Abstract
Transwomen frequently undergo androgen deprivation therapy (ADT) incorporated with oestrogen, but they are still prone to the occurrence of prostatic cancer since the prostate remains intact. The probability of this clinical condition reduces as compared with the general male population. This study aimed to study the occurrence of prostatic malignancy under hormonal therapy such as ADT in transwomen. An extensive literature search was performed using online searches on transgender health, centring on the incidence, diagnosis, treatment and management of prostate cancer in transgender women. Original articles from 1975 to 2022 were searched using PubMed, Scopus, EMBASE, DOAJ and Cochrane databases. Physical, mental and communal deliberation of health development is the major constituent of trans-health. It exhibits a fivefold reduction in prostatic malignancies in transwomen undergoing hormonal therapy contrasted with the extensive male community of indistinguishable age.
Keywords: Androgen deprivation therapy, prostate cancer, transgender
INTRODUCTION
Transgender and gender-variant individuals constitute approximately 0.3–0.5% of the world population, and the number of people recognising this community is constantly increasing.[1]
An incompatibility between gender allocation and sex demonstration is experienced by them.[2] The people allocated as male at birth are known as cis-male, and the ones recognise as female are known as transwomen.[3] Pharmaceutical therapy for the alignment of corporeal features with the accomplished gender may be opted for by transgender people. This involved gender-affirming surgery and gender-affirming hormone therapy. For transgender women, the antiandrogen incorporated with oestrogen constitutes hormonal therapy.[4] There may be probability and intricacies associated with the gender-affirming hormonal therapy such as blood clot formation in the veins and the maturation of sex steroid-associated malignancies,[4,5,6] but gender-affirming hormone therapy is normally contemplated by bilateral castration, frequently integrated with colpoplasty and the augmentation mammoplasty, which together constitute the gender-affirming surgical procedures.[5,6,7] Due to the occurrence of probable remarkable issues such as involuntary urination, prostatectomy is not performed in the course of gender-affirming surgery despite the prostate being biologically male viscera.[8] Hence, the possibility of prostatic ailment prevails even after the surgery in transgender women. The collusion of the androgen and the sex steroids in the origination and development of prostatic malignancies has led to various suppositions due to the biological province of prostate cells on androgen for operation and expansion.[8,9] Androgen deprivation therapy (ADT) is adapted for unhurried development of the ailment in the progressed or enhanced prostate cancer.[10] There is an absence of any relation between the internal level of serum testosterone and the occurrence of prostatic malignancies, exhibited by large meta-analyses. It also exhibits the relation with the incidence of prostatic malignancies in incompetent male with testosterone replacement therapy.[11] Cis-men detected with progressive prostatic malignancy undergo androgen replacement therapy. The therapy is employed with different demonstrations such as to check sex impulse in individuals with critical paraphilias.[12] However, there are very less studies available about the treatment of androgen deprivation in the prevalence of prostate cancer.
The principal objective of this article was to study the prevalence of prostate cancer in transwomen undergoing hormone therapy such as ADT.
MATERIAL AND METHODS
An extensive literature search was performed using online searches on transgender health, centring on the incidence, diagnosis, treatment and management of prostate cancer in transgender women. Original articles from 1975 to 2022 were searched using PubMed, Scopus, EMBASE, MedlinePlus, WebMD, Google Scholar, ScienceDirect, EMBASE, BioMed Central, Web of Science, Medscape, DOAJ and Cochrane databases.
Role of androgen in prostate cancer
ADT is crucial in effectively impeding the progression of cancerous prostate cells by reducing gene expression, simultaneous tumour regression and prostate-specific antigen (PSA) levels. This is because androgens are accountable for the survival of malignant prostate cells.[13] It could be contended that the prevalence of prostate cancer is not solely impacted by androgen deprivation but also by the utilisation of oestrogens. Oestrogens play a part in reducing androgens by inhibiting the hypothalamic–pituitary–gonadal axis and were first employed in the treatment of prostate cancer before luteinising hormone-releasing hormone agonists were introduced.[10] Nevertheless, some hypotheses propose that oestrogens may also have a stimulating effect on the development of prostate cancer.[11,12] The therapy of androgen deprivation has the capability to repress the majority of prostate cancers; however, certain high-risk prostate cancers gradually advance to castration-resistant prostate cancer (CRPC), which can thrive even when androgen levels are castrated. Metastatic CRPC presents androgen receptor (AR) as the most commonly anomalous gene.[13] The development of CRPC is based on several mechanisms:
Amplification of androgen receptor (AR) gene
One-way cells compensate for the loss of a crucial cellular pathway is by over-activating or expressing an integral protein within that pathway. In the context of prostate cancer and AR signalling, cancerous cells have been found to overexpress AR at both the mRNA and protein levels in vitro and in vivo models.[14,15,16] Research has revealed that nearly 25–30% of AIPCs exhibit AR genetic amplifications.[16] Such genetic amplifications have not been observed in cases where ADT was not administered, which further supports the notion that AR gene amplification is a typical by-product of hormone therapy. Increased AR expression at both the mRNA and protein levels has been demonstrated to make cancer cells more sensitive to lower-than-normal levels of androgens.[17] Although ADT can effectively reduce endogenous androgen production, it does not completely halt its production. Thus, even a small amount of androgens can activate the AR, theoretically speaking. With excessive AR expression via genetic amplification, even minute amounts of androgens can activate the protein, leading to downstream signalling. Notably, AR overexpression at the protein and mRNA levels has also been observed without AR gene amplifications, which suggests that other modes of AR regulation, such as epigenetic factors and miRNAs, may be involved.[18] Clinical studies have clearly shown that AR overexpression is a common occurrence during the development of AIPC. Therefore, therapies targeting the blocking of AR expression or signalling cascade could potentially be used for clinical purposes.
AR gene mutations
The incidence of AR mutations is quite limited in individuals having initial-stage tumours.[19] Nevertheless, in cases of androgen-independent prostate cancer, the incidence increases to 10–20% in advanced-stage or malignant tumours.[20] This reinforces the idea that AR mutations serve as a widespread mechanism that prostate cells can exploit to acquire androgen-independent features.
AR gene co-regulators
Numerous proteins have been recorded as serving as co-regulators with AR.[21] Any alterations in the balance of these proteins can have a significant impact on the overall expression of AR-regulated genes. Some of the extensively researched coactivators of AR signalling are TIF2, GRIP1, SRC1 and a wide group referred to as AR-associated (ARA) proteins.[22,23] It was discovered by Gregory et al.[24] that levels of TIF2 and SCR1 were raised in AIPC samples that also had augmented AR expression. Conversely, NCoR and SMRT are two of the most common AR co-repressors.[25] These proteins are both capable of enlisting histone deacetylases, leading to chromatin condensation and reduced transcriptional activity.[25] During the development of AIPC, both of these corepressors have been shown to be downregulated, resulting in increased AR-mediated transcriptional activity.[26]
No reciprocation is elicited by the CRPC to the androgen ablation.[27,28,29,30,31] Demonstration of the PSA gene takes place during this stage,[29,30] and on selected repression of the AR, the PSA expression reduces and the generation of cells also reduces. This gives evidence of the generation and persistence of CRPC prostate malignant cells consistently through atypical AR invigoration.[29]
Effect of ADT in transgender women
There is a significant reduction probability of prostatic malignancies in transgender women acquiring ADT and oestrogen as compared with the extensive male population.[32] The outcomes promote the inference that there is an inhibitory consequence of ADT on the incorporation and growth of prostate cancer. Antiandrogen integrated with oestrogens is usually safe but may lead to complications such as blood clot formation and growth of sex steroid-associated malignancies (e.g., breast cancer).[5] A study exhibits a fivefold reduction in the probability of prostatic malignancies in transgender women receiving hormonal therapy such as ADT as compared to a male population of similar age.[32] According to our study, transgender women receiving ADT require further research.
Investigational approach and treatment for prostate cancer in transgender women
The remedy for prostatic malignancies should be similar to the remedy for cis-men in transgender women who had not undergone gender-affirming surgery. The extent of the prostate decreases remarkably after GAHT.[33] The vaginoplasty is attainable followed by radiotherapy, radical prostatectomy or brachytherapy.
For transgender women who earlier endured GAS, focal therapy is a probable choice as it has the merit of decreased ramification in contrast to radical therapy choices.[34]
Comprehensively, there is no convenient choice for the remedy of confined prostatic malignancy in a patient. A recommended accession with asserted prostate cancer in a transgender woman is manifested in the flow diagram [Figure 1].[35,36]
Figure 1.
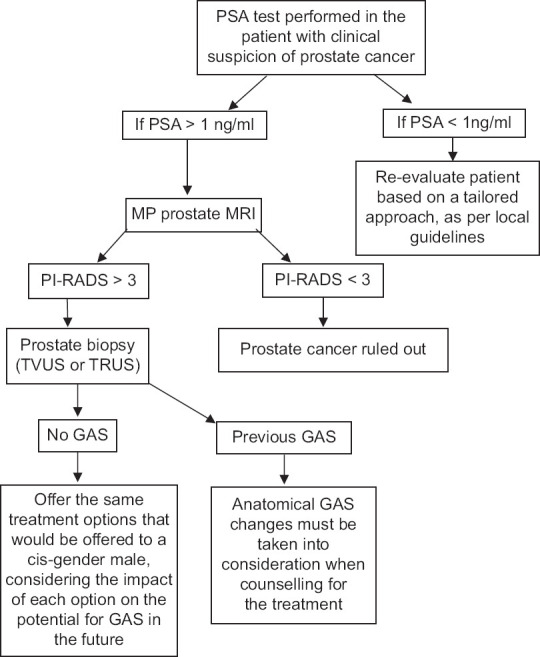
Investigational approach and treatment for prostate cancer in transgender women
CONCLUSION
The physical, psychological and social considerations involved in promoting health are crucial components of trans-health. All healthcare professionals should have knowledge of the healthcare needs of the transgender community. This research shows a significant fivefold decrease in prostate cancer in transwomen undergoing hormone therapy compared with the wider male population of the same age. This suggests that ADT has a suppressive effect on the initiation and growth of prostate cancer. Currently, abiraterone, cabazitaxel and enzalutamide are emerging as treatments, changing the way hormone-resistant cancers are treated. Therefore, transgender women should be aware of the possibility of developing prostate cancer despite lower levels of serum androgens.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Arcelus J, Bouman WP, Van Den Noortgate W, Claes I, Witcomb G, Fernandez-Arada F. Systematic review and meta-analysis of prevalence studies in transsexualism. Eur Psychiatry. 2015;30:807–15. doi: 10.1016/j.eurpsy.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 3.Wiepjes CM, Nota NM, de Blok CJM, Klaver M, de Vries ALC, Wensing-Kruger SA, et al. The Amsterdam Cohort of Gender Dysphoria Study (1972-2015): Trends in prevalence, treatment, and regrets. J Sex Med. 2018;15:582–90. doi: 10.1016/j.jsxm.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 4.Wierckx K, Van Caenegem E, Schreiner T, Haraldsen I, Fisher AD, Toye K, et al. Cross-sex hormone therapy in trans persons is safe and effective at short-time follow-up: Results from the European network for the investigation of gender incongruence. J Sex Med. 2014;11:1999–2011. doi: 10.1111/jsm.12571. [DOI] [PubMed] [Google Scholar]
- 5.den Heijer M, Bakker A, Gooren L. Long term hormonal treatment for transgender people. BMJ. 2017;359:j5027. doi: 10.1136/bmj.j5027. [DOI] [PubMed] [Google Scholar]
- 6.de Blok CJM, Wiepjes CM, Nota NM, van Engelen K, Adank MA, Dreijerink KMA, et al. Breast cancer risk in transgender people receiving hormone treatment: Nationwide cohort study in the Netherlands. BMJ. 2019;365:l1652. doi: 10.1136/bmj.l1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The World Professional Association for Transgender Health. Standards of Care for the Health of Transsexual, Transgender, and Gender Non Conforming People. Version 7. The World Professional Association for Transgender health; 2012 [Google Scholar]
- 8.Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349:366–81. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 9.Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–7. [Google Scholar]
- 10.Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: Treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71:630–42. doi: 10.1016/j.eururo.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Prins GS, Calderon-Gierszal EL, Hu WY. Stem cells as hormone targets that lead to increased cancer susceptibility. Endocrinology. 2015;156:3451–7. doi: 10.1210/en.2015-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonkhoff H. Estrogen receptor signaling in prostate cancer: Implications for carcinogenesis and tumor progression. Prostate. 2018;78:2–10. doi: 10.1002/pros.23446. [DOI] [PubMed] [Google Scholar]
- 13.Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–28. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown RS, Edwards J, Dogan A, Payne H, Harland SJ, Bartlett JM, et al. Amplification of the androgen receptor gene in bone metastases from hormone-refractory prostate cancer. J Pathol. 2002;198:237–44. doi: 10.1002/path.1206. [DOI] [PubMed] [Google Scholar]
- 15.Edwards J, Krishna NS, Grigor KM, Bartlett JM. Androgen receptor gene amplification and protein expression in hormone refractory prostate cancer. Br J Cancer. 2003;89:552–6. doi: 10.1038/sj.bjc.6601127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koivisto P, Kononen J, Palmberg C, Tammela T, Hyytinen E, Isola J, et al. Androgen receptor gene amplification: A possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 1997;57:314–9. [PubMed] [Google Scholar]
- 17.Waltering KK, Helenius MA, Sahu B, Manni V, Linja MJ, Jänne OA, et al. Increased expression of androgen receptor sensitizes prostate cancer cells to low levels of androgens. Cancer Res. 2009;69:8141–9. doi: 10.1158/0008-5472.CAN-09-0919. [DOI] [PubMed] [Google Scholar]
- 18.Powell SM, Christiaens V, Voulgaraki D, Waxman J, Claessens F, Bevan CL. Mechanisms of androgen receptor signalling via steroid receptor coactivator-1 in prostate. Endocr Relat Cancer. 2004;11:117–30. doi: 10.1677/erc.0.0110117. [DOI] [PubMed] [Google Scholar]
- 19.Newmark JR, Hardy DO, Tonb DC, Carter BS, Epstein JI, Isaacs WB, et al. Androgen receptor gene mutations in human prostate cancer. Proc Natl Acad Sci U S A. 1992;89:631923. doi: 10.1073/pnas.89.14.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taplin ME, Bubley GJ, Shuster TD, Frantz ME, Spooner AE, Ogata GK, et al. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N Engl J Med. 1995;332:1393–8. doi: 10.1056/NEJM199505253322101. [DOI] [PubMed] [Google Scholar]
- 21.Heemers HV, Tindall DJ. Androgen receptor (AR) coregulators: A diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev. 2007;28:778–808. doi: 10.1210/er.2007-0019. [DOI] [PubMed] [Google Scholar]
- 22.Bennett NC, Gardiner RA, Hooper JD, Johnson DW, Gobe GC. Molecular cell biology of androgen receptor signalling. Int J Biochem Cell Biol. 2009;42:813–27. doi: 10.1016/j.biocel.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Lemon B, Tjian R. Orchestrated response: A symphony of transcription factors for gene control. Genes Dev. 2000;14:2551–69. doi: 10.1101/gad.831000. [DOI] [PubMed] [Google Scholar]
- 24.Gregory CW, He B, Johnson RT, Ford OH, Mohler JL, French FS, et al. A mechanism for androgen receptor-mediated prostate cancer recurrence after androgen deprivation therapy. Cancer Res. 2001;61:4315–9. [PubMed] [Google Scholar]
- 25.Liao G, Chen LY, Zhang A, Godavarthy A, Xia F, Ghosh JC, et al. Regulation of androgen receptor activity by the nuclear receptor corepressor SMRT. J Biol Chem. 2003;278:5052–61. doi: 10.1074/jbc.M206374200. [DOI] [PubMed] [Google Scholar]
- 26.Godoy AS, Sotomayor PC, Villagran M, Yacoub R, Montecinos VP, McNerney EM, et al. Altered corepressor SMRT expression and recruitment to target genes as a mechanism that change the response to androgens in prostate cancer progression. Biochem Biophys Res Commun. 2012;423:564–70. doi: 10.1016/j.bbrc.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 28.Grossmann ME, Huang H, Tindall DJ. Androgen receptor signaling in androgen-refractory prostate cancer. J Natl Cancer Inst. 2001;93:1687–97. doi: 10.1093/jnci/93.22.1687. [DOI] [PubMed] [Google Scholar]
- 29.Dehm SM, Tindall DJ. Molecular regulation of androgen action in prostate cancer. J Cell Biochem. 2006;99:333–44. doi: 10.1002/jcb.20794. [DOI] [PubMed] [Google Scholar]
- 30.Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 31.Litvinov IV, De Marzo AM, Isaacs JT. Is the Achilles'heel for prostate cancer therapy a gain of function in androgen receptor signaling?J Clin Endocrinol Metab. 2003;88:2972–82. doi: 10.1210/jc.2002-022038. [DOI] [PubMed] [Google Scholar]
- 32.Gooren L, Morgentaler A. Prostate cancer incidence in orchidectomised male-to-female transsexual persons treated with oestrogens. Andrologia. 2014;46:1156–60. doi: 10.1111/and.12208. [DOI] [PubMed] [Google Scholar]
- 33.Jin B, Turner L, Walters WA, Handelsman DJ. The effects of chronic high dose androgen or estrogen treatment on the human prostate [corrected] [published correction appears in J Clin Endocrinol Metab 1997;82(2):413] Clin Endocrinol Metab. 1996;81:4290–5. doi: 10.1210/jcem.81.12.8954029. [DOI] [PubMed] [Google Scholar]
- 34.Weyers S, De Sutter P, Hoebeke S, Monstrey G, T’Sjoen G, Verstraelen H, et al. Gynaecological aspects of the treatment and follow-up of transsexual men and women. Facts Views Vis Obgyn. 2010;2:35–54. [PMC free article] [PubMed] [Google Scholar]
- 35.Guillaumier S, Peters M, Arya M, Afzal N, Charman S, Dudderidge T, et al. A multicentre study of 5-year outcomes following focal therapy in treating clinically significant nonmetastatic prostate cancer. Eur Urol. 2018;74:422–9. doi: 10.1016/j.eururo.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah TT, Peters M, Eldred-Evans D, Miah S, Yap T, Faure-Walker NA, et al. Early-medium-term outcomes of primary focal cryotherapy to treat nonmetastatic clinically significant prostate cancer from a prospective multicentre registry. Eur Urol. 2019;76:98–105. doi: 10.1016/j.eururo.2018.12.030. [DOI] [PubMed] [Google Scholar]


