Abstract
Background.
Local anesthesia is essential for pain control in dentistry. The authors assessed the comparative effect of local anesthetics on acute dental pain after tooth extraction and in patients with symptomatic irreversible pulpitis.
Types of Studies Reviewed.
The authors searched MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, and the US Clinical Trials registry through November 21, 2020. The authors included randomized controlled trials (RCTs) comparing long- vs short-acting injectable anesthetics to reduce pain after tooth extraction (systematic review 1) and evaluated the effect of topical anesthetics in patients with symptomatic pulpitis (systematic review 2). Pairs of reviewers screened articles, abstracted data, and assessed risk of bias using a modified version of the Cochrane risk of bias 2.0 tool. The authors assessed the certainty of the evidence using the Grading of Recommendations Assessment, Development and Evaluation approach.
Results.
Fourteen RCTs comparing long- vs short-acting local anesthetics suggest that bupivacaine may decrease the use of rescue analgesia and may not result in additional adverse effects (low certainty evidence). Bupivacaine probably reduces the amount of analgesic consumption compared with lidocaine with epinephrine (mean difference, −1.91 doses; 95% CI, −3.35 to −0.46; moderate certainty) and mepivacaine (mean difference, −1.58 doses; 95% CI, −2.21 to −0.95; moderate certainty). Five RCTs suggest that both benzocaine 10% and 20% may increase the number of people experiencing pain reduction compared with placebo when managing acute irreversible pulpitis (low certainty).
Practical Implications.
Bupivacaine may be superior to lidocaine with epinephrine and mepivacaine with regard to time to and amount of analgesic consumption. Benzocaine may be superior to placebo in reducing pain for 20 through 30 minutes after application.
Keywords: Short-acting local anesthetics, lidocaine, mepivacaine, articaine, long-acting local anesthetics, bupivacaine, benzocaine, post tooth extraction acute pain, symptomatic irreversible pulpitis
Local anesthesia for intraoperative pain control is an essential part of clinical practice in dentistry. An average dentist administers over 1,500 cartridges of dental local anesthetic per year.1 Local anesthesia is induced when propagation of action potentials has stopped such that sensation is not transferred from the source of stimulation (that is, tooth or periodontium) to the brain.2 Local anesthetics are classified according to the amide or ester linkages between the lipophilic group and a carbon chain.2 Bupivacaine, articaine, lidocaine, mepivacaine, and prilo-caine are local anesthetics of the amide classification.2 Benzocaine is a topical anesthetic of the ester classification.2
The pH of the tissue and the acid dissociation constant (pKa) of the drug are the most important factors affecting the onset and duration of action of local anesthetics.2 The onset of local anesthetics is delayed or even prevented when the pH decreases in sites of infection.2 There are no clinical differences in pKa among the amides, except for bupivacaine, which has a slightly higher pKa, leading to a slower onset of action.2 The duration of action of a local anesthetic is determined by the length of time that the drug spends in the nerve membrane to block the sodium channels.2 Injected local anesthetics cause vasodilation, which leads to a short duration of action intraorally when administered alone. This diffusion can be reduced by the addition of a vasoconstrictor such as epinephrine, available in formulations of 1:50,000, 1:100,000, and 1:200,000.2
In dentistry, long- and short-acting local anesthetics are used for intraoperative pain control and the management of postoperative pain, as in endodontic, periodontal, and oral surgical procedures.2 Topical anesthetics, such as benzocaine, have been prescribed to eliminate the need for needle insertion or for brief relief from pain caused by mucosal lesions or toothache in adults.3
A 2021 systematic review (SR) compared the different types of local anesthetics.4 The focus, however, was not on the comparison between long- vs short-acting local anesthetics, and the assessment of the certainty of evidence had important limitations.4 To our knowledge, there have been no high-quality SRs comparing different types of long- vs short-acting local anesthetics as well as SRs comparing benzocaine formulations to placebo.
Therefore, the first SR in our article aims to determine the effect of long- and short-acting local anesthetics for the management of acute pain after dental extractions (simple or surgical tooth extractions including impacted mandibular third-molar extractions) and temporary management of symptomatic pulpitis. The second SR addresses the effect of benzocaine compared with placebo for the management of acute pain associated with symptomatic irreversible pulpitis. These findings informed the recommendations of the upcoming evidence-based clinical practice guideline for the management of acute dental pain in adolescents and adults by the American Dental Association (ADA) Council on Scientific Affairs, the ADA Science and Research Institute, and the University of Pittsburgh’s and the University of Pennsylvania’s schools of dental medicine in partnership with the US Food and Drug Administration.
METHODS
This report follows the guidance of the preferred reporting items for SRs and meta-analyses checklist (eTable 1). We followed preestablished methodology outlined in the plan for guideline development and used eligibility criteria determined by the recommendation questions proposed by the guideline panel and outlined by the National Academy of Medicine’s Framing Opioid Prescribing Guidelines for Acute Pain: Developing the Evidence.5
Eligibility criteria
For both SRs, we only included articles published in the English language.
SR 1: Injected Local Anesthetics
We included randomized controlled trials (RCTs) comparing long-acting (that is, bupivacaine) to short-acting local (that is, lidocaine with epinephrine, articaine, and mepivacaine) anesthetics in adolescents and adults undergoing simple or surgical tooth extractions. The outcomes of interest included the use of rescue analgesia, time to analgesic consumption, amount of analgesic consumption, and adverse effects (for example, tissue trauma and prolonged paresthesia).
SR 2: Topical Local Anesthetics
We included RCTs comparing topical benzocaine doses head-to-head or against placebo (vehicle) in adolescents and adults with acute dental pain associated with symptomatic irreversible pulpitis. The outcomes of interest were the number of responders (that is, proportion of participants who had a reduced pain intensity score for at least 2 consecutive assessments measured at a follow-up time from 20–30 minutes), pain levels measured as the sum of pain relief combined with pain intensity difference at 60 minutes, and any adverse effects.
Information sources
We performed searches in MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, and the US Clinical Trials registry from inception through November 21, 2020. We conducted a broad search for both injectable and topical anesthetics (SR 1 and SR 2, respectively) for managing acute dental pain and concepts reflecting acute dental pain associated with tooth extraction and symptomatic irreversible pulpitis (eTable 2).
Study selection
Using Covidence software (Veritas Health Innovation), pairs of reviewers (A.M., S.I., M.A., Y.R., D.T., L.H.), after training and calibration exercises, independently screened titles and abstracts, followed by full texts of trials that we identified as potentially eligible. A third reviewer resolved conflicts (A.M.).
Data collection
For each eligible trial, pairs of reviewers (A.M., S.I., M.A., Y.R., D.T., L.H.), after training and calibration exercises, extracted data independently using a standardized, piloted data extraction form. Reviewers collected information on trial characteristics (for example, design, interventions, and comparisons) and participants (for example, age, sex, and country) and outcomes of interest. Reviewers resolved discrepancies via discussion and, when necessary, with final adjudication by a third reviewer.
Risk of bias of individual studies
For each eligible trial and outcome, reviewers, after training and calibration exercises, used a modified version of the Cochrane risk-of-bias tool for randomized trials (RoB 2, Version 2.0) and rated trials as at low risk of bias, probably low risk of bias, probably high risk of bias, or high risk of bias, across the following domains: bias arising from the randomization process; bias due to deviations from the intended intervention; bias due to missing data; bias due to outcome measurement; and bias in selection of the reported results. Reviewers resolved discrepancies via discussion and, when necessary, with final adjudication by a third reviewer.
Data synthesis
For dichotomous outcomes, we summarized the effect of interventions using risk ratio (relative effect). When the outcome incidence was low across studies (for example, there were no events in several study groups), we used risk difference. For continuous outcomes, we used mean difference (absolute effect). We calculated 95% CIs around all estimates. When studies reported the same outcome construct using a different scale with a different range, we converted the data to the scale most frequently reported across studies.6
For any outcome reported by more than 1 study, we conducted random-effect meta-analyses weighting studies according to the inverse of their variance, using the Cochrane Review Manager Version 5.4 (RevMan, Cochrane Collaboration) software. We used a wide approach to pooling and explored reasons if serious inconsistency (heterogeneity) was observed.
Certainty of the evidence
We assessed the certainty of evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. Two methodologists with experience in GRADE (A.M., S.I.) rated each domain for each comparison and outcome independently, resolving discrepancies via discussion. We rated the certainty as high, moderate, low, or very low, taking into consideration risk of bias, inconsistency (also known as heterogeneity),7 indirectness, publication bias, and imprecision. We used a minimally contextualized approach with a null effect threshold to rate the certainty that there is a benefit or a harm.8 When the point estimate was close to the null effect, we rated our certainty that there was a trivial effect (that is, no important difference) using a threshold of 10% of the baseline risk for dichotomous outcomes and 10% of the scale range for continuous outcomes,9 For dichotomous outcomes pooled using risk ratio, we presented absolute estimates of effect using the mean baseline risk across the trials. We created GRADE summary of findings tables using GRADEpro (McMaster University and Evidence Prime).
Subgroup and sensitivity analyses
We pooled all studies comparing bupivacaine with any short-acting anesthetic (SR 1), and when we observed important heterogeneity, we performed subgroup analyses to assess whether the specific type of short-acting anesthetic (lidocaine with epinephrine, articaine, mepivacaine) could be the source of heterogeneity.7 We did not plan any subgroup analyses for benzocaine (SR 2). We did not plan any sensitivity analyses.
RESULTS
SR 1: Long-acting vs short-acting injected local anesthetics
After screening 4,716 titles and abstracts, we included 14 RCTs (Figure10).11–24 Reasons for exclusion at the full-text screening stage (n = 844) are presented in the Figure. Dosages of long- and short-acting local anesthetics used in included studies are presented in Table 1.
Figure.
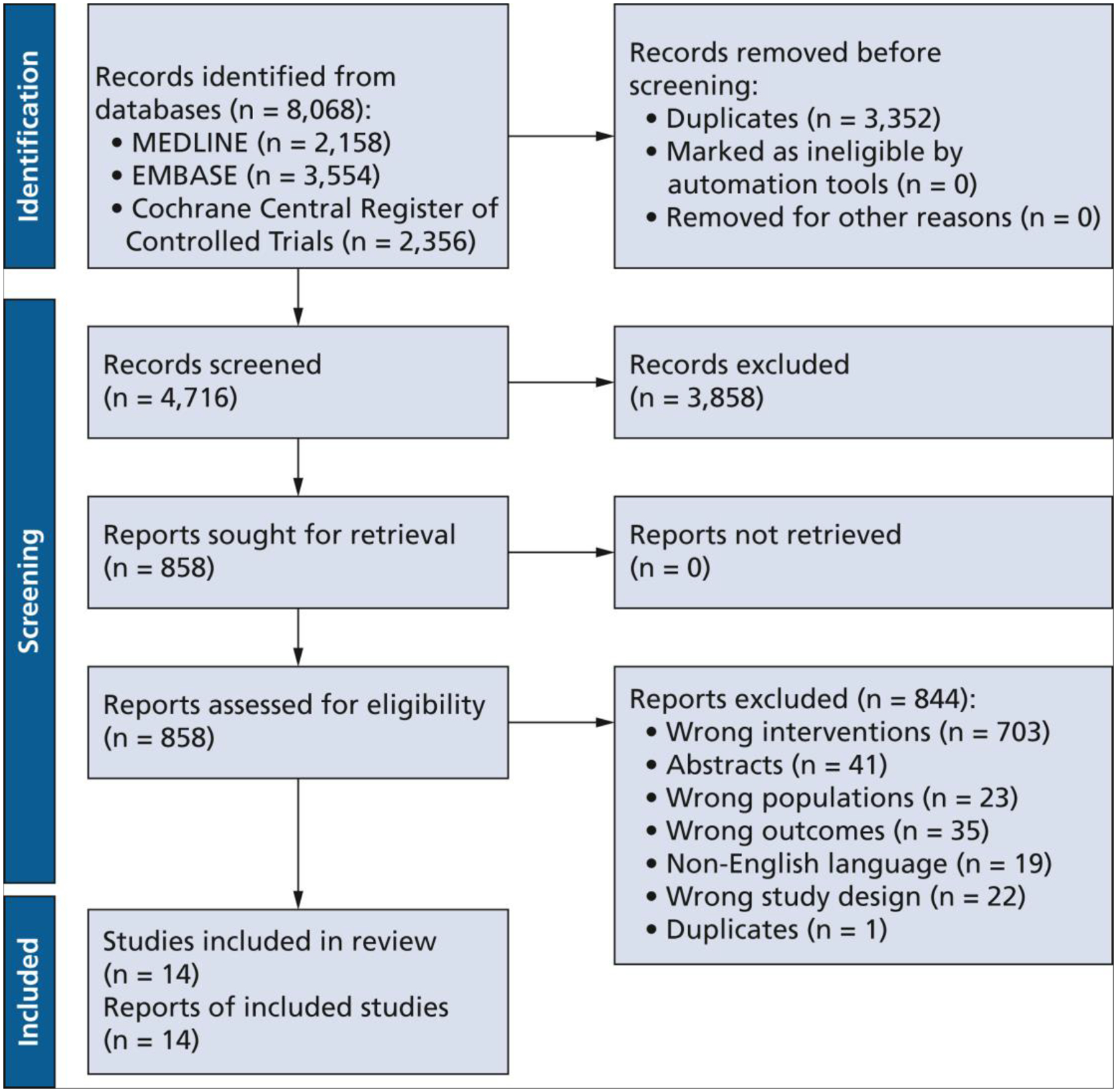
Study identification and selection flowchart of the studies including long-acting and short-acting local anaesthetics, following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 statement.10
Table 1.
Characteristics of the systematic review 1 studies comparing long-acting with short-acting local anesthetics.
| STUDY, YEAR | STUDY DESIGN | COUNTRY | PARTICIPANTS RANDOMIZED, NO. | AGE, Y, RANGE OR MEAN (SD) | SEX, FEMALE, % | TYPE OF EXTRACTION | LONG-ACTING ANESTHETIC | SHORT-ACTING ANESTHETIC |
|---|---|---|---|---|---|---|---|---|
| Trieger and Gillen,23 1979 | Parallel group | United States | 69 | 14–55 | Not reported | Surgical | (A) 0.5% bupivacaine with 1:200,000 epinephrine (B) 0.5% bupivacaine |
3% mepivacaine |
| Rosenquist and Colleagues,19 1988 | Split mouth | Hong Kong | 52 | 26.5 (not reported) | 50 | Surgical | 5 mg/mL of bupivacaine with 12.5 μg of epinephrine per mL | 20 mg/mL of lidocaine with 12.5 μg/mL of epinephrine |
| Hyrkas and Colleagues,15 1994 | Split mouth | Finland | 44 | 24.23 (2.94) | 59 | Surgical | 5 mg/mL of bupivacaine with 5 μg/mL of epinephrine | 20 mg/mL of lidocaine with 12.5 μg/mL of epinephrine |
| Bouloux and Punnia-Moorthy,12 1999 | Parallel group | Australia | 46 | 24 (not reported); range, 18–41 | 61 | Surgical | 0.5% bupivacaine with 1:200,000 epinephrine | 2% lidociane with 1:100,000 epinephrine |
| Markovic and Todorovi0ć,16 2006 | Split mouth | Serbia and Montenegro | 24 | Not reported | Not reported | Surgical | 0.5% bupivacaine | 2% lidocaine with 1:80,000 epinephrine |
| Gregorio and Colleagues,14 2008* | Split mouth | Brazil | 100 | 21.84 (4.60) | 58 | Surgical | 0.5% bupivacaine with 1:200,000 epinephrine | 4% articaine with 1:200,000 epinephrine |
| Trullenque-Eriksson and Guisado-Moya,24 2011 | Split mouth | Spain | 38 | 24.47 (not reported) | 68 | Surgical | 0.5% bupivacaine with 1:200,000 epinephrine | 4% articaine with 1:200,000 epinephrine |
| Sancho- Puchades and Colleagues,20 2012 | Split mouth | Spain | 36 | 23.8 (5) | 61.11 | Surgical | 0.5% bupivacaine with 1:200,000 epinephrine | 4% articaine with 1:200,000 epinephrine |
| Pellicer-Chover and Colleagues,18 2013 | Split mouth | Spain | 72 | 23.1 (6) | 66.66 | Surgical | 0.5% bupivacaine with 1:200,000 epinephrine | 4% articaine with 1:100,000 epinephrine |
| Thakare and Colleagues,21 2014 | Split mouth | India | 80 | 10–18 | Not reported | Surgical | 0.5% bupivacaine (no mention of addition of epinephrine) | 4% articaine (no mention of addition of epinephrine) |
| Brajkovic and Colleagues,13 2015 | Parallel group | Serbia | 57 | 23.7 (3.76) | 64.91 | Surgical | 0.5% bupivacaine | 2% lidocaine with 1:80,000 epinephrine |
| Olmedo-Gaya and Colleagues,17 2018 | Parallel group | Spain | 50 | 21.6 (5.86) | 48.00 | Surgical | 0.5% bupivacaine with 1:200,000 epinephrine | 4% articaine with 1:100,000 epinephrine |
| Adelusi and Colleagues,11 2019 | Parallel group | Nigeria | 252 | Not reported | Not reported | Simple (intra-alveolar) | 0.5% bupivacaine with 1:200,000 epinephrine | 2% lidocaine with 1:100,000 epinephrine |
| Tijanic and Buric,22 2019 | Parallel group | Serbia | 60 | 26.4 (3.32) | 51.67 | Surgical | 0.5% bupivacaine | 2% lidocaine with 1:100,000 epinephrine |
Gregorio and colleagues, 2008A included patients who underwent surgery with osteotomy; Gregorio and colleagues, 2008B included patients who underwent surgery without osteotomy.
Characteristics of Included Studies
The number of participants in the included studies ranged from 24 through 252. Fifty-seven percent of RCTs used a split mouth design. Mean (SD) age across studies ranged from 21.6 (5.86) through 26.5 (not reported) years. Participants underwent surgical tooth extractions in all studies except for 111 (Table 1).
Risk of Bias in Included Studies
The risk of bias domains judged as high or probably high risk of bias most frequently across included studies were bias arising from the randomization process and selective reporting of results (eTable 3).
Effects of Interventions
Proportion of participants requiring rescue analgesia from 8 through 48 hours
Meta-analysis of 8 RCTs suggests that when compared with short-acting anesthetics, bupivacaine may decrease the use of rescue analgesia by an important amount (risk ratio [RR], 0.48; 95% CI, 0.20 to 1.13; low certainty evidence) (Table 2).11,13,15,19,22 There was no evidence of a subgroup effect by type of short-acting local anesthetic (eFigure 1).
Table 2.
Bupivacaine vs short-acting local anesthetics for acute dental pain.
| OUTCOME | FOLLOW UP | PARTICIPANTS (RANDOMIZED CONTROLLED TRIALS), NO. | RELATIVE EFFECT* (95% CI) | ANTICIPATED ABSOLUTE EFFECTS, % (95% CI) | CERTAINTY | WHAT HAPPENS | ||
|---|---|---|---|---|---|---|---|---|
| With Short-Acting Local Anesthetics | With Bupivacaine | Difference | ||||||
| Use of Rescue Analgesia Assessed With Proportion of Patients Requiring Rescue Analgesia | 8–14 h | 638 (8) | Risk ratio, 0.48 (0.20 to 1.13) | 66.1 | 31.7 (13.2 to 74.7) | −34.4 (−52.9 to 8.6) | Low†,‡ | Bupivacaine may decrease the use of rescue analgesia by an important amount compared with short-acting local anesthetics. |
| Adverse Effects (Not Specified) Assessed with Proportion of Patients Experiencing Adverse Reactions | 7 d | 189 (4) | Not estimable | 0.0 | 0.0 | 0.0 (−4.0 to 4.0) | Moderate§ | There is probably no difference between bupivacaine and short-acting local anesthetics with regard to incidence of adverse effects. |
The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
There is high statistical heterogeneity (I2 = 96%, P < .00001). However, the heterogeneity is due to 2 studies that do not have an important impact on the pooled estimate; therefore, the authors did not rate down for inconsistency.
Using a threshold of 6.61% (based on 10% of the baseline risk, that is, the risk with short-acting local anesthetics), the lower bound of the 95% CI suggests an important difference favoring bupivacaine, whereas the upper bound suggests an important benefit of short-acting anesthetics. Therefore, the authors rated down 2 levels owing to imprecision.
Two of the 4 studies were at a high risk of bias. Both studies were at a high risk of selective outcome reporting because the number of participants analyzed was unclear. One study was also at a high risk of selection and detection bias because participants were randomized on the basis of alphabetization and there was no mention of allocation concealment or blinding of participants or personnel. Therefore, the authors rated down 1 level owing to risk of bias.
Adverse effects
Four RCTs suggest no difference between bupivacaine and short-acting local anesthetics with respect to risk of adverse effects (risk difference [RD], 0.0%; 95% CI, −4.0% to 4.0%; moderate certainty) (Table 2).16,20,22,23 There was no evidence of a subgroup effect by type of short-acting local anesthetic (eFigure 2).
Time to analgesic consumption
The relative effect of bupivacaine vs short-acting local anesthetics varied by type of short-acting anesthetic. One RCT suggests that bupivacaine increases the time to analgesic consumption compared with lidocaine with epinephrine (mean difference [MD], 2.56 hours; 95% CI, 2.07 to 3.05; high certainty evidence) (Table 3, eFigure 3).22 Meta-analysis of 3 RCTs resulted in very low certainty evidence regarding time to analgesic consumption for the comparison of bupivacaine and articaine (Table 4, eFigure 4).14,18,21 Bupivacaine probably increases time to analgesic consumption compared with mepivacaine (MD, 3.56 hours; 95% CI, 2.39 to 4.73; moderate certainty)23 (Table 5, eFigure 5).
Table 3.
Bupivacaine vs lidocaine for acute dental pain.
| OUTCOME | FOLLOW-UP | PARTICIPATINTS (RANDOMIZED CONTROLLED TRIALS), NO. | RELATIVE EFFECT* (95% CI) | ANTICIPATED ABSOLUTE EFFECTS, % (95% CI) | CERTAINTY | WHAT HAPPENS | ||
|---|---|---|---|---|---|---|---|---|
| With Lidocaine | With Bupivacaine | Difference | ||||||
| Use of Rescue Analgesia Assessed With Proportion of Patients Requiring Rescue Analgesia | 9–48 h | 517 (6) | Risk ratio, 0.36 (0.09 to 1.45) | 70.7 | 25.4 (6.4 to 100) | −45.2 (−64.3 to 31.8) | Low†,‡ | Bupivacaine may decrease the use of rescue analgesia by an important amount compared with lidocaine. |
| Time to Analgesic Consumption (Hours) | Not specified | 60 (1) | Not applicable | Mean time to analgesic consumption, 2.87 h | Not applicable | Mean difference, 2.56 (2.07 to 3.05) | High | Bupivacaine increases time to analgesic consumption compared with lidocaine. |
| Amount of Analgesic Consumption Assessed With Number of Doses | 24 h | 427 (5) | Not applicable | Mean amount of analgesic consumption, 3.10 doses | Not applicable | Mean difference, −1.91 (−3.35 to −0.46) | Moderate§,¶ | Bupivacaine probably decreases the amount of analgesic consumption compared with lidocaine. |
| Adverse Effects (Not Specified) Assessed With Proportion of Patients Experiencing Adverse Effects | 7 d | 84 (2) | Not estimable | 0.0 | 0.0 | 0.0 (−6.0 to 6.0) | Low#,** | There may be no difference between bupivacaine and lidocaine with regard to incidence of adverse effects. |
The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
There is high statistical heterogeneity (I2 = 98%, P < .00001). However, the heterogeneity is due to 2 studies that do not have an important impact on the pooled estimate; therefore, the authors did not rate down for inconsistency.
Using a threshold of 7.07% (based on 10% of the baseline risk, that is, the risk with lidocaine), the lower bound of the 95% CI suggests an important difference favoring bupivacaine, whereas the upper bound suggests an important benefit of lidocaine. Therefore, the authors rated down 2 levels owing to imprecision.
Four of the 5 studies were at a high risk of bias. One study was at a high risk of attrition bias owing to missing outcome data. Two studies were at a high risk of selective outcome reporting because they did not report exact measures of central tendency and variability and instead reported how many patients consumed a certain number of tablets. Another study was at a high risk of selective outcome reporting because data were extracted from a figure and it is unclear which measure of variability was reported. Therefore, the authors rated down 1 level owing to risk of bias.
There is high statistical heterogeneity (I2 = 98%, P < .00001). However, the heterogeneity is due to 1 study that does not have an important impact on the pooled estimate; therefore, the authors did not rate down for inconsistency.
One of the 2 studies was at a high risk of selective outcome reporting because the number of participants analyzed was unclear. Therefore, the authors rated down 1 level owing to risk of bias.
The optimal information size of 100 participants was not met. Therefore, the authors rated down 1 level owing to imprecision.
Table 4.
Bupivacaine vs articaine for acute dental pain.
| OUTCOME | FOLLOW-UP, D | PARTICIPANTS (RANDOMIZED CONTROLLED TRIALS), NO. | RELATIVE EFFECT* (95% CI) | ANTICIPATED ABSOLUTE EFFECTS, % (95% CI) | CERTAINTY | WHAT HAPPENS | ||
|---|---|---|---|---|---|---|---|---|
| With Articaine | With Bupivacaine | Difference | ||||||
| Use of Rescue Analgesia Assessed With Proportion of Patients Requiring Rescue Analgesia | 1 | 121 (2) | Risk ratio, 0.91 (0.46 to 1.79) | 46.7 | 42.5 (21.5 to 83.5) | −4.2 (−25.2 to 36.9) | Very low†,‡,§ | There is very low certainty evidence regarding the difference between bupivacaine and articaine for the use of rescue analgesia. |
| Time to Analgesic Consumption (Hours) | 1–4 | 331 (3) | Not applicable | Mean time to analgesic consumption, 6.37 h | Not applicable | Mean difference, −0.08 (−1.86 to 1.7) | Very low¶,#,** | There is very low certainty evidence regarding time to analgesic consumption for the comparison of bupivacaine and articaine. |
| Amount of Analgesic Consumption Assessed With Number of Doses | 1–4 | 136 (2) | Not applicable | Mean amount of analgesic consumption, 0.75 doses | Not applicable | Mean difference, 0.22 (−0.13 to 0.57) | Low††, ‡‡ | Bupivacaine may increase the amount of analgesic consumption compared with articaine. |
| Adverse Effects (Not Specified) Assessed With Proportion of Patients Experiencing Adverse Reactions | 7 | 36 (1) | Not estimable | 0.0 | 0.0 | 0.0 (−10.0 to 10.0) | Low§§ | There may be no difference between bupivacaine and articaine with regard to incidence of adverse effects. |
| Time to Analgesic Consumption | 7 | 88 (2) | Two studies reported on the time to analgesic intake without providing arm-level data. Trullenque-Eriksson and Guisado-Moya24 found no statistically significant differences between groups for the time elapsed until use of rescue analgesia. Olmedo-Gaya and colleagues17 also reported that there was no statistically significant tendency for earlier use of rescue analgesia in the articaine group (P = .183). | Very low¶¶,## | There is very low certainty evidence regarding the difference in time to analgesic consumption for bupivacaine and articaine. | |||
The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
One of the 2 studies was probably at a high risk of selective outcome reporting as it did not specify the follow-up time. Therefore, the authors rated down 1 level owing to risk of bias.
There is moderate statistical heterogeneity (I2 = 62%, P = .11). However, the 95% CI of the effect estimates overlap, so the authors did not rate down for inconsistency.
Using a threshold of 4.67% (based on 10% of the baseline risk, that is, the risk with articaine), the lower bound of the 95% CI suggests an important difference favoring bupivacaine, whereas the upper bound suggests an important difference favoring articaine. Therefore, the authors rated down 2 levels owing to imprecision.
One of the 3 studies was at a high risk of bias due to deviations from the intended interventions because there was no mention of blinding. Therefore, the authors rated down 1 level owing to risk of bias.
There is high statistical heterogeneity (I2 = 98%, P < .00001) and minimal overlap of 95% CIs. Therefore, the authors rated down 1 level owing to inconsistency.
Using the null as a threshold, the lower bound of the 95% CI suggests a difference favoring articaine, whereas the upper bound of the 95% CI suggests a difference favoring bupivacaine. Therefore, the authors rated down 1 level owing to imprecision.
One of the 2 studies was at a high risk of selection bias as no measure of variability was reported. Therefore, the authors rated down 1 level owing to risk of bias.
Using the null as a threshold, the lower bound of the 95% CI suggests a difference favoring bupivacaine, whereas the upper bound suggests a difference favoring articaine. Therefore, the authors rated down 1 level owing to imprecision.
The optimal information size of 100 participants was not met. Therefore, the authors rated down 2 levels owing to imprecision.
One study was at a high risk of bias arising from the randomization process because there was no mention of allocation concealment and the health care providers were not blinded. This study was also at high risk of bias owing to 46% missing outcome data. Both studies were at a high risk of bias owing to selection of the reported result because they did not report any arm-level data. Therefore, the authors rated down 2 levels owing to risk of bias.
The optimal information size of 100 participants was not met. Therefore, the authors rated down 1 level owing to imprecision.
Table 5.
Bupivacaine vs mepivacaine for acute dental pain.
| OUTCOME | FOLLOW-UP, H | PARTICIPANTS (RANDOMIZED CONTROLLED TRIALS), NO. | RELATIVE EFFECT* (95% CI) | ANTICIPATED ABSOLUTE EFFECTS (95% CI) | CERTAINTY | WHAT HAPPENS | ||
|---|---|---|---|---|---|---|---|---|
| With Mepivacaine | With Bupivacaine | Difference | ||||||
| Time to Analgesic Consumption Assessed With Duration (Hours) of Postoperative Analgesia | 24 | 69 (1) | Not applicable | Mean time to analgesic consumption, 2.9 h | Not applicable | Mean difference, 3.56 (2.39 4.73) | Moderate†,‡ | Bupivacaine probably increases time to analgesic consumption compared with mepivacaine. |
| Amount of Analgesic Consumption Assessed With Number of Doses | 24 | 69 (1) | Not applicable | Mean amount of analgesic consumption, 4.2 doses | Not applicable | Mean difference, −1.58 (−2.21 to −0.95) | Moderate§ | Bupivacaine probably decreases the amount of analgesic consumption compared with mepivacaine. |
| Adverse Effects (Not Specified) Assessed With Proportion of Patients Experiencing Adverse Effects or Complications | Not specified | 69 (1) | Not estimable | 0.0 | 0.0 | 0.0 (−9.0 to 9.0) | Low¶,# | There may be no difference between bupivacaine and mepivacaine with regard to incidents of adverse effects. |
The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
The study was at a high risk of bias because participants were randomized on the basis of alphabetization and there was no mention of allocation concealment or blinding of participants or personnel. Therefore, the authors rated down 1 level owing to risk of bias.
There is moderate statistical heterogeneity (I2 = 61%, P = .11). However, the 95% CIs of the effect estimates overlap, so the authors did not rate down for inconsistency.
The study was at a high risk of bias because participants were randomized on the basis of alphabetization and there was no mention of allocation concealment or blinding of participants or personnel. The study was also at a high risk of bias owing to selection of the reported results because no measure of variability was provided. Therefore, the authors rated down 1 level owing to risk of bias.
The study was at a high risk of bias because participants were randomized on the basis of alphabetization and there was no mention of allocation concealment or blinding of participants or personnel. It was also at a high risk of bias owing to selection of the reported results because the number of participants analyzed was unclear. Therefore, the authors rated down 1 level owing to risk of bias.
The optimal information size of 100 participants was not met. Therefore, the authors rated down 1 level owing to imprecision.
Amount of analgesic consumption
The mean difference in analgesic consumption varied between recipients of bupivacaine vs short-acting anesthetic depending on the type of short-acting anesthetic received. Five RCTs suggest that bupivacaine probably decreases the amount of analgesic consumption compared with lidocaine with epinephrine (MD, −1.91 doses; 95% CI, −3.35 to −0.46; moderate certainty) (Table 3, eFigure 6).11–13,16,22 Two RCTs suggest that bupivacaine may increase the amount of analgesic consumption at 1 to 4 days follow-up compared with articaine (MD, 0.22 doses; 95% CI, −0.13 to 0.57; low certainty) (Table 4, eFigure 7).14,20 In addition, bupivacaine probably decreases the amount of analgesic consumption measured at 24 hours compared with mepivacaine (MD, −1.58 doses; 95% CI, −2.21 to −0.95; moderate certainty) (Table 5, eFigure 8).23
SR 2: Topical anesthetics: benzocaine formulations
After screening 4,716 titles and abstracts, we included 5 RCTs (eFigure 9).25–29 Reasons for exclusion at the full-text screening stage are presented in eFigure 9.
Characteristics of Included Studies
All included RCTs had a parallel group design and were conducted in the United States. The number of participants ranged from 20 through 576. Mean (SD) age across studies ranged from 26.2 (not reported) through 31.1 (12.7) years. All studies included a population with symptomatic irreversible pulpitis (eTable 4).
Risk of Bias in Included Studies
The most common domains with risk-of-bias issues across studies were bias arising from the randomization process and bias from selective reporting of the results (eTable 5).
Effects of Interventions
Number of responders
The number of responders was assessed as the proportion of participants who had a reduced pain intensity score at 2 consecutive time points measured at a follow-up time from 20 through 30 minutes. Two RCTs suggest that there is probably a trivial benefit of using 20% benzocaine compared with 10% benzocaine with regard to the number of responders (RR, 0.93; 95% CI, 0.86 to 1.00; moderate certainty) (eTable 6, eFigure 10).25,26 Two RCTs also suggest that 10% benzocaine may increase the number of responders compared with placebo by an important amount (RR, 1.38; 95% CI, 0.74 to 2.56; low certainty) (eTable 7, eFigure 11).25,26 In addition, 3 RCTs suggest that 20% benzocaine may increase the number of responders from 20 through 30 minutes compared with placebo by an important amount (RR, 1.47; 95% CI, 1.03 to 2.10; low certainty) (eTable 8, eFigure 12).25,26,28
Adverse effects
Data from 2 RCTs suggest that there may be no important difference between 20% benzocaine and 10% benzocaine with respect to the proportion of participants experiencing any adverse effect measured from 90 through 120 minutes after application (RD, 0.0%; 95% CI, −3% to 3%; low certainty) (eTable 6, eFigure 13).25,26 Three RCTs also suggest that there may be an important difference favoring benzocaine 10% compared with placebo with regard to the incidence of adverse effects anytime from 10 through 120 minutes after application (RD, −1%; 95% CI, −4% to 3%; low certainty) (eTable 7, eFigure 14).25,26,29 In addition, evidence from 4 RCTs indicates that there may be an important difference favoring 20% benzocaine compared with placebo with regard to the risk of any adverse effect anytime from 10 through 120 minutes after application (RD, −1%; 95% CI, −4% to 3%; low certainty) (eTable 8, eFigure 15).25–28
Pain levels (measured as sum of pain relief combined with pain intensity difference) at 60 minutes
We did not find any evidence reporting this outcome.
DISCUSSION
We report 2 SRs to present a detailed picture of all the evidence used to inform the development of the upcoming evidence-based clinical practice guideline for the management of acute dental pain in adolescents and adults produced by the ADA Council on Scientific Affairs, ADA Science and Research Institute, and the University of Pittsburgh’s and the University of Pennsylvania’s schools of dental medicine in partnership with the US Food and Drug Administration. We found that bupivacaine is probably superior to lidocaine with epinephrine and mepivacaine regarding time to analgesic consumption, but there was very low certainty evidence on the difference between bupivacaine and articaine on this outcome. We also found low certainty evidence that bupivacaine decreases the need for rescue medication compared with short-acting local anesthetics, with likely no differences in adverse effects. Regarding topical anesthetics, 10% benzocaine and 20% benzocaine were superior to placebo with respect to the proportion of participants with a reduced pain intensity score for at least 2 consecutive time points from 20 through 30 minutes; 20% benzocaine was negligibly better than 10% benzocaine. The reason we may have seen fewer adverse effects in the benzocaine group compared with placebo groups could be because many of the observed adverse effects (that is, headache, increased heart rate, and increased blood pressure) were related to a lack of pain relief in the placebo groups. These symptoms are typically seen in patients who have pain due to symptomatic irreversible pulpitis.
The certainty of the evidence was low to very low for several comparisons and outcomes, including adverse effects of bupivacaine vs lidocaine with epinephrine, articaine, and mepivacaine, with similar certainty of the evidence for 10% benzocaine and 20% benzocaine compared with placebo. The most common reasons for rating down the certainty of the evidence were serious issues of risk of bias and imprecision. The risk of bias assessment showed shortcomings with randomization and selective reporting of results. Future research should focus on overcoming the methodological limitations identified, especially when designing trials in injected local anesthetics.
In 2014, a comparison of bupivacaine to lidocaine in an SR and meta-analysis containing 4 studies showed that in comparison with 2% lidocaine with 1:100,000 epinephrine, 0.5% bupivacaine with 1:200,000 epinephrine had a lower percentage of participants using postoperative analgesics, which is consistent with our results. In the same review, 0.5% bupivacaine with 1:200,000 epinephrine was superior to 2% lidocaine with 1:100,000 epinephrine in terms of postoperative pain control.30 In our SR and meta-analysis, 6 RCTs compared bupivacaine to lidocaine with epinephrine, and the evidence pertaining to proportion of patients requiring rescue analgesia from 8 through 48 hours was found to be of low certainty, according to the guidance from the GRADE Working Group. We did not find other SRs comparing bupivacaine to articaine and mepivacaine. To our knowledge, our study is the first SR assessing the effect of different doses of benzocaine.
Dosing and toxicity of injectable local anesthetics are cumulative. Sometimes 0.5% bupivacaine with 1:200,000 epinephrine is administered postoperatively after administration of 2% lidocaine with 1:100,000 epinephrine intraoperatively. In this case, from a safety standpoint, it is critical to consider the amount of lidocaine with epinephrine that is administered before bupivacaine to avoid the potential local anesthetics overdose.31 Furthermore, with respect to benzocaine, its overzealous use (typically overdose) can trigger methemoglobinemia. For this reason, topical benzocaine is no longer approved for teething pain in children younger than 2 years in the United States.32
The strengths of our SR and meta-analysis are numerous. Each stage of the review process was conducted in duplicate, and conflicts were resolved by a third reviewer. We assessed risk of bias for each RCT included in this study and the certainty of the evidence for each outcome of interest using widely accepted methods. We performed analyses and interpreted results using the latest methodological guidance from the GRADE Working Group. To make results easier to interpret, instead of using standardized mean difference, we reported continuous outcomes using mean difference via converting all scale scores to a single, most reported scale.6 This SR and meta-analysis, however, are limited to the inclusion of research studies published in English. Nevertheless, we believe it is unlikely that our conclusions would have differed if we had included studies in other languages. Because this SR was conducted to inform the recommendations of the upcoming evidence-based clinical practice guideline for the management of acute dental pain in adolescents and adults, our last date of search was November 21, 2020. We believe it is valuable to present the summary of the evidence as the guideline panel saw it, which is why we did not update this review for publication. However, through September 2022, there do not seem to be any new relevant studies that would change our conclusions.
CONCLUSIONS
Low certainty evidence suggests that long- vs short-acting local anesthetics may reduce the need for rescue analgesia, with probably no important difference between these interventions with regard to adverse effects when used postoperatively. Bupivacaine was superior in terms of time to analgesic consumption and amount of analgesic consumption compared with lidocaine with epinephrine and mepivacaine, but the evidence was of low and very low certainty for the comparison of bupivacaine and articaine. Regarding topical anesthetics, benzocaine 10% and benzocaine 20% were superior to placebo, and benzocaine 20% showed trivial differences compared with the 10% formulation.
Supplementary Material
Acknowledgments
This project was financially supported by the US Food and Drug Administration of the US Department of Health and Human Services. The content is that of the authors and does not necessarily represent the official views of, nor an endorsement by, the US Food and Drug Administration, the US Department of Health and Human Services, or the US Government.
ABBREVIATION KEY
- ADA
American Dental Association
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- pKa
Acid dissociation constant
- RCT
Randomized controlled trial
- SR
Systematic review
Biographies
Ms. Ibrahim was a bachelor of health sciences (honors) student, Faculty of Health Sciences, McMaster University, Hamilton, Ontario, Canada, when the work described in this article was conducted. She is now a master of science student, Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, Ontario, Canada.
Ms. Azab was a bachelor of health sciences (honors) student, Faculty of Health Sciences, McMaster University, Hamilton, Ontario, Canada, when the work described in this article was conducted. She is now a medical student, Temerty Faculty of Medicine, University of Toronto, Mississauga, Ontario, Canada.
Dr. Roldan was a research associate, Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, Ontario, Canada, when the work described in this article was conducted. She now is a master of science student, Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, Ontario, Canada.
Mr. Martinez is a research associate, Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, Ontario, Canada.
Ms. Tamilselvan is a bachelor of health sciences (honors) student, Faculty of Health Sciences, McMaster University, Hamilton, Ontario, Canada.
Mr. He was a bachelor of health sciences (honors) student, Faculty of Health Sciences, McMaster University, Hamilton, Ontario, Canada, when the work described in this article was conducted. He now is a medical student, Faculty of Medicine, University of Ottawa, Ottawa, Ontario, Canada.
Ms. Urquhart was a senior research associate, Evidence Synthesis and Translation Research, ADA Science and Research Institute, Chicago, IL, when the work described in this article was conducted. She now is an instructor, Department of Preventive and Restorative Sciences and the Center for Integrative Global Oral Health, School of Dental Medicine, University of Pennsylvania, Philadelphia, PA.
Ms. Tampi is an adjunct professor, Department of Cariology, University of Michigan School of Dentistry, Ann Arbor, MI.
Dr. Polk is an assistant professor, Department of Dental Public Health, University of Pittsburgh, Pittsburgh, PA.
Dr. Moore is a professor emeritus in pharmacology, Department of Dental Public Health, University of Pittsburgh, Pittsburgh, PA.
Dr. Hersh is a professor, Department of Oral Surgery and Pharmacology, University of Pennsylvania, Philadelphia, PA.
Dr. Carrasco-Labra is an associate professor, Department of Preventative and Restorative Sciences, and the Center for Integrative Global Oral Health, University of Pennsylvania, School of Dental Medicine, Philadelphia, PA.
Dr. Brignardello-Petersen is an assistant professor, Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, Ontario, Canada.
Footnotes
Disclosures. Dr. Hersh and the Trustees of the University of Pennsylvania have received grant funding from Pfizer Consumer Healthcare, Church & Dwight, and Bayer Healthcare. In addition, within the last 5 years, Dr. Hersh received consulting funds for reviewing data and sharing his expertise with the National Advertising Division of the Better Business Bureau concerning pain studies that compared Aleve and Extra Strength Tylenol. Dr. Moore has received grant funding from Pfizer Consumer and Church and Dwight. None of the other authors reported any disclosures.
The findings and conclusions in this article are those of the authors and do not necessarily reflect the official views, position, or policy of the American Dental Association, nor does this article constitute an endorsement by the American Dental Association.
This article has an accompanying online continuing education activity available at: http://jada.ada.org/ce/home.
Supplemental material is available online.
Contributor Information
Anna Miroshnychenko, Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, Ontario, Canada..
Sara Ibrahim, Faculty of Health Sciences, McMaster University, Hamilton, Ontario, Canada.
Maria Azab, Faculty of Health Sciences, McMaster University, Hamilton, Ontario, Canada.
Yetiani Roldan, Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, Ontario, Canada.
Juan Pablo Diaz Martinez, Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, Ontario, Canada..
Divyalakshmi Tamilselvan, Faculty of Health Sciences, McMaster University, Hamilton, Ontario, Canada..
Leon He, Faculty of Health Sciences, McMaster University, Hamilton, Ontario, Canada.
Olivia Urquhart, Evidence Synthesis and Translation Research, ADA Science and Research Institute, Chicago, IL.
Malavika Tampi, Department of Cariology, University of Michigan School of Dentistry, Ann Arbor, MI..
Deborah E. Polk, Department of Dental Public Health, University of Pittsburgh, Pittsburgh, PA..
Paul A. Moore, Department of Dental Public Health, University of Pittsburgh, Pittsburgh, PA..
Elliott V. Hersh, Department of Oral Surgery and Pharmacology, University of Pennsylvania, Philadelphia, PA..
Alonso Carrasco-Labra, Department of Preventative and Restorative Sciences, and the Center for Integrative Global Oral Health, University of Pennsylvania, School of Dental Medicine, Philadelphia, PA..
Romina Brignardello-Petersen, Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, Ontario, Canada..
References
- 1.Gaffen AS, Haas DA. Survey of local anesthetic use by Ontario dentists. J Can Dent Assoc. 2009;75(9):649. [PubMed] [Google Scholar]
- 2.Haas DA. An update on local anesthetics in dentistry. J Can Dent Assoc. 2002;68(9):546–551. [PubMed] [Google Scholar]
- 3.Hersh EV, Houpt MI, Cooper SA, Feldman RS, Wolff MS, Levin LM. Analgesic efficacy and safety of an intraoral lidocaine patch. JADA. 1996;127(11):1626–1634. [DOI] [PubMed] [Google Scholar]
- 4.Rossi MT, de Oliveira MN, Vidigal MTC, et al. Effectiveness of anesthetic solutions for pain control in lower third molar extraction surgeries: a systematic review of randomized clinical trials with network meta-analysis. Clin Oral Investig. 2021;25(1):1–22. [DOI] [PubMed] [Google Scholar]
- 5.National Academies of Sciences Engineering Medicine Health Medicine Division Board on Health Care Services Committee on Evidence-Based Clinical Practice Guidelines for Prescribing Opioids for Acute Pain. Framing Opioid Prescribing Guidelines for Acute Pain: Developing the Evidence. National Academies Press; 2019. [PubMed] [Google Scholar]
- 6.Thorlund K, Walter SD, Johnston BC, Furukawa TA, Guyatt GH. Pooling health-related quality of life outcomes in meta-analysis-a tutorial and review of methods for enhancing interpretability. Res Synth Methods. 2011;2(3):188–203. [DOI] [PubMed] [Google Scholar]
- 7.Guyatt GH, Oxman AD, Kunz R, et al. ; GRADE Working Group. GRADE guidelines, 7: rating the quality of evidence—inconsistency. J Clin Epidemiol. 2011;64(12): 1294–1302. [DOI] [PubMed] [Google Scholar]
- 8.Hultcrantz M, Rind D, Akl EA, et al. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017;87:4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng L, Brignardello-Petersen R, Hultcrantz M, et al. GRADE guidelines, 32: GRADE offers guidance on choosing targets of GRADE certainty of evidence ratings. J Clin Epidemiol. 2021;137:163–175. [DOI] [PubMed] [Google Scholar]
- 10.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adelusi EA, Abiose OB, Gbolahan OO. Post intra-alveolar extraction analgesia of bupivacaine and lidocaine: a randomized controlled clinical trial. Dent. 2019; 9(540):2. [Google Scholar]
- 12.Bouloux GF, Punnia-Moorthy A. Bupivacaine versus lidocaine for third molar surgery: a double-blind, randomized, crossover study. J Oral Maxillofac Surg. 1999; 57(5):510–514; discussion 15. [DOI] [PubMed] [Google Scholar]
- 13.Brajković D, Biočanin V, Milič M, Vučetić M, Petrović R, Brković B. Quality of analgesia after lower third molar surgery: a randomised, double-blind study of levobupivacaine, bupivacaine and lidocaine with epinephrine. Vojnosanit Pregl. 2015;72(1):50–56. [DOI] [PubMed] [Google Scholar]
- 14.Gregorio LV, Giglio FP, Sakai VT, et al. A comparison of the clinical anesthetic efficacy of 4% articaine and 0.5% bupivacaine (both with 1:200,000 epinephrine) for lower third molar removal. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106(1):19–28. [DOI] [PubMed] [Google Scholar]
- 15.Hyrkäs T, Ylipaavalniemi P, Oikarinen VJ, Paakkari I. Effective postoperative pain prevention through administration of bupivacaine and diclofenac. Anesth Prog. 1994;41(1):6–10. [PMC free article] [PubMed] [Google Scholar]
- 16.Marković AB, Todorović L. Postoperative analgesia after lower third molar surgery: contribution of the use of long-acting local anesthetics, low-power laser, and diclofenac. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(5):e4–e8. [DOI] [PubMed] [Google Scholar]
- 17.Olmedo-Gaya MV, Manzano-Moreno FJ, Muñoz-López JL, Vallecillo-Capilla MF, Reyes-Botella C. Double-blind, randomized controlled clinical trial on analgesic efficacy of local anesthetics articaine and bupivacaine after impacted third molar extraction. Clin Oral Investig. 2018; 22(9):2981–2988. [DOI] [PubMed] [Google Scholar]
- 18.Pellicer-Chover H, Cervera-Ballester J, Sanchis-Bielsa JM, Peñarrocha-Diago MA, Peñarrocha-Diago M, García-Mira B. Comparative split-mouth study of the anesthetic efficacy of 4% articaine versus 0.5% bupivacaine in impacted mandibular third molar extraction. J Clin Exp Dent. 2013;5(2):e66–e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenquist JB, Rosenquist KI, Lee PK. Comparison between lidocaine and bupivacaine as local anesthetics with diflunisal for postoperative pain control after lower third molar surgery. Anesth Prog. 1988;35(1):1–4. [PMC free article] [PubMed] [Google Scholar]
- 20.Sancho-Puchades M, Vílchez-Pérez M, Valmaseda-Castellón E, Paredes-García J, Berini-Aytés L, Gay-Escoda C. Bupivacaine 0.5% versus articaine 4% for the removal of lower third molars: a crossover randomized controlled trial. Med Oral Patol Oral Cir Bucal. 2012; 17(3):e462–e468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thakare A, Bhate K, Kathariya R. Comparison of 4% articaine and 0.5% bupivacaine anesthetic efficacy in orthodontic extractions: prospective, randomized crossover study. Acta Anaesthesiol Taiwan. 2014;52(2):59–63. [DOI] [PubMed] [Google Scholar]
- 22.Tijanic M, Buric N. A randomized anesthethic potency comparison between ropivacaine and bupivacaine on the perioperative regional anesthesia in lower third molar surgery. J Craniomaxillofac Surg. 2019;47(10):1652–1660. [DOI] [PubMed] [Google Scholar]
- 23.Trieger N, Gillen GH. Bupivacaine anesthesia and post-operative analgesia in oral surgery. Anesth Prog. 1979; 26(1):20–23. [PMC free article] [PubMed] [Google Scholar]
- 24.Trullenque-Eriksson A, Guisado-Moya B. Comparative study of two local anesthetics in the surgical extraction of mandibular third molars: bupivacaine and articaine. Med Oral Patol Oral Cir Bucal. 2011;16(3):e390–e396. [DOI] [PubMed] [Google Scholar]
- 25.Gangarosa LP Sr., Ciarlone AE, Neaverth EJ, Johnston CA, Snowden JD, Thompson WO. Use of verbal descriptors, thermal scores and electrical pulp testing as predictors of tooth pain before and after application of benzocaine gels into cavities of teeth with pulpitis. Anesth Prog. 1989;36(6):272–275. [PMC free article] [PubMed] [Google Scholar]
- 26.Hersh EV, Ciancio SG, Kuperstein AS, et al. An evaluation of 10 percent and 20 percent benzocaine gels in patients with acute toothaches: efficacy, tolerability and compliance with label dose administration directions. JADA. 2013;144(5):517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hersh EV, Cooper SA, Segal H, Greene J. Analgesic onset time as a measure of topical anesthetic efficacy in spontaneous toothache pain: a pilot study. J Clin Dent. 1993;4(2):52–54. [PubMed] [Google Scholar]
- 28.Hersh EV, Stoopler ET, Secreto SA, DeRossi SS. A study of benzocaine gel dosing for toothache. J Clin Dent. 2005;16(4):103–108. [PubMed] [Google Scholar]
- 29.Sveen OB, Yaekel M, Adair SM. Efficacy of using benzocaine for temporary relief of toothache. Oral Surg Oral Med Oral Pathol. 1982;53(6):574–576. [DOI] [PubMed] [Google Scholar]
- 30.Su N, Wang H, Zhang S, Liao S, Yang S, Huang Y. Efficacy and safety of bupivacaine versus lidocaine in dental treatments: a meta-analysis of randomised controlled trials. Int Dent J. 2014;64(1):34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore PA, Hersh EV. Local anesthetics: pharmacology and toxicity. Dent Clin North Am. 2010;54(4):587–599. [DOI] [PubMed] [Google Scholar]
- 32.US Food and Drug Administration. Safety information on benzocaine-containing products. Accessed March 20, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/safety-information-benzocaine-containing-products#:~:text=Due%20to%20the%20significant%20safety,children%20younger%20than%202%20years
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


