Abstract
Pasteurella multocida toxin (PMT) is a potent mitogen that also affects bone resorption. PMT acts intracellularly and is therefore postulated to have several domains involved in different aspects of its function. The toxin contains eight cysteine residues. Mutants with individual substitutions for each of these residues were constructed, and the effects of these on the biological activity of the toxin were determined by cultured-cell assays. Only the most C-terminal of the eight cysteines (C1165) was essential for full activity, although mutation of the cysteine residue at position 1159 caused a slight but reproducible loss of potency. In animal challenge experiments, mutant toxin (C1165S) was not toxic to piglets, even at doses exceeding a lethal dose of active PMT 1,000-fold. The mutant and wild-type toxins displayed identical purification characteristics, similar susceptibility to proteolytic digestion, and circular dichroism profiles, which indicated that no gross structural changes had taken place. The function of the essential C1165 residue is not yet known, although its most likely role is an enzymatic one at or near the catalytic center of the toxin.
Pasteurella multocida toxin (PMT) is a highly potent mitogen which at picomolar concentrations induces DNA synthesis leading to cell division in cultured and primary cells. The toxin affects several signal transduction pathways, resulting in increased inositol phosphate production, stimulation of protein kinase C activity, Ca2+ mobilization, and actin rearrangements (17, 20, 33, 38, 39).
PMT action stimulates bone resorption (13, 19, 40) by mitogenic activation of osteoblasts, leading to their growth and dedifferentiation (15, 28); these osteoblasts induce bone breakdown by osteoclasts (29). Experimental nasal infection of animals can also produce proliferation of bladder epithelium (18). This is of potential importance for human health, since toxigenic P. multocida has been isolated from some farm workers (9).
There is convincing evidence that PMT is internalized and processed via a low-pH compartment prior to modifying an intracellular target, which has yet to be identified. The earliest cellular events following exposure to PMT take several hours to arise, compared to the rapid response triggered by membrane-acting growth factors. Similarly, early, but not late, addition of lysosomotrophic agents or neutralizing antibody to PMT-treated cells inhibits DNA synthesis (33). PMT has no action on permeabilized Swiss 3T3 cells, which suggests that processing via the correct trafficking route might be required to release activated toxin or a fragment to the cytosol. Furthermore, PMT is highly resistant to proteolysis at a neutral pH but becomes susceptible at a pH of 5 or lower (36).
PMT is a large molecule (146 kDa). By analogy with other intracellularly acting toxins, it would be predicted to comprise individual domains for binding, internalization, and catalytic activity (26). There is little structural information, and individual domains have not been identified. An N-terminal region homologous to the cytotoxic necrotizing factors (CNF) from Escherichia coli (11, 30) is predicted to have a hydrophobic helical structure and thus is likely to be a transmembrane domain (24, 37). The C terminus of CNF has been shown to be catalytic, while a cell binding domain is located in the N terminus (25).
Many large toxins use disulfide bonds to link or stabilize multiple-domain structures and to enable the delivery of the catalytic fragment to the correct intracellular environment (6). The catalytic domains of diphtheria toxin (4), cholera toxin (14), ricin (3), pertussis toxin (27), Pseudomonas aeruginosa exotoxin A (2), and tetanus toxin (1) are all stabilized in this way. Reduction of the disulfide bond either releases active fragments or enables processing or conformational changes to take place. Moreover, the absence of disulfide bonds within the diphtheria toxin A fragment is crucial for translocation, which can be blocked by the inclusion of disulfide bridges by genetic modification (12). PMT has 8 cysteine residues, but their significance is not known. We have evaluated the role of each cysteine residue, using site-directed mutagenesis. An inactive mutant of PMT was identified, and a structural and biological analysis of this mutant is reported.
MATERIALS AND METHODS
Materials.
All chemical reagents used were of the highest grade available and were obtained from BDH Merck, unless stated otherwise. Microbiological media were prepared from Difco Laboratories products. Cell culture media and materials were from Flow Laboratories. Antibiotics, ethidium bromide, agarose, and acrylamide-bisacrylamide (premixed) were purchased from Sigma Ltd. N,N,N′,N′-tetramethylethylenediamine (TEMED) was purchased from Bio-Rad Laboratories. Restriction enzymes and DNA-modifying enzymes were purchased from Boehringer Mannheim, Promega Corporation, or New England Biolabs and used according to the manufacturer’s instructions. Oligonucleotides were synthesized in house on an Applied Biosystems 392 DNA/RNA synthesizer by Karen Mawditt. Radioisotopes were purchased from DuPont-New England Nuclear. Phenol was obtained from Rathburn Chemicals. DEAE Sephacel ion-exchange resin and Octyl Sepharose 4 Fast Flow medium were purchased from Pharmacia.
Bacterial strains, bacteriophage, plasmids, and growth conditions.
Full-length recombinant toxin is expressed from its own promoter in E. coli hosts at considerably higher levels than that observed in P. multocida, and for this reason all manipulations using infectious material were carried out under category 3+ regulations as defined by the United Kingdom Advisory Committee for Genetic Manipulation.
Two E. coli K-12 strains were used as hosts for recombinant PMT constructs: XL1-Blue (Stratagene) as a general purpose host and CJ236 (Dut− Ung−) (Boehringer Mannheim) for the incorporation of uracil in plasmid DNA. Bacteriophages VCS M13 and R408 (Stratagene) were employed as “helper phages” in the rescue of the single-stranded DNA mutagenesis template from CJ236. A ClaI fragment (of approximately 4 kb) containing the upstream and coding sequences of PMT was excised from the clone pAJL12 (23) and used to make a highly expressing construct, pTox2, in phagemid pBluescript II SK(−) (Stratagene).
E. coli strains were grown routinely, in Luria-Bertani broth (35) or solid medium, aerobically at 37°C. Enriched culture medium (2× yeast extract tryptone medium) was used during the generation of the mutagenesis template to obtain maximal bacterial growth rates. Antibiotic supplements to growth media were used as follows. Tetracycline was used at 10 μg/ml as a means to select for XL1-Blue containing the F′ episome, which enabled the formation of pili and subsequent superinfection by helper phage. Similarly, chloramphenicol was used at 15 μg/ml to select for the F′ episome in CJ236 cultures. All recombinant clones of PMT were grown in the presence of 50 μg of ampicillin/ml.
DNA isolation.
Plasmid DNA for use in subcloning and sequencing reactions was isolated by alkaline lysis (35). Single-stranded DNA was prepared from CJ236 transformed with pTox2, by using either the VCS M13 or the R408 helper bacteriophage (Stratagene), according to the manufacturer’s instructions.
Site-directed mutagenesis.
Negative-strand single-stranded DNA was prepared and used as a mutagenesis template under the conditions specified in the pBluescript II Exo/Mung DNA sequencing system protocol supplied by Stratagene. Oligonucleotides of 26 bases were made to span cysteine codons. Each oligonucleotide was made degenerate at the first and second positions corresponding to the cysteine codons. The oligonucleotides were complementary to the antisense strand of pTox2. All mutant clones were subjected to DNA sequence analysis around the region of the induced mutation by using the Sequenase version 2.0 system (U.S. Biochemical Corp.) in accordance with the manufacturer’s instructions.
Polyacrylamide gel electrophoresis (PAGE).
Proteins were separated in denatured and reduced from on 4% stacking and 8% resolving gels (22). Native protein gels were run under similar conditions but without denaturing or reducing agents.
Silver staining.
Following acrylamide gel electrophoresis, proteins were visualized by a silver-staining technique described by Heukeshoven and Dernick (16).
Toxin purification.
Cleared crude cell lysates and toxin purified for the Swiss 3T3 cell assay were prepared as described previously (41). The preparation of sufficient purified toxin for circular dichroism (CD) analysis and the pig toxicity study required a different approach. Initial fractionation of a cleared bacterial cell lysate by anion-exchange chromatography was carried out as described previously. Selected fractions were dialyzed overnight against 500 mM ammonium sulfate–25 mM sodium phosphate (pH 6.5) at room temperature and were further fractionated by hydrophobic interaction chromatography on Octyl Sepharose 4 Fast Flow medium by using a stepped ammonium sulfate gradient from 500 to 0 mM in 25 mM sodium phosphate (pH 6.5). Selected fractions were concentrated by using 30K Microsep microconcentrators (Filtron Technology Corp., Northborough, Mass.) according to the manufacturer’s instructions.
EBL cell cytotoxicity assay.
The cytotoxicity of toxin preparations was assessed by serial dilution on standard 96-well microtiter plates by a previously published method (34), with the following modifications. Ten-microliter amounts of cleared crude cell lysate were serially diluted 10-fold with 90 μl of Eagle’s minimum essential medium (Flow Laboratories) containing 10% fetal calf serum, and 90 μl of a suspension of embryonic bovine lung (EBL) cells at 3 × 105/ml was added to each well. After incubation for 2 days at 37°C under a 5% CO2 atmosphere, the cells were stained with 0.1% crystal violet in 1% acetic acid-neutral buffered formalin for 1 h and were examined microscopically to determine dilution endpoints for cytotoxicity. A minimum of three independent endpoint determinations were conducted for each cysteine mutant.
Swiss 3T3 fibroblast DNA synthesis assay.
Incorporation of [3H]thymidine into DNA was assessed by the method of Dicker and Rozengurt (8). Confluent, quiescent cultures of Swiss 3T3 cells were washed and incubated at 37°C under a 5% CO2 atmosphere in 2 ml of Dulbecco’s modified Eagle’s medium-Waymouth medium in a 1:1 (vol/vol) ratio, containing 0.037 MBq (1 μCi) of [3H]thymidine per ml and various concentrations of wild-type or mutant PMT in triplicate. After 40 h, DNA synthesis was assessed by measuring the level of [3H]thymidine incorporated into the acid-precipitable material. The average of three values for each sample was determined.
Pig toxicity experiment.
A litter of gnotobiotic piglets was split into five groups of two. At 2 weeks of age, the piglets were weighed and injected intraperitoneally with 2 ml of phosphate buffer containing toxin preparations as follows: group A, phosphate buffer control; group B, 400 ng of wild-type PMT/kg of body weight; group C, 40 μg of mutant PMT (C1165S)/kg; groups D and E, 500 μg of mutant PMT (C1165S)/kg. Following injection, the pigs were observed at regular intervals and their temperatures were recorded on a daily basis for 5 days. Animals in group B showed clinical signs of a toxic reaction and were killed for humane reasons 2 days after inoculation. The remaining piglets were all killed healthy 2 weeks after inoculation. At postmortem examination, the left and right nasal turbinate bones of each piglet were removed and weighed. Liver, kidney, ureter, bladder, and nasal turbinate samples from all piglets were fixed in neutral buffered formalin, embedded in paraffin wax, sectioned, and stained for microscopic examination by hematoxylin and eosin and by Alcian blue–periodic acid-Schiff stain.
Proteolysis of PMT.
Wild-type and mutant toxins were incubated with endoproteinase Glu-C (EC 3.4.21.19) (Sigma) at a 1:1 molar ratio in 25 mM phosphate (pH 6.5) or 50 mM NH4CH3CO2 for 1 h at 37°C. The reaction was stopped, neutralized, and visualized as described by Smyth et al. (36). Some digestions were carried out in the presence of a range of sodium dodecyl sulfate (SDS) concentrations or at different pHs.
Spectral analysis.
CD spectra were measured with a Jobin-Yvon CD6 spectropolarimeter at 22°C. The instrument, maintained under a constant nitrogen purge, was calibrated with an aqueous solution of d-10-camphosulfonic acid. Far-UV (180 to 260 nm) spectra were obtained from protein dissolved in 25 mM phosphate buffer (pH 6.5) at 1 mg/ml by using a 0.01-cm-pathlength sealed silica cell. Results shown are averages of three scans; the signal averaging time for each scan at each wavelength was 3 s. Data are expressed as mean residue ellipticities, [θ]M, derived from the molecular ellipticities, [θ], measured in millidegrees, by using the equation [θ]M = ([θ] × 100)/(C × d × N), where C is the molar protein concentration, d is the pathlength in centimeters, and N is the number of peptide bonds. Spectral deconvolution and secondary-structure analysis was accomplished by using the CONTIN analysis program of Provencher and Glockner (32).
RESULTS
Construction of cysteine mutants.
Oligonucleotides to mutate each of the eight cysteine residues were degenerate at the first and second positions of the cysteine codons in order to substitute a range of amino acids. The frequency of mutation was sufficiently high to enable candidate clones to be screened directly by sequence analysis. Twenty-six mutant clones were isolated and are listed in Table 1. A minimum of two different amino acid substitutions were found at all positions except C1159. Two of the clones contained deletions that resulted in frameshift mutations that encoded premature stop codons.
TABLE 1.
Expression of toxin by cysteine mutant clones
| Clonea | Codon mutation | ∼140-kDa proteinb |
|---|---|---|
| C26G1 | C26G | + |
| C26G2 | C26G | ++ |
| C26D | C26D | + |
| C113G1 | C113G | + |
| C113G2 | C113G | + |
| C113G3 | C113G | + |
| C113V | C113V | + |
| C113*c | Frameshift at C113 | − |
| C230G | C230G | (+) |
| C230D | C230D | (+) |
| C257S | C257S | + |
| C257I | C257I | + |
| C257Y | C257Y | + |
| C793G | C793G | + |
| C793Y | C793Y | + |
| C793* | Frameshift at C793 | − |
| C905D1 | C905D | + |
| C905D2 | C905D | (+) |
| C905D3 | C905D | + |
| C905Y | C905Y | + |
| C1159G1 | C1159G | + |
| C1159G2 | C1159G | ++ |
| C1165G1 | C1165G | + |
| C1165G2 | C1165G | + |
| C1165S | C1165S | + |
| C1165R | C1165R | + |
The independent isolation of identical mutant clones is indicated by subscript numbers 1 through 3.
The presence and intensity of a stained band on SDS-PAGE gels of cleared crude lysates corresponding to the expression of full-length PMT (∼140 kDa) from clone pTox2 was scored visually as follows: −, no ∼140-kDa band visible; (+), ∼50% less than pTox2; +, equivalent to pTox2; ++, ∼50% more than pTox2.
*, premature stop codon generated by a frameshift mutation.
Biological properties of mutant PMT.
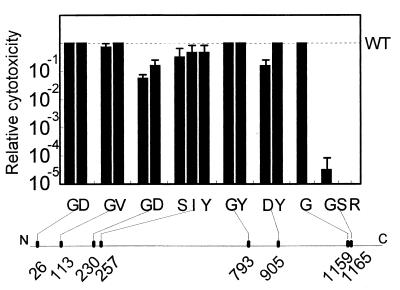
Cleared crude lysates of each mutant clone, with the exception of the two frameshift mutant clones, produced a protein of a molecular weight corresponding to that of PMT (Table 1), and this enabled rough comparisons of the amounts of toxin expressed by each clone to be made. The cytopathic effect of these samples on EBL cells was compared to that caused by wild-type toxin (Fig. 1). Point mutation of any of the first seven cysteine residues of PMT did not appreciably alter the cytopathic effect of the toxin on EBL cells. However, all four clones with mutations at the eighth cysteine residue (C1165) had either a dramatically reduced cytopathic effect or no cytopathic effect on EBL cells. These four independently derived mutants contained substitutions of glycine, serine, or arginine.
FIG. 1.
Cytotoxicity of PMT cysteine mutants for EBL cells. Cleared crude cell lysates of mutant clones were prepared from cultures grown to stationary phase (41). Tenfold serial dilutions of each lysate were made in microtiter plates with cell culture medium, and equal volumes of a suspension of EBL cells at 3 × 105/ml were added to each well. After incubation for 2 days, the cells were stained and examined microscopically to determine dilution endpoints for cytotoxicity, which was compared to that of wild-type (WT) PMT. A minimum of three independent endpoint determinations were conducted for each cysteine mutant. Amino acid substitutions are designated according to the single-letter code. The locations of mutations in the full-length toxin are indicated on the line below.
Toxin was purified from representative mutant clones encoding C26G1, C113G1, C230G, C257S, C793G, C905D1, C1159G1, and C1165G1. The purification characteristics of toxin from all these clones were unaltered during anion-exchange chromatography and on preparative gels. Toxin concentrations were standardized by comparison by eye of the staining intensities of purified mutant toxins on SDS-PAGE gels with that of a preparation of wild-type toxin of known concentration.
The mitogenicity of the purified mutant toxins for Swiss 3T3 cells was determined by using [3H]thymidine incorporation. The correlation between the mitogenic activity of wild-type toxin and those of mutants C26G1, C113G1, C230G, and C257S (data not shown) and mutants C793G and C905D1 (Fig. 2) was very close, while the C1159G1 mutant induced maximal DNA synthesis only at concentrations approximately 10-fold higher than those of wild-type toxin. Repetition using toxin repurified from the same clone (C1159G1) and from a different clone encoding the same mutation (C1159G2) gave similar results (data not shown). Mutant toxin encoding C1165G1 was not mitogenic at a concentration in excess of 100 ng/ml, i.e., at least 2 orders of magnitude greater than that required to achieve half-maximal stimulation by wild-type toxin (Fig. 2). Subsequent experiments showed that toxin purified from mutant C1165S also was not mitogenic (data not shown). These effects were all highly reproducible.
FIG. 2.
Stimulation of DNA synthesis by purified mutant and wild-type PMT in Swiss 3T3 cells. The abilities of mutant toxin preparations to stimulate incorporation of [3H]thymidine into the DNA of quiesced Swiss 3T3 fibroblasts were measured over a concentration range of 0.05 to 120 ng/ml and compared to that of wild-type PMT. Each result is expressed as a percentage of the level of [3H]thymidine incorporation stimulated by 10% fetal calf serum (fcs) (446 × 103 cpm ± 2.13%). Measurements were made in triplicate in two independent experiments, one of which is shown for the four cysteine residues located in the C terminus. Standard errors were calculated at the maximum level of stimulation to be ±3.57% for wild-type PMT, 3.96% for C1159G, and 3.77% for C1165G.
The toxicity of the C1165S mutant was also assessed in a whole-animal model, in order to examine the possibility that the C1165 mutants might have retained some biological activity that could not be identified by cultured-cell assays. The C1165S mutant was chosen because serine is the amino acid most biochemically similar to cysteine. Gnotobiotic piglets given an intraperitoneal dose of 400 ng of wild-type toxin per kg of body weight developed a toxic reaction, fluctuating temperatures, clinical signs of apathy, and anorexia over the 2 days following injection and had to be killed. Postmortem examination revealed pallor, congestion and slight jaundice of the liver, and thickening of bladder walls. Microscopic examination of the liver revealed congestion, foci of active Kupffer cells in one piglet, and vacuolation and moderate cloudy swelling of hepatocytes in both piglets. Hyperplasia of the epithelium of the renal pelvis was evident. Marked vacuolation and hyperplasia of the epithelia of both ureters and the bladder were observed (Fig. 3). There was no effect on turbinate bone growth, the overlying respiratory epithelium, or the tubular epithelium of the kidney. Control piglets and piglets dosed with 40 or 500 μg of the purified C1165S mutant toxin/kg appeared healthy for 2 weeks following injection. No differences or abnormalities were recognized macroscopically in these piglets or in the five tissues examined microscopically upon postmortem examination at 2 weeks. There was no significant difference between the weights of the turbinate bones from piglets treated with mutant toxin and those from controls.
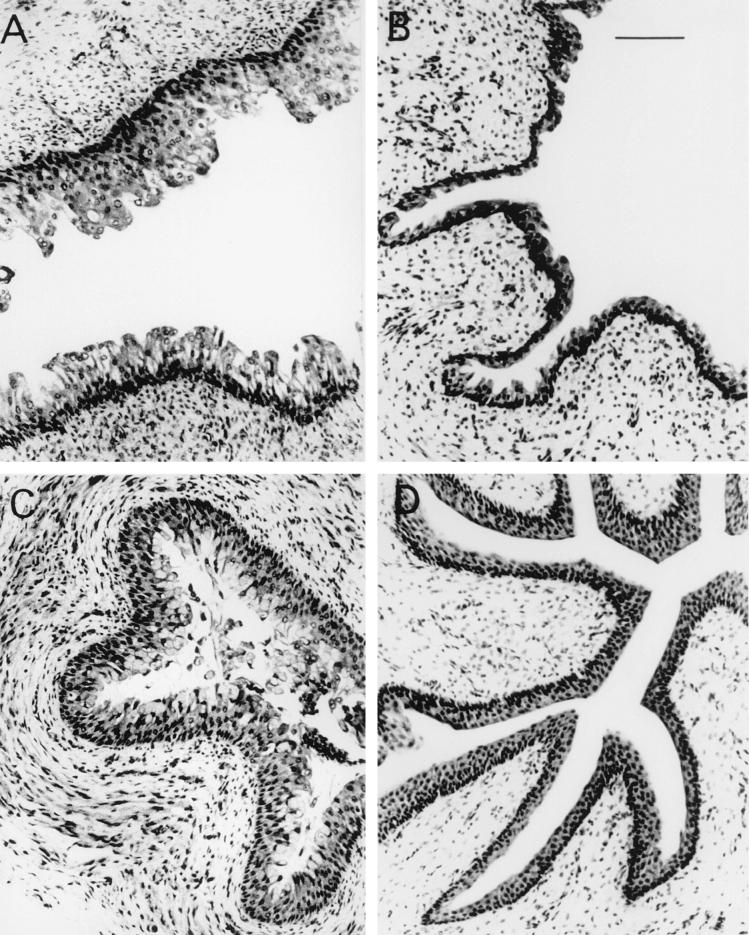
FIG. 3.
Effect of purified C1165S mutant toxin in a gnotobiotic pig model. A bladder (A) and a ureter (C) from a piglet dosed intraperitoneally with 400 ng of wild-type toxin/kg show marked epithelial hyperplasia compared with the normal stratified epithelia of a bladder (B) and a ureter (D) from a piglet treated with 1,250 times the equivalent dose of purified C1165S mutant toxin. Bar, 100 μm.
Biophysical properties of mutant PMT.
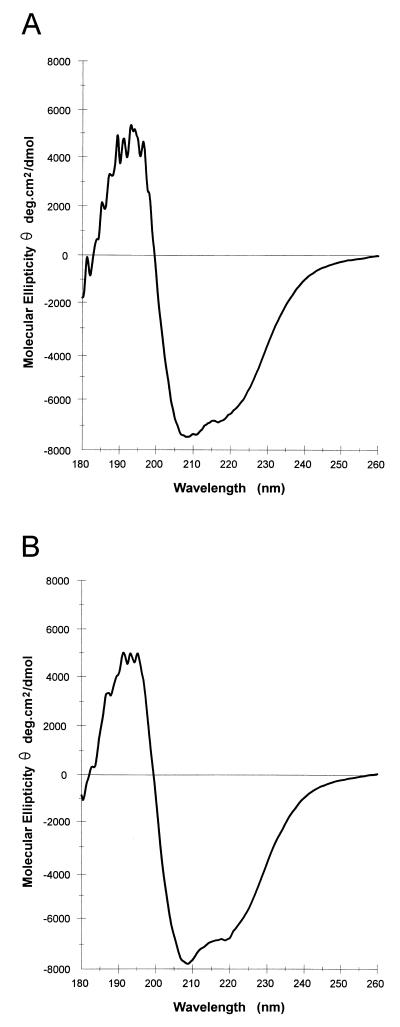
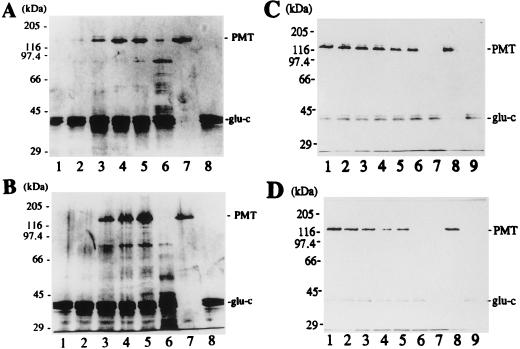
The CD profiles of wild-type PMT and the C1165G mutant were identical (Fig. 4), indicating that replacement of C1165 did not grossly affect structure. The possibility of more subtle changes that might not be distinguished by CD analysis was examined by comparison of the protease sensitivity of PMT under various conditions. At a neutral pH, mutant PMT (C1165S) was highly resistant to endoproteinase Glu-C, even at a 1:1 molar ratio of enzyme to substrate, although there was limited cleavage of a small proportion of the mutant protein that was not observed with wild-type toxin. Both wild-type and mutant toxins became highly susceptible to endoproteinase Glu-C at pH 5 (Fig. 5). Similarly, the mutant toxin appeared slightly more susceptible to proteolysis than wild-type toxin at the lowest SDS concentrations and became highly susceptible to proteolysis at a slightly lower concentration of denaturant than was required for wild-type toxin (Fig. 5).
FIG. 4.
CD spectra of wild-type PMT (A) and C1165G mutant PMT (B). Wild-type and mutant PMT were prepared as described in the text at 1 mg/ml in 25 mM phosphate buffer, pH 6.5, and far-UV (180 to 260 nm) spectra were obtained. The CD spectra shown were derived from three scans of each sample.
FIG. 5.
Susceptibility of wild-type and mutant PMT to proteolysis. (A and B) Wild-type PMT (A) and the C1165S mutant (B) incubated with endoproteinase Glu-C (glu-c) over a range of pH conditions. Lanes 1, pH 4.5; lanes 2, pH 5.0; lanes 3, pH 5.3; lanes 4, pH 6.7; lanes 5, pH 8.0; lanes 6, pH 6.7 plus 0.1% SDS; lanes 7, toxin alone; lanes 8, endoproteinase Glu-C alone. (C and D) Wild-type PMT (C) and the C1165S mutant (D) incubated with endoproteinase Glu-C over a range of SDS concentrations at pH 6.5. Lanes 1, no SDS; lanes 2, 0.005% SDS; lanes 3, 0.0075% SDS; lanes 4, 0.010% SDS; lanes 5, 0.0125% SDS; lanes 6, 0.015% SDS; lanes 7, 0.020% SDS; lanes 8, toxin alone; lanes 9, endoproteinase Glu-C alone. Silver-stained SDS-PAGE gels of the reaction products are shown.
DISCUSSION
We have shown that of the eight cysteine residues in PMT, only the most C-terminal residue is essential for its biological activity. Replacement of C1165 with any of three different amino acids completely abolished all cytotoxicity or mitogenicity for cultured cells. In addition, there were no toxic or proliferative changes in piglets given a dose 1,000 times in excess of a lethal dose of wild-type toxin. Furthermore, we have shown that the mutant toxin appeared to be correctly folded as judged by CD measurements. The transition to protease sensitivity at low pHs mirrored that found in wild-type toxin (36), although there was some limited proteolytic cleavage at a neutral pH. The mutant was also more susceptible to protease digestion in the presence of mild denaturants. Taken together, this indicates that the amino acid substitution has not affected gross structure, but the slight increase in proteolytic susceptibility suggests that the mutant toxin can adopt a more dynamic structure and is susceptible to proteolysis. The loss of biological activity in only one of these mutants showed that, unlike many other toxins, there were no essential disulfide bonds in PMT. Furthermore, since PMT is monomeric (42), C1165 cannot be postulated to form an interchain disulfide bond.
There are several possible roles for this crucial cysteine residue. Given that it is highly unlikely to form an essential disulfide bond, it could be involved in membrane interactions or play a role in the postulated catalytic mode of action of PMT. Several lines of evidence suggest that the latter is its most likely function. First, the region of highest homology to CNF is strongly predicted to be the translocation domain. In all intracellularly acting toxins in which structural assignments have been made, the receptor binding domain is contiguous with the transmembrane domain. Indeed, the correlation between the CNF and PMT sequences is poorest in the C-terminal region that contains the catalytic domain of CNF, which is known to have a target and a mode of action different from those of PMT (7, 21, 33). Injection of oocytes with PMT preincubated with antibodies against an N-terminal peptide reduced the mitogenic response (43). This could be due to blocking of catalytic activity or might reflect interference with toxin processing, or even steric hindrance by the attachment of the large antibody molecule. Although little is known about the domain architecture of PMT, recent detailed structural analysis using secondary-structure prediction algorithms and based on homology to proteins of known structure has given strong indications of the likely domain structure of PMT and would support our postulated role for the C terminus in catalysis (37). The C-terminal part of PMT is predicted to comprise an alternating alpha/beta structure, which is commonly found in the catalytic domain of proteins.
Cysteine can also contribute to catalysis through the formation of metal ion complexes like those in bacterial ferredoxins or zinc finger motifs (5). Petersen (31) suggested the location of a metal binding domain towards the C terminus of PMT, and copper ions have been reported to decrease the toxicity of PMT (10). However, despite a local abundance of histidine and methionine residues in proximity to C1159 and C1165, the C-terminal region of PMT does not encode classical metal binding motifs, and the molecular role of this essential residue has yet to be determined.
ACKNOWLEDGMENTS
We acknowledge the contribution of J. H. Morgan and B. Charleston to the animal experiments. In addition, we thank the staff of the gnotobiotic unit and of the histology and photography departments.
REFERENCES
- 1.Ahnert-Hilger G, Bader M F, Bhakdi S, Gratzl M. Introduction of macromolecules into bovine adrenal-medullary chromaffin cells and rat pheochromacytoma cells (PC12) by permeabilization with Streptolysin-O—inhibitory effect of tetanus toxin on catecholamine secretion. J Neurochem. 1989;52:1751–1758. doi: 10.1111/j.1471-4159.1989.tb07253.x. [DOI] [PubMed] [Google Scholar]
- 2.Allured V S, Collier R J, Carroll S F, McKay D B. Structure of exotoxin A of Pseudomonas aeruginosa at 3.0Å resolution. Proc Natl Acad Sci USA. 1986;83:1320–1324. doi: 10.1073/pnas.83.5.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbieri L, Battelli M G, Stirpe F. Reduction of ricin and other plant toxins by thiol:protein disulphide oxidoreductases. Arch Biochem Biophys. 1982;216:380–383. doi: 10.1016/0003-9861(82)90224-7. [DOI] [PubMed] [Google Scholar]
- 4.Collier R J, Kandel J. Structure and activity of diphtheria toxin. J Biol Chem. 1971;246:1496–1503. [PubMed] [Google Scholar]
- 5.Creighton T E. Proteins: structures and molecular properties. 2nd ed. New York, N.Y: W. H. Freeman and Co.; 1993. [Google Scholar]
- 6.de Paiva A, Poulain B, Lawrence G W, Shone C C, Tauc L, Dolly J O. A role for the interchain disulfide or its participating thiols in the internalization of botulinum neurotoxin A revealed by a toxin derivative that binds to ecto-acceptors and inhibits transmitter release intracellularly. J Biol Chem. 1993;268:20838–20844. [PubMed] [Google Scholar]
- 7.de Rycke J, Mazars P, Nougayrede J-P, Tasca C, Boury M, Herault F, Valette A, Oswald E. Mitotic block and delayed lethality in HeLa epithelial cells exposed to Escherichia coli BM2-1 producing cytotoxic necrotizing factor type 1. Infect Immun. 1996;64:1694–1705. doi: 10.1128/iai.64.5.1694-1705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dicker P, Rozengurt E. Phorbol esters and vasopressin stimulate DNA synthesis by a common mechanism. Nature. 1980;287:607–612. doi: 10.1038/287607a0. [DOI] [PubMed] [Google Scholar]
- 9.Donnio P Y, Avril J L, Andre P M, Vaucel J. Dermonecrotic toxin production by strains of Pasteurella multocida isolated from man. J Med Microbiol. 1991;34:333–337. doi: 10.1099/00222615-34-6-333. [DOI] [PubMed] [Google Scholar]
- 10.Erler W, Jacob B, Schlegel J. The influence of cations on the lethality and on the formation of the toxin of Pasteurella multocida. Microbiol Res. 1994;149:89–93. doi: 10.1016/s0944-5013(11)80146-1. [DOI] [PubMed] [Google Scholar]
- 11.Falbo V, Pace T, Picci L, Pizzi E, Caprioli A. Isolation and nucleotide sequence of the gene encoding cytotoxic necrotizing factor 1 of Escherichia coli. Infect Immun. 1993;61:4909–4914. doi: 10.1128/iai.61.11.4909-4914.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falnes P Ø, Choe S, Madshus I H, Wilson B, Olsnes S. Inhibition of membrane translocation of diphtheria toxin A-fragment by internal disulfide bridges. J Biol Chem. 1994;269:8402–8407. [PubMed] [Google Scholar]
- 13.Felix R, Fleisch H, Frandsen P L. Effect of Pasteurella multocida toxin on bone resorption in vitro. Infect Immun. 1992;60:4984–4988. doi: 10.1128/iai.60.12.4984-4988.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gill D M. Mechanism of action of cholera toxin. Adv Cyclic Nucleotide Res. 1977;8:85–118. [PubMed] [Google Scholar]
- 15.Gwaltney S M, Galvin R J S, Register K B, Rimler R B, Ackermann M R. Effects of Pasteurella multocida toxin on porcine bone marrow cell differentiation into osteoclasts and osteoblasts. Vet Pathol. 1997;34:421–430. doi: 10.1177/030098589703400506. [DOI] [PubMed] [Google Scholar]
- 16.Heukeshoven J, Dernick R. Simplified method for silver staining of proteins in polyacrylamide gels and the mechanism of silver staining. Electrophoresis. 1985;6:103–112. [Google Scholar]
- 17.Higgins T E, Murphy A C, Staddon J M, Lax A J, Rozengurt E. Pasteurella multocida toxin is a potent inducer of anchorage-independent cell growth. Proc Natl Acad Sci USA. 1992;89:4240–4244. doi: 10.1073/pnas.89.10.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoskins I C, Thomas L H, Lax A J. Nasal infection with Pasteurella multocida causes proliferation of bladder epithelium in gnotobiotic pigs. Vet Rec. 1997;140:22. doi: 10.1136/vr.140.1.22. [DOI] [PubMed] [Google Scholar]
- 19.Kimman T G, Lowik C W G M, van de Wee-Pals L J A, Thesingh C W, Defize P, Kamp E M, Bijvoet O L M. Stimulation of bone resorption by inflamed nasal mucosa, dermonecrotic toxin-containing conditioned medium from Pasteurella multocida, and purified dermonecrotic toxin from P. multocida. Infect Immun. 1987;55:2110–2116. doi: 10.1128/iai.55.9.2110-2116.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lacerda H M, Lax A J, Rozengurt E. Pasteurella multocida toxin, a potent intracellularly acting mitogen, induces p125FAK and paxillin tyrosine phosphorylation, actin stress fiber formation and focal contact assembly in Swiss 3T3 cells. J Biol Chem. 1996;271:439–445. doi: 10.1074/jbc.271.1.439. [DOI] [PubMed] [Google Scholar]
- 21.Lacerda H M, Pullinger G D, Lax A J, Rozengurt E. Cytotoxic necrotizing factor 1 from Escherichia coli and dermonecrotic toxin from Bordetella bronchiseptica induce p21rho-dependent tyrosine phosphorylation of focal adhesion kinase and paxillin in Swiss 3T3 cells. J Biol Chem. 1997;272:9587–9596. doi: 10.1074/jbc.272.14.9587. [DOI] [PubMed] [Google Scholar]
- 22.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Lax A J, Chanter N. Cloning of the toxin gene from Pasteurella multocida and its role in atrophic rhinitis. J Gen Microbiol. 1990;136:81–87. doi: 10.1099/00221287-136-1-81. [DOI] [PubMed] [Google Scholar]
- 24.Lax A J, Chanter N, Pullinger G D, Higgins T H, Rozengurt E. Sequence analysis of the potent mitogenic toxin of Pasteurella multocida. FEBS Lett. 1990;277:59–64. doi: 10.1016/0014-5793(90)80809-w. [DOI] [PubMed] [Google Scholar]
- 25.Lemichez E, Flatau G, Bruzzone M, Boquet P, Gauthier M. Molecular localization of the Escherichia coli cytotoxic necrotizing factor CNF1 cell-binding and catalytic domains. Mol Microbiol. 1997;24:1061–1070. doi: 10.1046/j.1365-2958.1997.4151781.x. [DOI] [PubMed] [Google Scholar]
- 26.Moss J, Vaughan M. ADP-ribosylating toxins and G proteins: insights into signal transduction. Washington, D.C: American Society for Microbiology; 1990. [DOI] [PubMed] [Google Scholar]
- 27.Moss J, Stanley S J, Burns D L, Hsia J A, Yost D A, Myers G A, Hewlett E L. Activation by thiol of the latent NAD glycohydrolase and ADP-ribosyltransferase activities of Bordetella pertussis toxin (islet activating protein) J Biol Chem. 1983;258:11879–11882. [PubMed] [Google Scholar]
- 28.Mullan P B, Lax A J. Pasteurella multocida toxin is a mitogen for bone cells in primary culture. Infect Immun. 1996;64:959–965. doi: 10.1128/iai.64.3.959-965.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mullan P B, Lax A J. Pasteurella multocida toxin stimulates bone resorption by osteoclasts via interaction with osteoblasts. Calcif Tissue Int. 1998;63:340–345. doi: 10.1007/s002239900537. [DOI] [PubMed] [Google Scholar]
- 30.Oswald E, Sugai M, Labigne A, Wu H C, Fiorentini C, Boquet P, O’Brien A D. Cytotoxic necrotizing factor type 2 produced by virulent Escherichia coli modifies the small GTP-binding proteins Rho involved in assembly of actin stress fibers. Proc Natl Acad Sci USA. 1994;91:3814–3818. doi: 10.1073/pnas.91.9.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petersen S K. The complete nucleotide sequence of the Pasteurella multocida toxin gene and evidence for a transcriptional repressor, TxaR. Mol Microbiol. 1990;4:821–830. doi: 10.1111/j.1365-2958.1990.tb00652.x. [DOI] [PubMed] [Google Scholar]
- 32.Provencher S W, Glockner J. Estimation of globular protein secondary structure from circular dichroism. Biochemistry. 1981;20:33–37. doi: 10.1021/bi00504a006. [DOI] [PubMed] [Google Scholar]
- 33.Rozengurt E, Higgins T E, Chanter N, Lax A J, Staddon J M. Pasteurella multocida toxin: potent mitogen for cultured fibroblasts. Proc Natl Acad Sci USA. 1990;87:123–127. doi: 10.1073/pnas.87.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rutter J M, Luther P D. Cell culture assay for toxigenic Pasteurella multocida. Vet Rec. 1984;114:393–396. doi: 10.1136/vr.114.16.393. [DOI] [PubMed] [Google Scholar]
- 35.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd Ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 36.Smyth M G, Pickersgill R W, Lax A J. The potent mitogen Pasteurella multocida toxin is highly resistant to proteolysis but becomes susceptible at lysosomal pH. FEBS Lett. 1995;360:62–66. doi: 10.1016/0014-5793(95)00077-m. [DOI] [PubMed] [Google Scholar]
- 37.Sowdhamini, R., P. N. Ward, and A. J. Lax. Unpublished data.
- 38.Staddon J M, Barker C J, Murphy A C, Chanter N, Lax A J, Michell R H, Rozengurt E. Pasteurella multocida toxin, a potent mitogen, increases inositol 1,4,5-triphosphate and mobilises Ca2+ in Swiss 3T3 cells. J Biol Chem. 1991;266:4840–4847. [PubMed] [Google Scholar]
- 39.Staddon J M, Chanter N, Lax A J, Higgins T E, Rozengurt E. Pasteurella multocida toxin, a potent mitogen, stimulates protein kinase C-dependent and -independent protein phosphorylation in Swiss 3T3 cells. J Biol Chem. 1990;265:11841–11848. [PubMed] [Google Scholar]
- 40.Sterner-Kock A, Lanske B, Überschär S, Atkinson M J. Effects of Pasteurella multocida toxin on osteoblastic cells in vitro. Vet Pathol. 1995;32:274–279. doi: 10.1177/030098589503200309. [DOI] [PubMed] [Google Scholar]
- 41.Ward P N, Higgins T E, Murphy A C, Mullan P B, Rozengurt E, Lax A J. Mutation of a putative ADP-ribosylation motif in the Pasteurella multocida toxin does not affect mitogenicity. FEBS Lett. 1994;342:81–84. doi: 10.1016/0014-5793(94)80589-x. [DOI] [PubMed] [Google Scholar]
- 42.Ward, P. N., M. G. Smyth, A. G. Rowe, and A. J. Lax. Unpublished data.
- 43.Wilson B A, Zhu X, Ho M, Lu L. Pasteurella multocida toxin activates the inositol triphosphate signaling pathway in Xenopus oocytes via Gqα-coupled phospholipase c-β1. J Biol Chem. 1997;272:1268–1275. doi: 10.1074/jbc.272.2.1268. [DOI] [PubMed] [Google Scholar]