Abstract
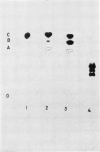
The metabolism of 2-chloro-4-ethylamino-6-isopropylamino-s-triazine (atrazine) in the resistant species, corn (Zea mays L.) and sorghum (Sorghum vulgare Pers.) was not the same. In corn, atrazine was metabolized via both the 2-hydroxylation and N-dealkylation pathways while sorghum metabolized atrazine via the N-dealkylation pathway. Atrazine metabolism in corn yielded the metabolites, 2-hydroxy-4-ethylamino-6-isopropylamino-s-triazine (hydroxyatrazine), 2-hydroxy-4-amino-6-isopropylamino-s-triazine (hydroxycompound I), and 2-hydroxy-4-amino-6-ethylamino-s-triazine (hydroxycompound II). None of these hydroxylated derivatives appeared as metabolites of atrazine in sorghum.
Hydroxycompounds I and II were formed in 2 ways in corn: (1) by benzoxazinone-catalyzed hydrolysis of 2-chloro-4-amino-6-isopropylamino-s-triazine (compound I) and 2-chloro-4-amino-6-ethylamino-s-triazine (compound II) that were formed by N-dealkylation of atrazine and (2) by N-dealkylation of hydroxyatrazine, the major atrazine metabolite in corn. The interaction of the 2-hydroxylation and N-dealkylation pathways in corn results in the formation of the 3 hydroxylated non-phytotoxic derivatives of atrazine.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hamilton R. H., Moreland D. E. Simazine: Degradation by Corn Seedlings. Science. 1962 Feb 2;135(3501):373–374. doi: 10.1126/science.135.3501.373. [DOI] [PubMed] [Google Scholar]
- Shimabukuro R. H. Atrazine metabolism and herbicidal selectivity. Plant Physiol. 1967 Sep;42(9):1269–1276. doi: 10.1104/pp.42.9.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]