Abstract
Background:
SGLT2 inhibitors (SGLT2is) and GLP-1 receptor agonists (GLP1-RAs) reduce major adverse cardiovascular events (MACE) in patients with type 2 diabetes mellitus (T2DM). However, their effectiveness relative to each other and other second-line antihyperglycemic agents is unknown, without any major ongoing head-to-head trials.
Methods:
Across the LEGEND-T2DM network, we included ten federated international data sources, spanning 1992–2021. We identified 1,492,855 patients with T2DM and established cardiovascular disease (CVD) on metformin monotherapy who initiated one of four second-line agents (SGLT2is, GLP1-RAs, dipeptidyl peptidase 4 inhibitor [DPP4is], sulfonylureas [SUs]). We used large-scale propensity score models to conduct an active comparator, target trial emulation for pairwise comparisons. After evaluating empirical equipoise and population generalizability, we fit on-treatment Cox proportional hazard models for 3-point MACE (myocardial infarction, stroke, death) and 4-point MACE (3-point MACE + heart failure hospitalization) risk, and combined hazard ratio (HR) estimates in a random-effects meta-analysis.
Findings:
Across cohorts, 16·4%, 8·3%, 27·7%, and 47·6% of individuals with T2DM initiated SGLT2is, GLP1-RAs, DPP4is, and SUs, respectively. Over 5·2 million patient-years of follow-up and 489 million patient-days of time at-risk, there were 25,982 3-point MACE and 41,447 4-point MACE events. SGLT2is and GLP1-RAs were associated with a lower risk for 3-point MACE compared with DPP4is (HR 0·89 [95% CI, 0·79–1·00] and 0·83 [0·70–0·98]), and SUs (HR 0·76 [0·65–0·89] and 0·71 [0·59–0·86]). DPP4is were associated with a lower 3-point MACE risk versus SUs (HR 0·87 [0·79–0·95]). The pattern was consistent for 4-point MACE for the comparisons above. There were no significant differences between SGLT2is and GLP1-RAs for 3-point or 4-point MACE (HR 1·06 [0·96–1·17] and 1·05 [0·97–1·13]).
Interpretation:
In patients with T2DM and established CVD, we found comparable cardiovascular risk reduction with SGLT2is and GLP1-RAs, with both agents more effective than DPP4is, which in turn were more effective than SUs. These findings suggest that the use of GLP1-RAs and SGLT2is should be prioritized as second-line agents in those with established CVD.
Funding:
National Institutes of Health, United States Department of Veterans Affairs
Keywords: Diabetes Mellitus, Type 2, Hypoglycemic Agents, Glucagon-Like Peptide-1 Receptor Agonists, Sodium-Glucose Transporter 2 Inhibitors, Comparative Effectiveness Research, Cardiovascular Diseases
INTRODUCTION
Over the past decade, the therapeutic options for type 2 diabetes mellitus (T2DM) have undergone a significant transformation.1,2 Sodium-glucose co-transporter-2 inhibitors (SGLT2is) and glucagon-like peptide-1 receptor agonists (GLP1-RAs) have expanded the role of antihyperglycemic agents from managing high blood glucose to addressing the elevated cardiovascular risk in patients with T2DM.3–6 In several large randomized clinical trials (RCTs), SGLT2is and GLP1-RAs reduced major adverse cardiovascular events (MACE), such as myocardial infarction (MI), hospitalization for heart failure, and cardiovascular mortality.7–14 While older antihyperglycemic agents, like sulfonylureas (SUs), have not undergone similarly comprehensive trials to evaluate their cardiovascular efficacy or safety, some studies suggested their neutral effect on cardiovascular outcomes and risk of hypoglycemia.15–17 Furthermore, direct comparisons of SGLT2is and GLP1-RAs with dipeptidyl peptidase-4 inhibitors (DPP4is), which are antihyperglycemic agents with neutral effects on MACE, have not been conducted and no major trials are in progress. Nevertheless, DPP4is and SUs continue to be used in clinical practice and are recommended as second-line T2DM agents in national clinical practice guidelines.18–22
Despite the availability of evidence from SGLT2i and GLP1-RA trials, several gaps in evidence challenge the development of treatment recommendations in T2DM.18,23,24 Specifically, trials of SGLT2is and GLP1-RAs were not designed as head-to-head comparisons with older agents but rather as additive treatments on top of commonly used T2DM agents.8,9,25 As a result, the relative cardiovascular efficacy of newer versus older agents is not known. Moreover, the comparative cardiovascular efficacy of SGLT2is and GLP1-RAs have not been evaluated. Thus far, comparative effectiveness assessments have been based on indirect estimates from clinical trials or comparative effectiveness drawn from a limited number of data sources.26–28 The findings from observational studies using single data sources are challenged by our observations that the uptake of these agents, and therefore, the selective pressures on the use of these agents, varies considerably across sites,21 with the potential to introduce bias in the evaluation of comparative effectiveness. These evidence gaps pose a significant challenge in designing treatment algorithms that rely on the comparative effectiveness and safety of drugs.24 As a result, there is a large variation in clinical practice guidelines and clinical practice with regard to these medications, with many patients initiated on the newer therapies and many others treated with older regimens.21,29
To address this, we conducted the Large-scale Evidence Generation and Evaluation across a Network of Databases for Type 2 Diabetes Mellitus (LEGEND-T2DM) initiative,30 a large-scale, systematic, federated evaluation of 4·7 million patients with T2DM across multiple international observational data sources, where we leveraged robust state-of-the-art methodological and analytic strategies to minimize residual confounding, publication bias, and p-hacking. Here, we compared the cardiovascular effectiveness of four second-line antihyperglycemic agents — SGLT2is, GLP1-RAs, DPP4is, or SUs — when initiated on the background of metformin therapy in T2DM.
METHODS
Data Sources
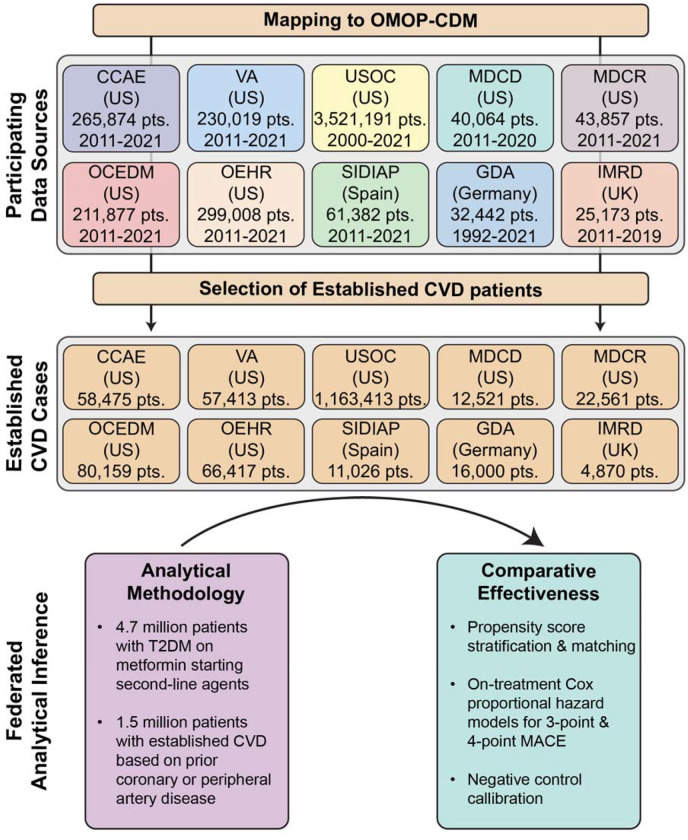
In this study, we included ten real-world data sources from the LEGEND-T2DM network, including six administrative claims and four EHR databases across four countries during 1992–2021 (figure 1). These represent data from six national-level and four health-system datasets from the US, as well as data sources from Germany, Spain, and the United Kingdom. The vast majority of patient records span from the mid-2000s to today, covering two decades of T2DM treatment as well as the introduction of many second-line antihyperglycemic agents. All LEGEND-T2DM data sources were previously standardized to the Observational Health Data Sciences and Informatics (OHDSI)’s Observational Medical Outcomes Partnership (OMOP) common data model (CDM) version 5,31 which mapped international coding systems into standard vocabulary concepts. The use of the OMOP CDM allows a federated analysis of data, without patient-level data sharing, using consistent cohort definitions and study design. All data partners received institutional approval or exemption for their participation. Details of data sources are presented in appendix pp 17–19.
Figure 1: Study Design and Analytical Methodology to Evaluate the Comparative Effectiveness of SGLT2is, GLP1-RAs, DPP4is, and SUs for Cardiovascular Outcomes.
Abbreviations: CCAE, IBM MarketScan® Commercial Claims and Encounters Data; CVD, cardiovascular disease; GDA, Germany Disease Analyser; IMRD, UK-IQVIA Medical Research Data; MACE, major adverse cardiovascular events; MDCD, IBM Health MarketScan® Multi-State Medicaid Database; MDCR, IBM Health MarketScan® Medicare Supplemental and Coordination of Benefits Database; OCEDM, Optum© Clinformatics Extended Data Mart - Date of Death; OEHR, Optum© de-identified Electronic Health Record Dataset; OMOP-CDM, Observational Medical Outcomes Partnership-common data model; SIDIAP, Information System for Research in Primary Care; T2DM, type 2 diabetes mellitus; USOC, United States Open Claims; VA, Department of Veterans Affairs Healthcare System.
Study Design
We identified patients with T2DM and established cardiovascular disease (CVD) on metformin monotherapy who initiated on any of the drug ingredients within one of the SGLT2i, GLP1-RA, DPP4i, or SU drug classes (appendix pp 2–10, 20).21 Each exposure cohort thus consists of new users of each drug class. We require patients with T2DM and CVD to have at least one year of prior observation in the database, with at least 3 months of prior metformin use before initiating a second-line agent, and no prior exposure to a comparator second-line or other antihyperglycemic agent or more than 30 days of insulin exposure (appendix pp 2–10). To ensure statistical power, we executed analyses for comparisons and data sources with at least 1,000 patients in each arm. To evaluate the comparative effectiveness of antihyperglycemic agents, we constructed target–comparator–database combinations, where we compared one antihyperglycemic agent (target) with another agent (comparator) across data sources. For this, we employed a new-user cohort design for target and comparator agents within each data source in order to emulate the hypothetical target trial.32,33 Methodological principles in the study design have been carefully constructed based on the evidence by experts and were leveraged previously to minimize bias and improve reproducibility.30,34
Study Outcomes
Across all data sources and pairwise exposure cohorts, we assess the relative risks of two primary and four secondary cardiovascular outcomes. The two primary outcomes of interest are (1) 3-point MACE, including acute MI, stroke, and sudden cardiac death, and (2) 4-point MACE, which additionally includes hospitalization for heart failure. Secondary outcomes of interest include the four individual MACE components. We construct outcome cohorts based on previously developed phenotypes validated and tested in prior work (appendix pp 11–14).34–39
For each outcome cohort, we included patients with no events prior to treatment initiation and defined continuous drug exposure as consecutive drug prescriptions with less than 30-day prescription gaps. We considered an on-treatment time-at-risk (TAR) definition that follows a patient from treatment initiation to treatment discontinuation, which captures direct treatment effects while allowing for escalation with additional T2DM agents.
Statistical Analysis
We employed a systematic federated analytic framework to address residual confounding, publication bias, and p-hacking.30,40 This framework uses data-driven, large-scale propensity score (PS) adjustment for measured confounding,41 a large set of negative control outcome experiments to address unmeasured confounding and systematic bias,42–44 study diagnostics to ensure validity and generalizability,44,45 and a principled meta-analysis approach to aggregate evidence across data sources. We used standardized vocabularies to construct consistent computable definitions of all study cohorts, covariates, and outcomes. We provide full disclosure of all hypotheses investigated and pre-specify and report all analytical procedures in the published protocol of LEGEND-T2DM.30 To promote open science and avoid publication bias, we have disseminated all results in a publicly available R ShinyApp (https://data.ohdsi.org/LegendT2dmClassEvidenceExplorer/), and all analytic code is publicly available on GitHub (https://github.com/ohdsi-studies/LegendT2dm).
Within each data source, we estimated the relative risks of all six outcomes between each pair of new-user cohorts, taking one exposure cohort as the target and the other as a comparator. For each pairwise comparison and each data source, we adjusted for measured confounding and improved balance between cohorts through both matching and stratifying on PS.46 We estimated the PS by a data-driven approach that adjusts for a broad range of predefined baseline patient characteristics through regularized regression.41 These characteristics included demographics, comorbidities, concomitant medication use, and healthcare utilization in the period before the initiation of the second-line antihyperglycemic agent. The choice of PS stratification vs. matching was based on the approach that achieves a standardized mean difference (SMD) <0·15 across all covariates.47 When both stratification and matching provide sufficient balance, we preferred stratification over matching and thus reported results based on stratification when available, as the former improves patient inclusion and, therefore, generalizability. We then used Cox proportional hazards models to estimate hazard ratios (HRs) of each outcome for each comparison, conditional on PS stratification or variable-ratio patient matching.
We also sought to address residual bias, which may persist in observational studies even after PS adjustment that controls for measured confounding.42,43 For this, we conducted negative control (falsification) outcome experiments for each comparison and outcome, where the null hypothesis of no differential effect (i.e., HR = 1) is believed to be true for each outcome. We selected 100 negative controls through a data-driven algorithm that identifies OMOP condition concept occurrences with similar prevalence to the outcomes of interest that lack evidence of association with exposures in published literature, drug–product labels, and spontaneous reports, which we then confirmed by expert review (appendix p 15).48 We list these negative controls in appendix pp 21–22. From these negative control experiments, we learn an empirical null distribution that informs residual study bias, i.e., a deviation from the empirical null across all outcomes represents a quantitative surrogate for the residual bias. We calibrate each original HR estimate to compute a calibrated HR estimate and 95% confidence interval (CI).42 We declare an HR as significantly different from the null if the calibrated p-value is <0·05 without considering multiple testing corrections.
We assessed, while blinded to the results, study diagnostics to ensure reliability and generalizability for all comparisons and only report estimates that pass the diagnostics.44,45 These study diagnostics included (1) minimum detectable risk ratio (MDRR) as a metric for statistical power, (2) preference score distributions between the target and comparator cohorts to evaluate empirical equipoise and population generalizability, and (3) cohort balance before and after propensity score (PS) adjustment, defined by the absolute SMDs on extensive patient characteristics. A study passed diagnostics if MDRR was less than 4, and >25% of patients had a preference score between 0·3 and 0·7 on both arms and maximum SMD <0·15 after PS adjustment. Additional diagnostics for visual examination included (4) calibration plots on negative control outcomes to examine residual bias, and (5) Kaplan-Meier plots to check proportionality assumptions for the Cox models.
We reported all HR estimates, their 95% CIs, and p-values post-calibration for studies that passed diagnostics. To aggregate evidence across non-overlapping data sources, we combined all calibrated HR estimates for each comparison using a random effects meta-analysis approach.49
RESULTS
Cohort Characteristics
Across ten federated longitudinal data sources from four countries, we identified 4,730,887 patients with T2DM. This included 1,492,855 patients with T2DM and established CVD on metformin monotherapy who initiated one of the four second-line antihyperglycemic agents and had no prior use of any other antihyperglycemic agents (figure 1, appendix p 17). Among these patients, 244,694 (16·4%) initiated SGLT2is, 123,991 (8·3%) GLP1-RAs, 413,236 (27·7%) DPP4is, and 710,934 (47·6%) SUs (table 1). US Open Claims (USOC) with 1,163,413 patients with T2DM and established CVD, followed by Optum Clinformatics Extended Data Mart - Date of Death (OCEDM) (N=80,159) and Optum de-identified Electronic Health Record Dataset (OEHR) (N=66,417) contributed the most patients to the study population. Median on-treatment time-at-risk for patients varied by drug class and database between 2·0 and 23·2 months. At least 25% of the patients were exposed to their first drug class for more than 12 months across a majority of databases (table 1).
Table 1:
Population Size and Follow-up Time for Initiators of SGLT2is, GLP1-RAs, DPP4is, and SUs Across Databases.
| Drug/Data Source | Patients, No. | On-treatment Follow-up Time (days), Median [IQR] |
|---|---|---|
|
| ||
| SGLT2i | ||
|
| ||
| CCAE | 10,135 | 161 [62 – 537] |
| GDA | 4,337 | 181 [97 – 537] |
| MDCD | 1,314 | 114 [30 – 383] |
| MDCR | 2,013 | 112 [34 – 541] |
| OCEDM | 11,597 | 118 [55 – 423] |
| OEHR | 8,996 | 71 [29 – 181] |
| SIDIAP | 2,281 | 322 [103 – 895] |
| USOC | 196,710 | 143 [61 – 525] |
| VA | 7,311 | 135 [71 – 443] |
| Total | 244,694 | |
|
| ||
| GLP1-RA | ||
|
| ||
| CCAE | 7,446 | 116 [45 – 441] |
| MDCR | 1,120 | 94 [29 – 443] |
| OCEDM | 6,546 | 98 [36 – 368] |
| OEHR | 4,525 | 60 [29 – 173] |
| USOC | 104,354 | 112 [52 – 432] |
| Total | 123,991 | |
|
| ||
| DPP4i | ||
|
| ||
| CCAE | 22,801 | 159 [63 – 597] |
| GDA | 10,428 | 221 [97 – 741] |
| IMRD | 4,024 | 354 [122 – 1,174] |
| MDCD | 4,449 | 142 [56 – 555] |
| MDCR | 10,541 | 187 [81 – 713] |
| OCEDM | 23,918 | 150 [59 – 569] |
| OEHR | 18,145 | 95 [89 – 312] |
| SIDIAP | 8,126 | 695 [204 – 1,916] |
| USOC | 299,835 | 157 [59 – 657] |
| VA | 10,969 | 220 [90 – 707] |
| Total | 413,236 | |
|
| ||
| SU | ||
|
| ||
| CCAE | 18,093 | 148 [59 – 559] |
| GDA | 1,235 | 216 [119 – 684] |
| IMRD | 846 | 350 [106 – 1312] |
| MDCD | 6,758 | 124 [33 – 503] |
| MDCR | 8,887 | 159 [59 – 632] |
| OCEDM | 38,098 | 177 [85 – 652] |
| OEHR | 34,751 | 110 [89 – 386] |
| SIDIAP | 619 | 600 [158 – 1,809] |
| USOC | 562,514 | 183 [89 – 752] |
| VA | 39,133 | 186 [90 – 689] |
| Total | 710,934 | |
Abbreviations: CCAE, IBM MarketScan® Commercial Claims and Encounters Data; DPP4i, dipeptidyl peptidase 4 inhibitor; GDA, Germany Disease Analyzer; GLP1-RA, glucagon-like peptide-1 receptor agonist; IMRD, UK-IQVIA Medical Research Data; MDCD, IBM Health MarketScan® Multi-State Medicaid Database; IQR, interquartile range; MDCR, IBM Health MarketScan® Medicare Supplemental and Coordination of Benefits Database; OCEDM, Optum© Clinformatics Extended Data Mart - Date of Death; OEHR, Optum© de-identified Electronic Health Record Dataset; SGLT2i, sodium-glucose co-transporter-2 inhibitor; SIDIAP, Information System for Research in Primary Care; SU, Sulfonylurea; USOC, United States Open Claims; VA, Department of Veterans Affairs Healthcare System.
Addressing Confounding Across Target-Comparator-Database Combinations
PS adjustment achieved pre-specified covariate balance in patient baseline characteristics for pairwise class comparisons across all databases (figure 2, appendix pp 23–108). The maximum SMD across target–comparator–database combinations consistently decreased after PS stratification (figure 2). For example, in comparison of patients initiating SGLT2is (target) with patients initiating DPP4is (comparator) in the IBM MarketScan Commercial Claims and Encounters (CCAE) database, before PS adjustment, patients initiating SGLT2is were more frequently men and had obesity and heart failure relative to patients initiating DPP4is. However, after PS adjustment, the SGLT2is or DPP4is populations were well balanced on all demographic and clinical patient characteristics (appendix pp 33–34).
Figure 2: Maximum Standardized Mean Difference Before and After Propensity Score Stratification for All Covariates Across Target-Comparator-Database Combinations.
Abbreviations: CCAE, IBM MarketScan® Commercial Claims and Encounters Data; DPP4i, dipeptidyl peptidase 4 inhibitor; GDA, Germany Disease Analyzer; GLP1-RA, glucagon-like peptide-1 receptor agonist; IMRD, UK-IQVIA Medical Research Data; MDCD, IBM Health MarketScan® Multi-State Medicaid Database; IQR, interquartile range; MDCR, IBM Health MarketScan® Medicare Supplemental and Coordination of Benefits Database; OCEDM, Optum© Clinformatics Extended Data Mart - Date of Death; OEHR, Optum© de-identified Electronic Health Record Dataset; PS, propensity score; SGLT2i, sodium-glucose co-transporter-2 inhibitor; SIDIAP, Information System for Research in Primary Care; SMD, standardized mean difference; SU, Sulfonylurea; USOC, United States Open Claims; VA, Department of Veterans Affairs Healthcare System.
Empirical Equipoise Across Target-Comparator-Database Combinations
For most data sources, all executed class comparisons were in empirical equipoise (>25% of patients had preference scores between 0·3 and 0·7 on both arms) (appendix pp 146–155). GDA, SIDIAP, and VA databases showed less equipoise for comparisons involving SGLT2is and DPP4is. However, in general, PS adjustment achieved sufficient covariate balance in terms of preference score distribution to reduce concerns that measured the estimated effects of baseline confounding biases (appendix pp 156–175). Furthermore, the study had limited residual systematic error, where calibration of effect estimates using negative control outcomes resulted in an increase in the proportion of nominal 95% CIs that included 1 for control outcomes across a majority of comparisons and databases (appendix pp 176–195). For example, for SGLT2is vs DPP4is comparison in SIDIAP using PS matching, before calibration, the nominal CIs covered 75·0% of control estimates; after calibration, they covered 91·7% (appendix p 192).
Comparative Effectiveness for Primary Endpoints
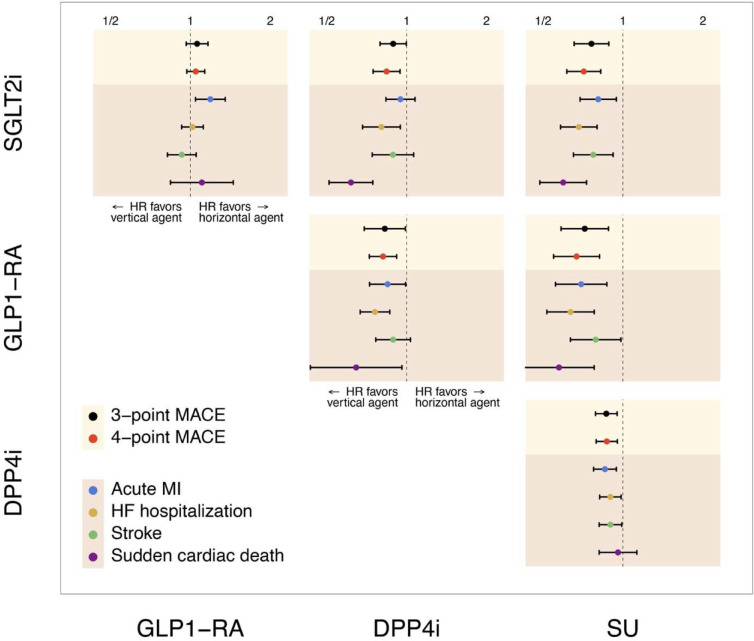
During 1,337,809 patient-years of follow-up, there were 25,982 3-point MACE and 41,447 4-point MACE events. SGLT2is and GLP1-RAs were associated with a lower hazard of 3-point MACE compared with DPP4is (HR 0·89 [95% CI, 0·79–1·00] and 0·83 [0·70–0·98], respectively), and with even lower HRs of 0·76 (95% CI, 0·65–0·89) and 0·72 (95% CI, 0·58–0·88) versus SUs, respectively. DPP4is were associated with a lower risk of 3-point MACE risk versus SUs (0·87 [0·79–0·95]). A consistent pattern was observed for 4-point MACE across the above-mentioned comparisons. In direct comparisons of SGLT2is and GLP1-RAs, there were no differences in either 3-point or 4-point MACE (1·06 [0·96–1·17], and 1·05 [0·97–1·13], respectively) (appendix pp 109–121, figure 3).
Figure 3: Meta-analytic Calibrated Hazard Ratio Estimates for Comparative Effectiveness of SGLT2is, GLP1-RAs, DPP4is, and SUs for Cardiovascular Outcomes.
Abbreviations: DPP4i, dipeptidyl peptidase 4 inhibitor; GLP1-RA, glucagon-like peptide-1 receptor agonist; HF, heart failure; HR, hazard ratio; MACE, major adverse cardiovascular events; MI, myocardial infarction; SGLT2i, sodium-glucose co-transporter-2 inhibitor; SU, Sulfonylurea.
Comparative Effectiveness for Secondary Endpoints
Acute MI
There were 13,536 episodes of acute MI across data sources during the follow-up. SGLT2is, GLP1-RAs, and DPP4is were associated with a lower hazard of acute MI compared with SUs (0·81 [0·69–0·95], 0·70 [0·56–0·87], and 0·86 [0·77–0·95], respectively). While GLP1-RAs were associated with a lower risk of acute MI compared with DPP4is (0·85 [0·73–0·99]), the hazard of acute MI was comparable for SGLT2is vs DPP4is (0·95 [0·83–1·08]). Compared with GLP1-RAs, SGLT2is were associated with a higher hazard of acute MI (1·19 [1·05–1·35]) (appendix pp 109, 122–127, figure 3).
Stroke
There were 13,999 episodes of stroke events across data sources during the follow-up. SGLT2is, GLP1-RAs, and DPP4is were associated with a lower hazard of stroke compared with SUs (0·77 [0·65–0·92], 0·79 [0·63–0·98], and 0·90 [0·81–0·99], respectively). Compared with DPP4is, SGLT2is and GLP1-RAs had a comparable hazard of stroke (0·89 [0·74–1·07] and 0·89 [0·77–1·03], respectively). There was no significant difference between SGLT2is and GLP1-RAs (0·93 [0·82–1·05]) regarding the hazard of stroke (appendix pp 109, 128–133, figure 3).
SCD
Over follow-up, 3,789 SCD occurred across data sources. SGLT2is and GLP1-RAs were associated with a lower hazard of SCD compared with DPP4is (0·62 (0·51–0·75), and 0·65 [0·44–0·96], respectively) and SUs (0·60 [0·48–0·73], and 0·57 [0·42–0·78], respectively). DPP4is versus SUs and SGLT2is versus GLP1-RAs had comparable hazards of SCD (0·96 [0·81–1·13], and 1·11 [0·84–1·45], respectively) (appendix pp 109, 134–139, figure 3).
HF Hospitalization
During the follow-up, 30,743 HF hospitalizations were recorded across all databases. SGLT2is and GLP1-RAs were associated with a lower risk of HF hospitalization compared with DPP4is (0·80 [0·68–0·95] and 0·76 [0·67–0·86], respectively) and SUs (0·68 [0·58–0·80] and 0·64 [0·52–0·78], respectively). DPP4is were associated with a lower hazard of HF hospitalization compared with SUs (0·90 [0·82–0·99]). Compared with GLP1-RAs, SGLT2is had a comparable hazard for HF hospitalization (1·02 [0·93–1·12]) (appendix pp 109, 140–145, figure 3).
Sensitivity Analyses
Across all outcomes, 98·4% (690/701) of calibrated relative risk estimates remained within the confidence intervals of the primary meta-analysis when systematically removing each data source from the leave-one-out meta-analysis. Within the primary endpoints of 3-point MACE and 4-point MACE and secondary endpoints of acute MI and hospitalization for heart failure, none of the leave-one-out analyses were outside of the confidence intervals of the meta-analysis (figure 4, appendix pp 196–201).
Figure 4: Swarm Plot of Calibrated Hazard Ratio Estimates of Major Outcome Meta-analysis and Leave-one-out Meta-analysis.
Circles depict the calibrated relative risk of each leave-one-out study in which one original data source is removed from the meta-analysis. Diamonds depict the original meta-analysis with all sources. Points are color-coded by major outcomes, y-axis includes the medication comparators, and x-axis on log-scale measures the calibrated relative risk of each outcome-comparator combination.
Abbreviations: DPP4i, dipeptidyl peptidase 4 inhibitor; GLP1-RA, glucagon-like peptide-1 receptor agonist; HF, heart failure; MACE, major adverse cardiovascular events; MI, myocardial infarction; SGLT2i, sodium-glucose co-transporter-2 inhibitor; SU, Sulfonylurea.
DISCUSSION
In this largest, multinational, federated study of 1·5 million patients with T2DM and established CVD across ten cohorts initiating a second-line antihyperglycemic agent after metformin monotherapy, SGLT2is and GLP1-RAs were associated with an 11% and 17% lower risk of cardiovascular events compared with DPP4is, respectively, the class of drugs approved before the US FDA-mandated cardiovascular outcomes trials for antihyperglycemic agents.50,51 All three agents were associated with lower cardiovascular events than SUs, with 24% and 28% lower risk with SGLT2is and GLP1-RAs and 12% lower risk with DPP4is. These patterns were consistent across both ischemic events, such as MI and stroke, as well as for heart failure. Between SGLT2is and GLP1-RAs, there was no difference in composite cardiovascular effectiveness outcomes – both 3-point and 4-point MACE, but there was a lower risk of acute MI with GLP1-RAs compared with SGLT2is. The study accounted for multiple null control outcomes and calibrated all estimates for residual bias on null control outcomes, with multiple sensitivity analyses confirming the findings of the primary analysis.
This builds on the evidence of comparative cardiovascular effectiveness of currently used antihyperglycemic therapies, especially in the absence of head-to-head RCTs of any of these agents. There are no ongoing or future trials expected to fill this knowledge gap. A few observational studies have used single data sources to evaluate the cardiovascular outcomes associated with these drugs.52–56 Moreover, some associations observed in the present study, such as those with DPP4i, contrast with others where the smaller study population may have necessitated studying a combination of drugs directly against SU but challenges isolating an effect.57 These analyses are limited by local patterns of treatment use and challenges with exposure and outcome ascertainment in individual sources that may confound the observed association of these agents with outcomes. Our recent work shows that the use of antihyperglycemic agents varies substantially in populations across the world.21 In the present study, we developed the target trial for head-to-head of these agents, focusing explicitly on the same disease stage, i.e. when escalation to a second-line agent is being initiated. We conducted the same study across ten different data sources, ensuring that the effects of the intervention were not limited to the selective pressures or outcome differences in a single population. We ensured the empirical equipoise by comparing SMDs before and after PS stratification. Finally, we ensured that unmeasured confounding, potentially manifesting as directional effects across several negative control outcomes, were used to calibrate the PS stratified effect estimates.
The findings have important implications for clinical practice. Current clinical practice guidelines offer the use of SGLT2is and GLP1-RAs as suggested agents to be used among those with existing cardiovascular risk.18,23,58 We find strong evidence supportive of that suggestion. However, our recent work shows a substantial proportion of patients with T2DM are often initiated on DPP4is and SUs despite their limited cardiovascular effectiveness or known superiority on other glycemic or other diabetes-related outcomes.59–61 Our observations would favor stronger support for exclusive second-line use of SGLT2is and GLP1-RAs among individuals with established CVD. There is also evidence that suggests a potentially larger reduction in risk of acute MI with GLP1-RAs compared with SGLT2is, which were not replicated for stroke, questioning whether the current guideline recommendation for preferential use of GLP1-RAs in ASCVD is appropriate. Moreover, we did not observe an exclusive role of decreased HF risk with SGLT2i, as suggested in current guidelines, as there was no observed difference in HF risk among those receiving SGLT2i or GLP1-RA. The original recommendations for HF were based on inference from RCTs of individual agents and emerging evidence, such as from the STEP2-HFpEF trial that found improvements in heart failure outcomes with semaglutide support the potential role of GLP1-RAs on HF outcomes as well.62 Our study favors the continued evaluation of GLP1-RA as a therapy to reduce HF risk and improve outcomes of those with HF.63,64
There are limitations that merit consideration. First, to enable the ability to capture information across data sources spanning administrative and EHR data sources necessitated the use of administrative definitions of both exposures and outcomes. However, to ensure the information is accurately captured, we used definitions that have been validated in prior studies.65–67 Second, we expect the identification of the exposure and outcomes to vary across data sources. However, the consistency of effect estimates across data sources further confirms the validity of the observations. Third, capturing outcomes for EHR-based datasets, such as for recurrent hospitalizations and deaths, differs from administrative sources that track events across institutions and capture out-of-hospital death events.68,69 However, we find that sources that capture cross-institution out-of-hospital information did not differ and had similar outcomes as those that focused on institutionally captured death events, further supporting the robustness of the drug association with outcomes. Fourth, we do not have information on adherence to therapies and whether differential adherence of agents would confound the observations. However, these agents are widely used without substantial adverse effects.18,19 Moreover, lack of adherence will likely bias these observations to the null, and the observed positive association across agents with known cardiovascular benefits suggests that it is not driving the observed associations. Finally, inference on all outcomes can still be subject to residual confounding. However, our use of calibrated estimates explicitly addresses the identification of residual confounding across a series of null control outcomes.
CONCLUSIONS
Our large, rigorous multinational network study of patients with T2DM and established CVD found cardiovascular risk reduction with SGLT2is and GLP1-RAs, with both agents more effective than DPP4is, which in turn were more effective than SUs. These findings suggest that the use of GLP1-RAs and SGLT2is should be prioritized as second-line agents in those with established CVD.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
Sodium-glucose co-transporter-2 inhibitors (SGLT2is) and glucagon-like peptide-1 receptor agonists (GLP1-RAs) exert cardiovascular benefits beyond blood glucose control, transforming the landscape of treating type 2 diabetes mellitus (T2DM) over the past decade. Large randomized clinical trials have substantiated the cardiovascular benefits of SGLT2is and GLP1-RAs in decreasing major adverse cardiovascular events. However, trials of SGLT2is and GLP1-RAs have not been designed as head-to-head comparisons with each other or older antihyperglycemic agents such as dipeptidyl peptidase-4 inhibitors (DPP4is) and sulfonylureas (SUs). Therefore, the comparative cardiovascular effectiveness of these antihyperglycemic agents is not established. Recent work also shows a substantial proportion of patients with T2DM are often initiated on DPP4is and SUs in the absence of information on their cardiovascular effectiveness. These knowledge gaps in evidence challenge the development of treatment recommendations based on cardiovascular risk profiles in T2DM.
Added value of this study
The Large-scale Evidence Generation and Evaluation across a Network of Databases for Type 2 Diabetes Mellitus (LEGEND-T2DM) initiative is a large-scale, systematic, federated evaluation of individuals with T2DM across multiple international observational data sources, where we leveraged robust state-of-the-art methodological and analytic strategies to minimize residual confounding, publication bias, and p-hacking. In this largest study of 1·5 million patients with T2DM and established cardiovascular disease (CVD) across ten cohorts from four countries initiating a second-line antihyperglycemic agent — SGLT2is, GLP1-RAs, DPP4is, or SUs — after metformin monotherapy, SGLT2is and GLP1-RAs were associated with an 11% and 17% lower risk of cardiovascular events compared with DPP4is, respectively. All three agents were associated with lower cardiovascular events than SUs, with 24% and 28% lower risk with SGLT2is and GLP1-RAs, and 12% lower risk with DPP4is. These patterns were consistent across both ischemic events, such as MI and stroke, as well as for heart failure.
Implications of all the available evidence
Our study defines strong empirical evidence in support of contemporary clinical practice guidelines that suggest SGLT2is and GLP1-RAs are used for those with T2DM and CVD. Our observations also provide support for exclusive second-line use of SGLT2is and GLP1-RAs among those with CVD.
Acknowledgments
This study was partially funded through the National Institutes of Health grants K23 HL153775, R01 LM006910, R01 HG006139 and R01 HL169954, and the US Department of Veterans Affairs under the research priority to “Put VA Data to Work for Veterans” (VA ORD 22-D4V). The funders had no role in the design and conduct of the protocol; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Declaration of interests
RK is an Associate Editor at JAMA and received support from the National Heart, Lung, and Blood Institute of the National Institutes of Health (under award K23 HL153775) and the Doris Duke Charitable Foundation (under award, 2022060). He also receives research support, through Yale, from Bristol-Myers Squibb, Novo Nordisk, and BridgeBio. He is a coinventor of U.S. Provisional Patent Applications 63/619,241, 63/606,203, 63/177,117, 63/346,610, 63/428,569, and 63/484,426, unrelated to current work. He is also a co-founder of Evidence2Health and Ensight-AI, both representing precision health platforms to improve evidence-based cardiovascular care. SLD reports grants from Alnylam Pharmaceuticals, Inc., AstraZeneca Pharmaceuticals LP, Biodesix, Inc, Celgene Corporation, Cerner Enviza, GSK PLC, Janssen Pharmaceuticals, Inc., Novartis International AG, Parexel International Corporation through the University of Utah or Western Institute for Veteran Research outside the submitted work. KKCM receives support from C W Maplethorpe Fellowship, National Institute of Health Research, UK, European Commission Framwork Horizon 2020, Hong Kong Research Grant Council, Innovation and Technology Commission of the Hong Kong Special Administration Region Government, outside the submitted work. DRM was supported by a Wellcome Trust Clinical Research Fellowship (214588/Z/18/Z). In the past three years, HMK received expenses and/or personal fees from UnitedHealth, Element Science, Aetna, Reality Labs, Tesseract/4Catalyst, F-Prime, the Siegfried and Jensen Law Firm, Arnold and Porter Law Firm, and Martin/Baughman Law Firm. He is a co-founder of Refactor Health and HugoHealth, and is associated with contracts, through Yale New Haven Hospital, from the Centers for Medicare & Medicaid Services and through Yale University from Johnson & Johnson. SCY reports being a Chief Technology Officer of PHI Digital Healthcare. JRT currently receives research support through Yale University from Johnson and Johnson to develop methods of clinical trial data sharing, from the Food and Drug Administration for the Yale-Mayo Clinic Center for Excellence in Regulatory Science and Innovation (CERSI) program (U01 FD005938), from the Agency for Healthcare Research and Quality (R01 HS022882), and from Arnold Ventures; formerly received research support from the Medical Device Innovation Consortium as part of the National Evaluation System for Health Technology (NEST) and from the National Heart, Lung and Blood Institute of the National Institutes of Health (NIH) (R01 HS025164, R01 HL144644); and in addition, JRT was an expert witness at the request of Relator’s attorneys, the Greene Law Firm, in a qui tam suit alleging violations of the False Claims Act and Anti-Kickback Statute against Biogen Inc. that was settled September 2022. PMT is a coinventor of a provisional patent 63/606,203 unrelated to the current work and is funded by 5T32 HL155000–03. CB, AO, and PBR are employees of Johnson & Johnson and MJS is an employee and shareholder of Johnson & Johnson. Other authors declare no conflicts of interest.
REFERENCES
- 1.Prattichizzo F, La Sala L, Rydén L, et al. Glucose-lowering therapies in patients with type 2 diabetes and cardiovascular diseases. Eur J Prev Cardiol 2019; 26: 73–80. [DOI] [PubMed] [Google Scholar]
- 2.Ansari MA, Chauhan W, Shoaib S, et al. Emerging therapeutic options in the management of diabetes: recent trends, challenges and future directions. Int J Obes 2023; : 1–21. [DOI] [PubMed] [Google Scholar]
- 3.Pablo A, Evelyn B, Claudia F, Yanina MA. GLP-1RA and SGLT2i: Cardiovascular Impact on Diabetic Patients. Curr Hypertens Rev 2021; 17: 149–58. [DOI] [PubMed] [Google Scholar]
- 4.Andrikou E, Tsioufis C, Andrikou I, Leontsinis I, Tousoulis D, Papanas N. GLP-1 receptor agonists and cardiovascular outcome trials: An update. Hellenic J Cardiol 2019; 60: 347–51. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 2018; 392: 1519–29· [DOI] [PubMed] [Google Scholar]
- 6.Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N Engl J Med 2022; 387: 1089–98. [DOI] [PubMed] [Google Scholar]
- 7.Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N Engl J Med 2015; 373: 2247–57. [DOI] [PubMed] [Google Scholar]
- 8.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2016; 375: 311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marso SP, Bain SC, Consoli A, et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016; 375: 1834–44. [DOI] [PubMed] [Google Scholar]
- 10.Holman RR, Bethel MA, Mentz RJ, et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2017; 377: 1228–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019; 394: 121–30. [DOI] [PubMed] [Google Scholar]
- 12.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2019; 380: 347–57. [DOI] [PubMed] [Google Scholar]
- 13.Zinman B, Bhosekar V, Busch R, et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 2019; 7: 356–67. [DOI] [PubMed] [Google Scholar]
- 14.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med 2017; 377: 644–57. [DOI] [PubMed] [Google Scholar]
- 15.Leiter LA. Latest Evidence on Sulfonylureas: What’s New? Diabetes Ther 2020; 11: 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaccaro O, Masulli M, Nicolucci A, et al. Effects on the incidence of cardiovascular events of the addition of pioglitazone versus sulfonylureas in patients with type 2 diabetes inadequately controlled with metformin (TOSCA.IT): a randomised, multicentre trial. Lancet Diabetes Endocrinol 2017; 5: 887–97. [DOI] [PubMed] [Google Scholar]
- 17.Rosenstock J, Kahn SE, Johansen OE, et al. Effect of Linagliptin vs Glimepiride on Major Adverse Cardiovascular Outcomes in Patients With Type 2 Diabetes: The CAROLINA Randomized Clinical Trial. JAMA 2019; 322: 1155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Diabetes Association Professional Practice Committee. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2024. Diabetes Care 2024; 47: S158–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2020; 41: 255–323. [DOI] [PubMed] [Google Scholar]
- 20.Buse JB, Wexler DJ, Tsapas A, et al. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020; 43: 487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khera R, Dhingra LS, Aminorroaya A, et al. Multinational patterns of second line antihyperglycaemic drug initiation across cardiovascular risk groups: federated pharmacoepidemiological evaluation in LEGEND-T2DM. BMJ Med 2023; 2: e000651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm – 2020 Executive Summary. Endocr Pract 2020; 26: 107–39. [DOI] [PubMed] [Google Scholar]
- 23.Blonde L, Umpierrez GE, Reddy SS, et al. American Association of Clinical Endocrinology Clinical Practice Guideline: Developing a Diabetes Mellitus Comprehensive Care Plan-2022 Update. Endocr Pract 2022; 28: 923–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tobias DK, Merino J, Ahmad A, et al. Second international consensus report on gaps and opportunities for the clinical translation of precision diabetes medicine. Nat Med 2023; 29: 2438–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 2015; 373: 2117–28. [DOI] [PubMed] [Google Scholar]
- 26.Fu EL, Clase CM, Janse RJ, et al. Comparative effectiveness of SGLT2i versus GLP1-RA on cardiovascular outcomes in routine clinical practice. Int J Cardiol 2022; 352: 172–9. [DOI] [PubMed] [Google Scholar]
- 27.Giugliano D, Longo M, Signoriello S, et al. The effect of DPP-4 inhibitors, GLP-1 receptor agonists and SGLT-2 inhibitors on cardiorenal outcomes: a network meta-analysis of 23 CVOTs. Cardiovasc Diabetol 2022; 21: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilbert MP, Pratley RE. GLP-1 Analogs and DPP-4 Inhibitors in Type 2 Diabetes Therapy: Review of Head-to-Head Clinical Trials. Front Endocrinol 2020; 11: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nargesi AA, Jeyashanmugaraja GP, Desai N, Lipska K, Krumholz H, Khera R. Contemporary National Patterns of Eligibility and Use of Novel Cardioprotective Antihyperglycemic Agents in Type 2 Diabetes Mellitus. J Am Heart Assoc 2021; 10: e021084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khera R, Schuemie MJ, Lu Y, et al. Large-scale evidence generation and evaluation across a network of databases for type 2 diabetes mellitus (LEGEND-T2DM): a protocol for a series of multinational, real-world comparative cardiovascular effectiveness and safety studies. BMJ Open 2022; 12: e057977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Overhage JM, Ryan PB, Reich CG, Hartzema AG, Stang PE. Validation of a common data model for active safety surveillance research. J Am Med Inform Assoc 2012; 19: 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dickerman BA, García-Albéniz X, Logan RW, Denaxas S, Hernán MA. Avoidable flaws in observational analyses: an application to statins and cancer. Nat Med 2019; 25: 1601–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hernán MA, Robins JM. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. Am J Epidemiol 2016; 183: 758–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suchard MA, Schuemie MJ, Krumholz HM, et al. Comprehensive comparative effectiveness and safety of first-line antihypertensive drug classes: a systematic, multinational, large-scale analysis. Lancet 2019; 394: 1816–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryan PB, Buse JB, Schuemie MJ, et al. Comparative effectiveness of canagliflozin, SGLT2 inhibitors and non-SGLT2 inhibitors on the risk of hospitalization for heart failure and amputation in patients with type 2 diabetes mellitus: A real-world meta-analysis of 4 observational databases (OBSERVE-4D). Diabetes Obes Metab 2018; 20: 2585–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.You SC, Rho Y, Bikdeli B, et al. Association of Ticagrelor vs Clopidogrel With Net Adverse Clinical Events in Patients With Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention. JAMA 2020; 324: 1640–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Desai M, Ryan PB, et al. Incidence of diabetic ketoacidosis among patients with type 2 diabetes mellitus treated with SGLT2 inhibitors and other antihyperglycemic agents. Diabetes Res Clin Pract 2017; 128: 83–90. [DOI] [PubMed] [Google Scholar]
- 38.Weinstein RB, Ryan PB, Berlin JA, Schuemie MJ, Swerdel J, Fife D. Channeling Bias in the Analysis of Risk of Myocardial Infarction, Stroke, Gastrointestinal Bleeding, and Acute Renal Failure with the Use of Paracetamol Compared with Ibuprofen. Drug Saf 2020; 43: 927–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan Z, DeFalco FJ, Ryan PB, et al. Risk of lower extremity amputations in people with type 2 diabetes mellitus treated with sodium-glucose co-transporter-2 inhibitors in the USA: A retrospective cohort study. Diabetes Obes Metab 2018; 20: 582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schuemie MJ, Ryan PB, Pratt N, et al. Principles of Large-scale Evidence Generation and Evaluation across a Network of Databases (LEGEND). J Am Med Inform Assoc 2020; 27: 1331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian Y, Schuemie MJ, Suchard MA. Evaluating large-scale propensity score performance through real-world and synthetic data experiments. Int J Epidemiol 2018; 47: 2005–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schuemie MJ, Hripcsak G, Ryan PB, Madigan D, Suchard MA. Empirical confidence interval calibration for population-level effect estimation studies in observational healthcare data. Proc Natl Acad Sci U S A 2018; 115: 2571–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schuemie MJ, Hripcsak G, Ryan PB, Madigan D, Suchard MA. Robust empirical calibration of p-values using observational data. Stat. Med. 2016; 35: 3883–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schuemie MJ, Ryan PB, Hripcsak G, Madigan D, Suchard MA. Improving reproducibility by using high-throughput observational studies with empirical calibration. Philos Trans A Math Phys Eng Sci 2018; 376. DOI: 10.1098/rsta.2017.0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schuemie MJ, Cepeda MS, Suchard MA, et al. How confident are we about observational findings in healthcare: A benchmark study. Harv Data Sci Rev 2020; 2. DOI: 10.1162/99608f92.147cc28e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983; 70: 41–55. [Google Scholar]
- 47.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009; 28: 3083–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Voss EA, Boyce RD, Ryan PB, van der Lei J, Rijnbeek PR, Schuemie MJ. Accuracy of an automated knowledge base for identifying drug adverse reactions. J Biomed Inform 2017; 66: 72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–88. [DOI] [PubMed] [Google Scholar]
- 50.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018; 41: 2669–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of Hyperglycemia in Type 2 Diabetes: A Patient-Centered Approach: Position Statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35: 1364–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richardson TL Jr, Halvorson AE, Hackstadt AJ, et al. Primary occurrence of cardiovascular events after adding sodium-glucose cotransporter-2 inhibitors or glucagon-like peptide-1 receptor agonists compared with dipeptidyl peptidase-4 inhibitors: A cohort study in veterans with diabetes. Ann Intern Med 2023; 176: 751–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kutz A, Kim DH, Wexler DJ, et al. Comparative Cardiovascular Effectiveness and Safety of SGLT-2 Inhibitors, GLP-1 Receptor Agonists, and DPP-4 Inhibitors According to Frailty in Type 2 Diabetes. Diabetes Care 2023; 46: 2004–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thein D, Christiansen MN, Mogensen UM, et al. Add-on therapy in metformin-treated patients with type 2 diabetes at moderate cardiovascular risk: a nationwide study. Cardiovasc Diabetol 2020; 19: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vashisht R, Patel A, Dahm L, et al. Second-Line Pharmaceutical Treatments for Patients with Type 2 Diabetes. JAMA Netw Open 2023; 6: e2336613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xie Y, Bowe B, Xian H, Loux T, McGill JB, Al-Aly Z. Comparative effectiveness of SGLT2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, and sulfonylureas on risk of major adverse cardiovascular events: emulation of a randomised target trial using electronic health records. Lancet Diabetes Endocrinol 2023; 11: 644–56. [DOI] [PubMed] [Google Scholar]
- 57.Wang H, Cordiner RLM, Huang Y, et al. Cardiovascular Safety in Type 2 Diabetes With Sulfonylureas as Second-line Drugs: A Nationwide Population-Based Comparative Safety Study. Diabetes Care 2023; 46: 967–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Samson SL, Vellanki P, Blonde L, et al. American Association of Clinical Endocrinology Consensus Statement: Comprehensive Type 2 Diabetes Management Algorithm - 2023 Update. Endocr Pract 2023; 29: 305–40. [DOI] [PubMed] [Google Scholar]
- 59.Khera R, Dhingra LS, Aminorroaya A, et al. Multinational patterns of second-line anti-hyperglycemic drug initiation across cardiovascular risk groups: A federated pharmacoepidemiologic evaluation in LEGEND-T2DM. bioRxiv. 2022; published online Dec 30. DOI: 10.1101/2022.12.27.22283968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McGuire DK, Alexander JH, Johansen OE, et al. Linagliptin Effects on Heart Failure and Related Outcomes in Individuals With Type 2 Diabetes Mellitus at High Cardiovascular and Renal Risk in CARMELINA. Circulation 2019; 139: 351–61. [DOI] [PubMed] [Google Scholar]
- 61.Rehman MB, Tudrej BV, Soustre J, et al. Efficacy and safety of DPP-4 inhibitors in patients with type 2 diabetes: Meta-analysis of placebo-controlled randomized clinical trials. Diabetes Metab 2017; 43: 48–58. [DOI] [PubMed] [Google Scholar]
- 62.Kosiborod MN, Abildstrøm SZ, Borlaug BA, et al. Semaglutide in Patients with Heart Failure with Preserved Ejection Fraction and Obesity. N Engl J Med 2023; 389: 1069–84. [DOI] [PubMed] [Google Scholar]
- 63.A Study of Tirzepatide (LY3298176) in Participants With Heart Failure With Preserved Ejection Fraction and Obesity (SUMMIT). Clinicaltrials.gov. https://classic.clinicaltrials.gov/ct2/show/NCT04847557 (accessed Jan 12, 2024). [Google Scholar]
- 64.Cardiac and Metabolic Effects of Semaglutide in Heart Failure With Preserved Ejection Fraction. Clinicaltrials.gov. https://classic.clinicaltrials.gov/ct2/show/NCT05371496 (accessed Jan 12, 2024). [Google Scholar]
- 65.Rubbo B, Fitzpatrick NK, Denaxas S, et al. Use of electronic health records to ascertain, validate and phenotype acute myocardial infarction: A systematic review and recommendations. Int J Cardiol 2015; 187: 705–11. [DOI] [PubMed] [Google Scholar]
- 66.Floyd JS, Blondon M, Moore KP, Boyko EJ, Smith NL. Validation of methods for assessing cardiovascular disease using electronic health data in a cohort of Veterans with diabetes. Pharmacoepidemiol Drug Saf 2016; 25: 467–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh S, Fouayzi H, Anzuoni K, et al. Diagnostic Algorithms for Cardiovascular Death in Administrative Claims Databases: A Systematic Review. Drug Saf 2019; 42: 515–27. [DOI] [PubMed] [Google Scholar]
- 68.Gliklich RE, Dreyer NA, Leavy MB, editors. Registries for Evaluating Patient Outcomes: A User’s Guide. Rockville (MD): Agency for Healthcare Research and Quality (US). [PubMed] [Google Scholar]
- 69.Gliklich RE, Leavy MB, Dreyer NA. Tools and Technologies for Registry Interoperability, Registries for Evaluating Patient Outcomes: A User’s Guide, 3rd Edition, Addendum 2. Agency for Healthcare Research and Quality (US), 2019. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.