Abstract
Using a quantitative dot blot overlay assay of polyvinylidene difluoride membranes, we investigated the ability of Escherichia coli heat-stable enterotoxin b (STb) to bind to various glycolipids of defined structure. STb bound strongly to acidic glycosphingolipids, including sulfatide (or 3′-sulfogalactosylceramide) and several gangliosides, but not significantly to their derivatives, galactosylceramide and asialogangliosides, respectively. STb exhibited the highest binding affinity for sulfatide. STb bound to pure sulfatide in a dose-dependent and saturable manner, with a detection level of a few nanograms. The binding was not inhibited by tetramethylurea, which is a strong disrupter of hydrophobic interactions, or by the anionic sulfated polymer of glucose, dextran sulfate, indicating that the binding is not due solely to either hydrophobic or ionic interactions via the sulfate group of the sulfatide. The specificity of the binding was confirmed by the finding that a 500-fold molar excess of sulfatide inhibited STb binding by approximately 45%, whereas no competition was obtained with galactosylceramide under the same conditions. Taken together, our data indicated that a galactose residue linked to a sulfate group is required for the binding specificity of STb. Then, total lipids extracted either from the mucous layer or from the epithelial cells of the pig jejunum brush border, the natural target of STb, were analyzed by thin-layer chromatography (TLC). Both extracts contained a lipidic molecule with a relative mobility on a TLC plate similar to that of the sulfatide standard. The migrated lipid extracted directly from a preparative TLC plate was confirmed to be sulfatide, as it was recognized by laminin, a sulfated glycolipid binding protein, and by a monoclonal antibody directed against sulfatide. In an overlay assay on PVDF membranes, STb bound to the sulfatide prepared from porcine jejunum as well as to the sulfatide standard. Thus, these findings suggest that the terminal oligosaccharide sequence Gal(3SO4)β1- on sulfatide could mediate binding of STb to its target cells and, in support of a recent report (E. Rousset, J. Harel, and J. D. Dubreuil, Microb. Pathog. 24:277–288, 1998), probably terminal sialic acid residue on another glycosphingolipid. Moreover, pretreatment in the ligated intestinal loop assay with laminin or sulfatase altered the biological activity of STb. In summary, we present data indicating that sulfatide represents a functional receptor for the STb toxin.
Enterotoxigenic Escherichia coli (ETEC) strains, which produce enterotoxins, are a worldwide, economically important agent of diarrhea in animals and humans. These enterotoxins are classified as heat-labile toxins (LT-I and LT-II), which are multimeric proteins that resemble cholera toxin in structure and function (28, 39), and heat-stable toxins (STa and STb), which are peptides with no sequence homology and distinct mechanism of action (10, 35). With respect to STb, the steps leading to diarrhea, especially those involving the initial binding of the toxin STb to intestinal epithelial cell surface, are poorly understood (11).
Early and later reports indicated that ETEC strains producing STb are closely associated with weaning pig diarrheal diseases (2, 4, 46). STb induced a rapid fluid accumulation in the small intestine leading to diarrhea, as demonstrated by in vivo studies in ligated intestinal loop in a variety of animal models (45), and caused increased short-circuit current across stripped porcine intestinal mucosa (43, 44). Unlike the case for other E. coli enterotoxins, intestinal secretion is independent of a cyclic nucleotide elevation (32, 35, 43). In vitro experiments with several different cell lines, of intestinal and nonintestinal origin, indicated that STb activates a pertussis toxin-sensitive GTP-binding regulatory protein (Gαi3), resulting in calcium ion entry through a receptor-dependent ligand-gated Ca2+ channel (9, 20). The elevated intracellular Ca2+ concentration in response to STb has been involved in activation of calmodulin-dependent protein kinase II through the Ca2+-calmodulin pathway (16). The protein kinase II might be implicated in activation of an undetermined channel conducting intestinal ion transport. In vivo, STb treatment results in a dose-dependent increase of prostaglandin E2 and 5-hydroxytryptamine, which are two intestinal secretagogues (35). STb has been reported to stimulate arachidonic acid release from membrane phospholipids, apparently through activity of phospholipases A2 and C via a Ca2+-dependent mechanism, and subsequent formation and secretion of prostaglandin E2 (15, 23, 35). In contrast, a Ca2+-independent mechanism was shown to mediate STb action via G-protein activation for the release of 5-hydroxytryptamine, presumably from intestinal enterochromaffin cells (19, 35). Furthermore, Chao and Dreyfus (6) have recently shown that after binding to the cell surface, STb is internalized into the cytoplasm following a stable association with lipids of the cell plasma membrane. They demonstrated that STb may penetrate into cells through an unknown receptor-mediated mechanism and suggested that STb may directly traverse the membrane bilayer.
However, the molecular details by which STb toxin could bind on the plasma membrane surface remain unknown. Mucosal surfaces are the ports of entry and major sites of many infectious agents. Many microbes, including viruses, bacteria, fungi, and parasites, bind to specific carbohydrate moieties on the mucosal surfaces (29). This property has been linked, in many cases, to the essential attachment to the host cells, enabling colonization and infection and potentially mediating a toxic effect on the host cells. These carbohydrate moieties may be present in either glycoproteins, proteoglycans, or glycolipids (30). We postulated that the intestinal epithelial cell membrane surface may expose specific attachment sites for STb toxin. In vitro, attachment of STb toxin to intestinal epithelial cells has been studied, but its host receptors have not been identified. Chao and Dreyfus (6) determined that STb does not bind to proteins, glycoproteins, or proteoglycans on the surface of cultured human epithelial cells but instead presumably binds directly to membrane lipids. Hitotsubashi et al. (22) showed that STb binds specifically to a protein of 25 kDa from mouse intestinal epithelial cell protein extracts. Nevertheless, Chao and Dreyfus (6) suggested that the identified 25-kDa protein does not appear to be exposed on the cell surface. In our previous study (38), treatment of porcine jejunal thin sections before the toxin binding assay with either sodium metaperiodate, organic solvents, or ceramide glycanase decreased STb binding, whereas no effect was observed after treatment with several specific proteases and O-glycosidase or N-glycosidase F. These data strongly indicated that glycosphingolipids present on the epithelial cell surface of the pig jejunum, the natural host tissue for STb, could be responsible for attachment of the toxin. Moreover, terminal sialic acid residues were presumed to be involved in binding to the pig jejunum, as pretreatment with neuraminidase from Clostridium perfringens prevented binding of STb to the cell surface.
In this work, binding of STb to various commercially available glycolipids was studied to examine the oligosaccharide structure recognized by STb toxin, using an overlay assay on polyvinylidene (PVDF) membranes. We report strong binding of STb toxin to terminal Gal(3SO4)β1 residue of sulfatide and weaker binding to terminal sialic acid residues found in several gangliosides. We further determined that sulfatide present in the total lipid extract of pig jejunum mucosal surface was bound by STb. Finally, the sulfatide appeared functional, as laminin, a sulfated glycolipid-binding protein, or sulfatase pretreatment of ligated rat intestinal loops inhibited significantly the in vivo action of STb. These data indicated that sulfatide represents a functional STb receptor.
MATERIALS AND METHODS
Materials.
Tetramethylurea (TMU) was purchased from American Chemicals Ltd. (Montreal, Québec, Canada). Orcinol ferric chloride spray reagent (Bial’s reagent), dextran sulfate (Mr, 10,000), dextran (Mr, 9,500), sulfatase (type VI) from Aerobacter aerogenes, laminin from basement membrane of Engelbreth-Holm-Swarm mouse sarcoma, rabbit antilaminin antibodies, NBT (nitro blue tetrazolium), BCIP (5-bromo-4-chloro-3-indolylphosphate), 4-chloro-1-naphthol and fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin G (IgG) were from Sigma-Aldrich Ltd. (Oakville, Ontario, Canada). Alkaline phosphatase-conjugated goat anti-rabbit IgG and horseradish peroxidase-conjugated goat anti-mouse Ig were obtained from Jackson ImmunoResearch Laboratories, Inc. (Mississauga, Ontario, Canada). Polyclonal antibodies to STb toxin were produced by immunizing rabbits with pure STb (12). Mouse monoclonal antibody to sulfatide was kindly provided by Pam Fredman, University of Göteborg, Göteborg, Sweden.
Lipids and glycolipids.
Cholesterol, phytosphingosine from yeast, lactosylceramide from bovine spleen, glucosylceramide from human (Gaucher’s) spleen, globotriosylceramide and globotetraosylceramide from human erythrocytes, monosialoganglioside GM3, and glycolipids from bovine brain (disialoganglioside GD3, monosialoganglioside GM2, monosialoganglioside GM1, trisialoganglioside GT1b, disialoganglioside GD1a, disialoganglioside GD1b, asialoganglioside GM1, galactosylceramide, and sulfatide) were purchased from Sigma-Aldrich.
STb purification.
STb enterotoxin was purified according to a previously described method (18). Briefly, the fusion protein MBP (maltose-binding protein)-STb was expressed from an Escherichia coli recombinant strain harboring plasmid pMal-STb, coding for a fusion protein composed of MBP and the mature form of STb (3). The fusion protein was affinity purified by using an amylose resin (New England Biolabs Ltd., Mississauga, Ontario, Canada). After cleavage of the fusion protein with factor Xa, STb was purified on a Poros R2/H-10/100 reverse-phase column (PerSeptive Biosystems, Cambridge, Mass.) coupled to a Waters (Milford, Mass.) model 625 liquid chromatography system. The peaks were monitored with a Waters model 990 photodiode array detector. Purified STb was lyophilized and stored at −20°C. The purity of the toxin was routinely verified by N-terminal sequence analysis using Edman degradation (Applied Biosystem model 470A gas-phase sequencer) as described before (18). The biological activity of STb was assessed by the rat ligated intestinal loop assay (12).
Extraction of total lipids from preparation of pig jejunal epithelial cells and mucus.
Pathogen-free pigs, about 8 weeks old, that had fasted for 24 h were euthanized. The jejunum was surgically excised and rinsed with ice-cold phosphate-buffered saline (PBS). To prepare jejunal epithelial cells, the jejunum was opened longitudinally, cut into pieces, and thoroughly rinsed with ice-cold PBS. The epithelial cells were harvested by scraping the mucosa with a microscope slide. The scraped cells were homogenized with a tissue grinder (Wheaton, Millville, N.J.) for 5 min. To prepare mucus from the porcine jejunum, the jejunum was also opened longitudinally and cut into pieces. The mucus was then harvested in PBS by gently scraping the mucosa with a rubber policeman stirrer. Crude mucus was subjected to ultracentrifugation at 27,000 × g for 30 min, and the supernatant was filter (0.22-μm pore size) sterilized.
Lipids were prepared by the chloroform-methanol extraction method (13). One volume of preparation corresponded to 1 ml of either the homogenized scraped jejunal epithelial cells or the ultracentrifugated and filtered jejunal mucus. Briefly, 20 volumes of a 2:1 (vol/vol) chloroform-methanol mixture containing 0.25% concentrated HCl was added and yielded a homogeneous single phase suspension after vortexing. The extract was allowed to stand for at least 20 min and was pelleted by centrifugation (15,000 × g for 20 min) to remove the nonsoluble material, mainly composed of proteins. The supernatant, corresponding to the total lipid extract, was decanted. The extract was concentrated by evaporation of the solvent in a fume hood under a stream of nitrogen. The quantity of lipid was estimated on the basis of dry weight.
Separation of lipids by TLC.
Various lipid standards and total lipids extracts were chromatographed on aluminum-backed thin-layer chromatography (TLC) plates coated with silica gel 60 (AL SIL G/ UV254; 250 mm; Whatman Ltd., Maidstone, Kent, England), using as the solvent system chloroform–methanol–0.02% CaCl2 in water (55:45:10 by volume). Chromatograms were developed by spraying with Bial’s reagent, as recommended by the manufacturer, to display sugars, glycolipids, sulfolipids, and gangliosides with different colorations. Two-dimensional TLC was carried out as follows. The first dimension was developed in the neutral solvent system of chloroform–methanol–0.02% CaCl2 in water (55:45:10 by volume), and the second dimension was developed in the acidic solvent system of chloroform-methanol-acetone-acetic acid-water (80:20:40:20:10 by volume).
Isolation of sulfatide following preparative TLC.
Total lipids extracted from pig jejunum epithelial cells were applied in a linear configuration across a 5-cm width of each aluminum-backed TLC plate. Sulfatide standards were spotted at each end of the TLC plate, and chromatography was performed with chloroform–methanol–0.02% CaCl2 in water (55:45:10 [vol/vol]) as the solvent. Following migration, standard lanes were cut out and stained with the Bial’s spray reagent. The regions corresponding to the band of sulfatide on the remaining unstained plates were scraped with a disposable scalpel, and the silica gel was collected. We also performed a blank control experiment using silica gel to which no lipid material was applied. The putative sulfatide and the control were eluted from the collected silica gel by extraction with 2 ml of chloroform-methanol (2:1 [vol/vol]) per mg of silica gel. Solvent was separated from the silica gel by centrifugation at 5,000 × g for 20 min. The extraction was conducted three times, and the solvent was pooled and evaporated. Then the eluted putative sulfatide was resuspended in methanol, and the control was resuspended in the same volume of methanol.
For identification of the eluted putative sulfatide, both extracts were submitted to either recognition with laminin, a glycoprotein which binds specifically to sulfated glycolipids (37), or immunodetection with mouse monoclonal antibody to sulfatide (14). Both extracts in methanol were dotted in triplicate on PVDF membranes (Millipore Corporation, Bedford, Mass.). The methanol was allowed to evaporate for 10 min. The membrane with dotted extracts was blocked for 1 h in PBS (0.1 M, pH 7.4) supplemented with 1% bovine serum albumin. The technique used to detect the binding of laminin was modified from the method described by Iida et al. (25). In brief, after blocking, the membrane was overlaid with laminin at a concentration of 1 or 100 μg/ml in PBS for 2 h. After being washed, the membrane was incubated for 1 h with rabbit antilaminin antibodies diluted 1/500 in PBS, washed, and incubated for 1 h with goat anti-rabbit IgG conjugated to alkaline phosphatase diluted 1/2,000 in PBS. After the membrane was washed, the bound laminin was detected by color development with 7.5 ml of the detection buffer containing 0.1 M Tris-HCl, 0.1 M NaCl, and 0.05 M MgCl2, 33 μl of NBT (75 μg/μl in 70% dimethylformamide), and 25 μl of BCIP (50 μg/μl in 100% dimethylformamide). The reaction was stopped after 10 min by addition of H2O. The immunodetection method with antisulfatide was carried out as follows. The blocked membrane was incubated with mouse monoclonal antisulfatide at a dilution of 1/100 in PBS for 2 h. Horseradish peroxidase-conjugated rabbit anti-mouse Ig, diluted 1/4,000 in PBS, was used as the second antibody. Solutions of 4-chloro-1-naphthol and H2O2 were used to reveal the peroxidase reaction product, using the method of Hawkes (21). Experiments omitting laminin and antisulfatide, respectively were done as negative controls.
Dot blot STb binding assay on PVDF membranes.
The assay was developed to quantify the binding of STb toxin to various glycolipids. Each step was performed at 25°C. A PVDF membrane was dotted with 1 μl of each glycolipid in methanol. The solvent was allowed to evaporate for 10 min, the membrane was blocked for 1 h with PBS (0.1 M, pH 7.4) supplemented with 1% bovine serum albumin and overlaid for 10 min with 50 μl/cm2 of STb at a concentration of 0.1 μg/ml in phosphate buffer (0.1 M, pH 5.8). The membrane was rinsed five times with PBS to remove unbound toxin and incubated for 1 h with a rabbit polyclonal antiserum directed against STb diluted 1/500 in PBS to detect bound toxin. The membrane was rinsed and incubated for 1 h with the alkaline phosphatase-conjugate diluted 1/2,000 in PBS. After washing the membrane, color development was carried out with NBT and BCIP as described above. Experiments omitting STb were done as negative controls.
Quantitative binding of STb to glycolipids was determined by densitometric analysis (Alpha Imager 2000; Canberra Packard Ltd., Montreal, Québec, Canada) of the PVDF membranes. For analysis, the background of the membrane was subtracted for each value (expressed as densitometric units [DU]) to allow for possible small differences in background within and between membranes. The mean densitometric value obtained for each glycolipid on the control membrane, without STb, was subtracted from the mean densitometric value obtained on the membrane with STb overlay. The results shown are the mean ± standard deviation of triplicate determinations and represent quantitative binding of STb.
Treatments of STb with various chemicals.
STb was incubated for 10 min in the presence of various chemicals prior to the STb binding assay as described above for the standard protocol. STb was preincubated with either 0.01 or 0.50 M TMU and with either 0.05 or 1 mg of dextran (Mr, 9,500) or dextran sulfate (Mr, 10,000) per ml. For the competition experiments, STb was preincubated with either sulfatide or galactosylceramide in 500-fold molar excess. For each experiment, a positive control experiment was done under the same conditions in the absence of the chemical tested.
Immunohistochemistry.
Freshly excised jejunum of a pig (about 6 weeks old) was cut into small pieces, immediately frozen in OCT compound (Miles Inc., Elkhard, Ind.), and stored at −70°C. Sections 5 μm thick were cut with a cryostat microtome (Leica model CM3050) and were thaw-mounted on poly-l-lysine-coated glass slides (Canlab, Mississauga, Ontario, Canada). After brief immersion of the slides in cold acetone, the diluted antisulfatide monoclonal antibody (1/50) in PBS was applied to tissue sections and incubated 10 h at 4°C. After five washes in PBS, diluted fluorescein isothiocyanate-conjugated anti-mouse IgG (1/64) in PBS was applied; these sections were incubated 10 h at 4°C in a dark chamber, and the latter was washed five times. Control slides without the first antibody were run in parallel. Slides were mounted in FA mounting fluid, pH 9.0 (Difco Laboratories, Detroit, Mich.), and observed in a Leitz microscope combined with a fluorescence epicondenser at a magnification of ×100 and under oil immersion at magnifications of ×500 and ×1,000.
Ligated rat intestinal loop assay.
In vivo action of STb was determined in a ligated rat intestinal loop assay as described previously (12). Briefly, 6- to 8-week-old white male rats (Sprague-Dawley) were fasted for 48 h and anesthetized. The small intestine was exteriorized following a midline abdominal incision and rinsed with 8 ml of 0.85% saline solution containing 300 μg of trypsin inhibitor (TI; Boerhinger GmbH, Mannheim, Germany) per ml. A series of 5-cm-long ligated segments (loops) was made in the small intestine and injected with 500 μl of the test material in 20 mM Tris-HCl buffer (pH 6.8) containing 300 μg of TI per ml. Each loop received either 2 U of sulfatase, 400 μg of laminin, or the buffer only and was then incubated for 20 min before the addition of 5 μg of pure STb. One rat also received either a dilution 1/100 of the antisulfatide monoclonal antibody, a mouse monoclonal antibody specific to the lipopolysaccharide O chain of Actinobacillus pleuropneumoniae serotype 1, or buffer only. The abdominal incision was closed, and the animals were kept alive for 4 h. After the rats were euthanized, the amount of fluid accumulated in the loop was measured. Results are expressed as the ratio of millimeters of fluid accumulated per centimeter of ligated intestinal segment. Values are the averages ± standard deviation of at least three experiments and were evaluated statistically by Student’s t test.
RESULTS
Binding of STb to glycolipids.
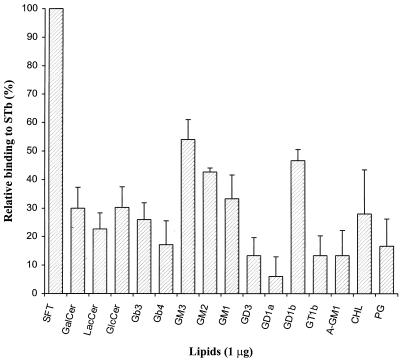
Various standards of neutral lipids and of acidic and neutral glycolipids were subjected to dot blot STb binding assay on a PVDF membrane to compare their affinities for STb (Table 1). As shown in Fig. 1, among the glycolipids tested, STb bound strongly to sulfatide but also more weakly to several gangliosides. The highest binding affinity for STb was obtained with sulfatide, and so the binding level of sulfatide was set arbitrarily at 100% for comparison purpose. A binding level relative to that of sulfatide of 30% or less was exhibited by all neutral glycolipids and every lipid tested, suggesting that neutral carbohydrate were not significantly involved in STb recognition.
TABLE 1.
Oligosaccharide structurea of glycosphingolipids used in this study
| Name | Abbreviation | Structure |
|---|---|---|
| 3-Sulfogalactosylceramide (sulfatide) | SFT | Gal(3SO4)β1Cer |
| Galactosylceramide | GalCer | Galβ1Cer |
| Lactosylceramide | LacCer | Galβ1-4Glcβ1Cer |
| Glucosylceramide | GlcCer | Glcβ1Cer |
| Globotriosylceramide (Pk antigen) | Gb3 | Galα1-4Galβ1-4Glcβ1Cer |
| Globotetraosylceramide (P antigen) | Gb4 | GalNAcβ1-3Galα1-4Galβ1-4Glcβ1Cer |
| Monosialoganglioside GM3 | GM3 | NeuAcα2-3Galβ1-4Glcβ1Cer |
| Monosialoganglioside GM2 | GM2 | GalNAcβ1-4[NeuAcα2-3]Galβ1-4Glcβ1Cer |
| Monosialoganglioside GM1 | GM1 | Galβ1-3GalNAcβ1-4[NeuAcα2-3]Galβ1-4Glcβ1Cer |
| Disialoganglioside GD3 | GD3 | [NeuAcα2-8,NeuAcα2-3]Galβ1-4Glcβ1Cer |
| Disialoganglioside GD1a | GD1a | NeuAcα2-3Galβ1-3GalNAcβ1-4[NeuAcα2-3]Galβ1-4Glcβ1Cer |
| Disialoganglioside GD1b | GD1b | Galβ1-3GalNAcβ1-4[NeuAcα2-8,NeuAcα2-3]Galβ1-4Glcβ1Cer |
| Trisialoganglioside GT1b | GT1b | NeuAcα2-3Galβ1-3GalNAcβ1-4[NeuAcα2-8,NeuAcα2-3]Galβ1-4Glcβ1Cer |
| Asialoganglioside GM1 | AGM1 | Galβ1-3GalNAcβ1-4Galβ1-4Glcβ1Cer |
FIG. 1.
Relative binding of STb to various lipids and glycolipids. Lipids tested (1 μg/μl in methanol) were immobilized on a PVDF membrane at 1 μg/spot, and the binding of STb (0.1 μg/ml) was measured after 10 min as described in Materials and Methods. Values represent averages of at least duplicate experiments (three spots per experiment) analyzed by densitometry. STb binding to sulfatide was taken as 100%, and relative binding to other lipids was calculated as a percentage as follows: (mean densitometric value of lipid × 100)/(mean densitometric value of sulfatide). The standard deviations are shown. For oligosaccharide structures of glycolipids, see Table 1.
The gangliosides GM3, GM2, and GD1b exhibited binding levels of at least 40% relative to the sulfatide. Binding was 54% to GM3 but less than 25% to lactosylceramide (asialo-GM3). The levels of GD1b and GM1 binding were approximately 46 and 33%, respectively, whereas binding was less than 15% for asialo-GM1. Thus, the relative binding levels between these related glycolipids of the sialo- and asialogangliosides series indicated that the absence of the sialic acid resulted in a marked difference in binding to STb. This result suggested that sialic acid residues are involved in the binding process. Interestingly, binding less than 30% of that for sulfatide was observed for galactosylceramide; the latter is the precursor structure of sulfatide where a terminal sulfate is added to the 3 position of the galactose. The difference obtained between these two structurally related structures suggested that the sulfate group on the glycolipid participates in the interaction with STb.
Binding epitope of sulfatide for STb.
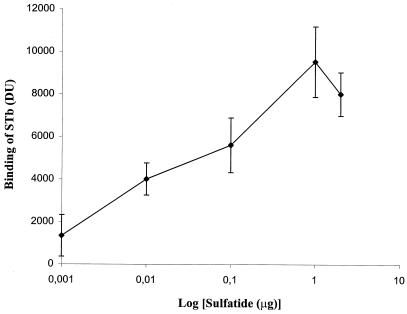
Since STb bound to sulfatide with the highest affinity among the lipids tested, further experiments were conducted to determine the nature of this interaction. The binding of STb (0.1 μg/ml) to increasing amounts of sulfatide was dose dependent (Fig. 2). This result shows that STb bound avidly to sulfatide, even at concentrations of less than 100 ng. Saturation was obtained around 1 μg of sulfatide within the concentration range used. The decreased binding may be due to superposition of glycolipid and ligand-glycolipid complexes occurring on the PVDF membrane, which might have been washed away as the amount of glycolipid increased.
FIG. 2.
Dose-dependent binding of STb to sulfatide. Sulfatide was immobilized on a PVDF membrane at various concentrations ranging from 0.001 to 2 μg in 1 μl of methanol, and the binding of STb (0.1 μg/ml) was measured after 10 min as described in Materials and Methods. Values represent the averages of duplicate experiments (three spots per experiment) analyzed by densitometry. The standard deviations from the mean are shown.
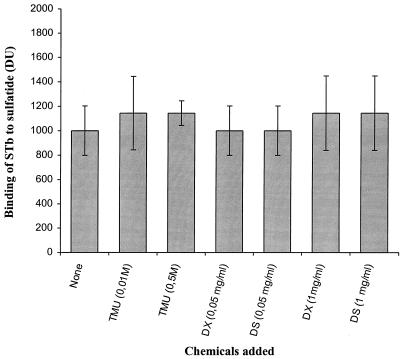
The nature of the interaction between STb and the sulfatide was further characterized by determining the effects of various chemicals on binding (Fig. 3). STb (0.1 μg/ml) was preincubated with a fixed concentration of the tested chemicals and subsequently added to 1 μg of immobilized sulfatide on a PVDF membrane. The toxin was treated with TMU, which strongly disrupts hydrophobic interactions. The binding of STb in the presence of TMU was the same as for the untreated control. Thus, the hydrophobic ceramide moiety of the sulfatide did not appear to be responsible for the interaction with STb.
FIG. 3.
Effects of various chemicals on STb binding to sulfatide. Sulfatide (1 μg) was immobilized on a PVDF membrane. Incubation of STb (0.1 μg/ml) was performed for 10 min with either 0.05 or 0.1 M TMU or 0.05 or 1 mg of dextran (DX; Mr, 9500) or dextran sulfate (DS; Mr, 10000) per ml before addition to immobilized sulfatide; binding of STb toxin was measured after 10 min as described in Materials and Methods. Values represent the averages of duplicate experiments (three spots per experiment) analyzed by densitometry. The standard deviations from the mean are shown.
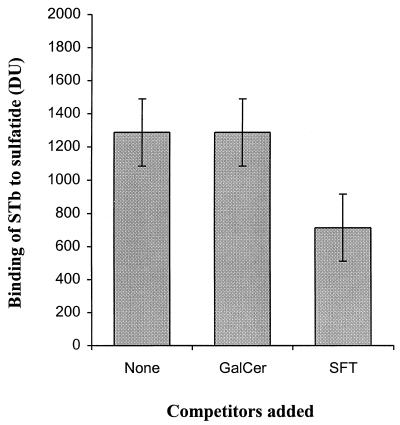
The difference of binding efficiencies observed between the sulfatide and galactosylceramide may be attributed to the charge of the sulfate group. As STb is a basic peptide (17, 18), it is possible that the binding was due simply to ionic charges. To determine the importance of the sulfate group, the toxin was incubated with either dextran or sulfated dextran. Those compounds had no significant effect on the binding of STb to sulfatide, suggesting that it is not solely the result of an ionic interaction (Fig. 3). Furthermore, binding to dextran and sulfated dextran was assayed by incubation with STb. No binding of STb was observed to 0.05 or 100 μg of dextran or dextran sulfate immobilized on nitrocellulose membranes (data not shown). The data indicate that recognition may also involved the stereospecificity between STb and both the sulfate group and the galactose residue that seems to constitute the recognized binding epitope. We also examined the ability of the sulfatide to compete for STb binding. Sulfatide in 500-fold molar excess was a potent competitor of STb binding, with approximately 45% inhibition of total binding obtained, whereas the corresponding amount of galactosylceramide showed no detectable inhibition (Fig. 4).
FIG. 4.
Competition assay of STb binding to sulfatide. After sulfatide (1 μg) was immobilized on a PVDF membrane, STb (0.1 μg/ml) preincubated for 10 min with a 500-fold molar excess of either the sulfatide or galactosylceramide was added; binding of STb was measured after 10 min as described in Materials and Methods. Values represent the binding of STb to sulfatide and are the averages of duplicate experiments (three spots per experiment) analyzed by densitometry. The standard deviations from the mean are shown.
Occurrence of sulfatide-like glycolipid in lipids extracted from pig jejunal epithelial cells and mucus.
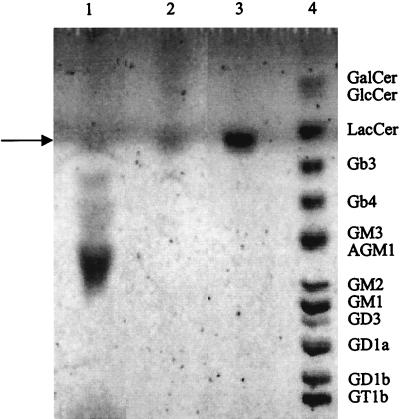
Total lipids were extracted from the mucosal cell surface and mucus of the pig jejunum and resolved by TLC. Many of the lipid molecules were detected by Bial’s reagent and were therefore regarded as glycolipids. One of them had a mobility similar to that of the standard sulfatide (Fig. 5). Identification of the lipid contained in this band was performed. We observed that the band comigrated with standard sulfatide when total lipids extract was submitted to two-dimensional TLC using a different elution system (data not shown). Thus, the band of interest did not correspond to a mixture of lipids, suggesting a composition and a structure similar to those of the standard sulfatide.
FIG. 5.
Occurrence of sulfatide-like glycolipid in pig jejunal mucus and epithelial cells. Total lipid extracts were separated by TLC developed in chloroform–methanol–0.02% CaCl2 in water (55:45:10 by volume) and visualized with Bial’s reagent. Lanes: 1, total lipids extracted from mucus of pig jejunum (200 μg); 2, total lipids extracted from epithelial cells of pig jejunum (200 μg); 3, sulfatide standard (5 μg); 4, glycolipid standards GalCer (10 μg), GlcCer (10 μg), LacCer (10 μg), Gb3 (2.5 μg), Gb4 (2.5 μg), GM3 (2.5 μg), GM2 (2.5 μg), GM1 (2.5 μg), GD3 (2.5 μg), GD1a (2.5 μg), GD1b (2.5 μg), GT1b (2.5 μg), and asialo-GM1 (2.5 μg). Reference glycolipids are indicated on the right. The arrow indicates the sulfatide. For abbreviations, see Table 1.
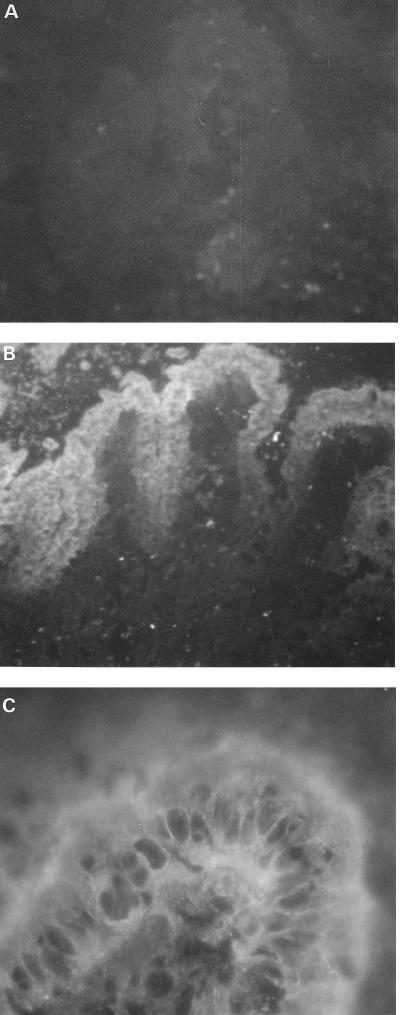
The jejunal lipid contained in this sulfatide-like band was extracted and purified to homogeneity from the TLC plate. The preparations of the jejunal lipid and of a silica gel control were overlaid with laminin, a glycoprotein which specifically binds to sulfated glycolipids. Laminin bound to the extracted lipid but not to the extracted silica gel control. Furthermore, the jejunal lipid was specifically recognized by a monoclonal antisulfatide antibody (data not shown). The epitope recognized by this monoclonal antibody, developed by Fredman et al. (14), is the terminal Gal(3SO4)β1- and part of the hydrophobic region of the ceramide. These results indicated that sulfatide was present in the total lipid extract of the pig jejunum mucosal surface. Moreover, an immunohistochemistry study of pig jejunum using the monoclonal antisulfatide antibody revealed that epithelial cells of the mucosa were specifically labeled, and no staining was observed in controls in which the first antibody was omitted (Fig. 6). Thus, sulfatide was shown to be accessible to STb toxin on the target cell surface.
FIG. 6.
Determination of the epithelial localization of sulfatide on pig jejunal mucosa, using immunohistochemistry and a monoclonal antisulfatide antibody. (A) Control experiment where the primary antibody was omitted (magnification, ×85); (B) pig jejunum showing immunofluorescence of the villi (×85); (C) pig jejunum showing immunofluorescence of the epithelial cells (×425).
Binding of STb to the sulfatide obtained from the pig jejunum mucosal cell surface.
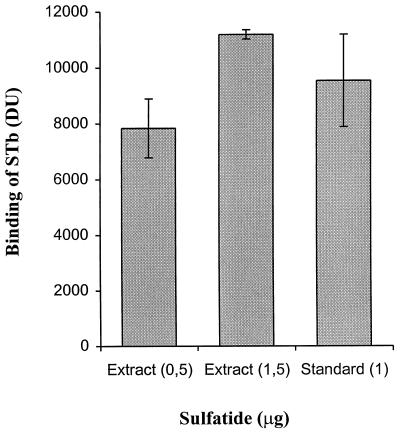
With TLC, the sulfatide band from the total lipids preparation of epithelial cells was four times less intense than the band corresponding to 5 μg of sulfatide standard as determined by densitometry (Fig. 5), indicating that approximately 1.25 μg of sulfatide is contained in 200 μg of total lipid extract (content of 0.62%). As shown in Fig. 7, the eluted epithelial cell sulfatide was bound by STb in the dot blot STb binding assay on a PVDF membrane. The amounts of eluted sulfatide dotted were from 80 and 240 μg of jejunal epithelial cell total lipids extract; the corresponding amounts of eluted sulfatide were thus estimated to 0.5 and 1.5 μg, respectively. The results indicate that the binding levels measured with the eluted sulfatide are similar to those with the sulfatide standard shown in Fig. 2, in which 0.5 and 1.5 μg resulted in binding levels of approximately 7,300 and 8,700 DU, respectively. Thus, the binding of STb to the sulfatide identified in the porcine jejunum seems dose dependent and saturable similarly to the sulfatide standard.
FIG. 7.
Binding of STb to the sulfatide isolated from pig jejunal epithelial cells. Following extraction from preparative TLC developed in chloroform–methanol–0.02% CaCl2 in water (55:45:10 by volume), the eluted pig jejunal sulfatide was immobilized on a PVDF membrane, and the binding of STb was measured after 10 min as described in Materials and Methods. Binding was calculated by subtracting the mean DU obtained for the silica gel control from the mean DU obtained for the eluted sulfatide. Values represent the averages of triplicate experiments (three spots per experiment) analyzed by densitometry. The standard deviations from the mean are shown.
Functional receptor activity of sulfated glycolipid for STb.
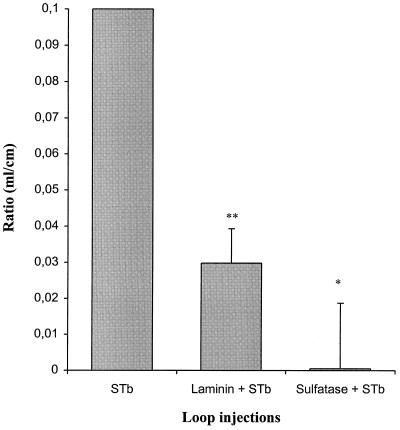
In the in vivo model, the biological activity of STb was strongly inhibited when the intestinal loops were pretreated with either sulfatase or laminin before addition of STb, while the ratio obtained when either sulfatase or laminin was injected alone into the loops was similar to that for the loop control containing only the buffer (Fig. 8). Approximately 100% inhibition of the STb-mediated secretion was observed following pretreatment with sulfatase. Thus, the sulfation of cell surface molecules seems crucial for the biological activity of STb. Pretreatment with laminin decreased the biological response of STb by approximately 71%, indicating the functionality of the sulfated glycolipids, including the sulfatide present in the cell membrane. Additional evidence demonstrated the functional activity of the sulfatide, as we observed a 35% reduction of secretion due to STb following pretreatment with the diluted monoclonal antisulfatide antibody (1/100) (data not shown).
FIG. 8.
Inhibition of the biological activity of STb. STb-mediated secretion was measured in ligated rat small intestinal loops. Each loop received 5 μg of STb diluted in 20 mM Tris-HCl buffer (pH 6.8) containing 300 μg of TI per ml following pretreatment with either laminin (400 μg) or sulfatase (2 U) for 20 min. The standard deviations are shown. Each ratio was significantly different from the STb maximal ratio (0.1 ml/cm), using the statistical Student t test analysis. ∗, P <0.01; ∗∗, P <0.005.
DISCUSSION
The interaction of most extracellular toxins, like E. coli STb, to host cells is thought to be an important first step in pathogenesis in order to elicit a specific toxic effect. This pathogenic event is most likely to take place through the binding of the toxin to specific receptors on target cells. In a search for molecules which serve as STb receptors, we recently investigated the interaction of STb at the surface of the epithelial cells of the pig jejunum brush border (38). Our study indicated that oxidation of the carbohydrates from the cell surface by sodium metaperiodate treatment significantly inhibited STb binding, suggesting that the binding could be mediated by a lectin-like activity of STb. We also showed that the glycoconjugate molecules bound by STb were lipid and not proteinaceous in nature.
In this study, we developed a simple binding assay for STb in which densitometric analysis provided a mean for quantitation of bound toxin to various purified lipids and glycolipids containing different oligosaccharide structures (Table 1). A PVDF membrane was used because of its stability in various organic solvents in addition to its capacity to immobilize glycolipids with high efficiency (5, 41). Several optimal parameters of the STb binding reaction to the pig jejunum thin sections were established in our previous study (38). Here, the binding assay was performed under the same optimal conditions (with STb at a final concentration of 0.1 μg/ml in 0.1 M phosphate buffer [pH 5.8] for 10 min). Of the many tested lipids and glycolipids, STb selectively bound to acidic glycolipids (Fig. 1). Sulfatide, which is a molecule carrying an anionic sulfate residue, showed the strongest binding affinity. STb also significantly bound to several gangliosides, and the negatively charged sialic acid residues appeared to be involved in this reaction. The implication of acidic glycolipids in STb binding was not surprising. Since mature STb is a basic peptide with a pI of 9.6 (18), the binding of STb to acidic molecules suggested that a strong stereospecific and electrostatic bond can be formed between a protonated nitrogen of STb and the anionic site on the glycolipid. In other words, most of the affinity of the acidic glycolipids with STb may require an intact three-dimensional structure containing some specific positively charged amino acids. Of interest, three positively charged amino acid residues (K45, K46, and R52) and one negatively charged amino acid residue (D53) contained in STb were previously shown to be crucial for enterotoxicity and were presumed to belong to a receptor-binding domain (10, 17).
The specificity of STb binding to sulfatide was demonstrated here by several indirect and direct pieces of evidence. We have shown that this binding was strong, dose dependent, and saturable and not due to either hydrophobic or sulfate group ionic interactions alone. Finally, the specificity was supported by the finding that binding was competed by an excess of the sulfatide. Differences in STb binding levels between sulfatide and galactosylceramide indicated that the sulfation of the carbohydrate chain was important for binding (Fig. 1). However, the sulfate group itself was not sufficient to explain STb binding, since sulfated dextran, a sulfated polymer of glucose, had no effect on binding of STb to sulfatide (Fig. 3). Sulfatide competition suggested that the binding was dependent on the sulfate group linked on the galactosyl moiety (Fig. 4). STb was not competed by galactosylceramide, which indicated that the unsubstituted galactosyl residue was not sufficient to allow STb binding. The sulfation of this carbohydrate seems important for binding. Thus, the binding goes beyond simple charge interactions with an anionic group and has also some specificity for the nature of the carbohydrate with a particular chain geometry and distribution of negative charge. These results indicate that STb binding specificity for sulfatide requires the Gal(3SO4)β1-residue.
A lipid preparation from the pig jejunum exhibited one lipid with a mobility on TLC similar to that of the standard sulfatide (Fig. 5), and the identity of the sulfatide was confirmed by specific detection with laminin binding and with an antisulfatide monoclonal antibody. Our findings indicated that substantial amounts of sulfatide were present in porcine epithelial cells and associated with the mucous layer. Glycolipids are membrane-bound molecules and do not appear in secretions, except when shed with epithelial cells. The small intestinal epithelium is continuously renewed by the proliferation of some mitotically active crypt cells, which migrate toward the top of the villi, where they desquamate. The presence of sulfatide in mucus could be explained in part by the abundance of this substance at the cell surface and also by the procedure retained for mucus preparation, in which the interstitial mucus was harvested and may therefore contain some epithelial cells.
The cellular surface localization and abundance of sulfatide was confirmed in pig jejunal mucosa by using immunohistochemistry (Fig. 6). Multiple functions have been proposed for sulfatide, including roles in sodium chloride transport (31), in myelin compaction by its association with the encephalitogen basic protein (8), as opiate receptors (7), and in cell adhesion (36, 40). Although this sulfated glycolipid is most abundant in the white matter of the brain, it is also found in the extracellular matrix, in mucus, and on the surface of epithelial cells of many mammalian tissues. Sulfatide has been already shown to be the most predominant acidic glycophingolipid in rabbit and human gastric mucosa (33, 34). Because of its acid-resistant nature and the high negative charge of the sulfated sugar moiety, it was assumed that sulfatide might play a role in protection of the mucosa. From this point of view, enrichment of the pig jejunum mucosa with sulfatide is consistent with the physiological relevance of this particular substance to mucosal function, because the pH of the jejunum is relatively acid, varying between 5.5 and 6.5. Moreover, the localization of sulfatide on the mucosal cell surface indicates that this molecule could also be recognized and utilized as a receptor. We showed that sulfatide was present in the total lipids extract of the pig jejunum mucosal surface and that this jejunal sulfatide was bound by STb toxin (Fig. 7). The binding activity of STb to sulfatide corroborates the inhibition of the secretory response evoked by STb in the ligated loop model observed with either sulfatase or laminin pretreatment (Fig. 8). The elimination of sulfate groups of various carbohydrate sequence, including the sulfate group on the galactose residue present in sulfatide, abolished completely STb activity. In addition, STb enterotoxicity was strongly affected following the action of the laminin, a 850-kDa cell adhesion protein which binds specifically to sulfated glycolipids and does not bind to other anionic lipids, including gangliosides, phospholipids, or cholesterol-3-sulfate (37). The monoclonal antisulfatide antibody also altered STb toxicity. Together, these data indicate that sulfatide molecule functions as a receptor for STb interaction in vivo.
Investigations will be pursued to determine if sulfatide is the unique functional receptor for STb. Effectively, the reported results also support that terminal sialic acid residues of gangliosides might be involved in the binding of STb. Significant binding was obtained with several gangliosides, including GM3 and GM1 whereas levels of binding to asialo-GM3 (lactosylceramide) and asialo-GM1 were more than two times less, respectively (Fig. 1). Because of the unique structural difference between these molecules in the presence or absence of sialic acid residues, it appears that sialic acid residues are important for STb binding to gangliosides. Binding of STb to gangliosides was expected because it had already been reported that pretreatment of pig jejunal epithelial cells surface with the neuraminidase from C. perfringens abolished the binding of STb (38). The effect of STb binding following the pretreatment of HT29 and T84 human intestinal epithelial cells with a neuraminidase from Vibrio cholerae was also studied by Chao and Dreyfus (6). The results showed that neuraminidase pretreatment did not reduce the binding of STb to the cells. While both the pH of the assay binding buffer and the nature of the cells used were different, the neuraminidase specificity cleavage appears to be the most important difference between the two studies. In fact, the enzyme from C. perfringens preferentially cleaves sialic acid in a terminal location and linked α2-3 to galactose, whereas the enzyme from V. cholerae used by Chao and Dreyfus (6) selectively cleaves terminal sialic acid linked α2-6 to galactose. Therefore, our data highlight the importance of sialic acid in α2-3 linkage to galactose for the binding of STb. Interestingly, the GM3 ganglioside, which exhibited the strongest binding to STb among all of gangliosides tested, contains a short carbohydrate sequence, where NeuAcα2-3Galβ1- is found in the terminal position (Table 1). However, the commercially available gangliosides tested in this study did not allow us to identify which ganglioside in the target tissue total lipids extract could be responsible for STb binding. Further analyses are in progress in our laboratory to examine the role of gangliosides in STb toxin binding, but one provocative hypothesis may be developed to describe the relevant binding of STb to its target cells.
First, since the C. perfringens neuraminidase treatment of pig jejunum abolished STb binding, it was thought that STb could not bind to glycosphingolipids other than gangliosides (38). Nevertheless, strong binding to sulfatide obtained from the pig jejunal epithelial cells was shown (Fig. 7). Thus, sulfatide and some gangliosides are present on the cell surface of the jejunum, and all could be bound by STb. Then, if these two attachment sites are required for STb on the target cells, the data let us hypothesize a multistep attachment process where sulfatide-mediated binding occurs only if the attachment to ganglioside takes place first. This hypothesis could explain why the binding of STb to sulfatide could not be detected after neuraminidase treatment that affects the ganglioside binding to the pig jejunum. Second, Chao and Dreyfus (6) observed that elimination of sulfated structures by pretreatment of human intestinal epithelial cell lines with sodium chlorate did not decrease the binding of STb to the cells. Sodium chlorate, which is a potent inhibitor of cellular sulfotransferases, induces expression of surface glycoproteins and glycolipids lacking sulfate groups (24). According to our proposed model, after elimination of surface sulfation, binding to sialic acid of gangliosides would be nevertheless possible. The observed binding might represent binding to ganglioside in absence of sulfated structures. Third, we present evidence that the sulfated structures act as biologically functional receptors for STb, as sulfatase pretreatment in ligated rat intestinal loop assay abolished STb enterotoxicity (Fig. 8). Overall in this study, cell surface sulfated glycolipids were shown to be required in both binding and biological activities of STb. We thus propose a multistep binding model for STb on epithelial cells: binding to ganglioside could represent the first step, which might be important to facilitate the subsequent binding to sulfatide. This second step could ensure an intimate contact of the toxin with the host cell membrane due to the shorter structure of the sulfatide molecule.
The competitive binding experiments performed by Chao and Dreyfus (6) indicated that unlabeled STb could specifically compete with 125I-STb binding to a receptor on T84 and HT29 cells but failed to reduce by more than 50% the binding in the presence of 5-, 100-, and 1,000-fold excess STb. The authors concluded that the STb-epithelial cell interactions could be characterized by binding to a high number of binding sites and that STb could interact with multiple receptors. Based on our previous study (38) and the results of this study, at least two glycolipid molecules could effectively be involved in STb recognition and binding to the pig jejunum. STb toxin could bind both sialic acid and sulfated galactose residues on gangliosides and sulfatide, respectively. These glycosphingolipids could serve as multiple attachment sites for STb toxin. Interestingly, the parallel occurrence of sialic acid and sulfate group specificities has been observed for some lectins. For example, the vascular adhesion family of selectins exhibited specificity for the blood group oligosaccharides sialyl-Lewisx and sialyl-Lewisa bearing either a sialic acid linked α2-3 or a sulfate group on carbon 3 of the nonreducing terminal galactose (42). Another example is the natural killer cell called lectin NKR-P1, which bound α2-3-linked sialic acid- or sulfate-modified galactosyl determinants on tumor cell membrane constituents, with greater affinity for the latter (1). Complete characterization of the STb-sulfatide and -ganglioside binding reactions should help to develop an STb-receptor model to study the process at the molecular level.
ACKNOWLEDGMENTS
This work was supported by grants to J.D.D. from the Natural Sciences and Engineering Research Council of Canada (OGP0139070) and Fonds pour la Formation de Chercheurs et l’Aide à la Recherche (93-ER-0214).
We express our appreciation to Pam Fredman, Göteborg University, Göteborg, Sweden, for providing the antisulfatide monoclonal antibody used in this study, to Maan Abul Milh for his helpful suggestions, and to Hans-E. Beausoleil and Vincent Labrie for their valuable collaboration with the rat loop assays.
REFERENCES
- 1.Bazouska K, Yuen C-T, O’Brien J, Childs R A, Chai W, Lawson A M, Drbal K, Fiserovà A, Popisil M, Feizi T. Oligosaccharide ligands for NKR-P1 protein activate NK cells and cytotoxicity. Nature. 1994;372:150–157. doi: 10.1038/372150a0. [DOI] [PubMed] [Google Scholar]
- 2.Blanco M, Blanco J E, Gonzales E A, Mora A, Jansen W, Gomes T A T, Zerbini F, Yano T, de Castro A F P, Blanco J. Genes coding for enterotoxins and verotoxins in porcine Escherichia coli strains belonging to different O:K:H serotypes: relationship with toxic phenotypes. J Clin Microbiol. 1997;35:2958–2963. doi: 10.1128/jcm.35.11.2958-2963.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bossé M, Handl C E, Lortie L A, Harel J, Dubreuil J D. Fusion of the genes encoding Escherichia coli heat-stable enterotoxin b (STb) and the maltose-binding protein to obtain mature STb enterotoxin. J Gen Microbiol. 1993;139:631–638. doi: 10.1099/00221287-139-3-631. [DOI] [PubMed] [Google Scholar]
- 4.Burgess M N, Bywater R J, Cowley C M, Mullan N A, Newsome P M. Biological evaluation of a methanol-soluble, heat-stable Escherichia coli enterotoxin in infant mice, pigs, rabbits, and calves. Infect Immun. 1978;21:526–531. doi: 10.1128/iai.21.2.526-531.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chabraoui F, Derrington E A, Mallie-Didier F, Confavreux C, Quincy C, Caudie C. Dot-blot immunodetection of antibodies against GM1 and other gangliosides on PVDF-P membranes. J Immunol Methods. 1993;165:225–230. doi: 10.1016/0022-1759(93)90348-b. [DOI] [PubMed] [Google Scholar]
- 6.Chao K L, Dreyfus L A. Interaction of Escherichia coli heat-stable enterotoxin B with cultured human intestinal epithelial cells. Infect Immun. 1997;65:3209–3217. doi: 10.1128/iai.65.8.3209-3217.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Craves F B, Zalc B, Leybin L, Baumann N, Loh H H. Antibodies to cerebroside sulfate inhibit the effects of morphine and β-endorphin. Science. 1980;207:75–76. doi: 10.1126/science.6243189. [DOI] [PubMed] [Google Scholar]
- 8.Demel D A, London U, Geurts Van Hessel W S M, Vossenberg F G A, Van Deenen L L M. The specific interaction of myelin basic protein with lipids at the air-water interface. Biochim Biophys Acta. 1973;311:506–519. doi: 10.1016/0005-2736(73)90126-0. [DOI] [PubMed] [Google Scholar]
- 9.Dreyfus L A, Harville B, Howard D E, Shaban R, Beatty D M, Morris S J. Calcium influx mediated by the Escherichia coli heat-stable enterotoxin B (STB) Proc Natl Acad Sci USA. 1993;90:3202–3206. doi: 10.1073/pnas.90.8.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dreyfus L A, Urban R G, Whipp S C, Slaughter C, Tachias K, Kupersztoch Y M. Purification of the STB enterotoxin of Escherichia coli and the role of selected amino acids on its secretion, stability and toxicity. Mol Microbiol. 1992;6:2397–2406. doi: 10.1111/j.1365-2958.1992.tb01414.x. [DOI] [PubMed] [Google Scholar]
- 11.Dubreuil J D. Escherichia coli STb enterotoxin. Microbiology. 1997;143:1783–1795. doi: 10.1099/00221287-143-6-1783. [DOI] [PubMed] [Google Scholar]
- 12.Dubreuil J D, Fairbrother J M, Lallier R, Larivière S. Production and purification of heat-stable enterotoxin b from a porcine Escherichia coli strain. Infect Immun. 1991;59:198–203. doi: 10.1128/iai.59.1.198-203.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folch J, Lees M, Sloane Stanley G H. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 14.Fredman P, Mattson L, Andersson K, Davidsson P, Ishizuka I, Jeansson S, Mansson J E, Svennerholm L. Characterization of the binding epitope of a monoclonal antibody to sulphatide. Biochem J. 1988;251:17–22. doi: 10.1042/bj2510017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujii Y, Kondo Y, Okamoto K. Involvement of prostaglandin E2 synthesis in the intestinal secretory action of Escherichia coli heat-stable enterotoxin II. FEMS Microbiol Lett. 1995;130:259–265. doi: 10.1111/j.1574-6968.1995.tb07729.x. [DOI] [PubMed] [Google Scholar]
- 16.Fujii Y, Nomura T, Yamanaka H, Okamoto K. Involvement of Ca(2+)-calmodulin-dependent protein kinase II in the intestinal secretory action of Escherichia coli heat-stable enterotoxin II. Microbiol Immunol. 1997;41:633–636. doi: 10.1111/j.1348-0421.1997.tb01904.x. [DOI] [PubMed] [Google Scholar]
- 17.Fujii Y, Okamuro Y, Hitotsubashi S, Saito A, Akashi N, Okamoto K. Effect of alterations of basic amino acid residues of Escherichia coli heat-stable enterotoxin II on enterotoxicity. Infect Immun. 1994;62:2295–2301. doi: 10.1128/iai.62.6.2295-2301.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Handl C E, Harel J, Flock J I, Dubreuil J D. High yield of active STb enterotoxin from a fusion protein (MBP-STb) expressed in Escherichia coli. Protein Expression Purif. 1993;4:275–281. doi: 10.1006/prep.1993.1035. [DOI] [PubMed] [Google Scholar]
- 19.Harville B A, Dreyfus L A. Release of serotonin from RBL-2H3 cells by the Escherichia coli peptide toxin STb. Peptides. 1996;17:363–366. doi: 10.1016/0196-9781(96)00033-2. [DOI] [PubMed] [Google Scholar]
- 20.Harville B A, Morris B J, Dreyfus L A. Abstracts of the 95th General Meeting of the American Society for Microbiology 1995. Washington, D.C: American Society for Microbiology; 1995. Involvement of heterotrimeric G-protein (Gαi3) in the mechanism of action of Escherichia coli enterotoxin B, abstr. B-215. [Google Scholar]
- 21.Hawkes R. Identification of concanavalin A-binding protein after sodium dodecyl sulfate gel electrophoresis and protein blotting. Anal Biochem. 1982;123:143–146. doi: 10.1016/0003-2697(82)90634-0. [DOI] [PubMed] [Google Scholar]
- 22.Hitotsubashi S, Fujii Y, Okamoto K. Binding protein for Escherichia coli heat-stable enterotoxin II in mouse intestinal membrane. FEMS Microbiol Lett. 1994;122:297–302. doi: 10.1111/j.1574-6968.1994.tb07183.x. [DOI] [PubMed] [Google Scholar]
- 23.Hitotsubashi S, Fujii Y, Yamanaka H, Okamoto K. Some properties of purified Escherichia coli heat-stable enterotoxin II. Infect Immun. 1992;60:4468–4474. doi: 10.1128/iai.60.11.4468-4474.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoogewerf A J, Cisar L A, Evans D C, Bensadoun A. Effect of chlorate on the sulfation of lipoprotein lipase and heparan sulfate proteoglycans. Sulfation of heparan sulfate proteoglycans affects lipoprotein lipase degradation. J Biol Chem. 1991;266:16564–16571. [PubMed] [Google Scholar]
- 25.Iida N, Toida T, Kushi Y, Handa S, Fredman P, Svennerholm L, Ishizuka I. A sulfated glucosylceramide from rat kidney. J Biol Chem. 1989;264:5974–5980. [PubMed] [Google Scholar]
- 26.IUPAC-IUB Commission on Biochemical Nomenclature. The nomenclature of lipids. Biochem J. 1978;171:21–35. doi: 10.1042/bj1710021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.IUPAC-IUB Joint Commission on Biochemical Nomenclature. Nomenclature of glycoproteins, glycopeptides and peptidoglycans. Recommendations 1985. Eur J Biochem. 1986;159:1–6. doi: 10.1111/j.1432-1033.1986.tb09825.x. [DOI] [PubMed] [Google Scholar]
- 28.Kaper J B, Morris J G, Jr, Levine M M. Cholera. Clin Microbiol Rev. 1995;8:48–86. doi: 10.1128/cmr.8.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karlsson K A. Microbial recognition of target-cell glycoconjugates. Curr Opin Struct Biol. 1995;5:622–635. doi: 10.1016/0959-440x(95)80054-9. [DOI] [PubMed] [Google Scholar]
- 30.Karlsson K A, Ångstrom J, Bergstrom J, Lanne B. Microbial interaction with animal cell surface carbohydrates. APMIS Suppl. 1992;100:71–83. [PubMed] [Google Scholar]
- 31.Karlsson K A, Samuelson B E, Steen G O. The lipid composition and Na+-K+-dependent adenosine triphosphatase activity of the salt (nasal) gland of eider duck and herring hull. Eur J Biochem. 1974;46:243–258. doi: 10.1111/j.1432-1033.1974.tb03617.x. [DOI] [PubMed] [Google Scholar]
- 32.Kennedy D J, Greenberg R N, Dunn J A, Abernathy R, Ryerse J S, Guerrant R L. Effects of Escherichia coli heat-stable enterotoxin STb on intestines of mice, rats, rabbits, and piglets. Infect Immun. 1984;46:639–643. doi: 10.1128/iai.46.3.639-643.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Natomi H, Saitoh T, Sugano K, Iwamori M, Fukayama M, Nagai Y. Systematic analysis of glycosphingolipids in the human gastrointestinal tract: enrichment of sulfatides with hydroxylated longer-chain fatty acids in the gastric and duodenal mucosa. Lipids. 1993;28:737–742. doi: 10.1007/BF02535996. [DOI] [PubMed] [Google Scholar]
- 34.Natomy H, Sugano K, Iwamori M, Takaku F, Nagai Y. Region-specific distribution of glycosphingolipids in the rabbit gastrointestinal tract: preferential enrichment of sulfoglycolipids in the mucosal regions exposed to acid. Biochim Biophys Acta. 1988;961:213–222. [PubMed] [Google Scholar]
- 35.Peterson J W, Whipp S C. Comparison of the mechanisms of action of cholera toxin and the heat-stable enterotoxins of Escherichia coli. Infect Immun. 1995;63:1452–1461. doi: 10.1128/iai.63.4.1452-1461.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberts D D, Ginsburg V. Sulfated glycolipids and cell adhesion. Arch Anal Biochem Biophys. 1988;267:405–415. doi: 10.1016/0003-9861(88)90046-x. [DOI] [PubMed] [Google Scholar]
- 37.Roberts D D, Rao C N, Magnani J L, Spitalnik S L, Liotta L A, Ginsburg V. Laminin binds specifically to sulfated glycolipids. Proc Natl Acad Sci USA. 1985;82:1306–1310. doi: 10.1073/pnas.82.5.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rousset E, Harel J, Dubreuil J D. Binding characteristics of Escherichia coli enterotoxin b (STb) to the pig jejunum and partial characterization of the molecule involved. Microb Pathog. 1998;24:277–288. doi: 10.1006/mpat.1997.0193. [DOI] [PubMed] [Google Scholar]
- 39.Spangler B D. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol Rev. 1992;56:622–647. doi: 10.1128/mr.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suzuki Y, Toda Y, Tamatani T, Watanabe T, Suzuki T, Nakao T, Murase K, Kiso M, Hasegawa A, Tadano-Aritomi K, Ishizuka I, Miyasaka M. Sulfated glycolipids are ligands for a lymphocyte homing receptor, L-selectin (LECAM-1), binding epitope in sulfated sugar chain. Biochem Biophys Res Commun. 1993;190:426–434. doi: 10.1006/bbrc.1993.1065. [DOI] [PubMed] [Google Scholar]
- 41.Taki T, Handa S, Ishikawa D. Blotting of glycolipids and phospholipids from a high-performance thin-layer chromatogram to a polyvinylidene difluoride membrane. Anal Biochem. 1994;221:312–316. doi: 10.1006/abio.1994.1418. [DOI] [PubMed] [Google Scholar]
- 42.Varky A. Selectin ligands. Proc Natl Acad Sci USA. 1994;91:7390–7397. doi: 10.1073/pnas.91.16.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weikel C S, Guerrant R L. STb enterotoxin of Escherichia coli: cyclic nucleotide-independent secretion. Ciba Found Symp. 1985;112:94–115. doi: 10.1002/9780470720936.ch6. [DOI] [PubMed] [Google Scholar]
- 44.Weikel C S, Nellans H N, Guerrant R L. In vivo and in vitro effects of a novel enterotoxin, STb, produced by Escherichia coli. J Infect Dis. 1986;153:893–901. doi: 10.1093/infdis/153.5.893. [DOI] [PubMed] [Google Scholar]
- 45.Whipp S C. Protease degradation of Escherichia coli heat-stable, mouse-negative, pig-positive enterotoxin. Infect Immun. 1987;55:2057–2060. doi: 10.1128/iai.55.9.2057-2060.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whipp S C, Moon H W, Argenzio R A. Comparison of enterotoxic activities of heat-stable enterotoxins from class 1 and class 2 Escherichia coli of swine origin. Infect Immun. 1981;31:245–251. doi: 10.1128/iai.31.1.245-251.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]