sepsis and molecular actions of glucocorticoids. Crit Care Lond Engl. 2004;8:243–52. doi: 10.1186/cc2878. [DOI] [PMC free article] [PubMed] [Google Scholar]