Abstract
Based on the concept of the common mucosal immune system, immunization at various inductive sites can induce an immune response at other, remote mucosal surfaces. The immune responses elicited through rectal and oral routes of antigen delivery were compared with respect to (i) measurement of antibody responses in serum and various external secretions of the vaccinees and (ii) characterization of the nature and homing potentials of circulating antibody-secreting cells (ASC). Specific ASC appeared in the circulation in 4 of 5 volunteers after oral and 9 of 11 volunteers after rectal immunization with Salmonella typhi Ty21a. The kinetics, magnitude, and immunoglobulin isotype distribution of the ASC responses were similar in the two groups. In both groups, almost all ASC (99 or 95% after oral or rectal immunization, respectively) expressed α4β7, the gut homing receptor (HR), whereas l-selectin, the peripheral lymph node HR, was expressed only on 22 or 38% of ASC, respectively. Oral immunization elicited a more pronounced immune response in saliva and vaginal secretion, while rectal immunization was more potent in inducing a response in nasal secretion, rectum, and tears. No major differences were found in the abilities of the two immunization routes to induce a response in serum or intestinal secretion. Thus, the rectal antigen delivery should be considered as an alternative to the oral immunization route. The different immune response profiles found in various secretions after oral versus rectal antigen administration provide evidence for a compartmentalization within the common mucosal immune system in humans.
Mucosal delivery of antigens is one of the main goals of current vaccine development. Mucosal immunization has several advantages over the conventional parenteral route: it is safer, less expensive, and easier to carry out in developing countries, and the antigen can be introduced to the body through the same routes as in a natural infection. It seems appealing to administer antigens through the gastrointestinal route, as the intestine contains a large accumulation of lymphoid tissues with lymphoepithelial structures involved in the induction of mucosal immune responses (4). Accordingly, the oral route of antigen delivery is the most common and most frequently explored among the mucosal immunization routes. However, oral antigen delivery poses some problems, such as the denaturation of stomach acid and digestion of antigens due to long exposure to gastrointestinal proteolytic enzymes. Alternative gastrointestinal routes include rectal antigen delivery, which so far has not been extensively explored in humans (9, 14, 29, 35, 36). However, the rectal mucosa is known to be rich in lymphoepithelial structures analogous to Peyer’s patches (37).
The different mucosal surfaces in the body are believed to be interconnected via circulating lymphocytes, as recognized by the concept of the common mucosal immune system (CMIS) (32): immunization at one mucosal inductive site (e.g., intestinal Peyer’s patches) can lead to an immune response at another, anatomically remote mucosal effector site (e.g., saliva or genital tract secretions). Consistent with this concept, mucosal immunization is known to be followed by a transient appearance of antibody-secreting cells (ASC) in the peripheral blood (11, 22), and antibody responses have been found on mucosal surfaces distant from the original site of mucosal immunization (7, 16, 32, 33). However, recent data suggest that some degree of compartmentalization may exist within the CMIS (18, 34); therefore, the general routes of lymphocyte homing from each mucosal site need to be explored.
Recently, it has become possible to investigate the homing potentials of circulating ASC by examining their expression of homing receptors (HR) (24–26, 40, 41). Homing of lymphocytes into tissues is currently understood as a multistep process in which a cascade of events described as initial contact and rolling, activation, arrest, and finally diapedesis follow each other (5, 38, 45, 46). Many different molecules participate in the process, yet the organ specificity is regarded to be contributed by a small selection of them. HR are cell surface receptors that specifically bind to their ligands, addressins on the endothelial cells of the target tissues: this binding is a prerequisite for the penetration of the cell through the endothelial cell wall. The organ specificity of lymphocyte homing is based on a differential expression of the addressins in the target tissues. Examination of HR expression on lymphocytes reveals the homing potentials of the cells. Among the HR contributing to the organ specificity of the homing process are α4β7 integrin (guiding cells to the gut mucosa) (3, 13, 17), l-selectin (guiding cells to the peripheral lymph node) (6, 19, 20, 28), and cutaneous lymphocyte antigen (guiding cells to the skin) (2, 39). It has been suggested that the respiratory tract may have its own, still unidentified HR (1).
α4β7 integrin is known as a gut-specific HR, yet the homing mechanisms of cells to other mucosal surfaces are still obscure: this information is currently obtained by analyzing antibody responses in various secretions. To characterize the human immune responses elicited by oral versus rectal antigen administration in different compartments of the immune system, we studied the ASC response with special attention to the homing potentials of the cells and the induction of immune response in serum and various external secretions.
MATERIALS AND METHODS
The immune response to oral and rectal typhoid immunization was evaluated by characterizing the circulating specific ASC with respect to their kinetics, immunoglobulin (Ig) isotype distribution (enzyme-linked immunospot [ELISPOT] assay) and expression of HR (immunomagnetic cell sorting plus ELISPOT assay) and by measuring specific antibody responses in serum and various secretions (enzyme-linked immunosorbent assay [ELISA]).
Volunteers and vaccinations.
Sixteen healthy women (ages 24 to 41) participated in the study. None of the vaccinees had a history of typhoid fever, and none had received a typhoid vaccine during the last 10 years. They all had regular menstrual cycles; two were on birth control pills.
The volunteers received, 2 days apart, a total of four doses of the live, attenuated Salmonella typhi Ty21a vaccine (Vivotif Berna, Swiss Serum and Vaccine Institute, Berne, Switzerland) starting on day 14 of the menstrual cycle; 5 volunteers received the vaccine orally, and 11 received it rectally. Each dose contained at least 2 × 109 to 6 × 109 live bacteria. The orally administered vaccine was in enteric-coated capsules. The rectally administered vaccine was obtained from the enteric-coated capsule by opening the capsule, suspending the contents of it in 1 ml of sterile saline, and administering this suspension in the rectum with a 10-cm-long 1-ml syringe. The vaccine vial and the syringe were washed with an additional 0.5 ml of saline, and this washing solution was administered similarly to the original vaccine dose. To avoid leakage, the rectal vaccine was taken only at bedtime after lying down. The oral vaccine was taken in the morning, preceded and followed by at least a 2-h fast. Informed consent was received from all participants. The study protocol was approved by the Ethical Committee of the University of Alabama at Birmingham.
Collection of samples of blood and secretions.
Samples of heparinized blood were collected from the volunteers on days 0, 7 to 9, 14, and 28 after vaccination. Four orally and six rectally immunized volunteers were screened for the kinetics of the ASC response by drawing blood samples at 2- to 3-day intervals until spots were no longer found.
Sera and external secretions (tears, parotid saliva, nasal wash, intestinal lavage, and cervicovaginal secretions) were collected on days 0, 14, and 28 except for the intestinal lavage, which was collected only on day 28. Sera and the external secretions were frozen immediately after collection and kept at −70°C until assay.
Tears were collected via capillary tube from the medial corner of the eye after stimulation with mist expressed from an orange peel. Parotid saliva was collected with use of Schaefer cups (44). Nasal wash was obtained by instillation of prewarmed saline in the nostrils and expelling after 30 s.
Intestinal secretions were obtained by the oral administration of Golytely (buffered polyethylene glycol solution; Braintree Laboratories, Braintree, Mass.), 2 liters taken within 1 h. The effluent was collected and filtered through cheesecloth, protease inhibitors were added, and the fluid was centrifuged twice as previously described (15). Rectal wicks (Polyfiltronics, Inc., Rockland, Mass.) were used in some volunteers to obtain intestinal samples. The elution of proteins from the wick was performed as described elsewhere (29).
Cervicovaginal secretions were collected by a gynecologist by instillation of 2 to 3 ml of saline into the vagina, rinsing, and aspiration with a plastic pipette (30). The fluid collected was transferred in a tube placed on ice and centrifuged to remove particulate material.
Measurement of antibodies in biological fluids.
ELISA was used for determination of vaccine antigen-specific as well as total levels of Igs in sera and secretions collected from study participants. The assay was performed in 96-well Maxisorp plates (A/S Nunc, Roskilde, Denmark). The inactivated Salmonella strain SL2404 (see below) at a concentration of 106 microorganisms/ml or F(ab′)2 fragment of goat anti-human IgA, IgG, or IgM (Jackson ImmunoResearch Laboratories, Inc., Avondale, Pa.) was used as the capture antigen. Ig trapped by the coating antigens or antibodies was detected by use of biotin-labeled F(ab′)2 of goat IgG anti-human IgA, IgG, or IgM (Biosource International, Camarillo, Calif.) followed by horseradish peroxidase-labeled ExtrAvidin and the ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)]–H2O2 substrate (Sigma Immunochemicals, St. Louis, Mo.). Pooled calibrated human serum, Monitrol (Dade International, Miami, Fla.), and secretory IgA (S-IgA) were used to construct the standard curves. As positive control in each ELISA plate, we used a human serum with high S. typhi-specific antibody titer (a gift from Carol Tacket, University of Maryland School of Medicine). The absorbance was measured in a Vmax photometer (Molecular Devices, Palo Alto, Calif.) at 414 nm. Salmonella-specific antibodies were calculated like total IgA or IgG levels by converting the optical densities in nanograms per milliliter by means of calibration curves constructed from optical density values obtained from dilutions of standardized S-IgA or serum IgG as previously described (42). The calibration curves were constructed, and the results were calculated with of a Delta Soft computer program. Because of the marked variability in the level of Igs in external secretions (due to the unknown dilution factor especially in nasal, vaginal, and rectal lavages), the specific antibody levels were reported as the percentage of the total isotype level.
Isolation of mononuclear cells.
Mononuclear cells were isolated from heparinized venous blood by Ficoll-Hypaque density gradient centrifugation as described earlier (21).
Separation of the receptor-negative and -positive cell populations.
The separation of the cells into receptor-negative and -positive populations has been described in detail elsewhere (24–26). Briefly, the cells were incubated in the first stage with the mouse monoclonal antibodies (MAbs) specific to l-selectin (Leu8; Becton Dickinson, Mountain View, Calif.) and α4β7 (ACT-1; LeukoSite Inc., Cambridge, Mass.) for 30 min on ice. The cells were then washed twice with 1% fetal calf serum–phosphate-buffered saline (PBS) and incubated with Dynal M-450 magnetic beads coated with sheep anti-mouse IgG (Dynal, Oslo, Norway) on ice for 30 min. The beads with the attached cells were then separated from the suspension by applying a magnet outside the test tubes, and the supernatants with the receptor-negative cells were collected. Next, the beads were washed, the magnetic separation was repeated, and the supernatant was pooled with the original population of negatively selected cells. The receptor-positive cells attached to the beads were then suspended in medium. Both receptor-positive and receptor-negative cell populations were immediately analyzed by the ELISPOT assay for numbers of ASC.
Fluorescence-activated cell sorting analyses and efficiency of cell separation.
The efficiency of cell separation was checked in pilot experiments as described earlier (24). The flow cytometric profiles of the original cell population were compared to those of the receptor-negative cells obtained after immunomagnetic separation. Indirect immunofluorescence staining was performed as described previously (24). Briefly, cells were first incubated with the primary mouse MAb ACT-1 or Leu8 or, for control purposes, with an irrelevant MAb of matching isotype, then washed twice, and incubated with the fluorescein isothiocyanate-conjugated goat F(ab′)2 anti-mouse Ig secondary antibody (Tago, Burlingame, Calif.). After being washed twice, the cells were fixed in 2% paraformaldehyde and analyzed with a FACScan (Becton Dickinson). For both α4β7 and l-selectin, over 90% of the brightly staining cells were removed by the immunomagnetic separation.
Assay of specific ASC.
The receptor-positive and -negative cell populations were each tested for specific ASC by ELISPOT assay as described in detail previously (21). In brief, 96-well microtiter plates (Maxisorp; Nunc) were coated with a whole-cell preparation of formalin-killed Salmonella strain SL2404 (108 bacteria/ml of PBS) sharing with the vaccine strain the O-9,12 antigen. The wells were blocked with 1% bovine serum albumin in PBS. Then the cells were incubated in the wells for 3 h, and antibodies secreted during this time were detected with alkaline phosphatase-conjugated swine anti-human IgA, IgM (diluted 1:100; Orion Diagnostica, Espoo, Finland), or goat anti-human IgG (diluted 1:500; Sigma) antiserum followed by application of substrate (5-bromo-4-chloro-3-indolylphosphate) in melted agarose. For each Ig isotype-surface marker combination, 0.8 × 106 to 4.8 × 106 cells were screened. Specific ASC were enumerated by counting the spots in the wells in a light microscope.
Assay of ISC.
Ig-secreting cells (ISC) were enumerated as described in detail previously (21). Microtiter plate wells were coated with human IgA-, IgG-, or IgM-specific antiserum. The next steps were carried out as described above for specific ASC.
Statistics.
The proportions of receptor-positive ASC or ISC were calculated as 100 × (number of ASC or ISC in receptor-positive population)/(sum of number of ASC or ISC in receptor-positive and receptor-negative populations). Percentages of cells expressing the different receptors were determined as arithmetic means of the proportions of ASC or ISC expressing the given cell surface marker ± standard deviation (SD). Statistical comparisons were carried out by Student’s t test.
For each secretion sample assayed, the concentration of specific antibodies was expressed as a percentage of the total antibody concentration (IgA or IgG) in order to compensate for the dilution factor. The data were logarithmically transformed to obtain geometric means ± standard errors of the means (SEM). The paired t test of logarithmically transformed data was used to determine whether the highest postimmunization proportions of specific antibodies were statistically greater than those in preimmune samples of the same immunization group.
Results of all statistical analyses were considered significant only when P was < 0.05.
RESULTS
ASC response. (i) General characteristics.
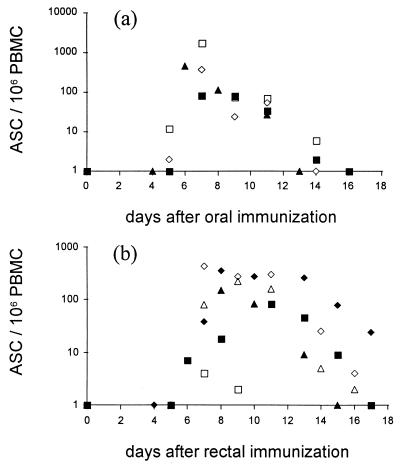
A response of circulating specific ASC was found in 4 of the 5 orally and in 9 of the 11 rectally vaccinated volunteers. The kinetics of the ASC response were studied in four orally and six rectally immunized volunteers (Fig. 1). The kinetics of the response in the two groups appeared to be similar: in most volunteers the ASC appeared in the blood several days after vaccination, peaked on days 7 to 8, and declined on days 14 to 18. The results are consistent with earlier studies on the kinetics of an ASC response after various numbers of oral vaccine doses (21).
FIG. 1.
Kinetics of the circulating specific ASC response after oral (a; n = 4) or rectal (b; n = 6) immunization of humans with live S. typhi Ty21a vaccine. Each volunteer received one dose (at least 109 live bacteria/dose) of vaccine on each of days 0, 2, 4, and 6. Each value represents the sum of IgA-, IgG-, and IgM ASC of one individual on a given day. PBMC, peripheral blood mononuclear cells.
The geometric means of the number of ASC at the peak of the response were 119 ± 18 and 51 ± 9/106 cells after oral and rectal immunization, respectively. This difference was statistically not significant (P < 0.57). The dominating Ig class in the ASC response was IgA in three of four and five of nine subjects after oral or rectal immunization, respectively. In three of nine subjects, rectal immunization induced an IgM-dominated response. This isotype distribution is similar to what has been found earlier with oral Ty21a vaccine (21–23).
(ii) Expression of α4β7.
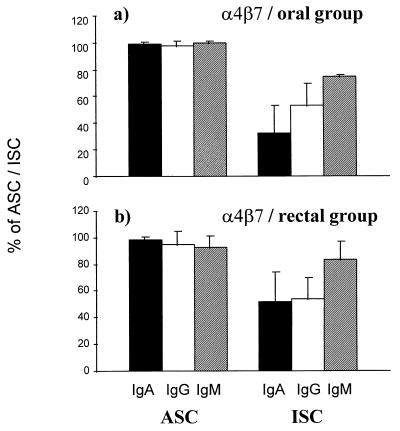
The α4β7 HR was expressed on 99% of ASC in the orally immunized volunteers and on 95% of ASC in the rectally immunized volunteers (Fig. 2). These proportions were significantly higher than those of α4β7-expressing cells among all ISC of the same vaccinees (averages of 43 and 56% for orally and rectally immunized groups, respectively).
FIG. 2.
Expression of the gut HR α4β7 on circulating specific ASC and total ISC in volunteers 7 days after oral (a; n = 4) or rectal (b; n = 9) vaccination with S. typhi Ty21a. The bars indicate arithmetic means (±SD) of percentages of cells expressing α4β7 among IgA (black bars), IgG (white bars), and IgM (hatched bars) ASC and ISC.
(iii) Expression of l-selectin.
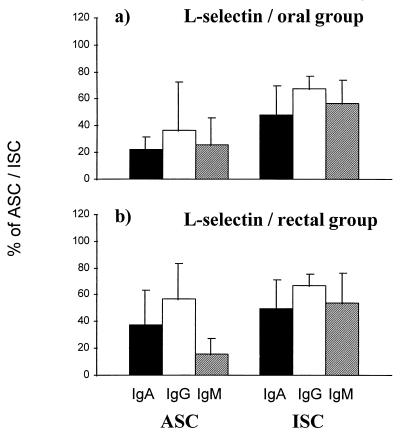
l-Selectin was expressed on similar proportions of ASC after oral (22%) and rectal (38%) immunizations (Fig. 3). The proportions were significantly lower than those among all ISC of the same volunteers (averages of 57 and 54% for orally and rectally immunized groups, respectively) irrespective of the route of immunization.
FIG. 3.
Expression of the peripheral lymph node HR l-selectin on circulating specific ASC and total ISC in volunteers 7 days after oral (a; n = 4) or rectal (b; n = 9) vaccination with S. typhi Ty21a. The bars indicate arithmetic means (±SD) of percentages of cells expressing l-selectin among IgA (black bars), IgG (white bars), and IgM (hatched bars) ASC and ISC.
Antigen-specific antibodies in serum and external secretions. (i) In tears.
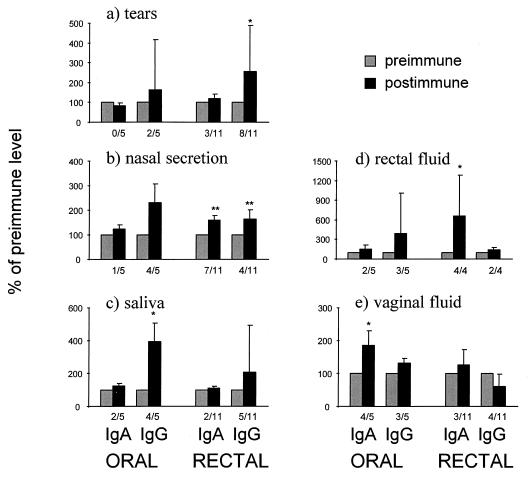
Rectal but not oral immunization elicited an IgG antibody response in tears. No significant increase in specific IgA antibodies was found after rectal or oral immunizations (Fig. 4a).
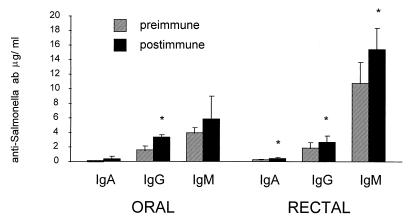
FIG. 4.
Maximal S. typhi-specific antibody responses in tears (a), nasal secretion (b), saliva (c), rectal fluid (d), and vaginal fluid (e) after oral (n = 5) or rectal (n = 11) immunization with S. typhi Ty21a. The data are expressed as percentages (geometric means ± SEM) of preimmune levels of specific antibodies relative to the corresponding isotype. Significant increases in proportions of specific antibodies are indicated with asterisks. Numbers of responders (at least 1.5-fold increase in the proportion of specific antibodies) are indicated below each postimmune bar.
(ii) In nasal mucosa.
In the orally immunized group, no significant increase in the proportion of specific antibodies was found in the nasal wash. In the rectally immunized volunteers, by contrast, a marked response was found both in IgA (P < 0.01) and IgG (P < 0.01) (Fig. 4b).
(iii) In saliva.
The oral route of immunization was more potent than the rectal route in inducing antibodies in saliva. A significant responses was found in IgG (Fig. 4c).
(iv) In rectum.
The postimmunization proportions of specific IgA antibodies in rectal mucosa were significantly elevated in the rectally immunized group, whereas in the orally immunized group no change from the preimmunization level was found after immunization (Fig. 4d).
(v) In vaginal secretions.
Oral immunization was found to be more potent than rectal immunization in inducing an antibody response in the vagina. The proportion of S. typhi-specific IgA antibodies was found to be significantly elevated after oral immunization, whereas no increase was found in the IgG isotype (Fig. 4e).
(vi) In intestinal secretions.
A postimmunization intestinal wash was obtained from five orally and four rectally immunized volunteers. When the proportions of specific IgA antibodies in the postimmunization samples in the orally and rectally immunized volunteers were compared, no statistically significant difference was found (geometric means of the proportions of specific IgA, 1.6 and 6.3% for oral and rectal immunization groups, respectively). Unfortunately, preimmunization samples were not available from the volunteers.
(vii) In serum.
The concentration of S. typhi-specific antibodies in serum increased in both groups following immunization (Fig. 5). After rectal immunization, significant changes were seen in all isotypes, after oral immunization in IgG. Individual responders (>1.5-fold increase in concentrations of specific antibodies) were found in both groups for all isotypes: in the orally immunized group, responses were found in three of five, in two of five, and in one of five volunteers for IgA, IgG, and IgM isotypes, respectively. In the group of 11 rectally immunized volunteers, 7, 1, and 2 responded with specific IgA, IgG, and IgM antibodies, respectively.
FIG. 5.
S. typhi-specific antibodies in serum after oral (n = 5) or rectal (n = 11) immunization with S. typhi Ty21a. The bars indicate geometric mean concentrations (±SEM) of specific IgA, IgG, or IgM antibodies (ab) in serum before and after immunization. Significant increases from preimmune levels are indicated with asterisks.
DISCUSSION
The rectal route of antigen delivery is an alternative to oral immunization for inducing protective immunity via the intestinal route (9, 14, 29, 35). Administration of a vaccine via the rectal route circumvents some pitfalls encountered with oral vaccine administration, such as degradation and denaturation of antigens by enzymes and gastric fluid. An important but seldom recognized problem is developing oral vaccine formulations that can be administered to infants and very young children. This issue is of major concern, since diarrheal diseases are known to occur most frequently in children less than 2 years of age in underdeveloped countries (12). The rectal route is frequently used for administering drugs to small children.
Previous studies have shown that both a mucosal and a systemic immune response can be induced by local administration of an antigen in the colon (36) or rectum (9, 14, 29, 35) in humans. Rectal immunization with Ty21a vaccine has also been shown to induce specific ASC in the peripheral blood of vaccinees (14), similar to that found with orally administered Ty21a (21, 22). However, the contribution of these circulating ASC to the mucosal and systemic immune defense depends on the final effector site to which these cells home. After oral immunization, all circulating ASC have been shown to express the gut mucosal HR α4β7 (24), implying homing to the intestinal mucosa. Because some degree of compartmentalization exists within the CMIS (34), and because differences in the immune system between the various parts of the intestine have been reported (8, 27, 31), it was of interest to see if some differences between immune responses induced at different parts of the gastrointestinal tract could be found.
The ASC responses induced via the oral and rectal immunization routes were found to be similar in magnitude; the responses were dominated by IgA, and practically all cells in both cases expressed α4β7. However, despite the similar ASC responses, the two immunization routes did not induce identical antibody responses in various secretions. This may imply that in addition to α4β7 and l-selectin expression other factors should be studied when the organ specificity of the mucosal homing event is evaluated. The oral route was found to be more effective in inducing a response in saliva and vaginal secretions, whereas tears, rectal mucosal secretions, and nasal wash exhibited a higher response after rectal immunization. Hence, the data indicate that the oral and rectal immunization routes do indeed exhibit certain differences as induction sites, thus providing further evidence for a compartmentalization within the CMIS.
It is generally held that local mucosal immunization at a given site is the most potent way of inducing an immune response at that site (10). Consistently, in the present study rectal immunization was more potent than the oral route in inducing a response in the rectal mucosa. It also appeared that oral immunization may more effectively induce antibodies in the intestinal wash; yet the present study failed to find a statistical significance between the two immunization groups.
The rectal route was found to be more effective than the oral route in inducing a response in the nasal wash. This finding might have a practical application: the nasal mucosa is constantly exposed to many different antigens, and an immune response at this site of entry would certainly be beneficial. The rectal route can easily be used for administration of live, attenuated microbes and could thus represent a useful alternative for induction of an immune response in the nasal as well as other mucosal secretions.
Induction of antibodies in tears has not been a subject of extensive research. Interestingly, the rectal route of immunization was found to elicit IgG and IgA immune responses in tears of approximately half of the volunteers. In previously reported studies (11, 33), the oral route of immunization with formalin-inactivated Streptococcus mutans resulted in the induction of S-IgA but not IgG antibodies in tears. It is possible that the rectal immunization with another vaccine (live attenuated Salmonella) stimulates IgG as well as IgA responses. Whether this is the case for other live vaccines given rectally requires further studies.
Induction of antibodies in saliva is desirable for vaccines against oral infectious diseases such as dental caries (43). Oral immunization seems to be more effective than rectal immunization in the induction of a salivary immune response. This finding is consistent with previous studies (14).
Induction of an immune response in the genital tract has been a subject of increasing interest, especially since the genital tract and the rectal mucosa are portals of entry of human immunodeficiency virus into the body. Consistent with previous studies (29, 35), the present study suggests that oral delivery of antigen would be more potent than rectal delivery in inducing an immune response in the genital tract. In fact, vaginal immunization may be even more potent than immunization by the oral or rectal route in inducing vaginal antibody responses in humans (29).
In conclusion, this study shows that a significant immune response can be induced in humans via the rectal route of immunization, and rectal immunization thus represents an alternative for induction of mucosal immune responses. The differences found in the antibody responses induced by the oral versus rectal routes of antigen delivery provide evidence of compartmentalization within the CMIS in humans. This emphasizes the importance of exploring each mucosal site as a potential route for vaccine delivery.
ACKNOWLEDGMENTS
This work was carried out at the University of Alabama at Birmingham.
This study was supported by Public Health Service grants AI 28147, DE 08182, and AI 34970 and training grant 5-T32-AI 07051 and by the Finnish Academy, Maud Kuistila Foundation, and Sigrid Jusélius Foundation.
REFERENCES
- 1.Abitorabi M A, Mackay C R, Jerome E H, Osorio O, Butcher E C, Erle D J. Differential expression of homing molecules on recirculating lymphocytes from sheep gut, peripheral, and lung lymph. J Immunol. 1996;156:3111–3117. [PubMed] [Google Scholar]
- 2.Berg E L, Yoshino T, Rott L S, Robinson M K, Warnock R A, Kishimoto T K, Picker L J, Butcher E C. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991;174:1461–1466. doi: 10.1084/jem.174.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berlin C, Berg E L, Briskin M J, Andrew D P, Kilshaw P J, Holzmann B, Weissman I L, Hamann A, Butcher E C. α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–195. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 4.Brandtzaeg P. Role of J-chain and secretory component in receptor-mediated glandular and hepatic transport of immunoglobulins in man. Scand J Immunol. 1985;22:111–146. doi: 10.1111/j.1365-3083.1985.tb01866.x. [DOI] [PubMed] [Google Scholar]
- 5.Butcher E C, Picker L J. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 6.Camerini D, James S P, Stamenkovic I, Seed B. Leu-8/TQ1 is the human equivalent of the MEL-14 lymph node homing receptor. Nature. 1989;342:78–82. doi: 10.1038/342078a0. [DOI] [PubMed] [Google Scholar]
- 7.Clancy R L, Cripps A W, Husband A J, Buckley D. Specific immune response in the respiratory tract after administration of an oral polyvalent bacterial vaccine. Infect Immun. 1983;39:491–496. doi: 10.1128/iai.39.2.491-496.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crago S S, Kutteh W H, Moro I, Allansmith M R, Radl J, Haaijman J J, Mestecky J. Distribution of IgA1-, IgA2-, and J-chain containing cells in human tissues. J Immunol. 1984;132:16–18. [PubMed] [Google Scholar]
- 9.Crowley-Nowick P A, Bell M C, Brockwell R, Edwards R P, Chen S, Partridge E E, Mestecky J. Rectal immunization for induction of specific antibody in the genital tract of women. J Clin Immunol. 1997;17:370–379. doi: 10.1023/a:1027312223474. [DOI] [PubMed] [Google Scholar]
- 10.Czerkinsky C, Holmgren J. The mucosal immune system and prospects for anti-infectious and anti-inflammatory vaccines. Immunologist. 1995;3:97–103. [Google Scholar]
- 11.Czerkinsky C, Prince S J, Michalek S M, Jackson S, Russell M W, Moldoveanu Z, McGhee J R, Mestecky J. IgA antibody-producing cells in peripheral blood after antigen ingestion: evidence for a common mucosal immune system in humans. Proc Natl Acad Sci USA. 1987;84:2449–2453. doi: 10.1073/pnas.84.8.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edelman R, Sack R B. Summary of the 21st United States-Japan joint cholera conference. J Infect Dis. 1986;154:377–380. doi: 10.1093/infdis/154.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erle D J, Briskin M J, Butcher E C, Garcia-Pardo A, Lazarovits A I, Tidswell M. Expression and function of the MAdCAM-1 receptor, integrin α4β7, on human leukocytes. J Immunol. 1994;153:517–525. [PubMed] [Google Scholar]
- 14.Forrest B D, Shearman D J C, LaBrooy J T. Specific immune response in humans following rectal delivery of live typhoid vaccine. Vaccine. 1990;8:209–212. doi: 10.1016/0264-410x(90)90047-p. [DOI] [PubMed] [Google Scholar]
- 15.Gaspari M M, Brennan P T, Solomon S N, Elson C O. A method for obtaining, processing, and analyzing human intestinal secretions for antibody content. J Immunol Methods. 1988;110:85–91. doi: 10.1016/0022-1759(88)90086-5. [DOI] [PubMed] [Google Scholar]
- 16.Goldblum R M, Ahlstedt S, Carlsson B, Hanson L-Å, Jodal U, Lidin-Janson G, Sohl-Åkerlund A. Antibody-forming cells in human colostrum after oral immunization. Nature. 1975;257:797–799. doi: 10.1038/257797a0. [DOI] [PubMed] [Google Scholar]
- 17.Hamann A, Andrew D P, Jablonski-Westrich D, Holzmann B, Butcher E C. Role of α4 integrins in lymphocyte homing to mucosal tissues in vivo. J Immunol. 1994;152:3282–3293. [PubMed] [Google Scholar]
- 18.Haneberg B, Kendall D, Amerongen H M, Apter F M, Kraehenbuhl J-P, Neutra M R. Induction of specific immunoglobulin A in the small intestine, colon-rectum, and vagina measured by a new method for collection of secretions from local mucosal surfaces. Infect Immun. 1994;62:15–23. doi: 10.1128/iai.62.1.15-23.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jutila M A. Leukocyte traffic to sites of inflammation. APMIS. 1992;100:191–201. doi: 10.1111/j.1699-0463.1992.tb00861.x. [DOI] [PubMed] [Google Scholar]
- 20.Kansas G S. Structure and function of l-selectin. APMIS. 1992;100:287–293. doi: 10.1111/j.1699-0463.1992.tb00874.x. [DOI] [PubMed] [Google Scholar]
- 21.Kantele A. Antibody secreting cells in the evaluation of the immunogenicity of an oral vaccine. Vaccine. 1990;8:321–326. doi: 10.1016/0264-410x(90)90088-4. [DOI] [PubMed] [Google Scholar]
- 22.Kantele A, Arvilommi H, Jokinen I. Specific immunoglobulin-secreting human blood cells after peroral immunization against Salmonella typhi. J Infect Dis. 1986;153:1126–1131. doi: 10.1093/infdis/153.6.1126. [DOI] [PubMed] [Google Scholar]
- 23.Kantele A, Arvilommi H, Kantele J M, Rintala L, Mäkelä P H. Comparison of the human immune response to live oral, killed oral or killed parenteral Salmonella typhi Ty21a vaccines. Microb Pathog. 1991;10:117–126. doi: 10.1016/0882-4010(91)90072-i. [DOI] [PubMed] [Google Scholar]
- 24.Kantele A, Kantele J M, Savilahti E, Westerholm M, Arvilommi H, Lazarovits A, Butcher E C, Mäkelä P H. Homing potentials of circulating lymphocytes in humans depend on the site of activation: oral, but not parenteral, typhoid vaccination induces circulating antibody-secreting cells that all bear homing receptors directing them to the gut. J Immunol. 1997;158:574–579. [PubMed] [Google Scholar]
- 25.Kantele J M, Arvilommi H, Kontiainen S, Salmi M, Savilahti E, Westerholm M, Jalkanen S, Kantele A. Mucosally activated circulating human B-cells in diarrhoea express homing receptors directing them back to the gut. Gastroenterology. 1996;110:1061–1067. doi: 10.1053/gast.1996.v110.pm8612994. [DOI] [PubMed] [Google Scholar]
- 26.Kantele J M, Kantele A, Arvilommi H. Circulating immunoglobulin-secreting cells are heterogeneous in their expression of maturation markers and homing receptors. Clin Exp Immunol. 1996;104:525–530. doi: 10.1046/j.1365-2249.1996.47751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kett K, Brandtzaeg P, Radl J, Haaijman J F. Different subclass distribution of IgA-producing cells in human lymphoid organs and various secretory tissues. J Immunol. 1986;136:3631–3635. [PubMed] [Google Scholar]
- 28.Kishimoto J K, Jutila M A, Butcher E C. Identification of a human peripheral lymph node homing receptor: a rapidly down-regulated adhesion molecule. Proc Natl Acad Sci USA. 1990;87:2244–2248. doi: 10.1073/pnas.87.6.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kozlowski P A, Cu-Uvin S, Neutra M R, Flanigan T P. Comparison of the oral, rectal, and vaginal immunization routes for the induction of antibodies in the rectal and genital tract secretions of women. Infect Immun. 1997;65:1387–1394. doi: 10.1128/iai.65.4.1387-1394.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kutteh W H, Prince S J, Hammonds K R, Kutteh C C, Mestecky J. Variations in immunoglobulins and IgA subclasses of human uterine cervical secretions around the time of ovulation. Clin Exp Immunol. 1996;104:538–542. doi: 10.1046/j.1365-2249.1996.36742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langman J M, Rowland R. The number and distribution of lymphoid follicles in the human large intestine. J Anat. 1986;194:189–194. [PMC free article] [PubMed] [Google Scholar]
- 32.Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987;7:265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- 33.Mestecky J, McGhee J R, Arnold R R, Michalek S M, Prince S J, Babb J L. Selective induction of an immune response in human external secretions by ingestion of bacterial antigen. J Clin Investig. 1978;61:731–737. doi: 10.1172/JCI108986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moldoveanu Z, Russell M W, Wu H-Y, Mestecky J. Compartmentalization within the mucosal immune system. Adv Exp Med Biol. 1995;371A:97–101. doi: 10.1007/978-1-4615-1941-6_17. [DOI] [PubMed] [Google Scholar]
- 35.Nardelli-Haefliger D, Kraehenbuhl J-P, Curtiss III R, Schodel F, Potts A, Kelly S, De Grandi P. Oral and rectal immunization of adult female volunteers with a recombinant attenuated Salmonella typhi vaccine strain. Infect Immun. 1996;64:5219–5224. doi: 10.1128/iai.64.12.5219-5224.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogra P L, Karzon D T. Distribution of poliovirus antibody in serum, nasopharynx and alimentary tract following segmental immunization of lower alimentary tract with poliovirus. J Immunol. 1969;102:1423–1430. [PubMed] [Google Scholar]
- 37.O’Leary A D, Sweeney E C. Lympho-glandular complexes of the colon: structure and distribution. Histology. 1986;10:267–283. doi: 10.1111/j.1365-2559.1986.tb02481.x. [DOI] [PubMed] [Google Scholar]
- 38.Picker L J. Control of lymphocyte homing. Curr Opin Immunol. 1994;6:394–406. doi: 10.1016/0952-7915(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 39.Picker L J, Kishimoto T K, Smith C W, Warnock R A, Butcher E C. ELAM-1 is an adhesion molecule for skin homing T-cells. Nature. 1991;349:796–811. doi: 10.1038/349796a0. [DOI] [PubMed] [Google Scholar]
- 40.Quiding-Järbrink M, Lakew M, Nordström I, Banchereau J, Butcher E, Holmgren J, Czerkinsky C. Human circulating specific antibody-forming cells after systemic and mucosal immunizations: differential homing commitments and cell surface differentiation markers. Eur J Immunol. 1995;25:322–327. doi: 10.1002/eji.1830250203. [DOI] [PubMed] [Google Scholar]
- 41.Quiding-Järbrink M, Nordström I, Granström G, Kilander A, Jertborn M, Butcher E C, Lazarovits I, Holmgren J, Czerkinsky C. Differential expression of tissue-specific adhesion molecules on human circulating antibody-forming cells after systemic, enteric and nasal immunization. A molecular basis for the compartmentalization of effector B cell responses. J Clin Investig. 1997;99:1281–1286. doi: 10.1172/JCI119286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Russell M W, Brown T A, Radl J, Haaijman J J, Mestecky J. Assay of human IgA subclass antibodies in serum and secretions by means of monoclonal antibodies. J Immunol Methods. 1986;87:87–93. doi: 10.1016/0022-1759(86)90347-9. [DOI] [PubMed] [Google Scholar]
- 43.Russell M W, Mestecky J. Potential for immunological intervention against dental caries. J Biol Buccale. 1986;14:159–175. [PubMed] [Google Scholar]
- 44.Schaefer M E, Rhodes M, Prince S J, Michalek S M, McGhee J R. A plastic intraoral device for collection of human parotid saliva placed over the parotid duct. J Dent Res. 1977;56:728–733. doi: 10.1177/00220345770560070401. [DOI] [PubMed] [Google Scholar]
- 45.Springer T A. Adhesion receptors of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 46.Yednock T A, Rosen S D. Lymphocyte homing. Adv Immunol. 1989;44:313–378. doi: 10.1016/s0065-2776(08)60645-8. [DOI] [PubMed] [Google Scholar]