Abstract
Protein misfolding, aggregation, and spread through the brain are primary drivers of neurodegenerative diseases pathogenesis. Phagocytic glia are responsible for regulating the load of pathogenic protein aggregates in the brain, but emerging evidence suggests that glia may also act as vectors for aggregate spread. Accumulation of protein aggregates could compromise the ability of glia to eliminate toxic materials from the brain by disrupting efficient degradation in the phagolysosomal system. A better understanding of phagocytic glial cell deficiencies in the disease state could help to identify novel therapeutic targets for multiple neurological disorders. Here, we report that mutant huntingtin (mHTT) aggregates impair glial responsiveness to injury and capacity to degrade neuronal debris in male and female adult Drosophila expressing the gene that causes Huntington’s disease (HD). mHTT aggregate formation in neurons impairs engulfment and clearance of injured axons and causes accumulation of phagolysosomes in glia. Neuronal mHTT expression induces upregulation of key innate immunity and phagocytic genes, some of which were found to regulate mHTT aggregate burden in the brain. Finally, a forward genetic screen revealed Rab10 as a novel component of Draper-dependent phagocytosis that regulates mHTT aggregate transmission from neurons to glia. These data suggest that glial phagocytic defects enable engulfed mHTT aggregates to evade lysosomal degradation and acquire prion-like characteristics. Together, our findings reveal new mechanisms that enhance our understanding of the beneficial and potentially harmful effects of phagocytic glia in HD and potentially other neurodegenerative diseases.
INTRODUCTION
Neuron-glia crosstalk is critical for maintaining homeostasis in the central nervous system (CNS), and disruption of these intercellular interactions is increasingly recognized as a central component of many neurological disorders, including neurodegenerative diseases. Glia perform immune surveillance functions in the CNS and respond to neuronal injury by altering gene expression (Magaki et al., 2018) and clearing damaged cells (Raiders et al., 2021; Zheng and Tuszynski, 2023). These glial responses may initially be neuroprotective, but prolonged glial reactivity propels the neurodegenerative disease state, for example, by driving premature loss of living neurons or functional synapses (Neniskyte et al., 2011; Hong et al., 2016; Dejanovic et al., 2022) and inducing neuroinflammation (Liddelow et al., 2017). Expanding our understanding of how glia influence neuron function and survival could reveal promising new therapeutic strategies for neurodegenerative diseases.
A pathological hallmark of most neurodegenerative diseases is the accumulation of misfolded proteins into intra- or extracellular amyloid aggregates in vulnerable regions of the CNS (Knowles et al., 2014). Protein aggregates form due to age-associated decline in cellular protein folding capacity (Santra et al., 2019; Stein et al., 2022) and overwhelming of degradative pathways, including the ubiquitin-proteasome system, autophagy, and phagocytosis (Aman et al., 2021; Duong et al., 2021; Wodrich et al., 2022). As professional phagocytes of the brain, microglia and astrocytes clear damaged and dysfunctional cells (Paolicelli et al., 2011; Wakida et al., 2018; Herzog et al., 2019; Lee et al., 2021) and other pathogenic material, such as protein aggregates (Liu et al., 2017; Choi et al., 2020). Defective glial clearance of debris may be a driving force underlying neurodegenerative disease, highlighted by a growing list of genetic risk variants associated with phagocytic and endolysosomal pathways (Podleśny-Drabiniok et al., 2020). Endolysosomal impairment promotes protein aggregate accumulation; in turn, aggregates can drive further endolysosomal dysfunction, including deacidification and membrane permeabilization of intracellular vesicles (Krasemann et al., 2017; Heckmann et al., 2019; Burbidge et al., 2022; Lee et al., 2022). Aggregates that escape degradation may gain the ability to spread and seed soluble proteins in a prion-like manner (Jucker and Walker, 2018; Monaco and Fraldi, 2020).
Mechanisms by which pathogenic aggregates dysregulate endolysosomal processing remain largely unknown. Intracellular membrane fusion events that regulate endo/phagosome maturation are catalyzed by Rab GTPases (Ng and Tang, 2008; Chan et al., 2011; Langemeyer et al., 2018), enzymes that cycle between active (GTP-bound) and inactive (GDP-bound) states to organize endomembranes into distinct functional domains (Hall, 1990; Chan et al., 2011). Rab dysfunction is implicated in several neurodegenerative diseases—e.g., upregulation of Rab4, Rab5, Rab7, and Rab27 has been observed in Alzheimer’s disease (AD) (Ginsberg et al., 2011), and several Rabs are known substrates of leucine rich repeat kinase 2 (LRRK2), a genetic risk factor in familial Parkinson’s disease (PD) (Jeong et al., 2018). Intriguingly, spread of ɑ-synuclein between cultured neuronal and enteroendocrine cells is mediated by the LRRK2 substrate Rab35 (Bae et al., 2018; Rodrigues et al., 2022), suggesting that Rab-dependent processes may contribute to formation and propagation of pathogenic aggregate seeds.
Here, we investigated the impact of protein aggregates generated in neurons on phagocytic glial cell functions in a Drosophila model of Huntington’s disease (HD). We report that neuronal expression of aggregation-prone mutant huntingtin (mHTT) protein reduces the ability of glia to clear axonal debris and to mount phagocytic and innate immunity transcriptional responses following acute nerve injury. We also observed mHTT-induced changes to numbers of glial lysosomes and Rab+ vesicles in uninjured brains, and identify Rab10 as a novel modifier of prion-like spreading of mHTT aggregates in adult fly brains. Together, these studies shed light on mechanisms by which phagocytic glia respond to and are impaired by accumulation of pathogenic aggregates in neurons.
MATERIALS AND METHODS
Fly husbandry
Fly stocks and crosses were raised on standard cornmeal/molasses media on a 12 hr light/12 hr dark cycle at 25°C, unless otherwise noted. No sex-specific differences were observed in any experiments, so both males and females were utilized in this study. Transgenic or mutant flies were either generated for this study (described below), purchased from Bloomington Drosophila Stock Center, or kindly provided by collaborators. Genotypes and sources of all fly stocks used in this study are listed in Table 1, and complete genotypes of flies used in each figure are listed in Table 2.
Table 1.
Drosophila genotype and source information.
| Name | ID (if available) | Reference | Notes |
|---|---|---|---|
| Or67d-QF | Liang et al., 2013 | ||
| repo-Gal4 | RRID:BDSC_7415 | Sepp et al., 2001 | |
| mz0709-Gal4 | Ito et al., 1995 | a gift from Marc Freeman (Vollum Institute) | |
| alrm-Gal4 | RRID:BDSC_67032 | ||
| orco-Gal4 | RRID:BDSC_23292 | ||
| Or47b-Gal4 | RRID:BDSC_9983 | ||
| QUAS-HTTex1Q25-mCherry attP24 | Donnelly et al., 2020 | ||
| QUAS-HTTex1Q91-mCherry attP24 | Donnelly et al., 2020 | ||
| QUAS-HTTex1Q91-mCherry attP3 | Donnelly et al., 2020 | ||
| QUAS-HTTex1Q91-mCherry suHw(attP8) | This study | ||
| UAS-HTT ex1 Q25-GFP | Donnelly et al., 2020 | ||
| UAS-HTT ex1 Q91-GFP | Donnelly et al., 2020 | ||
| UAS-GFP | Donnelly et al., 2020 | ||
| QUAS-HTT ex1 Q25-GFP | This study | ||
| QUAS-HTT ex1 Q91-GFP | This study | ||
| UAS-mCherry-LGals3 | This study | ||
| UAS-mCherry-LGals8 | This study | ||
| QUAS-HTT ex1 Q25-V5 | This study | ||
| QUAS-HTT ex1 Q91-V5 | This study | ||
| UAS-pHluorin-HTT ex1 Q25-tdTomato | This study | ||
| UAS-pHluorin-HTT ex1 Q91-tdTomato | This study | ||
| UAS-mCD8-GFP | RRID:BDSC_5137 | ||
| UAS-Draper RNAi | Logan et al., 2012 | a gift from Marc Freeman (Vollum Institute) | |
| tubP-Gal80 ts | RRID:BDSC_7017 | ||
| UAS-Drs RNAi | RRID:BDSC_55391 | ||
| UAS-PGRP-SA RNAi | RRID:BDSC_60037 | ||
| UAS-MMP1 RNAi | RRID:BDSC_31489 | ||
| UAS-ets21c RNAi | RRID:BDSC_39069 | ||
| UAS-mCherry RNAi | RRID:BDSC_35785 | ||
| UAS-rel RNAi | RRID:BDSC_33661, RRID BDSC_28943 |
||
| UAS-dl RNAi | RRID:BDSC_38905, RRID:BDSC_32934, RRID:BDSC_34938, RRID:BDSC_27650, RRID:BDSC_36650 |
||
| UAS-NinjurinA RNAi | RRID:BDSC_50632 RRID:BDSC_51358 |
||
| UAS-Toll-6 RNAi | RRID:BDSC_56048, RRID:BDSC_64968 |
||
| Orco-QF2 | RRID:BDSC_91997 | ||
| TI{TI}Rab10 YFP | RRID:BDSC_62548 | ||
| TI{TI}Rab5 YFP | RRID:BDSC_62543 | ||
| TI{TI}Rab7 YFP | RRID:BDSC_62545 | ||
| UAS-MApHS | Han et al., 2014 | a gift from Chun Han (Cornell University) | |
| Repo-Cas9[6A] | Koreman et al., 2021 | a gift from Chun Han (Cornell University) | |
| gRNA-drpr(BR) | Sapar et al., 2018 | a gift from Chun Han (Cornell University) | |
| Jra-GFP.FLAG | RRID:BDSC_50755 | ||
| OK107-Gal4 | |||
| UAS-GFP-LAMP1 | Pulipparacharuvil et al., 2005 | A gift from Helmut Kramer (UT Southwestern) | |
| UAS-LAMP1-GFP | RRID:BDSC_42714 | nSyb-Gal4 was removed from stock | |
| UAS-Rbcn-3a RNAi | RRID:BDSC_34612 | ||
| UAS-Spinster RNAi | RRID:BDSC_27702 | ||
| UAS-Vha100–2 RNAi | RRID:BDSC_64859 | ||
| UAS-Vha16–1 RNAi | RRID:BDSC_40923 | ||
| UAS-Vha68–3 RNAi | RRID:BDSC_42954 | ||
| UAS-YFP-Rab5 | RRID:BDSC_9775 | ||
| UAS-YFP-Rab7 | RRID:BDSC_23641 | ||
| UAS-YFP-Rab10 | RRID:BDSC_24097 | ||
| UAS-YFP-Rab10 (T23N) | RRID:BDSC_9788 | ||
| UAS-YFP-Rab10 (Q68L) | RRID:BDSC_23259 | ||
| UAS-Rab7 RNAi | RRID:BDSC_27051 | ||
| UAS-Rab10 RNAi | RRID:BDSC_26289 | ||
| UAS-Rab2 RNAi | RRID:BDSC_28701 RRID:BDSC_34922 |
||
| UAS-Rab6 RNAi | RRID:BDSC_27490 RRID:BDSC_35744 |
||
| UAS-Rab11 RNAi | RRID:BDSC_27730 | ||
| UAS-Rab3 RNAi | RRID:BDSC_34655 | ||
| UAS-Rab14 RNAi | RRID:BDSC_28708 | ||
| UAS-Rab4 RNAi | RRID:BDSC_33757 | ||
| UAS-Rab5 RNAi | RRID:BDSC_30518 | ||
| UAS-Rab8 RNAi | RRID:BDSC_27519 RRID:BDSC_34373 |
||
| UAS-Rab9 RNAi | RRID:BDSC_42942 RRID:BDSC_34374 |
||
| UAS-luciferase RNAi | RRID:BDSC_31603 | ||
| UAS-Rab9Db RNAi | RRID:BDSC_38269 | ||
| UAS-Rab32 RNAi | RRID:BDSC_38956 RRID:BDSC_28002 |
||
| UAS-Rab18 RNAi | RRID:BDSC_34734 RRID:BDSC_27665 |
||
| UAS-RabX4 RNAi | RRID:BDSC_44070 | ||
| UAS-Rab27 RNAi | RRID:BDSC_50537 | ||
| UAS-RabX2 RNAi | RRID:BDSC_53928 | ||
| UAS-Rab39 RNAi | RRID:BDSC_53995 | ||
| UAS-Rab23 RNAi | RRID:BDSC_55352 RRID:BDSC_63689 RRID:BDSC_36091 RRID:BDSC_28025 |
||
| UAS-Rab35 RNAi | RRID:BDSC_67952 RRID:BDSC_80457 |
||
| UAS-Rab40 RNAi | RRID:BDSC_80472 | ||
| UAS-RabX6 RNAi | RRID:BDSC_26281 RRID:BDSC_53252 |
||
| UAS-Rab19 RNAi | RRID:BDSC_34607 | ||
| UAS-Rab21 RNAi | RRID:BDSC_29403 | ||
| UAS-Rab27 RNAi | RRID:BDSC_35774 | ||
| UAS-Draper-I | Logan et al., 2012 | a gift from Marc Freeman (Vollum Institute) | |
| Rab10(CRISPR-KO) | Kohrs et al., 2021 | a gift from P. Robin Heisinger (Freie Universitat Berlin) |
Table 2.
Genotypes of flies used in all figures.
| Figure | Genotype |
|---|---|
| Figure 1A & C–E | Or67d-QF/Y;QUAS-HTT ex1 Q25-mCherry/QUAS-mCD8-GFP |
| Figure 1B–E | Or67d-QF/Y;QUAS-HTT ex1 Q91-mCherry/QUAS-mCD8-GFP |
| Figure 2A,D–E | Or67d-QF/Y;UAS-HTT ex1 Q25-V5/QUAS-mCD8-GFP;repo-gal4/+ |
| Figure 2B,D–E | Or67d-QF/Y;UAS-HTT ex1 Q91-V5/QUAS-mCD8-GFP;repo-gal4/+ |
| Figure 2C | +/Y;UAS-HTTex1Q25-V5/+;repo-gal4/+ & +/Y;UAS-HTTex1Q91-V5/+;repo-gal4/+ |
| Figure 3A & C–D | w1118/Y;UAS-HTT ex1 Q25-V5/Or47b-Gal4;UAS-MapHS/+ |
| Figure 3B–D | w1118/Y;UAS-HTTex1Q91-V5/Or47b-Gal4;UAS-MapHS/+ ; |
| Figure 3C | w1118/Y;repo-Cas9/Or47b-Gal4;UAS-MapHS/gRNA-draper |
| Figure 4A & C | w1118/Y;+/+;UAS-pHluorin-HTTex1Q25-tdTomato/Orco-Gal4 |
| Figure 4B–C | w1118/Y;+/+;UAS-pHluorin-HTTex1Q91-tdTomato/Orco-Gal4 |
| Figure 4D & F | w1118/Y;+/+;UAS-pHluorin-HTTex1Q25-tdTomato/+;OK107-Gal4/+ |
| Figure 4E–F | w1118/Y;+/+;UAS-pHluorin-HTTex1Q91-tdTomato/+;OK107-Gal4/+ |
| Figure 4G & I | W1118/Y;Repo-Cas9/+;+/+ |
| Figure 4H–I | W1118/Y;Repo-Cas9/+;gRNA-draper/+ |
| Figure 4J & L | W1118/Y;Or47b-Gal4/repo-Cas9;+/UAS- UAS-pHluorin-HTT ex1 Q91-tdTomato |
| Figure 4J–L | W1118/Y;Or47b-Gal4/repo-Cas9;gRNA-draper/UAS- UAS-pHluorin-HTT ex1 Q91-tdTomato |
| Figure 5A |
w1118; UAS-GFP/+;orco-Gal4/+ & w1118; UAS-HTTex1Q25-GFP/+; orco-Gal4/+ & w1118; UAS-HTTex1Q91-GFP/+;orco-Gal4/+ |
| Figure 5C & E | w1118/Y; UAS-HTTex1Q25-V5/+;orco-Gal4/Toll6 MIMICGFP |
| Figure 5D–E | w1118/Y; UAS-HTTex1Q91-V5/+;orco-Gal4/Toll6 MIMICGFP |
| Figure 5F & H | w1118/Y; UAS-HTTex1Q25-V5/+;orco-Gal4/Jra.GFP |
| Figure 5G–H | w1118/Y; UAS-HTTex1Q91-V5/+;orco-Gal4/Jra.GFP |
| Figure 6A–H |
w1118; UAS-HTTex1Q25-GFP/+;orco-Gal4/+ & w1118; UAS-HTTex1Q91-GFP/+;orco-Gal4/+ |
| Figure 7A, A, C, E | w1118; QUAS-HTT ex1 Q25-mCherry/+;orco-QF2/+ |
| Figure 7B–D, F–H | w1118; QUAS-HTT ex1 Q91-mCherry/+;orco-QF2/+ |
| Figure 8A & C–D | or67d-QF,QUAS-HTT ex1 Q91-mCherry/+;QUAS-HTT ex1 Q25-GFP/+;mz0709-Gal4/+ |
| Figure 8B–D | or67d-QF,QUAS-HTT ex1 Q91-mCherry/+;QUAS-HTT ex1 Q25-GFP/+;alrm-Gal4/+ |
| Figure 9A–B, E | (control) or67d-QF,QUAS-HTTex1Q91-mCherry/+;+/+;mz0709-Gal4 /+ & or67d-QF,QUAS-HTTex1Q91-mCherry/+;+/+;mz0709-Gal4/UAS-RNAi-X & or67d-QF,QUAS-HTTex1Q91-mCherry/+; UAS-RNAi-X /+;mz0709-Gal4/+ |
| Figure 9C–E | (control) or67d-QF,QUAS-HTTex1Q91-mCherry/+;+/+;repo-Gal4,Gal80ts/UAS-RNAi-mCherry & or67d-QF,QUAS-HTTex1Q91-mCherry/+;+/+;repo-Gal4, Gal80ts/UAS-RNAi-Ets21c |
| Figure 10A & C–D & F | w1118/Y;UAS-HTT ex1 Q25-GFP/+;orco-Gal4/+ |
| Figure 10B–C & E–H | w1118/Y;UAS-HTTex1Q91-GFP/+;orco-Gal4/+ |
| Figure 11A & G–I | w1118/Y;QUAS-HTTex1Q25-mCherry/UAS-LAMP1-GFP;orco-QF2/repo-Gal4 |
| Figure 11B–C & G–I | w1118/Y;QUAS-HTTex1Q91-mCherry/UAS-LAMP1-GFP;orco-QF2/repo-Gal4 |
| Figure 11D & G–I | w1118/Y;QUAS-HTTex1Q25-mCherry/UAS-GFP-LAMP1;orco-QF2/repo-Gal4 |
| Figure 11E–I | w1118/Y;QUAS-HTTex1Q91-mCherry/UAS-GFP-LAMP1;orco-QF2/repo-Gal4 |
| Figure 12A–B & E–G | Control: QUAS-HTTex1Q25-GFP/+/+;repo-Gal4,orco-QF2/UAS-mCherry-LGals3 & QUAS-HTTex1Q91-GFP/+/+;repo-Gal4,orco-QF2/UAS-mCherry-LGals3 |
| Figure 12C–G | Control: QUAS-HTTex1Q25-GFP/+/+;repo-Gal4,orco-QF2/UAS-mCherry-LGals8 & QUAS-HTTex1Q91-GFP/+/+;repo-Gal4,orco-QF2/UAS-mCherry-LGals8 |
| Fig 13A–C | Control: UAS-HTTex1Q25-GFP/Or67d-QF,QUAS-HTTex1Q91-mCherry;+/+;repo-Gal4/UAS-RNAi.mCherry & UAS-HTTex1Q25-GFP/Or67d-QF,QUAS-mHTT-mCherry;+/+;repo-Gal4/UAS-RNAi-X or UAS-HTTex1Q25-GFP//Or67d-QF,QUAS-mHTT-mCherry /+;UAS-RNAi-X/+ ; repo-Gal4/+ |
| Figure 14A–C | Or67d-QF, QUAS-HTTex1Q91-mCherry /+; QUAS- HTTex1Q25-GFP/UAS-RNAi-X;repo-Gal4/+ & Or67d-QF,QUAS-HTTex1Q91-mCherry /+;QUAS-HTTex1Q25-GFP/+;repo-Gal4/UAS-RNAi-X |
| Figure 14D–E | Or67d-QF,UAS-HTTex1Q25-GFP/+ ; GH146-Gal4,QUAS-HTTex1Q91-mCherry/+ & Or67d-QF,UAS-HTTex1Q25-GFP/+ ; GH146-Gal4,QUAS-HTTex1Q91-mCherry/+ ; drprD5/+ & Or67d-QF,UAS-HTTex1Q25-GFP/rab10{CRISPR-KO} ; GH146-Gal4,QUAS-HTTex1Q91-mCherry/+ & Or67d-QF,UAS-HTTex1Q25-GFP/rab10{CRISPR-KO} ; GH146-Gal4,QUAS-HTTex1Q91-mCherry/+ ; drprD5/+ & Or67d-QF,UAS-HTTex1Q25-GFP/rab10CRISPR ; GH146-Gal4,QUAS-HTTex1Q91-mCherry/rab14{CRISPR-KO} & Or67d-QF,UAS-HTTex1Q25-GFP/rab10CRISPR ; GH146-Gal4,QUAS-HTTex1Q91-mCherry/rab14{CRISPR-KO} ; drprD5/+ |
| Figure 14F | UAS-HTTex1Q25-GFP, QUAS-HTTex1Q91-mCherry/Or67d-QF;+/+;repo-Gal4/+ & UAS-HTTex1Q25-GFP,QUAS-HTTex1Q91-mCherry/Or67d-QF;+/+;repo-Gal4/UAS-RNAi-Rab10 & UAS-HTTex1Q25-GFP,QUAS-HTTex1Q91-mCherry/Or67d-QF;UAS-draper-I/+;repo-Gal4/UAS-RNAi-Rab10 |
| Figure 14G | w1118/w1118;+/+;+/+ (w1118) & Rab10(CRISPR-KO)/w1118;+/+;+/+ & Rab10(CRISPR-KO)/ Rab10(CRISPR-KO);+/+;+/+ |
| Figure 15A | controls: w1118; UAS-GFP/+;orco-Gal4/+ & w1118; UAS-HTTex1Q25-GFP/+;orco-Gal4/+ & w1118; UAS-HTTex1Q91-GFP/+;orco-Gal4/+ |
| Figure 15B–C & H–I | TI{TI}Rab10[EYFP]/Y; UAS-HTTex1Q25-V5/+; orco-Gal4/+ & TI{TI}Rab10[EYFP]/Y;UAS-HTTex1Q91-V5/+;orco-Gal4/+ |
| Figure 15D–E & H–I | w1118/Y;UAS-HTTex1Q25-V5/TI{TI}Rab5[EYFP];orco-Gal4/+ & w1118/Y;UAS-HTTex1Q91-V5/TI{TI}Rab5[EYFP];orco-Gal4/+ |
| Figure 15F–I | w1118/Y;UAS-HTTex1Q25-V5/+;orco-Gal4/TI{TI}Rab7[EYFP] & w1118/Y;UAS-HTTex1Q91-V5/+;orco-Gal4/ TI{TI}Rab7[EYFP] |
| Figure 16A–B & G–I | w1118/Y;QUAS-HTTex125-mCherry/UAS-YFP-Rab10;orco-QF2,repo-Gal4/+ & w1118/Y;QUAS-HTTex1Q91-mCherry/UAS-YFP-Rab10;orco-QF2,repo-Gal4/+ |
| Figure 16C–D & 16G–I | w1118/Y;QUAS-HTTex125-mCherry/UAS-YFP-Rab5;orco-QF2,repo-Gal4/+ & w1118/Y;QUAS-HTTex1Q91-mCherry/UAS-YFP-Rab5;orco-QF2,repo-Gal4/+ |
| Figure 16E–I | w1118/Y;QUAS-HTTex125-mCherry/UAS-YFP-Rab7;orco-QF2,repo-Gal4/+ & w1118/Y;QUAS-HTTex1Q91-mCherry/UAS-YFP-Rab7;orco-QF2,repo-Gal4/+ |
| Figure 17A1–3 | w1118/Y;QUAS-HTTex1Q91-mCherry/UAS-YFP-Rab10;orco-QF2, repo-Gal4/+ |
| Figure 17B1–2 | w1118/Y;QUAS-HTTex1Q91-mCherry/UAS-YFP-Rab5;orco-QF2,repo-Gal4/+ |
| Figure 17C1–2 | w1118/Y;QUAS-HTTex1Q91-mCherry/UAS-YFP-Rab7;orco-QF2,repo-Gal4/+ |
Acute antennal nerve injury was performed by bilateral removal of the second and third antennal segments from anesthetized adult flies (MacDonald et al., 2006). For quantitative PCR analyses, maxillary palps were removed in addition to third antennal segments to sever all olfactory receptor neuron (ORN) axons. Axotomized flies were incubated for the indicated times on standard media prior to dissection and processing for imaging.
Cloning and Drosophila transgenesis
pUASTattB(HTTex1Q25-V5) and pUASTattB(HTTex1Q91-V5) plasmids were cloned by PCR amplification of HTT exon 1 (HTTex1) cDNA using a reverse primer that inserted an in-frame, C-terminal V5 epitope tag (GKPIPNPLLGLDST) and ligation into the pQUASTattB vector backbone via XhoI and XbaI restriction sites. pQUASTattB(HTTex1Q25-GFP) and pQUASTattB(HTTex1Q91-GFP) were generated by replacing the mCherry sequence in pQUASTattB(HTTex1Q25-mCherry) and pQUASTattB(HTTex1Q91-mCherry) plasmids previously generated by our lab (Pearce et al., 2015) with an in-frame, C-terminal GFP sequence via Gibson Assembly (New England Biolabs, Inc. Ipswich, MA). pUASTattB(pHluorin-HTTex1Q25-tdTomato) and pUASTattB(pHluorin-HTTex1Q91-tdTomato) plasmids were generated by PCR amplification of HTTex1 cDNAs and subcloning to replace the CD4 sequence in the pUASTattB(MApHS) plasmid (Han et al., 2014) via In-Fusion cloning (Takara Bio USA, Inc., Mountain View, CA). pUASTattB(mCherry-Galectin-3) and pUASTattB(mCherry-Galectin-8) were cloned by PCR amplification of human LGals3 and LGals8 cDNAs from the pHAGE-mKeima-LGALS3 and pHAGE-FLAG-APEX2-LGALS8 plasmids (Addgene plasmids #175780 and #175758, Watertown, MA) (Eapen et al., 2021) and insertion downstream of an in-frame mCherry sequence in the pUASTattB vector backbone via Gibson Assembly.
Plasmids were microinjected into w- embryos with the su(Hw)attP8, attP24, VK19, or VK27 φC31 attP integration sites at BestGene, Inc (Chino Hills, CA). Table 1 lists detailed genotype information for all transgenic flies generated in this study.
Drosophila brain dissection and sample preparation
Adult fly brains were dissected in ice-cold phosphate-buffered saline (PBS) containing either no detergent or 0.03% (PBS/0.03T), 0.1% (PBS/0.1T), or 0.3% (PBS/0.3T) Triton X-100. Dissected brains were fixed in 4% paraformaldehyde (PFA) in the dark at room temperature (RT) for 20 minutes. For imaging of GFP or mCherry fluorescence signals, brains were washed 7X in PBS/0.03T buffer before incubation in Slowfade Gold Antifade Mountant (Invitrogen, Carlsbad, CA). When imaging pHluorintagged and other pH-sensitive constructs, such as GFP-LAMP1, brains were washed 7X in PBS for at least 50 minutes at RT before incubation in Slowfade. For immunostaining, brains were washed 7X in PBS/0.3T after fixation, blocked in PBS/0.3T containing 5% normal goat serum (Lampire Biological Laboratories, Pipersville, PA) for 30 min at RT, incubated in primary antibodies diluted in blocking solution for 24–48 hours at 4°C, washed 7X in PBS/0.3T, and then incubated in secondary antibodies diluted in blocking solution for 16–20 hours at 4°C. After a final set of 7X PBS/0.3T washes, dissected brains were incubated in Slowfade. For Magic Red and LysoTracker staining, brains were dissected in PBS and incubated in 1:1,000 LysoTracker Red DND-99 (Invitrogen, Carlsbad, CA) or 1:1,250 Magic Red (ImmunoChemistry, Davis, CA) diluted in PBS for 20 minutes at RT. Brains were washed 5X in PBS for 15 min total, fixed in 4% PFA in PBS/0.1T for 20 minutes at RT, washed 6X in PBS/0.1T for 30 min total, and incubated in Slowfade. Following incubation in Slowfade for 1–24 hours at 4°C in the dark, all brains were bridge-mounted in Slowfade on glass microscopy slides under #1.5 cover glass, and edges were sealed using clear nail polish.
Primary antibodies used in this study include: chicken anti-GFP (RRID: AB_300798; 1:1,000; Abcam, Cambridge, UK), chicken anti-GFP (RRID: AB_2534023; 1:500; Thermo Fisher Scientific Inc., Waltham, MA), rat anti-N-cadherin (clone DN-Ex #8; RRID: AB_528121; 1:75; Developmental Studies Hybridoma Bank, Iowa City, IA), mouse anti-Repo (clone 8D12; RRID: AB_528448; 1:25; Developmental Studies Hybridoma Bank, Iowa City, IA), mouse anti-V5 (RRID: AB_2556564; 1:125; Thermo Fisher Scientific Inc., Waltham, MA), and rabbit anti-Draper (1:500; a kind gift from Marc Freeman, Vollum Institute, Portland, OR). Secondary antibodies include: AlexaFluor 405 goat anti-rabbit (RRID: AB_221605; 1:250), FITC-conjugated donkey anti-chicken (RRID: AB_2340356; 1:250; Jackson Immuno Research Labs, West Grove, PA), AlexaFluor 568 goat anti-mouse (RRID: AB_2534072; 1:250), AlexaFluor 647 goat anti-rabbit (RRID: AB_2535812; 1:250), and AlexaFluor 647 goat anti-rat IgGs (RRID: AB_141778; 1:250) (Invitrogen, Carlsbad, CA).
Image acquisition
All microscopy data were collected on a Leica SP8 laser-scanning confocal system equipped with 405 nm, 488 nm, 561 nm, and 633 nm lasers and 40X 1.3 NA or 63X 1.4 NA oil objective lenses. Leica LAS-X software was used to establish optimal settings during each microscopy session and to collect optical z-slices of whole-mounted brain samples with Nyquist-optimized sampling criteria. Optical zoom was used to magnify and establish a region of interest (ROI) in each sample. For images showing a single glomerulus, confocal slices were collected to generate ~73 × 73 × 20 μm (xyz) stacks, with z-axis boundaries established using fluorescent signal in DA1 or VA1lm ORN axons. For images of a single antennal lobe, confocal slices were collected to generate ~117 × 117 × 26 μm (xyz) stacks, which were located using HTTex1 fluorescence in ORN axons.
Post-imaging analysis
Raw confocal data were analyzed in 2D using ImageJ/FIJI (RRID:SCR_002285; NIH, Bethesda, MD) or in 3D using Imaris image analysis software (RRID:SCR_007370; Bitplane, Zürich, Switzerland). Methods used for image segmentation and semi-automated quantification of fluorescent signals were previously described (Donnelly et al., 2020). Briefly, raw confocal data were cropped to establish an ROI for further analysis and displayed as a 2D sum intensity projection (ImageJ) or a 3D volume (Imaris). Background fluorescence was subtracted from raw confocal images. mCD8-GFP and HTTex1-mCherry fluorescent signals was segmented using the ‘Surfaces’ function in Imaris (surface detail = 0.25 μm, background subtraction = 0.75 μm). Using the ‘split touching objects’ option, seed point diameter was set to 0.85 μm. pHluorin- and tdTomato-labeled VA1lm ORN axons were quantified in central 30 × 30 pixels, 50-slice ROIs from sum intensity projections of each VA1lm glomerulus. pHluorin- and tdTomato-labeled Or83b+ ORN axons or MBN soma were quantified from central 100 × 100 pixels, 75-slice ROIs of each antennal lobe or mushroom body calyx, and background intensity was subtracted from sum intensity projections. Quantification of Toll-6MIMICGFP fluorescence was performed using the ‘Spots’ function in Imaris (xy diameter = 0.5 μm, z-diameter = 1.0 μm), and glial nucleiassociated GFP-Jra signal was quantified using the “Surfaces” function to identify Repo+/GFP+ nuclei (surface detail = 0.2 μm, background subtraction = 1.6 μm, number of voxels >10).
mCherry-tagged mHTTex1 and GFP-tagged wtHTTex1 aggregates were identified and quantified as previously reported (Donnelly et al., 2020). Briefly, mCherry+ surfaces were segmented and measured in Imaris (surface detail = 0.2 μm, background subtraction = 0.45 μm, seed point diameter = 0.85 μm). Seeded wtHTTex1 aggregates were identified as mHTTex1-mCherry+ objects that overlapped with wtHTTex1-GFP signal. Intracellular vesicles were identified using the ‘Surfaces’ algorithm in Imaris (surface detail = 0.2 μm, background subtraction = 0.4 μm, volume >0.001 μm3) to segment fluorescent signals associated with lysosomes (MR+, LTR+, LAMP1-GFP+, GFP-LAMP1+, mCherry-Galectin-3+, or mCherry-Galectin-8+) or phagosomes (YRab+ or YFP-Rab+). Intracellular vesicles associated with mHTTex1 aggregates were identified by filtering for lysosomal or phagosomal surfaces within 0.2 μm of mHTTex1 objects using the “Shortest Distance” calculation in Imaris.
qPCR
Transgenic Drosophila were flash frozen in liquid nitrogen, and heads were collected using a microsieve with a 230 nm filter to separate bodies from heads and 170 nm filter to separate heads from appendages. Total RNA was extracted from isolated fly heads using the Zymo Direct-Zol RNA miniprep kit (Zymo Research, Irvine, CA). Extracted RNA was quantified on a Nanodrop 2000 (Thermo Fisher Scientific), and equal quantities of each sample were subjected to cDNA synthesis using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Waltham, MA). qPCR was performed on a T100 Thermal Cycler with a CFX384 Real-Time System (Bio-Rad Laboratories, Hercules, CA) using 10 ng of input RNA per replicate and TB Green Premix Ex Taq II (Takara Bio, Kusatsu, Japan). Sequences of all qPCR primers used in this study are listed in Table 3.
Table 3.
Primer sequences used for qPCR analyses.
| Gene Target | Forward (F) and Reverse (R) Primer Sequences (5’-3’) |
|---|---|
| rpl32 | F: CCGCTTCAAGGGACAGTATCTG R: ATCTCGCCGCAGTAAACGC |
| toll-6 | F: AATATTGTGGAGTGCTCGGG R: GCGTTTAAGGCCACTGAAAG |
| dorsal | F: ATGCGAGCGGTGTTCAGTAA R: ACGATGCGAAAAGCCAGTCT |
| relish | F: ACAGGACCGCATATCG R: GTGGGGTATTTCCGGC |
| draper-I | F: TGTGATCATGGTTACGGAGGAC R: CAGCCGGGTGGGCAA |
| mmp1 | F: GAAGGCTCGGACAACGAGT R: GTCGTTGGACTGGTGATCG |
| ets21c | F: CAACGACGACGAACCAAAT R: GTTCGCGTTGGACGAATC |
| rab10 | F: ACATCCGCCAAGTCGAACAT R: CTGGTTCCGGCGATCGATAA |
| rab5 | F: AGTCCGCTGTGGGCAAGTC R: CTCCTGGTACTCGTGGAACTGTC |
| rab7 | F: AATTTTGCACGCAACCGCTG R: GAGTAGCCAATTCGATGGTGC |
| drosomycin | F: GCTGTCCTGATGCTGGTGGT R: CGGAAAGGGACCCTTGTATCTTC |
| attacin-a | F: CTCGTTTGGATCTGACCAAGG R: CCATGACCAGCATTGTTGTAG |
| attacin-d | F:CGTTGAGGTTGAGATTGCCACT R: CGGTCCCTCAGTTCGGCATGAC |
| diptericinA | F: CCACCGCAGTACCCACTCAAT R: CGATGACTGCAAAGCCAAAACCA |
| metchnikowin | F: CAGTGCTGGCAGAGCCTCAT R: CGTCGGTTAGGATTGAAGGGCGA |
Experimental design and statistical analyses
All quantified data were organized and analyzed in Prism 9 software (Graphpad, San Diego, CA). Power analyses to determine appropriate sample sizes for each experiment were calculated using a Sample Size calculator available at ClinCalc.com (ɑ = 0.05, β = 0.2). All quantifications are graphed as mean ± standard error of the mean (s.e.m). A single glomerulus or antennal lobe represented one biological replicate. Statistical comparisons were performed using the following tests where appropriate: unpaired, two-tailed t-test when comparing two samples and ANOVA followed by post hoc multiple comparison tests when comparing ≥3 samples. Detailed statistical information for each experiment, including sample sizes (n), means, and test results are summarized in Table 4.
Table 4.
Statistical information for all quantitative results.
RESULTS
Expression of mHTT exon 1 fragments in neurons inhibits phagocytic clearance of axonal debris
HD is a monogenic neurodegenerative disease caused by expansion of a CAG repeat region in exon 1 of the huntingtin (HTT/IT15) gene beyond a pathogenic threshold of 37 repeats, leading to production of mHTT proteins containing expanded N-terminal polyglutamine (polyQ≥37) tracts (Scherzinger et al., 1999). mHTT proteins are prone to misfolding and accumulate into insoluble aggregates (Wanker, 2000), whereas wild-type HTT (wtHTT) proteins containing polyQ≤36 tracts remain soluble unless seeded by a pre-formed HTT aggregate (Preisinger et al., 1999; Chen et al., 2001). To recapitulate these molecular features of HD, we generated transgenic flies that express N-terminal exon 1 fragments of human mHTT (mHTTex1) containing a polyQ91 tract or wtHTT (wtHTTex1) with a polyQ25 tract via the GAL4-UAS (Brand and Perrimon, N., 1993) or QF-QUAS binary expression systems (Potter and Luo, 2011). As we have previously reported (Pearce et al., 2015; Donnelly et al., 2020), fluorescent protein fusions of mHTTex1 and wtHTTex1 appeared punctate (i.e., aggregated or insoluble) or diffuse (i.e., soluble), respectively, in axon termini of the DA1 type of olfactory receptor neurons (ORNs), which synapse in the DA1 glomerulus of the antennal lobe in the central brain of adult flies (Couto et al., 2005) (Fig. 1A and B).
Figure 1. mHTTex1 expression in ORNs impairs clearance of injured axons.
(A-B) Maximum intensity projections of mCD8-GFP-labeled DA1 ORN axons expressing (A) HTTex1Q25- (wtHTTex1) or (B) HTTex1Q91-mCherry (mHTTex1) in 7 day-old uninjured flies (left) or 14 day-old flies subjected to bilateral antennal nerve axotomy 7 days earlier (right). Scale bars = 5 μm. (C-D) Quantification of (C) mCD8-GFP+ and (D) HTTex1-mCherry+ DA1 ORN axons remaining in 7, 14, and 28 day-old uninjured flies or flies at 1, 3, and 5 days post-injury. (E) Quantification of mCD8-GFP+ DA1 ORN axons in 7, 14, and 28 day-old flies expressing HTTex1Q25- or HTTex1Q91-mCherry in DA1 ORNs. All quantified data were normalized to uninjured 1 day-old adults and graphed as mean ± s.e.m.; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 by unpaired two-tailed t-test.
To monitor the capacity of glia to maintain CNS homeostasis in the presence of mHTTex1 aggregates, we performed a series of experiments that quantified glial responses to acute injury in the adult fly CNS. Surgical removal of the second and third antennal segments initiates Wallerian degeneration of ORN axons, inducing a robust phagocytic glial response that involves upregulation of the scavenger receptor, Draper, and clearance of axonal debris within 7 days (MacDonald et al., 2006). To determine if neuronal mHTTex1 expression affects the ability of glial cells to efficiently clear axonal debris, we co-expressed mCherry-tagged HTTex1 transgenes with membrane-targeted GFP (mCD8-GFP) in DA1 ORNs and measured GFP and mCherry fluorescence intensities following antennal nerve axotomy (Fig. 1A–D). Interestingly, mHTTex1 expression was associated with reduced steady-state mCD8-GFP levels in DA1 ORN axons in 2 and 4 week-old flies (Fig. 1E), likely due to neurotoxicity caused by accumulation of mHTTex1 aggregates over time. Quantification of DA1 ORN axons remaining after antennal nerve injury revealed that clearance of axonal debris was reduced in flies co-expressing neuronal mHTTex1 compared with controls expressing wtHTTex1 (Fig. 1C). This effect could be observed as early as 1 day post-injury, suggesting that mHTTex1 causes defects in both clearance and engulfment of axonal debris. Delayed clearance of axonal debris was exacerbated in older (14 and 28 day-old) mHTTex1-expressing flies (Fig. 1C), possibly related to a decline in Draper activity during normal aging (Purice et al., 2016) and/or enhanced glial dysfunction compounded by mHTTex1 aggregate accumulation. Quantification of mCherry fluorescence indicated that clearance of axonal mHTTex1 was also slowed compared to wtHTTex1 (Fig. 1D), suggesting that glia are deficient in degrading both axonal debris and neuronal mHTTex1 aggregates.
We also tested whether formation of mHTTex1 aggregates in glial cells impacts ORN axonal debris clearance following acute injury. Restricting expression of mHTTex1 to glia resulted in appearance of heterogeneously-sized mHTTex1 aggregates throughout the brain (Fig. 2A). Glial mHTTex1 aggregates also slowed injury-induced clearance of mCD8-GFP-labeled axons compared with wtHTTex1-expressing controls (Fig. 2B–D), though glial mHTTex1 expression did not affect DA1 ORN axon abundance in 1 day-old flies (Fig. 2E). Together, these findings indicate that mHTTex1 aggregates originating in either neurons or glia slow efficient clearance of injured ORN axons by phagocytic glia.
Figure 2. mHTTex1 expression in glia is associated with reduced ORN axon clearance post-injury.
(A-B) Maximum intensity projections of antennal lobes from 5–6 day-old flies expressing HTTex1Q25- (top) or HTTex1Q91-V5 (bottom) in glia and immunostained with anti-V5. Scale bars = 10 μm. (B-C) Maximum intensity projections of mCD8-GFP-labeled DA1 ORN axons in 1 day-old flies expressing (B) HTTex1Q25- or (C) HTTex1Q91-V5 in repo+ glia. Scale bars = 5 μm. (D) Quantification of mCD8-GFP+ DA1 ORN axons in flies expressing HTTex1Q25- or HTTex1Q91-V5 in repo+ glia, either uninjured or at 1 and 3 days post-injury. (E) Quantification of mCD8-GFP+ DA1 ORN axons in 1 day-old flies expressing HTTex1Q25- or HTTex1Q91-mCherry in repo+ glia. All data were normalized to uninjured 1 day-old adult flies and graphed as mean ± s.e.m.; ***p<0.001 by unpaired two-tailed t-test.
Neuronal mHTT aggregates impair nascent phagosome formation
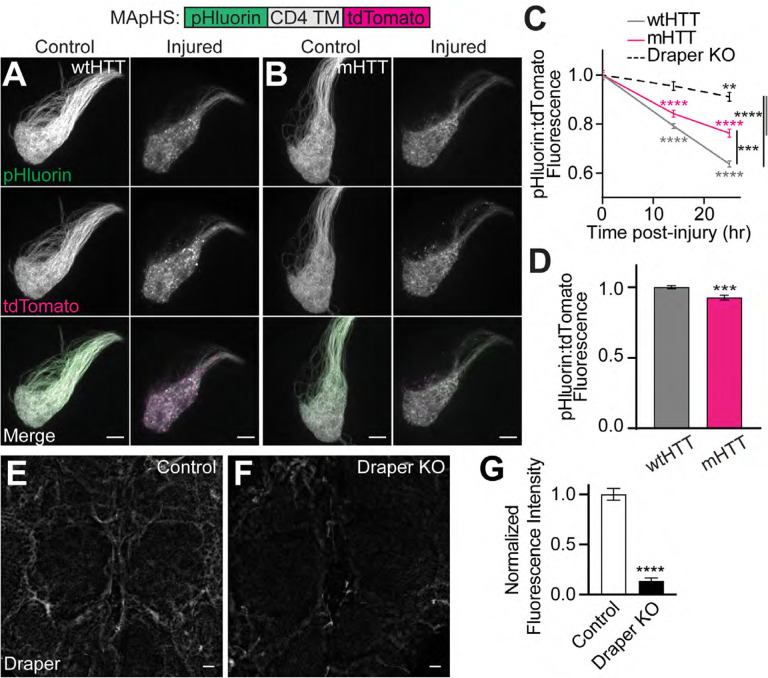
Phagocytosis occurs via 4 major steps: (1) extension of phagocyte membranes toward extracellular “find me” cues, (2) recognition of “eat me” signals by scavenger receptors, (3) cytoskeletal and plasma membrane reorganization to surround and internalize extracellular material, and (4) maturation of nascent phagosomes through sequential endomembrane fusions that culminate at the lysosome (Vieira et al., 2002). We have previously reported that the conserved scavenger receptor, Draper/Ced-1/MEGF10 (MacDonald et al., 2006; Evans et al., 2015; Iram et al., 2016), regulates engulfment, clearance, and intercellular spreading of mHTTex1 aggregates originating in ORN axons (Pearce et al., 2015; Donnelly et al., 2020). To determine whether delayed clearance of injured axons containing mHTTex1 aggregates (Fig. 1C and 2D) could result from defective Draper-dependent engulfment, we co-expressed V5-tagged mHTTex1 or wtHTTex1 with the ratiometric membrane-associated pH sensor (MApHS), consisting of the transmembrane domain of CD4 flanked by N-terminal ecliptic pHluorin and C-terminal tdTomato (Han et al., 2014), in the VA1lm type of ORNs. Ecliptic pHluorin is brightest at pH 7.5 and dims as pH drops, quenching at pH <6.0 (Miesenböck et al., 1998), whereas tdTomato fluorescence is pH resistant. Thus, internalization of MApHS-labeled ORN axonal debris into a rapidly-acidified nascent phagosome can be monitored by calculating pHluorin:tdTomato fluorescence intensity ratios (Han et al., 2014). Indeed, pHluorin:tdTomato ratios in VA1lm axons were decreased at 14 and 25 hours post-axotomy (Fig. 3A–C), and this effect was lost when draper was deleted from glia using the tissue-specific CRISPR/Cas9-TriM method (Fig. 3C and E–G) (Poe et al., 2019), demonstrating that this construct accurately reports nascent phagosome acidification following engulfment. Notably, the decrease in pHluorin:tdTomato ratio following injury was less pronounced in VA1lm ORN axons co-expressing mHTTex1 compared with wtHTTex1-expressing controls (Fig. 3A–C), suggesting that mHTTex1 aggregates impair nascent phagosome formation following engulfment.
Figure 3. mHTTex1 expression inhibits engulfment of injured ORN axons.
(A-B) Maximum intensity projections of VA1lm ORN axons co-expressing the ratiometric phagocytic indicator, MApHS, and (A) HTTex1Q25- or (B) HTTex1Q91-V5 from 7 day-old uninjured flies (left) and flies 25 hours post-injury (right). Scale bars = 10μm. (C) pHluorin:tdTomato fluorescence intensity ratios calculated in VA1lm glomeruli from 7-day-old uninjured flies and flies at 14 or 25 hours post-injury. Data were normalized to the uninjured condition for each genotype. (D) pHluorin:tdTomato fluorescence intensity ratios in 7 day-old flies co-expressing MApHS with HTTex1Q25- or HTTex1Q91-V5 in VA1lm ORNs, normalized to wtHTTex1 controls. Data are shown as mean ± s.e.m.; **p<0.01, ***p<0.001, ****p<0.0001 by unpaired two-tailed t-test. (E-F) Maximum intensity projections of the central brain from 6–7 day-old flies expressing (E) repo-Cas9 and (F) repo-Cas9 plus gRNAs targeting draper (“Draper KO”). Brains were immunostained with anti-Draper. Scale bars = 10 μm. (G) Quantification of Draper immunofluorescence in brains from flies shown in (E-F), normalized to control.
Neuronal mHTT accumulates in low pH intracellular compartments
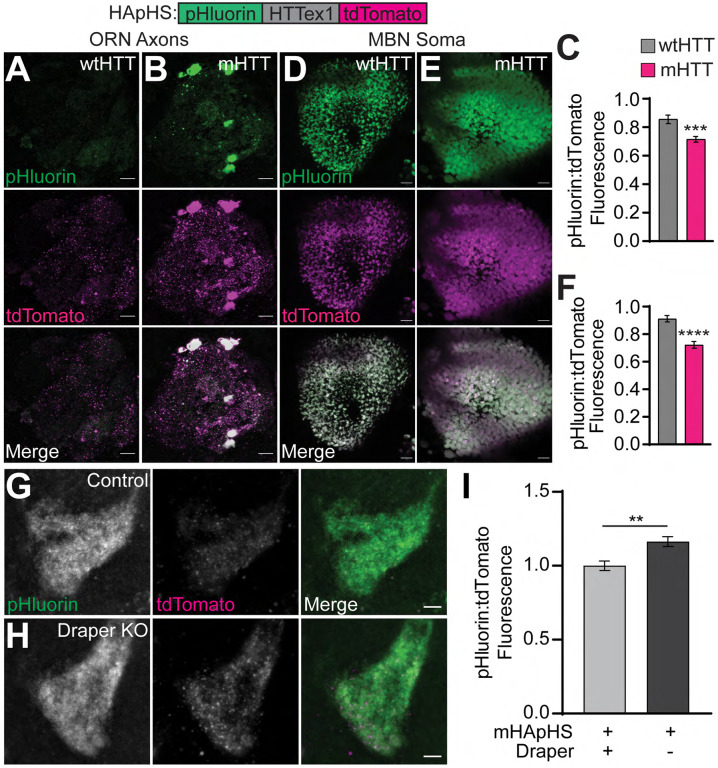
mHTTex1 expression was associated with a slight but significant decrease in steady-state pHluorin:tdTomato ratios in MApHS-labeled axons compared with wtHTTex1-expressing controls (Fig. 3D), suggesting that even in the absence of acute injury, mHTTex1 signals for ORN axon engulfment. To test this, we generated transgenic flies that express mHTTex1 or wtHTTex1 fused to N-terminal pHluorin and C-terminal tdTomato fluorescent proteins, herein referred to as mHTT-associated pH sensor (mHApHS) or wtHTT-associated pH sensor (wtHApHS). Accumulation of HTTex1 protein in low pH cellular compartments would cause a decrease in the ratio of pHluorin:tdTomato fluorescence for these constructs. wtHApHS and mHApHS transgenes were expressed in either Or83b+ ORNs, which encompass 70–80% of all adult ORNs (Fig. 4A–B) (Larsson et al., 2004), or mushroom body neurons (MBNs; Fig. 4D–E), which are downstream in the fly olfactory circuit and innervate the learning and memory center of the fly CNS (McGuire et al., 2001). In both ORN axons and MBN soma, pHluorin:tdTomato ratios associated with mHApHS were significantly decreased compared with wtHApHS controls (Fig. 4C and F), suggesting that mHTTex1 proteins accumulate in acidified cellular compartments. To discriminate mHTTex1 proteins engulfed by glia from mHTTex1 internalized into neuronal autophagolysosomes, we measured VA1lm ORN axonal mHApHS-associated fluorescence in animals with glial draper loss-of-function (Fig. 4G–H). Glial draper knockout increased pHluorin:tdTomato ratios for axonal mHApHS (Fig. 4I), suggesting that at least some portion of mHTTex1 aggregates accumulate in acidic portions of the glial phagolysosomal system.
Figure 4. Neuronal mHTTex1 accumulates in acidic cellular compartments.
(A-B, D-E) Maximum intensity projections of (A-B) Or83b+ ORN axons or (D-E) OK107+ MBN soma expressing (A,D) HTTex1Q25- or (B,E) HTTex1Q91-associated pH sensor (HApHS) from 13–14 day-old flies. Scale bars = 10 μm. (C,F) pHluorin:tdTomato fluorescence intensity ratios of data shown in (A-B and D-E), normalized to wtHTTex1 controls. (G-H) Maximum intensity projections of VA1lm ORN axons co-expressing mHApHS and (G) repo-Cas9 or (H) repo-Cas9 and gRNAs targeting draper. Scale bars = 5 μm. (I) pHluorin:tdTomato fluorescence intensity ratios calculated in VA1lm glomeruli from 9–10 day-old flies as shown in (G-H). Data were normalized to flies not expressing gRNAs. All quantified data are shown as mean ± s.e.m.; **p<0.01, ***p<0.001, ****p<0.0001 by unpaired two-tailed t-test.
Neuronal mHTT impairs injury-responsive gene upregulation
Glia alter their transcriptional profile to elicit cellular responses to insult or injury in the brain. For example, acute CNS injury in Drosophila increases transcription of many genes involved in phagocytosis and innate immunity, including the cell surface receptors, Draper and Toll-6, and components of their downstream signaling pathways (Fig. 5A) (Purice et al., 2017; Byrns et al., 2021; Alphen et al., 2022). To test whether the reduced ability of glia to clear mHTTex1 -containing axonal debris correlates with reduced injury responsiveness at a transcriptional level, we used qPCR and GFP-tagged reporters to quantify changes in gene expression of key components of these phagocytic and innate immunity pathways following mHTTex1 accumulation in neurons. mHTTex1 expression in uninjured Or83b+ ORNs increased relative expression of toll-6, relish, and drpr-I transcripts between 1.2- and 1.5-fold (Fig. 5B) and levels of GFP-tagged Toll-6 and Jra proteins in the CNS (Fig. 5C–H), suggesting that neuronal mHTTex1 aggregates activate a mild injury response in the brain. Jra-GFP expression increased throughout the CNS and in Repo+ nuclei (Fig. 5F–H), suggesting that mHTTex1-induced upregulation of this subunit of the AP-1 transcription factor can be at least partially attributed to glia. To test whether mHTTex1 impairs the ability of glia to respond to acute neural injury, we monitored gene expression changes after bilateral antennal and maxillary palp nerve ablation. Similar to previous reports (Purice et al., 2017), expression of toll-6, dorsal, relish, drpr-I, mmp1, and ets21c genes was significantly increased 3 hrs after injury to ORN axons expressing either mCD8-GFP or wtHTTex1 (Fig. 5B). However, injury-induced upregulation of each of these genes was significantly reduced in animals expressing mHTTex1 in Or83b+ ORNs (Fig. 5B), suggesting that mHTTex1 aggregation attenuates glial transcriptional responses to injury. We further analyzed the impact of mHTTex1 on downstream immune responses in the brain by measuring induction of antimicrobial peptide (AMP) genes, well-established transcriptional targets of activated Relish/NFkB following Toll-6 or immune-deficiency pathway activation (Swanson et al., 2020b, 2020a; Alphen et al., 2022). Levels of five AMP genes, including drosomycin, attacinA, attacinD, diptericinA, and metchnikowin, were significantly increased 3 hours after ORN axotomy (Fig. 6A–E), similar to previous reports (Katzenberger et al., 2013; Swanson et al., 2020b; Marischuk et al., 2021). Interestingly, mHTTex1 expression in ORNs alone was sufficient to induce upregulation of drosomycin and attacinD (Fig. 6A & C), albeit to a lesser extent than following acute injury. Further, injury-induced upregulation of drosomycin and attacinA was significantly reduced by mHTTex1 expression in ORNs, suggesting that the activity of Relish-dependent signaling is altered by accumulation of mHTTex1 aggregates. Together, these data indicate that neuronal mHTTex1 aggregates trigger a mild immune response in the brain, but also inhibit the ability of glia to mount robust transcriptional responses to neural injury.
Figure 5. mHTTex1 expression in ORNs upregulates phagocytic and innate immunity genes and impairs injury-induced transcriptional responses.
(A) Diagrams of Toll-6 (purple) and Draper (blue) signaling pathways. (B) qPCR analysis of the indicated genes in 8–11 day-old flies expressing GFP, HTTex1Q25-, or HTTex1Q91-GFP in Or83b+ ORNs. RNA was isolated from heads of uninjured flies or flies 3 hours after bilateral antennal and maxillary palp nerve injury. Data are shown as mean ± s.e.m and normalized to the housekeeping gene rpl32. *p<0.05, **p<0.01, ***p<0.001 by one-way ANOVA; asterisks and hashtags indicate statistical significance comparing −/+ injury or genotypes, respectively. (C-D and F-G) Maximum intensity projections of Or83b+ ORN axons from 14–15 day-old flies expressing (C,F) HTTex1Q25- or (D,G) HTTex1Q91-V5 in (C-D) Toll-6MIMICGFP (Toll-6-GFP) or (F-G) Jra-GFP genetic backgrounds. Brains were immunostained with GFP, V5, and N-Cadherin (C-D) or GFP, Repo, and N-Cadherin (F-G) antibodies. In panel (C), diffuse wtHTTex1 signal was adjusted post-acquisition for increased visibility. Scale bars = 10 μm. (E,H) Quantification of (E) Toll-6-GFP or (H) Jra-GFP expression from flies show in (C-D and F-G). Data are graphed as mean ± s.e.m.; *p<0.05, ****p<0.0001 by unpaired two-tailed t-test.
Figure 6. mHTTex1 expression in ORNs increases expression and impairs injury-induced upregulation of some AMP genes.
(A-E) qPCR analysis of the indicated AMP genes in 8–11 day-old flies expressing HTTex1Q25- or HTTex1Q91-GFP in Or83b+ ORNs. RNA was isolated from heads of uninjured flies or flies 3 hours after bilateral antennal and maxillary palp nerve injury. Data are shown as mean ± s.e.m and normalized to the housekeeping gene rpl32. **p<0.01, ***p<0.001 by unpaired two-tailed t-test; asterisks and hashtags indicate statistical significance comparing −/+ injury or genotypes, respectively.
Our findings are in agreement with previously published work demonstrating that expression of mHTTex1 and Aβ in neurons activates Draper-dependent phagocytosis, likely in an effort to reduce levels of these pathogenic proteins in the brain (Pearce et al., 2015; Ray et al., 2017). We further investigated this by immunostaining adult fly brains expressing mHTTex1 in ORNs to monitor expression levels and localization of endogenous Draper protein. In 1 week-old adult flies expressing neuronal mHTTex1, Draper immunolabeling increased ~1.2-fold in the vicinity of ORN axons compared with age-matched controls expressing wtHTTex1 (Fig. 7A–C). Closer analysis revealed that in some cases, Draper immunofluorescence was directly adjacent to or surrounding mHTTex1-mCherry fluorescence (Fig. 7D). To further examine these interactions, we used image segmentation and three-dimensional reconstruction of confocal stacks to represent mHTTex1-mCherry+ aggregates and Draper+ glial membranes as individual “surfaces” (Fig. 7E–F), as previously described (Donnelly et al., 2020). Close physical association of mHTTex1 with Draper and other glial proteins was defined as surfaces located ≤0.2 μm from a mHTTex1 object. Interestingly, whereas almost no Draper signal was detected near wtHTTex1 (Fig. 7E), ~14% of mHTTex1 aggregates were closely associated with Draper surfaces (Fig. 7F1–3). These findings are consistent with Draper+ glial membranes being recruited to neuronal mHTTex1 aggregates to facilitate engulfment.
Figure 7. A subset of mHTTex1 aggregates are closely associated with Draper+ glial membranes.
(A-B) Maximum intensity projections of Or83b+ ORN axons from 7–8 day-old flies expressing (A) HTTex1Q25- or (B) HTTex1Q91-mCherry and immunostained for Draper. Scale bars = 10 μm. (C) Quantification of Draper immunofluorescence from flies show in (A-B). Data are normalized to control and graphed as mean ± s.e.m.; *p<0.05 by unpaired two-tailed t-test; n=12. (D) Single 0.35 μm confocal slice showing magnified HTTex1Q91-mCherry aggregate and associated Draper signal. Scale bar = 0.5 μm. (E-F) High-magnification confocal stacks of Draper signal within ≤0.2 μm of either (E) HTTex1Q25- or (F1–3) HTTex1Q91-mCherry surfaces. Raw data are shown to the left of segmented surfaces generated from each fluorescence signal. In (E), diffuse wtHTTex1 signal was adjusted post-acquisition for increased visibility. Scale bars = 1 μm.
Synaptic neuropil in the Drosophila brain is primarily inhabited by two glial subtypes, ensheathing glia and astrocytic glia (Doherty et al., 2009). In adult flies, ensheathing glia compartmentalize synaptic regions and respond to CNS injury by upregulating Draper and clearing debris via phagocytosis (Doherty et al., 2009). Consistent with our previous findings in all repo+ glia (Pearce et al., 2015), mz0709+ ensheathing glia were vulnerable to prion-like conversion of glial wtHTTex1 proteins by mHTTex1 aggregates generated in DA1 ORN axons (Fig. 8A, C–D). Seeded wtHTTex1 aggregates were defined as wtHTTex1 signal that colocalized with mHTTex1 objects identified by image segmentation of confocal stacks (Donnelly et al., 2020). Conversely, seeding of wtHTTex1 expressed in alrm+ astrocytic glia, which lack detectable Draper expression in the adult fly brain (Doherty et al., 2009), was not observed (Fig. 8B–D). Interestingly, ensheathing glial-specific RNAi knockdown of Toll-6, Relish, and NijA increased mHTTex1 aggregate numbers in DA1 ORN axons, similar to the effects of Draper-I knockdown (Fig. 9A–B). Further, adult-specific, pan-glial knockdown of Ets21c, which was found to be required for normal development, also increased numbers of mHTTex1 aggregates in DA1 ORN axons (Fig. 9C–D). Mean mHTTex1 aggregate volume was not affected by ensheathing glial knockdown of these genes except for a ~20% increase following NijA depletion (Fig. 9E), suggesting that aggregate or vesicle size was unaffected by these genetic manipulations. Thus, several glial genes with established roles in phagocytic and innate immune signaling regulate basal turnover of mHTTex1 aggregates in ORN axons.
Figure 8. Seeded aggregation of wtHTTex1 protein expressed in ensheathing glia by neuronal mHTTex1 aggregates.
(A-B) Maximum intensity projections of DA1 glomeruli from 4–5 day-old flies expressing HTTex1Q91-mCherry in DA1 ORNs and HTTex1Q25-GFP in (A) mz0709+ ensheathing glia or (B) Alrm+ astrocytic glia. Scale bars = 5 μm. (C-D) Quantification of (C) HTTex1Q91-mCherry (“mHTT”) and (D) seeded HTTex1Q25-GFP (“mHTT+wtHTT”) aggregates from flies shown in (A-B). Data are shown as mean ± s.e.m.; ***p<0.001 by unpaired two-tailed t-test.
Figure 9. Glial phagocytic and innate immunity genes regulate numbers of mHTTex1 aggregates in ORN axons.
(A) Maximum intensity projections of DA1 glomeruli from 7 day-old flies expressing HTTex1Q91-mCherry in DA1 ORNs and siRNAs targeting the indicated genes in ensheathing glia. Scale bars = 5 μm. (B) Quantification of HTTex1Q91-mCherry aggregates detected in DA1 glomeruli from flies shown in (A). (C) Maximum intensity projections of DA1 glomeruli from 7 day-old flies expressing HTTex1Q91-mCherry in DA1 ORNs and siRNAs targeting mCherry or Ets21c in repo+ glia in the presence of tubP-Gal80ts. Adult flies were raised at the permissive (18°C, top) or restrictive (29°C, bottom) temperatures to regulate siRNA expression in adults. Scale bars = 5 μm. (D) Quantification of HTTex1Q91-mCherry aggregates detected in DA1 glomeruli from flies shown in (C). (E) Mean volumes for HTTex1Q91-mCherry aggregates detected in DA1 glomeruli from flies shown in (A and C). All graphed data are shown as mean ± s.e.m.; *p<0.05, **p<0.01, ***p<0.001 by one-way ANOVA or unpaired two-tail t-test compared to no RNAi or mCherryRNAi controls.
Neuronal mHTT aggregates are associated with defects in multiple endolysosomal compartments
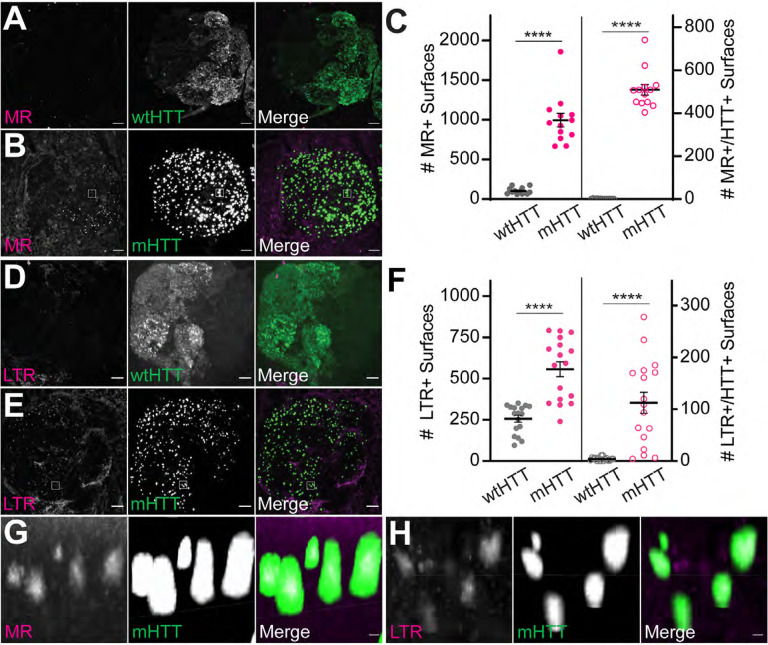
Our findings thus far indicate that neuronal mHTTex1 aggregates elicit mild injury responses in the brain and reduce the ability of phagocytic glia to respond transcriptionally and functionally to acute nerve injury. We have previously postulated that prion-like spreading of mHTTex1 in the fly CNS could be facilitated by escape of engulfed aggregates from the endolysosomal compartment of phagocytic glia (Pearce et al., 2015; Donnelly et al., 2020). Therefore, we sought to determine whether neuronal mHTTex1 aggregates are associated with defects in endolysosomal processing in non-injured brains. We first measured effects of neuronal mHTTex1 expression on the quantity, size, and function of lysosomes using dyes that label active cathepsins and low pH cellular compartments. Expression of mHTTex1 in Or83b+ ORNs increased numbers of lysosomes labeled by the active cathepsin dye, Magic Red (MR) (Fig. 10A–C), and the low pH sensor, LysoTracker Red (LTR) (Fig. 10D–F), compared with control brains expressing wtHTTex1. High resolution analysis of confocal stacks and filtering for segmented MR+ and LTR+ surfaces within 0.2 μm of a mHTTex1 object revealed close association of MR+ and LTR+ signals with mHTTex1 aggregates (Fig. 10C and F–H).
Figure 10. Neuronal mHTTex1 expression increases numbers of acidified and active lysosomes in adult brains.
(A-B) Maximum intensity projections of antennal lobes from 9–10 day-old adult flies expressing (A) HTTex1Q25- or (B) HTTex1Q91-GFP in Or83b+ ORNs and stained with Magic Red (MR) to label active cathepsins. Scale bars = 10 μm. (C) Quantification of MR+ surfaces (left) and HTTex1-associated MR+ surfaces (right) from flies shown in (A and B); HTTex1-associated vesicles were defined by filtering for MR+ surfaces ≤0.2 μm from HTTex1 fluorescent signal in confocal stacks. (D-E) Maximum intensity projections of antennal lobes from 15 day-old flies expressing (D) HTTex1Q25- or (E) HTTex1Q91-GFP in Or83b+ ORNs and stained with Lysotracker Red (LTR) to label low pH compartments. Scale bars = 10 μm. Diffuse wtHTTex1 signal was adjusted post-acquisition for increased visibility in panels (A and D). (F) Quantification of LTR+ surfaces (left) and HTTex1-associated LTR+ surfaces (right) from flies shown in (D and E); HTTex1-associated vesicles were defined by filtering for LTR+ surfaces ≤0.2 μm from HTTex1 fluorescent signal in confocal stacks. Data are shown as mean ± s.e.m.; ****p<0.0001 by unpaired two-tailed t-test. (G-H) High-magnification images of boxes indicated in (B and E) showing co-localization of MR (G) or LTR (H) with mHTTex1 fluorescent signals. Scale bars = 1 μm.
We next employed Drosophila genetic tools to assess the impact of neuronal mHTTex1 specifically on glial lysosomes by driving expression of lysosomal-associated membrane protein 1 (LAMP1) tagged at its cytosolic C-terminus with GFP (LAMP1-GFP) in all glia. Neuronal mHTTex1 expression increased the overall number of glial LAMP1-GFP+ vesicles and the number of LAMP1-GFP+ vesicles in close proximity to HTTex1 signal (Fig. 11A–C and G–H). We typically observed only partial overlap of LAMP1-GFP signal with mHTTex1 aggregate surfaces identified by this method, possibly due to incomplete labeling or rupture of lysosomal membranes as a result of aggregate size or structural features. Interestingly, this subpopulation of LAMP1-GFP+ lysosomes closely associated with mHTTex1 were enlarged compared with all lysosomes (Fig. 11I). To examine whether neuronal mHTTex1 aggregates affect lysosome integrity, we expressed a transgene encoding LAMP1 fused at its N-terminus to GFP in glia. This construct integrates into the lysosomal membrane such that GFP is exposed to the lumen, and loss of GFP signal can thus be used to monitor LAMP1+ lysosome degradative activity (Pulipparacharuvil et al., 2005). Interestingly, the quantity and mean volume of GFP-LAMP1+ vesicles were significantly increased in brains expressing mHTTex1 in Or83b+ ORNs (Fig. 11D–G), suggesting lysosomal enlargement and dysfunction due to mHTTex1 aggregates. GFP-LAMP1+ surfaces were also more associated with mHTTex1 aggregates compared to wtHTTex1 controls (Fig. 11F and I).
Figure 11. LAMP1+ vesicle accumulation in fly brains expressing mHTTex1 in ORNs.
(A-B) Confocal stacks showing antennal lobes from 19–22 day-old flies expressing (A) HTTex1Q25- or (B) HTTex1Q91-mCherry in Or83b+ ORNs and LAMP1 tagged at its cytoplasmic C-terminus with GFP (LAMP1-GFP) in glia. Brains were immunostained with anti-GFP to amplify LAMP1-GFP signal. LAMP1-GFP+ or HTTex1+ segmented surfaces are shown to the right of each set of raw fluorescence images. Insets show magnified regions of interest from each image. Scale bars = 10 μm. (C) High-magnification confocal stack showing a LAMP1-GFP+ surface within 0.2 μm of two HTTex1Q91-mCherry+ aggregates. Scale bar = 1 μm. (D-E) Confocal stacks showing antennal lobes from 21–22 day-old flies expressing (D) HTTex1Q25- or (E) HTTex1Q91-mCherry in Or83b+ ORNs and LAMP1 tagged at its luminal N-terminus with GFP (GFP-LAMP1) in glia. GFP-LAMP1+ or HTTex1+ segmented surfaces are shown to the right of each set of raw fluorescence images. Insets show a single GFP-LAMP1+ surface of interest from each image. Scale bars = 10 μm. Diffuse wtHTTex1 signal was adjusted post-acquisition for increased visibility in panels (A and D). (F) High-magnification confocal stack showing a GFP-LAMP1+ surface within 0.2 μm of a HTTex1Q91-mCherry+ aggregate. Scale bar = 1μm. (G-I) Quantification of total LAMP1-GFP+ or GFP-LAMP1+ surfaces (G), LAMP1-GFP+ or GFP-LAMP1+ surfaces ≤0.2 μm from HTTex1 surfaces (H), and mean LAMP1-GFP+ or GFP-LAMP1+ surface volume (I) in brains expressing HTTex1Q25- or HTTex1Q91-mCherry. The dark red bars in (I) represent LAMP1+ surfaces that co-localized with mHTTex1. All graphed data are shown as mean ± s.e.m.; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 by unpaired two-tailed t-test.
To directly test whether glial endolysosomes experience increased membrane damage due to neuronal mHTTex1 expression, we generated transgenic flies expressing mCherry-tagged Galectin-3 or Galectin-8, lectins that translocate from the cytoplasm to the lumen of ruptured lysosomes and endosomes, respectively (Aits et al., 2015; Daussy and Wodrich, 2020; Jia et al., 2020). Neuronal mHTTex1 expression increased overall numbers of glial Galectin-3/8+ surfaces (Fig. 12A–E), Galectin-3/8+ surfaces closely associated with mHTTex1 aggregates (Fig. 12F), and mean volume of mHTTex1-associated Galectin-3/8+ vesicles (Fig. 12G). Together, these data suggest that neuronal mHTTex1 aggregates induce non cell-autonomous accumulation, enlargement, and membrane damage of endolysosome vesicles in glial cells.
Figure 12. Increased association of Galectins-3 and -8 expressed in glia with neuronal mHTTex1 aggregates.
(A-D) Confocal stacks showing antennal lobes from 16–18 day-old flies expressing HTTex1Q25- (A and C) or HTTex1Q91-GFP (B and D) in Or83b+ ORNs together with Galectin-3 (A-B) or Galectin-8 (C-D) tagged with mCherry in glia. Segmented Galectin+ or HTTex1+ surfaces are shown to the right of each set of raw fluorescence images. Insets show Galectin+ surfaces of interest from each image. Scale bars = 10 μm. (E-G) Quantification of total Galectin+ surfaces (E), Galectin+ surfaces ≤0.2 μm from HTTex1 surfaces (F), and mean Galectin+ surface volume (G) in brains expressing HTTex1Q25- or HTTex1Q91-mCherry. The dark red bars in (G) represent Galectin+ surfaces that colocalized with mHTTex1. All graphed data are shown as mean ± s.e.m.; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 by unpaired two-tailed t-test.
“Seeding-competent” mHTT aggregates are defined by their ability to nucleate or “seed” the aggregation of normally-soluble wtHTT proteins, a defining characteristic of infectious prion and other prion-like proteins (Jucker and Walker, 2018; Donnelly et al., 2022). Many studies have pointed to a role for defective clearance by endolysosomal pathways in promoting the propagation of pathogenic aggregates (Freeman et al., 2013; Jiang et al., 2017; Chen et al., 2019; Jiang and Bhaskar, 2020; Polanco and Götz, 2022). To test whether altered glial lysosome function affects seeding competency of mHTTex1, we used RNAi to individually knockdown proteins with known roles in lysosome degradation in glia and examined the ability of neuronal mCherry-tagged mHTTex1 to seed aggregation of glial, GFP-tagged wtHTTex1. Depletion of two subunits of the vacuolar ATPase (V-ATPase), Vha68–3 (Portela et al., 2018) and rabconnectin-3A (Yan et al., 2009), and Spinster, a late-endosomal and lysosomal efflux permease (Rong et al., 2011), increased numbers of glial wtHTTex1 aggregates detected as GFP+ surfaces that colocalized with mHTTex1 aggregates (Fig. 13A–C) (Donnelly et al., 2020). Knockdown of Vha16–1, a V-ATPase subunit that regulates endolysosome membrane fusion (Finbow et al., 1994; Dunlop et al., 1995), did not affect wtHTTex1 seeding; however, it did cause accumulation of mHTTex1 aggregates in DA1 ORN axons (Fig. 13A and B). Together, these findings suggest that disruption of normal glial lysosome acidification and/or degradative capacity promotes formation of seeding-competent mHTTex1 aggregates.
Figure 13. Knockdown of genes regulating lysosome acidification alters seeded aggregation of glial wtHTTex1 protein by neuronal mHTTex1 aggregates.
(A) Confocal stacks showing DA1 glomeruli from 8–9 day-old flies expressing HTTex1Q91-mCherry in DA1 ORNs and HTTex1Q25-GFP plus siRNAs targeting the indicated genes in repo+ glia. Negative controls expressed siRNAs targeting mCherry. Scale bars = 5 μm. (B-C) Quantification of (B) HTTex1Q91-mCherry or (C) seeded HTTex1Q25-GFP aggregates from brains shown in (A). Data are shown as mean ± s.e.m.; *p<0.05, ***p<0.005 by unpaired two-tailed t-test.
The GTPase Rab10 mediates prion-like transmission of mHTT aggregates
Lysosome dysfunction could occur secondary to upstream defects in endo/phagosome maturation. Our prior work supports a model in which a portion of mHTTex1 aggregates engulfed by glia evade degradation during phagosome maturation and/or phagolysosome formation (Pearce et al., 2015; Donnelly et al., 2020). To test this model, we used forward genetic screening to interrogate roles for glial Rab GTPases in prion-like conversion of cytoplasmic wtHTTex1 proteins by engulfed neuronal mHTTex1 aggregates. The Drosophila genome encodes 31 Rab and Rab-like proteins, all of which have mammalian orthologs, and most of these GTPases are implicated in vesicle and target membrane fusion in cells (Zhang et al., 2007). To determine whether any Drosophila Rabs mediate escape of phagocytosed mHTTex1 aggregates and seeding of wtHTTex1 in the glial cytoplasm, we individually knocked down each Rab in repo+ glia using RNAi. Glial-restricted silencing of 23 of the 31 Rabs produced viable adults, and these flies were used to monitor effects of Rab knockdown on mHTTex1-induced aggregation of wtHTTex1 in glia. Only two Rab RNAi lines, Rab10RNAi and Rab23RNAi#2, significantly altered numbers of induced wtHTTex1 aggregates (Fig. 14A–C). Of note, 3 additional Rab23 RNAi lines had no significant effects on numbers of wtHTTex1 aggregates, suggesting that Rab23RNAi#2 may cause off-target effects. Strikingly, Rab10 depletion reduced numbers of seeded wtHTTex1 aggregates, phenocopying effects of Draper knockdown (Fig. 14A and C) and suggesting that Draper and Rab10 function in the same pathway. To test whether this effect of Rab10 loss-of-function was mediated via a reduction in Draper expression, we measured endogenous Draper immunofluorescence in rab10 null flies. Draper protein levels were ~18% lower in rab10 −/− animals compared to wild-type controls (Fig. 14D). This reduction is unlikely to fully account for decreased seeding of wtHTTex1 following Rab10 knockdown, as numbers of wtHTTex1 aggregates are similar between wild-type and draper heterozygotes (Fig. 14G) (Pearce et al., 2015). To further explore this, we attempted to restore Draper function via overexpression of Draper-I, which rescues loss of Draper function in aged flies (Purice et al., 2016). However, transgenic expression of Draper-I in glia failed to rescue the effects of Rab10 knockdown on wtHTTex1 aggregate seeding (Fig. 14E). These findings suggest that Rab10 acts downstream of Draper rather than by regulating Draper activity. We further tested for interactions between drpr and rab10 using loss-of-function alleles to examine effects on mHTTex1 aggregate transmission from presynaptic DA1 ORNs to postsynaptic projection neurons (PNs), a process previously reported to require transport though Draper+ glia (Donnelly et al., 2020). Because reduced survival of rab10 null flies (Kohrs et al., 2021) was exacerbated by transgenic HTTex1 expression, we tested for genetic interaction between drpr and rab10 in heterozygous and trans-heterozygous animals. While we detected no change in mHTTex1 aggregate numbers (Fig. 14F), significantly fewer wtHTTex1 aggregates formed in PNs of rab10+/− drpr+/− transheterozygotes than in individual heterozygotes (Fig. 14G). This same effect was not observed in rab14+/− drpr+/− transheterozygous animals (Fig. 14G), indicating that the genetic interaction is specific to drpr and rab10. Thus, Draper and Rab10 appear to function in the same phagocytic pathway that regulates prion-like transmission of phagocytosed mHTTex1 aggregates in the fly brain.
Figure 14. Rab10 is required for seeded aggregation of glial wtHTTex1 by neuronal mHTTex1 aggregates.
(A) Confocal stacks of DA1 glomeruli from 7 day-old flies expressing HTTex1Q91-mCherry in DA1 ORNs and HTTex1Q25-GFP plus siRNAs targeting firefly luciferase (FFLuc), Draper, or Rab10 in repo+ glia. Surfaces representing mHTTex1 aggregates (red) and seeded wtHTTex1 aggregates are superimposed on the raw data. Scale bars = 5μm. (B-C) mHTTex1 (B) or mHTTex1+wtHTTex1 (C) aggregates quantified from flies expressing HTTex1Q91-mCherry in DA1 ORNs and HTTex1Q25-GFP plus siRNAs targeting Draper-I (Drpr) or 23 different Rab GTPases in repo+ glia. Negative controls expressed no siRNAs or siRNAs targeting FFLuc or mCherry (black bars). (D) Normalized Draper immunofluorescence in the central brain of 4–5 day-old wild-type (rab10 +/+) and rab10 null (rab10 −/−) flies. (E) Quantification of seeded wtHTTex1 aggregates in flies expressing HTTex1Q91-mCherry in DA1 ORNs and HTTex1Q25-GFP plus siRNAs targeting Rab10, without or with Draper-I cDNAs in repo+glia. (F-G) Quantification of mHTTex1 (F) or mHTTex1+wtHTTex1 (G) aggregates from 10 day-old flies expressing HTTex1Q91-mCherry in DA1 ORNs and HTTex1Q25-GFP in PNs, either heterozygous or trans-heterozygous for draper (drpr/+), rab10 (rab10/+), or rab14 (rab14/+) mutant alleles. All graphed data are shown as mean ± s.e.m.; *p<0.05, **p<0.01, ***p<0.005 by one-way ANOVA or unpaired two-tailed t-test comparing to controls.
Neuronal mHTT aggregates alter numbers of early and late glial phagosomes
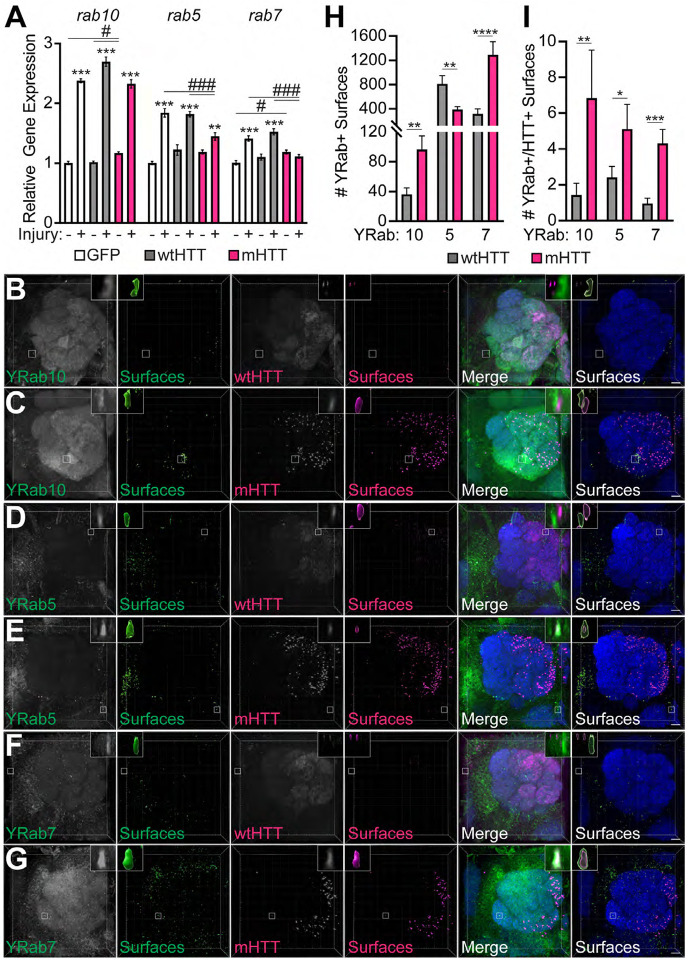
Our forward genetic screen indicates that at least one glial Rab GTPase, Rab10, regulates the seeding capacity of neuronal mHTTex1 aggregates. Interestingly, Rab10 has been reported to regulate phagosome maturation in mammalian cells (Cardoso et al., 2010; Seto et al., 2011; Lee et al., 2020; Wang et al., 2023), and Rab10 expression and activity are altered in neurodegenerative diseases, including AD and PD (Eguchi et al., 2018; Tavana et al., 2018; Yan et al., 2018). Because little is known about Rab10’s role in glia, we sought to characterize this GTPase alongside two additional Rabs with well-established roles in endocytosis, Rab5 and Rab7, markers of early and late endo/phagosomes, respectively (Hutagalung and Novick, 2011). Interestingly, we found that rab10, rab5, and rab7 were upregulated between ~1.4 and 2.4-fold following acute injury to ORN axons, and mHTTex1 expression in Or83b+ ORNs alone caused upregulation of rab10 and rab7 genes by ~1.2-fold (Fig. 15A). Interestingly, injury-induced upregulation of rab5 and rab7 was inhibited by ~50% and ~100%, respectively, in flies expressing mHTTex1 compared to controls (Fig. 15A). Altogether, these results identify rab5, rab7, and rab10 as novel injury-response genes in the fly CNS and suggest that neuronal mHTTex1 aggregates impair injury-induced responses of rab5 and rab7.
Figure 15. Association of neuronal mHTTex1 aggregates with Rab GTPases that label early, maturing, and late phagosomes.
(A) qPCR analysis of rab10, rab5, and rab7 expression in 8–11 day-old flies expressing GFP, HTTex1Q25-, or HTTex1Q91-GFP in Or83b+ ORNs. RNA was isolated from heads of uninjured flies or flies 3 hours after bilateral antennal and maxillary palp nerve injury. Data are shown as mean ± s.e.m and normalized to the housekeeping gene rpl32. *p<0.05, **p<0.01, ***p<0.001 by one-way ANOVA, asterisks and hashtags indicate statistical significance comparing −/+ injury or genotypes, respectively. (B-G) Confocal stacks of the antennal lobe from 7 day-old flies expressing (B, D, & F) HTTex1Q25- or (C, E, & G) HTTex1Q91-V5 in Or83b+ ORNs and endogenously-tagged (B-C) YRab10, (D-E) YRab5, or (F-G) YRab7 in all cells. Brains were immunostained using YFP (green), V5 (magenta), and N-Cadherin (blue) antibodies. Segmented YRab+ or HTT+ surfaces are shown to the right of each set of raw fluorescent images. Insets show magnified YFP+ surfaces of interest from each image. Diffuse wtHTTex1 signal was adjusted post-acquisition for increased visibility in panels (B, D, and F). Scale bars = 10 μm. (H-I) Quantification of YRab+ surfaces (H) and YRab+ surfaces within 0.2μm of HTTex1+ surfaces (I). Data are shown as mean ± s.e.m.; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 by unpaired two-tailed t-test.
We next examined effects of neuronal mHTTex1 expression on the localization of Rab10, Rab5, and Rab7 proteins endogenously-tagged with YFP-myc at their N-termini, herein referred to as YRab10, YRab5, and YRab7 (Dunst et al., 2015). YRab+ vesicles were identified in confocal stacks as segmented YFP+ surfaces with a mean diameter of 0.3–8 μm (Fig. 15B–G), consistent with vesicle sizes reported in other fly tissues (Prince et al., 2019). Expression of mHTTex1 in Or83b+ ORN axons caused an overall increase in numbers of YRab10+ and YRab7+ vesicles, but a decrease in the number of YRab5+ vesicles compared with wtHTTex1 controls (Fig. 15H). Further, each of these YRab+ vesicles subpopulations were closely associated with mHTTex1 aggregates more frequently than with wtHTTex1 (Fig. 15I), suggesting that mHTTex1 protein interacts with each of these intracellular vesicle subpopulations in the brain.
To assess effects of neuronal mHTTex1 aggregates specifically on glial Rab+ compartments, we expressed YFP-tagged Rab5, 7, and 10 transgenes in all glia. Similar to our findings with endogenous YRabs, expression of mHTTex1 in Or83b+ ORN axons was associated with increased numbers of glial YFP-Rab10+ and -Rab7+ vesicles (Fig. 16A–B, E–F, and G); however, we observed a significant decrease in glial YFP-Rab5+ vesicle abundance (Fig. 16C–D and G). YFP-Rab10+, -5+, and -7+ vesicles increased their association with axonal mHTTex1 aggregates compared with wtHTTex1 (Fig. 16H), in many cases with closely associated YFP-Rab+ signal partially surrounding a mHTTex1 aggregate (Fig. 17). Of note, only a small fraction of YFP-Rab+ vesicles were identified as associated with mHTTex1 aggregates, possibly due to transient interactions with vesicle compartments as aggregates transit the glial phagolysosomal system, heterogeneous labeling of phagosomes by these markers in intact brain tissue, or because our selection filter excluded YFP-Rab+ surfaces located >0.2 μm away from an aggregate. The mean volume of YFP-Rab7+ vesicles that interacted with mHTTex1 aggregates was significantly increased compared with wtHTTex1 controls (Fig. 16I), suggesting that mHTTex1 leads to enlargement of Rab7+ late phagolysosomes. Together, these data suggest that accumulation of phagocytosed mHTTex1 aggregates in Rab7+ or Rab10+ late phagosomes and decreased association with early Rab5+ phagosomes could be a key mechanism underlying protein aggregate-induced toxicity and spreading in HD.
Figure 16. Association of neuronal mHTT aggregates with glial Rab GTPases that label early, maturing, and late phagosomes.
(A-F) Confocal stacks of the antennal lobe from 16–19 day-old flies expressing (A, C, E) HTTex1Q25- or (B, D, F) HTTex1Q91-V5 in Or83b+ ORNs together with (A-B) YFP-Rab10, (C-D) YFP-Rab5, or (E-F) YFP-Rab7 in repo+ glia. Brains were immunostained with anti-GFP to amplify YFP-Rab signals. Segmented Rab+ or HTTex1+ surfaces are shown to the right of each set of raw fluorescence images. Insets show magnified YFP-Rab+ surfaces of interest from each image. Diffuse wtHTTex1 signal was adjusted post-acquisition for increased visibility in panels (A, C, and E). Scale bars = 10 μm. (G-I) Quantification of total YFP-Rab+ surfaces (G), YFP-Rab+ surfaces ≤0.2 μm from HTTex1 surfaces (H), and mean YFP-Rab+ surface volume (I) in brains expressing HTTex1Q25- or HTTex1Q91-mCherry. The dark red bars in (I) represent YFP-Rab+ surfaces that co-localized with mHTTex1. All graphed data are shown as mean ± s.e.m.; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 by unpaired two-tailed t-test.
Figure 17. Glial YFP-Rab+ surfaces are closely associated with neuronal mHTTex1 aggregates.
High magnification confocal stacks showing examples of individual YFP+ surfaces within 0.2 μm of HTTex1Q91 aggregates from 21–22 day-old adult brains expressing HTTex1Q91-mCherry in Or83b+ ORNs and YFP-Rab10 (A1–3), YFP-Rab5 (B1–2), or YFP-Rab7 (C1–2) in repo+ glia. Brains were immunostained with anti-GFP to amplify YFP-Rab signals. Scale bars = 1 μm.
DISCUSSION
Toxic amyloid aggregates have been a primary target in neurodegenerative disease drug development for decades, with some recent promise using immunotherapy to reduce aggregate loads in the brain (Karran and De Strooper, 2022). Microglia and astrocytes have also emerged as attractive therapeutic targets in efforts to boost neuroprotective glial functions or reduce neuroinflammation. However, approaches that target glial cells must effectively strike a balance between amplifying beneficial and reducing harmful effects of these cells in the brain. Here, we tested for interactions between phagocytic glia and pathogenic protein aggregates in a Drosophila model of HD. We report that aggregates formed by mHTTex1 protein fragments impair glial transcriptional and functional responses to CNS injury, induce upregulation of stress response and innate immunity genes, and alter numbers of endolysosomal vesicles detected in uninjured brains. A targeted forward genetic screen revealed that Rab10, a GTPase previously reported to regulate phagosome maturation, mediates prion-like conversion of cytoplasmic wtHTTex1 proteins by phagocytosed mHTTex1 aggregates. Together, these findings suggest that neuronal mHTTex1 aggregates compromise intracellular membrane integrity as they transit endolysosomal systems, generating toxic, seeding-competent aggregates that propagate disease phenotypes.
Glia respond to neural injury by altering their transcriptional, morphological, and metabolic profiles to promote neuronal survival and clear debris from the brain; however, failure of glia to return to a resting state elicits harmful neuroinflammatory consequences (Liddelow et al., 2020). We have previously reported that activated phagocytic glia can have both beneficial (i.e., elimination of toxic aggregates) and harmful (i.e., as vectors for aggregate spread) effects in the brain (Pearce et al., 2015; Donnelly et al., 2020). We report here that key glial injury-responsive pathways, i.e., Draper-mediated phagocytosis and Toll-6-mediated innate immune signaling, are induced in the presence mHTTex1 aggregation in the adult fly brain. These findings are in line with studies from other labs demonstrating that Drosophila Toll-6 and mammalian Toll-Like Receptor signaling pathways are upregulated in response to dying neurons during development (McLaughlin et al., 2019) and in patient and mammalian models of neurodegenerative disease (Casula et al., 2011; Miron et al., 2018; Kouli et al., 2020). Interestingly, increased microglial NF-κB signaling mediates tau spread and toxicity in mice, further linking innate immunity to prion-like mechanisms of disease progression (Wang et al., 2022). Thus, activation of glial immune pathways may contribute to feed-forward mechanisms involving aggregate formation, pathology propagation, and neuroinflammatory signaling.
Genome-wide association studies have revealed numerous genes associated with increased risk of AD and other neurodegenerative diseases, and many of these risk variants are enriched in pathways that control key glial cell functions. For example, rare risk-associated variants of the microglial TREM2 gene alter amyloid aggregate accumulation and seeding in cells and animal models (Leyns et al., 2019; Parhizkar et al., 2019; Jain et al., 2023). A number of additional genes involved in endolysosomal processing are associated with increased risk of AD, PD, FTD, and/or ALS, such as the phagocytic receptor CD33, endosomal genes BIN1 and RIN3, and GRN, which encodes the lysosomal progranulin protein (Podleśny-Drabiniok et al., 2020; Welikovitch et al., 2023). Although Draper/MEGF10 variants are not known risk factors in neurodegenerative disease, MEGF10 is highly expressed in phagocytic astrocytes (Chung et al., 2013), mediates Ab aggregate engulfment (Singh et al., 2010; Fujita et al., 2020), and acts as a receptor for C1q, a mediator of early synapse loss in AD mouse models (Hong et al., 2016; Iram et al., 2016). Our finding that upregulation of draper and other phagocytic genes is inhibited by mHTTex1 expression suggests that glial responsiveness and phagocytic capacity is attenuated in the presence of protein aggregate pathology in neurons. Genetic or environmental risk factors that impact glial health could accelerate these defects and exacerbate aggregate-induced neurotoxicity in the brain.
Lysosomes are essential for cell survival, not only to clear damaged or toxic materials from cells, but also as intracellular centers for macromolecule recycling, energy metabolism, and cell-cell communication. Lysosomal abnormalities, including vesicle enlargement, deacidification, and membrane leakiness, have been reported in patient brains and animal and cell models of multiple neurodegenerative diseases, suggesting that these defects play a central role in disease progression (Bonam et al., 2019; Polanco and Götz, 2022; Udayar et al., 2022). We report here that Rab7+, Rab10+, and Lamp1+ phagolysosomes accumulate in glia as a result of mHTTex1 expression in neurons and that lysosome dysfunction and membrane permeabilization increase in the presence of mHTTex1 aggregates. We also observed that neuronal aggregates were associated with decreased nascent phagosome formation and reduced numbers of early (Rab5+) phagosomes in glia. These findings are consistent with a “traffic jam” model (Small et al., 2017) in which mHTTex1 aggregates accumulate over time in glial endolysosomal compartments, preventing proper flow of materials into (i.e., engulfment) and out of (i.e., degradation) the pathway (Fig. 18). We postulate that persistence of aggregates in faulty endolysosomes promotes formation and/or release of degradation-resistant aggregates with enhanced toxicity and seeding capacity. This hypothesis is in agreement with recent studies in tauopathy models showing that Ab aggregation occurs secondary to lysosome deacidification and membrane permeabilization (Lee et al., 2022), and hypophagocytic glia contribute to tau aggregate propagation (Hopp et al., 2018; Brelstaff et al., 2021). Release of seeding-competent aggregates from dysfunctional lysosomes could occur via active exocytosis, perhaps in an effort to alleviate cell toxicity, or passively due to vesicle rupture (Flavin et al., 2017; Falcon et al., 2018; Yuste-Checa et al., 2021).
Figure 18. “Traffic jam” model illustrating effects of neuronal mHTTex1 aggregates on phagocytic glia.
mHTTex1 aggregates (magenta circles) generated in axons cause phagolysosomal defects in neighboring glial cells. Neuronal mHTTex1 aggregates activate glial Draper and Toll-6 signaling pathways, but impair normal phagocytic responses to injury, including reduced nascent phagosome formation and decreased numbers of Rab5+ early phagosomes (red arrow). A buildup of engulfed mHTTex1 aggregates in glia leads to accumulation of maturing (Rab10+ or Rab7+) phagosomes and lysosomes (LAMP1+) (green arrows), possibly further slowing Draper-dependent engulfment and early phagosome formation. Defective phagocytic clearance could enhance leak or ejection of some mHTTex1 aggregates from phagolysosomes to increase their toxicity and capacity to seed soluble HTT proteins (magenta + green circles).
Among the growing list of genes linked to neurodegenerative disease pathogenesis are Rab GTPases and genes that modify Rab functions (Kiral et al., 2018). Rab proteins are essential for vesicle sorting and trafficking in all cells and use GTP hydrolysis to differentially associate with and organize intracellular membranes (Homma et al., 2021). In a forward genetic screen aimed at identifying Rabs that regulate phagocytic processing of neuronal mHTTex1 aggregates, we uncovered glial Rab10 as a modifier of prion-like spreading of mHTTex1 from neurons to glia. Interestingly, several Rab proteins have been previously reported to alter secretion or cell-to-cell propagation of tau or α-synuclein aggregates (Rodriguez et al., 2017; Bae et al., 2018; Ugbode et al., 2019; Rodrigues et al., 2022), suggesting that Rab-dependent endomembrane fusion could be exploited by prion-like aggregates. Our data suggest that Rab10 acts downstream of Draper to enable engulfed mHTTex1 aggregates to evade lysosomal degradation, perhaps escaping to the cytoplasm during Rab10-dependent vesicle fusion. While relatively understudied compared with other Rabs, Rab10 has been reported to localize to and regulate maturing endo/phagosomes and lysosomes in human macrophages and microglia (Cardoso et al., 2010; Lee et al., 2020). Interestingly, Rab10 has already been implicated in multiple neurodegenerative diseases: (1) RAB10 gene expression is altered in human AD brains (Ridge et al., 2017), (2) rare RAB10 polymorphisms are linked to protection against AD (Ridge et al., 2017; Tavana et al., 2018), (3) Rab10 depletion is associated with reduced Ab levels (Udayar et al., 2013; Ridge et al., 2017), and (4) Rab10 is a substrate of the PD risk factor gene leucine-rich repeat kinase 2 (LRRK2) (Steger et al., 2016; Seol et al., 2019). Mutant LRRK2-mediated Rab phosphorylation leads to endocytic defects (Liu et al., 2020; Rivero-Ríos et al., 2020; Streubel-Gallasch et al., 2021), and LRRK2-modified Rab10 localizes to and promotes secretion from stressed lysosomes (Eguchi et al., 2018; Kluss et al., 2022). Interestingly, phosphorylated Rab10 (pRab10) levels are elevated in the CNS of AD and PD patients, and pRab10 has been reported to colocalize with pathological tau (Yan et al., 2018; Tezuka et al., 2022) suggesting that post-translational modification of Rab10 modifies the disease state. Whether Rab10’s phosphorylation status affects its ability to regulate prion-like activity of mHTTex1 or other amyloid aggregates warrants further investigation.
In conclusion, our findings demonstrate that axonal mHTTex1 aggregates activate glial phagocytosis but also impair normal glial responses to acute neural injury. Aggregate engulfment-induced defects in endolysosomal processing, such as Rab-mediated vesicle membrane fusion, could facilitate the formation and spread of prion-like aggregate seeds in the brain. These findings point to central roles for phagocytic glia in regulating pathological aggregate burden in HD and highlight the importance of exploring therapeutic interventions that target non-neuronal cells.
SIGNIFICANCE STATEMENT.
Deposition of amyloid aggregates is strongly associated with neurodegenerative disease progression and neuronal cell loss. Many studies point to glial cells as dynamic mediators of disease, capable of phagocytosing toxic materials, but also promoting chronic inflammation and proteopathic aggregate spread. Thus, glia have emerged as promising therapeutic targets for disease intervention. Here, we demonstrate in a Drosophila model of Huntington’s disease that neuronal mHTT aggregates interfere with glial phagocytic engulfment, phagolysosomal processing, and innate immunity transcriptional responses. We also identify Rab10 as a novel modifier of prion-like transmission of mHTT aggregates. Our findings add to a growing narrative of glia as double-edged players in neurodegenerative diseases.
ACKNOWLEDGMENTS:
We would like to thank Sabrina Abbruzzese, Ryan Buist, Georgiana Moore, and David Tomlinson for experimental assistance, and all former and current members of the Pearce Lab for their support and many fruitful discussions about this project. This work was supported by funding from University of the Sciences, Rowan University, the W.W. Smith Charitable Trusts, and the National Institutes of Health (awards NS128847 and AG063295).
Footnotes
CONFLICT OF INTEREST STATEMENT: The authors declare no competing financial interests.
REFERENCES
- Aits S., Kricker J., Liu B., Ellegaard A.-M., Hämälistö S., Tvingsholm S., et al. (2015). Sensitive detection of lysosomal membrane permeabilization by lysosomal galectin puncta assay. Autophagy 11, 1408–1424. doi: 10.1080/15548627.2015.1063871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alphen B. van, Stewart S., Iwanaszko M., Xu F., Li K., Rozenfeld S., et al. (2022). Glial immune-related pathways mediate effects of closed head traumatic brain injury on behavior and lethality in Drosophila. PLOS Biology 20, e3001456. doi: 10.1371/journal.pbio.3001456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aman Y., Schmauck-Medina T., Hansen M., Morimoto R. I., Simon A. K., Bjedov I., et al. (2021). Autophagy in healthy aging and disease. Nat Aging 1, 634–650. doi: 10.1038/s43587-021-00098-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae E.-J., Kim D.-K., Kim C., Mante M., Adame A., Rockenstein E., et al. (2018). LRRK2 kinase regulates α-synuclein propagation via RAB35 phosphorylation. Nat Commun 9, 3465. doi: 10.1038/s41467-018-05958-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonam S. R., Wang F., and Muller S. (2019). Lysosomes as a therapeutic target. Nat Rev Drug Discov 18, 923–948. doi: 10.1038/s41573-019-0036-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A. H., and Perrimon N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes | Development | The Company of Biologists. Available at: https://journals.biologists.com/dev/article/118/2/401/37970/Targeted-gene-expression-as-a-means-of-altering (Accessed September 19, 2022). [DOI] [PubMed] [Google Scholar]
- Brelstaff J. H., Mason M., Katsinelos T., McEwan W. A., Ghetti B., Tolkovsky A. M., et al. (2021). Microglia become hypofunctional and release metalloproteases and tau seeds when phagocytosing live neurons with P301S tau aggregates. Science Advances 7, eabg4980. doi: 10.1126/sciadv.abg4980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbidge K., Rademacher D. J., Mattick J., Zack S., Grillini A., Bousset L., et al. (2022). LGALS3 (galectin 3) mediates an unconventional secretion of SNCA/α-synuclein in response to lysosomal membrane damage by the autophagic-lysosomal pathway in human midbrain dopamine neurons. Autophagy 18, 1020–1048. doi: 10.1080/15548627.2021.1967615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrns C. N., Saikumar J., and Bonini N. M. (2021). Glial AP1 is activated with aging and accelerated by traumatic brain injury. Nat Aging 1, 585–597. doi: 10.1038/s43587-021-00072-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso C. M. P., Jordao L., and Vieira O. V. (2010). Rab10 regulates phagosome maturation and its overexpression rescues Mycobacterium-containing phagosomes maturation. Traffic 11, 221–235. doi: 10.1111/j.1600-0854.2009.01013.x [DOI] [PubMed] [Google Scholar]
- Casula M., Iyer A. M., Spliet W. G. M., Anink J. J., Steentjes K., Sta M., et al. (2011). Toll-like receptor signaling in amyotrophic lateral sclerosis spinal cord tissue. Neuroscience 179, 233–243. doi: 10.1016/j.neuroscience.2011.02.001 [DOI] [PubMed] [Google Scholar]
- Chan C.-C., Scoggin S., Wang D., Cherry S., Dembo T., Greenberg B., et al. (2011). Systematic Discovery of Rab GTPases with Synaptic Functions in Drosophila. Current Biology 21, 1704–1715. doi: 10.1016/j.cub.2011.08.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. J., Nathaniel D. L., Raghavan P., Nelson M., Tian R., Tse E., et al. (2019). Compromised function of the ESCRT pathway promotes endolysosomal escape of tau seeds and propagation of tau aggregation. J. Biol. Chem., jbc.RA119.009432. doi: 10.1074/jbc.RA119.009432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Berthelier V., Yang W., and Wetzel R. (2001). Polyglutamine aggregation behavior in vitro supports a recruitment mechanism of cytotoxicity11Edited by F. Cohen. Journal of Molecular Biology 311, 173–182. doi: 10.1006/jmbi.2001.4850 [DOI] [PubMed] [Google Scholar]
- Choi I., Zhang Y., Seegobin S. P., Pruvost M., Wang Q., Purtell K., et al. (2020). Microglia clear neuron-released α-synuclein via selective autophagy and prevent neurodegeneration. Nat Commun 11, 1386. doi: 10.1038/s41467-020-15119-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung W.-S., Clarke L. E., Wang G. X., Stafford B. K., Sher A., Chakraborty C., et al. (2013). Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504, 394–400. doi: 10.1038/nature12776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto A., Alenius M., and Dickson B. J. (2005). Molecular, Anatomical, and Functional Organization of the Drosophila Olfactory System. Current Biology 15, 1535–1547. doi: 10.1016/j.cub.2005.07.034 [DOI] [PubMed] [Google Scholar]
- Daussy C. F., and Wodrich H. (2020). “Repair Me if You Can”: Membrane Damage, Response, and Control from the Viral Perspective. Cells 9, 2042. doi: 10.3390/cells9092042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejanovic B., Wu T., Tsai M.-C., Graykowski D., Gandham V. D., Rose C. M., et al. (2022). Complement C1q-dependent excitatory and inhibitory synapse elimination by astrocytes and microglia in Alzheimer’s disease mouse models. Nat Aging 2, 837–850. doi: 10.1038/s43587-022-00281-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty J., Logan M. A., Taşdemir O. E., and Freeman M. R. (2009). Ensheathing glia function as phagocytes in the adult Drosophila brain. J Neurosci 29, 4768–4781. doi: 10.1523/JNEUROSCI.5951-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly K. M., Coleman C. M., Fuller M. L., Reed V. L., Smerina D., Tomlinson D. S., et al. (2022). Hunting for the cause: Evidence for prion-like mechanisms in Huntington’s disease. Frontiers in Neuroscience 16. Available at: https://www.frontiersin.org/articles/10.3389/fnins.2022.946822 (Accessed January 31, 2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly K. M., DeLorenzo O. R., Zaya A. D., Pisano G. E., Thu W. M., Luo L., et al. (2020). Phagocytic glia are obligatory intermediates in transmission of mutant huntingtin aggregates across neuronal synapses. eLife 9, e58499. doi: 10.7554/eLife.58499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop J., Jones P. C., and Finbow M. E. (1995). Membrane insertion and assembly of ductin: a polytopic channel with dual orientations. EMBO J 14, 3609–3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunst S., Kazimiers T., von Zadow F., Jambor H., Sagner A., Brankatschk B., et al. (2015). Endogenously Tagged Rab Proteins: A Resource to Study Membrane Trafficking in Drosophila. Dev Cell 33, 351–365. doi: 10.1016/j.devcel.2015.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong L., Radley H., Lee B., Dye D., Pixley F., Grounds M., et al. (2021). Macrophage function in the elderly and impact on injury repair and cancer. Immunity & Ageing 18, 4. doi: 10.1186/s12979-021-00215-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eapen V. V., Swarup S., Hoyer M. J., Paulo J. A., and Harper J. W. (2021). Quantitative proteomics reveals the selectivity of ubiquitin-binding autophagy receptors in the turnover of damaged lysosomes by lysophagy. eLife 10, e72328. doi: 10.7554/eLife.72328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi T., Kuwahara T., Sakurai M., Komori T., Fujimoto T., Ito G., et al. (2018). LRRK2 and its substrate Rab GTPases are sequentially targeted onto stressed lysosomes and maintain their homeostasis. Proc Natl Acad Sci U S A 115, E9115–E9124. doi: 10.1073/pnas.1812196115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans I. R., Rodrigues F. S. L. M., Armitage E. L., and Wood W. (2015). Draper/CED-1 Mediates an Ancient Damage Response to Control Inflammatory Blood Cell Migration In Vivo. Curr Biol 25, 1606–1612. doi: 10.1016/j.cub.2015.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon B., Noad J., McMahon H., Randow F., and Goedert M. (2018). Galectin-8–mediated selective autophagy protects against seeded tau aggregation. J Biol Chem 293, 2438–2451. doi: 10.1074/jbc.M117.809293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finbow M. E., Goodwin S. F., Meagher L., Lane N. J., Keen J., Findlay J. B. C., et al. (1994). Evidence that the 16 kDa proteolipid (subunit c) of the vacuolar H+-ATPase and ductin from gap junctions are the same polypeptide in Drosophila and Manduca: molecular cloning of the Vha16k gene from Drosophila. Journal of Cell Science 107, 1817–1824. doi: 10.1242/jcs.107.7.1817 [DOI] [PubMed] [Google Scholar]
- Flavin W. P., Bousset L., Green Z. C., Chu Y., Skarpathiotis S., Chaney M. J., et al. (2017). Endocytic vesicle rupture is a conserved mechanism of cellular invasion by amyloid proteins. Acta Neuropathol 134, 629–653. doi: 10.1007/s00401-017-1722-x [DOI] [PubMed] [Google Scholar]
- Freeman D., Cedillos R., Choyke S., Lukic Z., McGuire K., Marvin S., et al. (2013). Alpha-Synuclein Induces Lysosomal Rupture and Cathepsin Dependent Reactive Oxygen Species Following Endocytosis. PLOS ONE 8, e62143. doi: 10.1371/journal.pone.0062143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y., Maeda T., Sato C., Sato M., Hatakeyama H., Ota Y., et al. (2020). Engulfment of Toxic Amyloid β-protein in Neurons and Astrocytes Mediated by MEGF10. Neuroscience 443, 1–7. doi: 10.1016/j.neuroscience.2020.07.016 [DOI] [PubMed] [Google Scholar]
- Ginsberg S. D., Mufson E. J., Alldred M. J., Counts S. E., Wuu J., Nixon R. A., et al. (2011). Upregulation of select rab GTPases in cholinergic basal forebrain neurons in mild cognitive impairment and Alzheimer’s disease. J Chem Neuroanat 42, 102–110. doi: 10.1016/j.jchemneu.2011.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. (1990). The Cellular Functions of Small GTP-Binding Proteins. doi: 10.1126/science.2116664 [DOI] [PubMed] [Google Scholar]
- Han C., Song Y., Xiao H., Wang D., Franc N. C., Jan L. Y., et al. (2014). Epidermal cells are the primary phagocytes in the fragmentation and clearance of degenerating dendrites in Drosophila. Neuron 81, 544–560. doi: 10.1016/j.neuron.2013.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann B. L., Teubner B. J. W., Tummers B., Boada-Romero E., Harris L., Yang M., et al. (2019). LC3-associated endocytosis facilitates β-amyloid clearance and mitigates neurodegeneration in murine Alzheimer’s Disease. Cell 178, 536. doi: 10.1016/j.cell.2019.05.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog C., Pons Garcia L., Keatinge M., Greenald D., Moritz C., Peri F., et al. (2019). Rapid clearance of cellular debris by microglia limits secondary neuronal cell death after brain injury in vivo. Development 146, dev174698. doi: 10.1242/dev.174698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma Y., Hiragi S., and Fukuda M. (2021). Rab family of small GTPases: an updated view on their regulation and functions. FEBS J 288, 36–55. doi: 10.1111/febs.15453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S., Beja-Glasser V. F., Nfonoyim B. M., Frouin A., Li S., Ramakrishnan S., et al. (2016). Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352, 712–716. doi: 10.1126/science.aad8373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp S. C., Lin Y., Oakley D., Roe A. D., DeVos S. L., Hanlon D., et al. (2018). The role of microglia in processing and spreading of bioactive tau seeds in Alzheimer’s disease. J Neuroinflammation 15, 269. doi: 10.1186/s12974-018-1309-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutagalung A. H., and Novick P. J. (2011). Role of Rab GTPases in Membrane Traffic and Cell Physiology. Physiol Rev 91, 119–149. doi: 10.1152/physrev.00059.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iram T., Ramirez-Ortiz Z., Byrne M. H., Coleman U. A., Kingery N. D., Means T. K., et al. (2016). Megf10 Is a Receptor for C1Q That Mediates Clearance of Apoptotic Cells by Astrocytes. J Neurosci 36, 5185–5192. doi: 10.1523/JNEUROSCI.3850-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain N., Lewis C. A., Ulrich J. D., and Holtzman D. M. (2023). Chronic TREM2 activation exacerbates Aβ-associated tau seeding and spreading. J Exp Med 220, e20220654. doi: 10.1084/jem.20220654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong G. R., Jang E.-H., Bae J. R., Jun S., Kang H. C., Park C.-H., et al. (2018). Dysregulated phosphorylation of Rab GTPases by LRRK2 induces neurodegeneration. Molecular Neurodegeneration 13, 8. doi: 10.1186/s13024-018-0240-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J., Claude-Taupin A., Gu Y., Choi S. W., Peters R., Bissa B., et al. (2020). Galectin-3 coordinates a cellular system for lysosomal repair and removal. Dev Cell 52, 69–87.e8. doi: 10.1016/j.devcel.2019.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P., Gan M., Yen S.-H., McLean P. J., and Dickson D. W. (2017). Impaired endo-lysosomal membrane integrity accelerates the seeding progression of α-synuclein aggregates. Sci Rep 7, 7690. doi: 10.1038/s41598-017-08149-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S., and Bhaskar K. (2020). Degradation and Transmission of Tau by Autophagic-Endolysosomal Networks and Potential Therapeutic Targets for Tauopathy. Frontiers in Molecular Neuroscience 13. Available at: https://www.frontiersin.org/articles/10.3389/fnmol.2020.586731 (Accessed October 31, 2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jucker M., and Walker L. C. (2018). Propagation and spread of pathogenic protein assemblies in neurodegenerative diseases. Nat. Neurosci. 21, 1341–1349. doi: 10.1038/s41593-018-0238-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karran E., and De Strooper B. (2022). The amyloid hypothesis in Alzheimer disease: new insights from new therapeutics. Nat Rev Drug Discov 21, 306–318. doi: 10.1038/s41573-022-00391-w [DOI] [PubMed] [Google Scholar]
- Katzenberger R. J., Loewen C. A., Wassarman D. R., Petersen A. J., Ganetzky B., and Wassarman D. A. (2013). A Drosophila model of closed head traumatic brain injury. Proceedings of the National Academy of Sciences 110, E4152–E4159. doi: 10.1073/pnas.1316895110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiral F. R., Kohrs F. E., Jin E. J., and Hiesinger P. R. (2018). Rab GTPases and Membrane Trafficking in Neurodegeneration. Curr Biol 28, R471–R486. doi: 10.1016/j.cub.2018.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluss J. H., Beilina A., Williamson C. D., Lewis P. A., Cookson M. R., and Bonet-Ponce L. (2022). Lysosomal positioning regulates Rab10 phosphorylation at LRRK2+ lysosomes. Proc Natl Acad Sci U S A 119, e2205492119. doi: 10.1073/pnas.2205492119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles T. P. J., Vendruscolo M., and Dobson C. M. (2014). The amyloid state and its association with protein misfolding diseases. Nat Rev Mol Cell Biol 15, 384–396. doi: 10.1038/nrm3810 [DOI] [PubMed] [Google Scholar]
- Kohrs F. E., Daumann I.-M., Pavlovic B., Jin E. J., Kiral F. R., Lin S.-C., et al. (2021). Systematic functional analysis of rab GTPases reveals limits of neuronal robustness to environmental challenges in flies. eLife 10, e59594. doi: 10.7554/eLife.59594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouli A., Camacho M., Allinson K., and Williams-Gray C. H. (2020). Neuroinflammation and protein pathology in Parkinson’s disease dementia. Acta Neuropathologica Communications 8, 211. doi: 10.1186/s40478-020-01083-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasemann S., Madore C., Cialic R., Baufeld C., Calcagno N., El Fatimy R., et al. (2017). The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity 47, 566–581.e9. doi: 10.1016/j.immuni.2017.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langemeyer L., Fröhlich F., and Ungermann C. (2018). Rab GTPase Function in Endosome and Lysosome Biogenesis. Trends in Cell Biology 28, 957–970. doi: 10.1016/j.tcb.2018.06.007 [DOI] [PubMed] [Google Scholar]
- Larsson M. C., Domingos A. I., Jones W. D., Chiappe M. E., Amrein H., and Vosshall L. B. (2004). Or83b Encodes a Broadly Expressed Odorant Receptor Essential for Drosophila Olfaction. Neuron 43, 703–714. doi: 10.1016/j.neuron.2004.08.019 [DOI] [PubMed] [Google Scholar]
- Lee H., Flynn R., Sharma I., Haberman E., Carling P. J., Nicholls F. J., et al. (2020). LRRK2 Is Recruited to Phagosomes and Co-recruits RAB8 and RAB10 in Human Pluripotent Stem Cell-Derived Macrophages. Stem Cell Reports 14, 940–955. doi: 10.1016/j.stemcr.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.-H., Kim J.-Y., Noh S., Lee H., Lee S. Y., Mun J. Y., et al. (2021). Astrocytes phagocytose adult hippocampal synapses for circuit homeostasis. Nature 590, 612–617. doi: 10.1038/s41586-020-03060-3 [DOI] [PubMed] [Google Scholar]
- Lee J.-H., Yang D.-S., Goulbourne C. N., Im E., Stavrides P., Pensalfini A., et al. (2022). Faulty autolysosome acidification in Alzheimer’s disease mouse models induces autophagic build-up of Aβ in neurons, yielding senile plaques. Nat Neurosci 25, 688–701. doi: 10.1038/s41593-022-01084-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyns C. E. G., Gratuze M., Narasimhan S., Jain N., Koscal L. J., Jiang H., et al. (2019). TREM2 function impedes tau seeding in neuritic plaques. Nat Neurosci 22, 1217–1222. doi: 10.1038/s41593-019-0433-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow S. A., Guttenplan K. A., Clarke L. E., Bennett F. C., Bohlen C. J., Schirmer L., et al. (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487. doi: 10.1038/nature21029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow S. A., Marsh S. E., and Stevens B. (2020). Microglia and Astrocytes in Disease: Dynamic Duo or Partners in Crime? Trends Immunol 41, 820–835. doi: 10.1016/j.it.2020.07.006 [DOI] [PubMed] [Google Scholar]
- Liu C.-C., Hu J., Zhao N., Wang J., Wang N., Cirrito J. R., et al. (2017). Astrocytic LRP1 Mediates Brain Aβ Clearance and Impacts Amyloid Deposition. J Neurosci 37, 4023–4031. doi: 10.1523/JNEUROSCI.3442-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Xu E., Zhao H. T., Cole T., and West A. B. (2020). LRRK2 and Rab10 coordinate macropinocytosis to mediate immunological responses in phagocytes. EMBO J 39, e104862. doi: 10.15252/embj.2020104862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald J. M., Beach M. G., Porpiglia E., Sheehan A. E., Watts R. J., and Freeman M. R. (2006). The Drosophila Cell Corpse Engulfment Receptor Draper Mediates Glial Clearance of Severed Axons. Neuron 50, 869–881. doi: 10.1016/j.neuron.2006.04.028 [DOI] [PubMed] [Google Scholar]
- Magaki S. D., Williams C. K., and Vinters H. V. (2018). Glial function (and dysfunction) in the normal & ischemic brain. Neuropharmacology 134, 218–225. doi: 10.1016/j.neuropharm.2017.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marischuk K., Crocker K. L., Ahern-Djamali S., and Boekhoff-Falk G. (2021). Innate immunity pathways activate cell proliferation after penetrating traumatic brain injury in adult Drosophila. 2021.09.01.458615. doi: 10.1101/2021.09.01.458615 [DOI] [Google Scholar]
- McGuire S. E., Le P. T., and Davis R. L. (2001). The Role of Drosophila Mushroom Body Signaling in Olfactory Memory. Science 293, 1330–1333. doi: 10.1126/science.1062622 [DOI] [PubMed] [Google Scholar]
- McLaughlin C. N., Perry-Richardson J. J., Coutinho-Budd J. C., and Broihier H. T. (2019). Dying neurons utilize innate immune signaling to prime glia for phagocytosis during development. Dev Cell 48, 506–522.e6. doi: 10.1016/j.devcel.2018.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesenböck G., De Angelis D. A., and Rothman J. E. (1998). Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394, 192–195. doi: 10.1038/28190 [DOI] [PubMed] [Google Scholar]
- Miron J., Picard C., Frappier J., Dea D., Théroux L., and Poirier J. (2018). TLR4 Gene Expression and Pro-Inflammatory Cytokines in Alzheimer’s Disease and in Response to Hippocampal Deafferentation in Rodents. Journal of Alzheimer’s Disease 63, 1547–1556. doi: 10.3233/JAD-171160 [DOI] [PubMed] [Google Scholar]
- Monaco A., and Fraldi A. (2020). Protein Aggregation and Dysfunction of Autophagy-Lysosomal Pathway: A Vicious Cycle in Lysosomal Storage Diseases. Front Mol Neurosci 13, 37. doi: 10.3389/fnmol.2020.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neniskyte U., Neher J. J., and Brown G. C. (2011). Neuronal Death Induced by Nanomolar Amyloid β Is Mediated by Primary Phagocytosis of Neurons by Microglia*. Journal of Biological Chemistry 286, 39904–39913. doi: 10.1074/jbc.M111.267583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng E. L., and Tang B. L. (2008). Rab GTPases and their roles in brain neurons and glia. Brain Research Reviews 58, 236–246. doi: 10.1016/j.brainresrev.2008.04.006 [DOI] [PubMed] [Google Scholar]
- Paolicelli R. C., Bolasco G., Pagani F., Maggi L., Scianni M., Panzanelli P., et al. (2011). Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–1458. doi: 10.1126/science.1202529 [DOI] [PubMed] [Google Scholar]
- Parhizkar S., Arzberger T., Brendel M., Kleinberger G., Deussing M., Focke C., et al. (2019). Loss of TREM2 function increases amyloid seeding but reduces plaque-associated ApoE. Nat Neurosci 22, 191–204. doi: 10.1038/s41593-018-0296-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce M. M. P., Spartz E. J., Hong W., Luo L., and Kopito R. R. (2015). Prion-like transmission of neuronal huntingtin aggregates to phagocytic glia in the Drosophila brain. Nat Commun 6, 6768. doi: 10.1038/ncomms7768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podleśny-Drabiniok A., Marcora E., and Goate A. M. (2020). Microglial Phagocytosis: A DiseaseAssociated Process Emerging from Alzheimer’s Disease Genetics. Trends Neurosci 43, 965–979. doi: 10.1016/j.tins.2020.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poe A. R., Wang B., Sapar M. L., Ji H., Li K., Onabajo T., et al. (2019). Robust CRISPR/Cas9-Mediated Tissue-Specific Mutagenesis Reveals Gene Redundancy and Perdurance in Drosophila. Genetics 211, 459–472. doi: 10.1534/genetics.118.301736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanco J. C., and Götz J. (2022). Exosomal and vesicle-free tau seeds—propagation and convergence in endolysosomal permeabilization. The FEBS Journal 289, 6891–6907. doi: 10.1111/febs.16055 [DOI] [PubMed] [Google Scholar]
- Portela M., Yang L., Paul S., Li X., Veraksa A., Parsons L. M., et al. (2018). Lgl reduces endosomal vesicle acidification and Notch signaling by promoting the interaction between Vap33 and the V-ATPase complex. Sci Signal 11, eaar1976. doi: 10.1126/scisignal.aar1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter C. J., and Luo L. (2011). Using the Q system in Drosophila. Nat Protoc 6, 1105–1120. doi: 10.1038/nprot.2011.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisinger E., Jordan B. M., Kazantsev A., and Housman D. (1999). Evidence for a recruitment and sequestration mechanism in Huntington’s disease. Philos Trans R Soc Lond B Biol Sci 354, 1029–1034. doi: 10.1098/rstb.1999.0455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince E., Kroeger B., Gligorov D., Wilson C., Eaton S., Karch F., et al. (2019). Rab-mediated trafficking in the secondary cells of Drosophila male accessory glands and its role in fecundity. Traffic 20, 137–151. doi: 10.1111/tra.12622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulipparacharuvil S., Akbar M. A., Ray S., Sevrioukov E. A., Haberman A. S., Rohrer J., et al. (2005). Drosophila Vps16A is required for trafficking to lysosomes and biogenesis of pigment granules. Journal of Cell Science 118, 3663–3673. doi: 10.1242/jcs.02502 [DOI] [PubMed] [Google Scholar]
- Purice M. D., Ray A., Münzel E. J., Pope B. J., Park D. J., Speese S. D., et al. (2017). A novel Drosophila injury model reveals severed axons are cleared through a Draper/MMP-1 signaling cascade. eLife 6, e23611. doi: 10.7554/eLife.23611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purice M. D., Speese S. D., and Logan M. A. (2016). Delayed glial clearance of degenerating axons in aged Drosophila is due to reduced PI3K/Draper activity. Nat Commun 7. doi: 10.1038/ncomms12871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiders S., Han T., Scott-Hewitt N., Kucenas S., Lew D., Logan M. A., et al. (2021). Engulfed by Glia: Glial Pruning in Development, Function, and Injury across Species. J. Neurosci. 41, 823–833. doi: 10.1523/JNEUROSCI.1660-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A., Speese S. D., and Logan M. A. (2017). Glial Draper Rescues Aβ Toxicity in a Drosophila Model of Alzheimer’s Disease. J Neurosci 37, 11881–11893. doi: 10.1523/JNEUROSCI.0862-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge P. G., Karch C. M., Hsu S., Arano I., Teerlink C. C., Ebbert M. T. W., et al. (2017). Linkage, whole genome sequence, and biological data implicate variants in RAB10 in Alzheimer’s disease resilience. Genome Med 9, 100. doi: 10.1186/s13073-017-0486-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero-Ríos P., Romo-Lozano M., Fernández B., Fdez E., and Hilfiker S. (2020). Distinct Roles for RAB10 and RAB29 in Pathogenic LRRK2-Mediated Endolysosomal Trafficking Alterations. Cells 9, 1719. doi: 10.3390/cells9071719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues P. V., de Godoy J. V. P., Bosque B. P., Amorim Neto D. P., Tostes K., Palameta S., et al. (2022). Transcellular propagation of fibrillar α-synuclein from enteroendocrine to neuronal cells requires cell-to-cell contact and is Rab35-dependent. Sci Rep 12, 4168. doi: 10.1038/s41598-022-08076-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez L., Mohamed N.-V., Desjardins A., Lippé R., Fon E. A., and Leclerc N. (2017). Rab7A regulates tau secretion. J Neurochem 141, 592–605. doi: 10.1111/jnc.13994 [DOI] [PubMed] [Google Scholar]
- Rong Y., McPhee C. K., Deng S., Huang L., Chen L., Liu M., et al. (2011). Spinster is required for autophagic lysosome reformation and mTOR reactivation following starvation. Proc Natl Acad Sci U S A 108, 7826–7831. doi: 10.1073/pnas.1013800108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra M., Dill K. A., and de Graff A. M. R. (2019). Proteostasis collapse is a driver of cell aging and death. Proceedings of the National Academy of Sciences 116, 22173–22178. doi: 10.1073/pnas.1906592116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzinger E., Sittler A., Schweiger K., Heiser V., Lurz R., Hasenbank R., et al. (1999). Self-assembly of polyglutamine-containing huntingtin fragments into amyloid-like fibrils: Implications for Huntington’s disease pathology. Proc Natl Acad Sci U S A 96, 4604–4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seol W., Nam D., and Son I. (2019). Rab GTPases as Physiological Substrates of LRRK2 Kinase. Exp Neurobiol 28, 134–145. doi: 10.5607/en.2019.28.2.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto S., Tsujimura K., and Koide Y. (2011). Rab GTPases regulating phagosome maturation are differentially recruited to mycobacterial phagosomes. Traffic 12, 407–420. doi: 10.1111/j.1600-0854.2011.01165.x [DOI] [PubMed] [Google Scholar]
- Singh T. D., Park S.-Y., Bae J., Yun Y., Bae Y.-C., Park R.-W., et al. (2010). MEGF10 functions as a receptor for the uptake of amyloid-β. FEBS Letters 584, 3936–3942. doi: 10.1016/j.febslet.2010.08.050 [DOI] [PubMed] [Google Scholar]
- Small S. A., Simoes-Spassov S., Mayeux R., and Petsko G. A. (2017). Endosomal traffic jams represent a pathogenic hub and therapeutic target in Alzheimer’s disease. Trends Neurosci 40, 592–602. doi: 10.1016/j.tins.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger M., Tonelli F., Ito G., Davies P., Trost M., Vetter M., et al. (2016). Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. Elife 5, e12813. doi: 10.7554/eLife.12813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein K. C., Morales-Polanco F., van der Lienden J., Rainbolt T. K., and Frydman J. (2022). Ageing exacerbates ribosome pausing to disrupt cotranslational proteostasis. Nature 601, 637–642. doi: 10.1038/s41586-021-04295-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streubel-Gallasch L., Giusti V., Sandre M., Tessari I., Plotegher N., Giusto E., et al. (2021). Parkinson’s Disease–Associated LRRK2 Interferes with Astrocyte-Mediated Alpha-Synuclein Clearance. Mol Neurobiol 58, 3119–3140. doi: 10.1007/s12035-021-02327-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson L. C., Rimkus S. A., Ganetzky B., and Wassarman D. A. (2020a). Loss of the Antimicrobial Peptide Metchnikowin Protects Against Traumatic Brain Injury Outcomes in Drosophila melanogaster. G3 (Bethesda) 10, 3109–3119. doi: 10.1534/g3.120.401377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson L. C., Trujillo E. A., Thiede G. H., Katzenberger R. J., Shishkova E., Coon J. J., et al. (2020b). Survival Following Traumatic Brain Injury in Drosophila Is Increased by Heterozygosity for a Mutation of the NF-κB Innate Immune Response Transcription Factor Relish. Genetics 216, 1117–1136. doi: 10.1534/genetics.120.303776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavana J. P., Rosene M., Jensen N. O., Ridge P. G., Kauwe J. S., and Karch C. M. (2018). RAB10: an Alzheimer’s disease resilience locus and potential drug target. Clin Interv Aging 14, 73–79. doi: 10.2147/CIA.S159148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezuka T., Taniguchi D., Sano M., Shimada T., Oji Y., Tsunemi T., et al. (2022). Pathophysiological evaluation of the LRRK2 G2385R risk variant for Parkinson’s disease. npj Parkinsons Dis. 8, 1–7. doi: 10.1038/s41531-022-00367-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udayar V., Buggia-Prévot V., Guerreiro R. L., Siegel G., Rambabu N., Soohoo A. L., et al. (2013). A Paired RNAi and RabGAP Overexpression Screen Identifies Rab11 as a Regulator of β-Amyloid Production. Cell Rep 5, 1536–1551. doi: 10.1016/j.celrep.2013.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udayar V., Chen Y., Sidransky E., and Jagasia R. (2022). Lysosomal dysfunction in neurodegeneration: emerging concepts and methods. Trends in Neurosciences 45, 184–199. doi: 10.1016/j.tins.2021.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugbode C., Fort-Aznar L., and Sweeney S. T. (2019). Leaky endosomes push tau over the seed limit. Journal of Biological Chemistry 294, 18967–18968. doi: 10.1074/jbc.H119.011687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira O. V., Botelho R. J., and Grinstein S. (2002). Phagosome maturation: aging gracefully. Biochem J 366, 689–704. doi: 10.1042/BJ20020691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakida N. M., Cruz G. M. S., Ro C. C., Moncada E. G., Khatibzadeh N., Flanagan L. A., et al. (2018). Phagocytic response of astrocytes to damaged neighboring cells. PLOS ONE 13, e0196153. doi: 10.1371/journal.pone.0196153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Fan L., Khawaja R. R., Liu B., Zhan L., Kodama L., et al. (2022). Microglial NF-κB drives tau spreading and toxicity in a mouse model of tauopathy. Nat Commun 13, 1969. doi: 10.1038/s41467-022-29552-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Arnold M. L., Smart A. J., Wang G., Androwski R. J., Morera A., et al. (2023). Large vesicle extrusions from C. elegans neurons are consumed and stimulated by glial-like phagocytosis activity of the neighboring cell. eLife 12, e82227. doi: 10.7554/eLife.82227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanker E. E. (2000). Protein Aggregation and Pathogenesis of Huntingtons Disease: Mechanisms and Correlations. 381, 937–942. doi: 10.1515/BC.2000.114 [DOI] [PubMed] [Google Scholar]
- Welikovitch L. A., Dujardin S., Dunn A. R., Fernandes A. R., Khasnavis A., Chibnik L. B., et al. (2023). Rate of tau propagation is a heritable disease trait in genetically diverse mouse strains. iScience 26, 105983. doi: 10.1016/j.isci.2023.105983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodrich A. P. K., Scott A. W., Shukla A. K., Harris B. T., and Giniger E. (2022). The Unfolded Protein Responses in Health, Aging, and Neurodegeneration: Recent Advances and Future Considerations. Front Mol Neurosci 15, 831116. doi: 10.3389/fnmol.2022.831116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T., Wang L., Gao J., Siedlak S. L., Huntley M. L., Termsarasab P., et al. (2018). Rab10 Phosphorylation is a Prominent Pathological Feature in Alzheimer’s Disease. J Alzheimers Dis 63, 157–165. doi: 10.3233/JAD-180023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Denef N., and Schüpbach T. (2009). The vacuolar proton pump (V-ATPase) is required for Notch signaling and endosomal trafficking in Drosophila. Dev Cell 17, 387–402. doi: 10.1016/j.devcel.2009.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste-Checa P., Trinkaus V. A., Riera-Tur I., Imamoglu R., Schaller T. F., Wang H., et al. (2021). The extracellular chaperone Clusterin enhances Tau aggregate seeding in a cellular model. Nat Commun 12, 4863. doi: 10.1038/s41467-021-25060-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Schulze K. L., Hiesinger P. R., Suyama K., Wang S., Fish M., et al. (2007). Thirty-One Flavors of Drosophila Rab Proteins. Genetics 176, 1307–1322. doi: 10.1534/genetics.106.066761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B., and Tuszynski M. H. (2023). Regulation of axonal regeneration after mammalian spinal cord injury. Nat Rev Mol Cell Biol 24, 396–413. doi: 10.1038/s41580-022-00562-y [DOI] [PubMed] [Google Scholar]