Abstract
Background:
The risk of fetal atrioventricular block in anti-Ro/SSA antibody exposed pregnancies with no prior affected offspring approximates 2%. High antibody titer is necessary but not sufficient for atrioventricular block and specific antibody titers do not predict risk. However, there are no data on the negative predictive value of antibody titer to identify pregnancies at low risk for fetal atrioventricular block and thus do not require surveillance.
Objective(s):
To define anti-Ro52 and anti-Ro60 antibody thresholds for identification of fetuses unlikely to develop atrioventricular block using clinically validated and research laboratory tests.
Study Design:
We performed a multicenter review of pregnant subjects who tested positive in their local commercial laboratories for anti-Ro/SSA antibodies at University of Colorado Children’s Hospital (2014–2021), Phoenix Children’s Hospital (2014–2021), and enrolled in the Research Registry for Neonatal Lupus at New York University Langone Medical Center (2002–2021). Subjects were referred based on rheumatologic symptoms or history of atrioventricular block in a previous pregnancy and were retrospectively grouped based on pregnancy outcome. Group 1: No fetal atrioventricular block in current or past pregnancies; Group 2: Fetal atrioventricular block in the current pregnancy; Group 3: Normal current pregnancy but fetal atrioventricular block in a previous pregnancy. Maternal sera were analyzed for anti-Ro52 and anti-Ro60 antibodies using a clinically validated multiplex bead assay (Associated Regional and University Pathologists Laboratories, Inc, Salt Lake City, Utah) and a research enzyme linked immunosorbent immunoassay (New York University). We calculated the negative predictive value separately for anti-Ro52 and anti-Ro60 antibodies and for the two combined using a logistic regression model and a parallel testing strategy.
Results:
We recruited 270 subjects (141 Group 1, 66 Group 2, 63 Group 3). Eighty-nine subjects in Group 1 had data on hydroxychloroquine treatment: Anti-Ro/SSA antibody titers were no different between those treated (n=46) and untreated (n=43). Mean anti-Ro52 and anti-Ro60 titers were lowest in Group 1 and not different between Groups 2 and 3. No cases of fetal atrioventricular block developed among subjects with anti-Ro52 and anti-Ro60 titers <110 arbitrary units per milliliter using the multiplex bead assay of the Associated Regional and University Pathologists Laboratories (n=141). No cases of fetal atrioventricular block developed among subjects with research laboratory anti-Ro52 titers < 650 and anti-Ro60 < 4060 enzyme linked immunosorbent immunoassay units (n=94). Using these 100% negative predictive value thresholds, over 50% of the anti-Ro/SSA antibody pregnancies that ultimately had no f-AVB could be excluded from surveillance based on clinical and research titers, respectively.
Conclusions:
Our data suggest there is a clinical immunoassay level of maternal anti-Ro/SSA antibodies below which the pregnancy is at low risk for fetal atrioventricular block. We speculate that prospectively applying these data may avert the costly serial echocardiograms currently recommended for all anti-Ro/SSA antibody positive pregnancies and guide future management.
Keywords: Cardiac neonatal lupus, fetal arrhythmia, fetal heart block, anti-SSA/Ro antibodies, Sjogren’s syndrome
Condensation:
In mothers with no prior affected children, there is a threshold for anti-Ro/SSA antibody titers below which fetal atrioventricular block is unlikely to occur.
Introduction
The risk of fetal atrioventricular block (AVB) is a critical aspect in counseling anti-Ro/SSA positive women regarding pregnancy. To date, the strongest factor predicting fetal AVB is a prior affected child, which increases the risk from 1–2% in primiparous mothers or those with healthy offspring to 18%.1–4 However, except for mothers with a previously affected child who are usually serially monitored due to the high recurrence rate, recommendations for surveillance are often applied without consideration of risk and based on anecdote and institutional culture with the anticipation that identification of early disease will improve outcomes. Surveillance can vary from routine obstetrical care without added monitoring to weekly fetal echocardiograms between 17–26 weeks of gestation and in some cases extending beyond the generally accepted vulnerable period for fetal AVB.5–7 The cost and time burdens of weekly surveillance echocardiograms are significant and, in most cases, have not been shown to detect the transition period from potentially reversible incomplete (1° and 2°) fetal AVB to irreversible complete (3°) fetal AVB.6 Even ambulatory fetal heart rate monitoring, which has successfully identified this transition period, is time consuming for mothers.8, 9 Risk stratifying to reduce or even eliminate surveillance for subjects at minimal or no risk would be a major advance in the management of anti-Ro/SSA exposed pregnancies.
Evidence from translational research laboratories suggests that lower anti-Ro/SSA antibody levels are associated with a reduced risk of fetal AVB,10, 11 but few commercially available tests report anti-Ro52 and anti-Ro60 titers, specificities which have both been associated with the development of fetal AVB dating back to 1989.12 For example, many commercial clinical laboratory improvement amendment (CLIA) approved laboratories use the BioPlex assay, which provides crude ranges of <1, 1–8, or >8 units and do not distinguish between reactivity to anti-Ro60 or anti-Ro52 separately. Our study was initiated to address whether commercially available testing of anti-Ro52 (recombinant protein) and anti-Ro60 (native antigen) and/or testing in a research laboratory using similar sources of these antigens could define a threshold titer below which mothers were highly unlikely to develop fetal AVB, thus reducing the burden of costly and unnecessary surveillance.
Materials and Methods
Subjects
Pregnant subjects from 3 centers (University of Colorado Children’s Hospital Colorado (UCD), New York University (NYU), and Phoenix Children’s Hospital (PCH)) who tested positive for anti-Ro/SSA (either anti-Ro52 or anti-Ro60 or both) in their local commercial assay between 2014 and 2021 (UCD and PCH) and between 2002 and 2021 (NYU) were included in this retrospective cohort study. Maternal serum was retested in the ARUP laboratory. Subjects were divided into 3 groups based on pregnancy outcomes. Group 1: No 1°, 2°, or 3° fetal AVB in the current pregnancy and either primigravid or no history of fetal AVB in a previous pregnancy; Group 2: 2° or 3° Fetal AVB in the current pregnancy; Group 3: No 2° or 3° fetal AVB in the current pregnancy but a history of 2° or 3° fetal AVB in a previous pregnancy.
Hydroxychloroquine use at the time of pregnancy was recorded for most subjects. Pregnancies with fetal 1° AVB or isolated endocardial fibroelastosis were excluded. This study was approved by the institutional review boards at NYU, UCD and PCH.
Fetal 2° and 3° AVB were diagnosed in utero by Doppler and M-mode fetal echocardiography according to standard techniques.13, 14 Second degree AVB was defined as periods of atrial conduction to the ventricle, and 3° AVB was diagnosed if atrial and ventricular beats were unrelated, and the ventricle contracted at its own intrinsic rate. After birth, infants with fetal AVB underwent 12-lead postnatal electrocardiogram (ECG) to confirm the prenatal rhythm.
Laboratory testing
Sera from pregnant subjects undergoing clinical care at UCD and PCH were sent directly to the clinically validated Associated Regional and University Pathologists (ARUP) Laboratories, Salt Lake City, UT, a CLIA-approved laboratory. Sera from pregnant NYU subjects were collected either as part of the Research Registry for Neonatal Lupus or the Lupus Biorepository (both NYU ethics board-approved) and frozen until time of shipment to the ARUP Laboratories. Testing for anti-Ro52 and anti-Ro60 IgG antibody titers at the ARUP Laboratories was done using the fluorescent microsphere immunodetection system (FIDIS) Connective 10 multiplex bead assay (Theradiag, Marne La Vallée, France).15 A positive result was defined as > 41 arbitrary units per milliliter (AU/mL) for anti-Ro52 or anti-Ro60, per the manufacturer’s recommendation. Only positive assays were included in the analysis, and all assays were performed without knowledge of the subjects’ outcome group.
Maternal serum was also evaluated in the research laboratory of JPB and RMC at NYU Grossman School of Medicine. Recombinant human Ro52 (1 μg/μl)10 or native bovine Ro60 (Arotec Diagnostics) (0.1 μg/μl) in phosphate buffered saline (PBS) were coated onto 96-well microtiter plates (flat bottom polystrene microplates, Crystalgen) overnight at 4°C. Next, the plates were washed with PBS/0.1% (Tween 20) to block all non-specific sites. Serial dilutions (1:1,000 – 1:100,000) of human sera containing the primary antibody were applied for 1 hour at room temperature and run in duplicate. Enzyme-labeled secondary antibody, alkaline-phosphatase conjugated rabbit anti-human IgG (γ-chain specific) (Sigma, St Louis, MO), was applied (1:2,000) to bind the primary antibody. Finally, the phosphatase substrate was added, and optical density (OD) was measured at 405 nm. High and low positive sera from fetal AVB pregnancies were used as positive controls. Controls were diluted 1:1,000 (and 1:150,000 for the high positive) to ensure that measurements were captured across the assay’s range. Reactions were stopped when the low positive control reached an OD of 1. A dilution factor was applied to all test samples to give an OD between 0.3–0.8.
Statistical methods
Summaries used median (minimum, maximum) or count (%) for continuous and categorical variables, respectively. Boxplots were created to visualize the distribution of titers within the 3 groups.
Quantile regression of the median was used to evaluate whether the distribution of anti-Ro52 or anti-Ro60 differed significantly among Group 1 subjects who were taking hydroxychloroquine versus those who were not. Those with unknown hydroxychloroquine use were excluded from this analysis. Quantile regression was also used to determine if the median titer level differed significantly across the 3 groups.
Given the interest in identifying those pregnant subjects unlikely to develop fetal AVB, we calculated titer thresholds with 100% negative predictive value (NPV) using anti-Ro52 only, anti-Ro60 only, and anti-Ro52 and anti-Ro60 in combination. These analyses were conducted separately for those subjects with ARUP titers and those with NYU titers. Because the purpose of our study was to use titers to screen subjects who would not need surveillance, healthy pregnancies with a history of fetal AVB (Group 3) are described but excluded from further analysis, as this group should always be surveilled due to their very high risk of recurrence. Accordingly, comparisons were made only between pregnant subjects with no history of fetal AVB and those with fetal AVB in the current pregnancy only. All analyses were completed using R v4.1.0 (Vienna, Austria). The “quantreg” package in R was used to implement the quantile regression with the bootstrap method used for calculating p-values.16
Results
Anti-Ro52 and Anti-Ro60 Antibody Titers
Maternal sera were obtained from 270 anti-Ro/SSA antibody positive pregnancies (157 from NYU and 113 from UCD and PCH; Figure 1). Anti-Ro52 and anti-Ro60 titers were evaluated either by ARUP (N=113) or NYU (N= 70) or both (N=87). Titers are summarized in Table 1 and Figure 1. Compared to Group 1 (no current or past fetal AVB, N=141), median titers were significantly higher in Group 2 (current fetal AVB, N=66; p<0.001 for both ARUP anti-Ro52 and anti-Ro60, p=0.043 for NYU anti-Ro52, and p=0.004 for NYU anti-Ro60) and Group 3 (past fetal AVB, N=63; p<0.001 for all comparisons).
Figure 1:
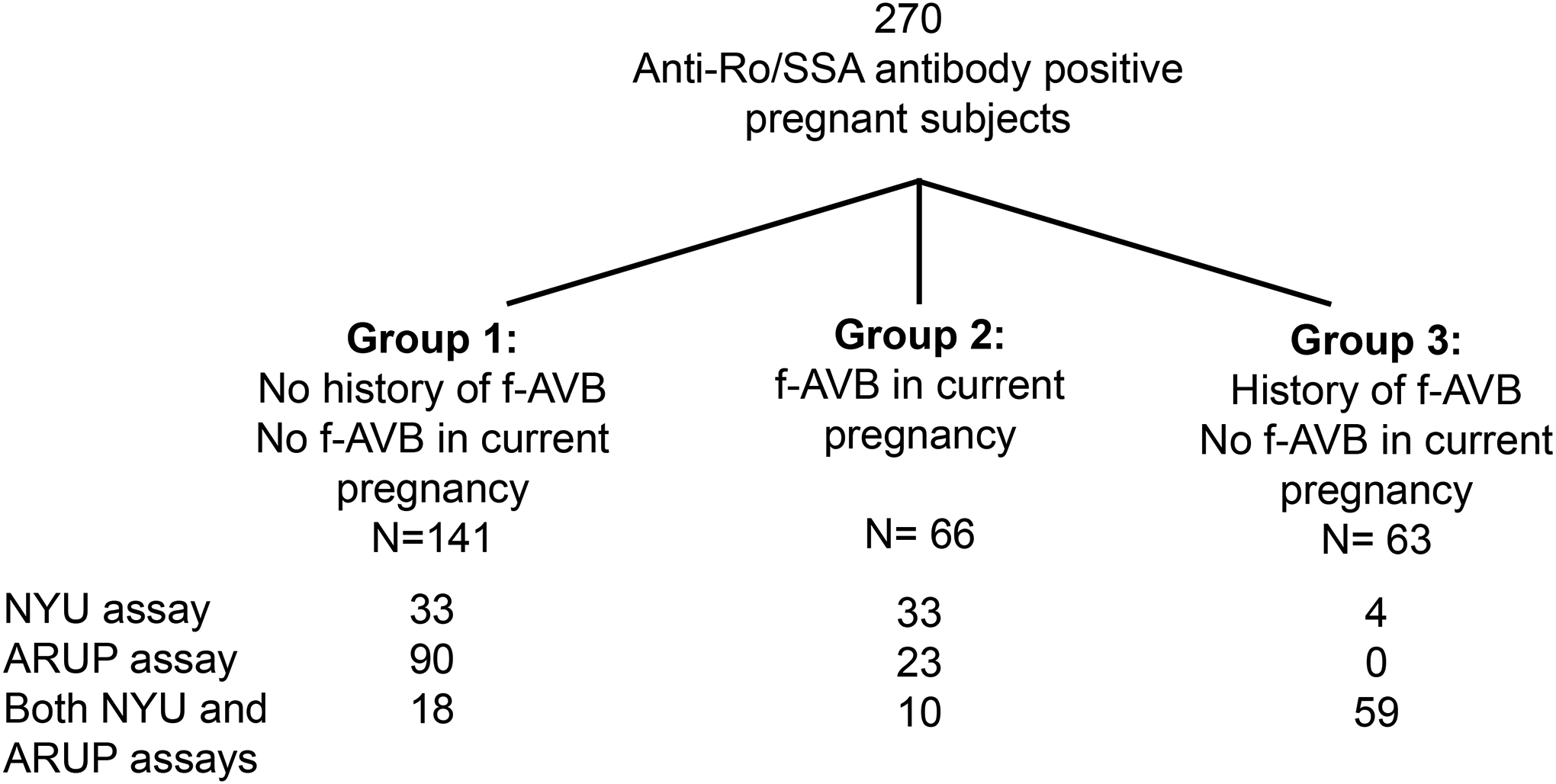
Study sample sizes by outcome group and assay used. F-AVB = fetal atrioventricular block
Table 1:
Summary table by outcome and anti-Ro/SSA antibody titer presented as median (minimum, maximum).
| Antibody Specificity | Overall (n=270) | Group 1. No hx fetal AVB, no AVB in current pregnancy (N=141) | Group 2. Fetal AVB in current pregnancy (N=66) | Group 3. Hx of fetal AVB; no AVB in current pregnancy (N=63) |
|---|---|---|---|---|
| ARUP anti-Ro52 (AU/mL) | 124.5 (0.0, 429.0) | 41.0 (0.0, 429.0) | 180.0 (44.0, 293.0) | 205.0 (0.0, 265.0) |
| ARUP anti-Ro60 (AU/mL) | 123.0 (0.0, 199.0) | 106.5 (0.0, 199.0) | 133.0 (58.0, 199.0) | 131.0 (0.0, 172.0) |
| NYU anti-Ro52 (EU) | 9860.0 (13.0, 190516.0) | 409.0 (13.0, 185800.0) | 12439.0 (440.0, 190516.0) | 23393.0 (202.0, 157515.0) |
| NYU anti-Ro60 (EU) | 13151.0 (3.0, 72780.0) | 1720.0 (3.0, 68516.0) | 16736.0 (168.0, 72780.0) | 15399.0 (724.0, 57800.0) |
Key: ARUP = Associated Regional and University Pathologists; AVB = atrioventricular block; Hx = history; NYU = New York University; EU = ELISA units; AU = Arbitrary units.
Anti-Ro/SSA antibody titers from both NYU and ARUP were more similar between subjects with current or past pregnancies affected by fetal AVB (Groups 2 and 3) than when compared to subjects with currently healthy pregnancies and no history of fetal AVB (Group 1). Forty-six of 89 (51.7%) Group 1 pregnancies were treated with hydroxychloroquine. There was no statistical difference in ARUP anti-Ro52 or anti-Ro60 antibody titers between treated and untreated subjects (p=0.486 and 0.679, respectively). The median (1st, 3rd quartile) ARUP titers for subjects not treated with hydroxychloroquine were – anti-Ro52: 22.0 AU/mL (4.0, 106.0) and anti-Ro60: 106.0 AU/mL (64.5, 126.0).
Models determining the negative predictive value for fetal AVB
Three approaches were compared using either ARUP or NYU titers to target the goals of 100% NPV (i.e., only identifying fetuses that do not develop AVB based on the threshold) and maximizing the number of unaffected fetuses who would be correctly predicted as not developing AVB and able to forgo surveillance. The first approach tested the predictive value of anti-Ro52 alone, the second approach tested the predictive value of anti-Ro60 alone, and the third approach tested successful prediction of anti-Ro52 and anti-Ro60 together. Table 2 summarizes the performance of each of the 3 approaches for ARUP and NYU anti-Ro52 and anti-Ro60 titers.
Table 2:
Comparison of results reported for each approach for both ARUP and NYU titers.
| Summary | ARUP Titers (AU/mL) | NYU Titers (EU) |
|---|---|---|
| N with Titers | N=141 | N=94 |
| N (%) with f-AVB | 33 (23.4%) | 43 (45.7%) |
| N (%) with No f-AVB | 108 (76.6%) | 51 (54.3%) |
| Anti-Ro52 Alone | ||
| Threshold | <44 | <440 |
| N Pregnancies Below (%) | 55/141 (39.0%) | 26/94 (27.7%) |
| Percent No f-AVB Pregnancies Below | 55/108 (50.9%) | 26/51 (51.0%) |
| Anti-Ro60 Alone | ||
| Threshold | <58 | <168 |
| N Pregnancies Below (%) | 19/141 (13.5%) | 13/94 (13.8%) |
| Percent No f-AVB Pregnancies Below | 19/108 (17.6%) | 13/51 (25.5%) |
| Anti-Ro52 and Anti-Ro60 Together | ||
| Threshold | Anti-Ro52<110 & Anti-Ro60<110 | Anti-Ro52<650 & Anti-Ro60<4060 |
| N Pregnancies Below (%) | 54/141 (38.3%) | 27/94 (28.7%) |
| Percent No f-AVB Pregnancies Below | 54/108 (50.0%) | 27/51 (52.9%) |
Key: ARUP = Associated Regional and University Pathologists; AVB = atrioventricular block; NYU = New York University; EU = ELISA units; AU = Arbitrary units.
ARUP negative predictive titers for fetal AVB
For ARUP measurements, two strategies performed well for the target of 100% NPV (i.e., only identifying fetuses that do not develop AVB based on the threshold) and similarly maximizing the number of healthy fetuses who would be correctly predicted as healthy. Figure 3A summarizes the overall performance of each strategy.
Figure 3A:

ARUP anti-Ro/SSA antibody titer threshold with 100% negative predictive value for fetal AVB including percent of pregnancies within fetal AVB and no fetal AVB groups (not including those with history of fetal AVB but no fetal AVB in current pregnancy) that would be excluded from surveillance.
Anti-Ro52 on its own performed well at maximizing the proportion of healthy pregnancies. Specifically, the threshold of < 44 AU/mL identified 55 of 108 (51%) healthy pregnancies as healthy while predicting 0 of 33 (0%) AVB pregnancies as healthy.
The model using anti-Ro60 alone demonstrated less specificity; a 100% NPV threshold of < 58 AU/mL correctly categorized only 19 of 108 (18%) of healthy pregnancies and incorrectly categorized 0 of 33 (0%) of fetal AVB pregnancies.
The model using both anti-Ro52 and anti-Ro60 resulted in a joint 100% NPV threshold of < 110 AU/mL (for both anti-Ro52 and anti-Ro60) which correctly categorized 54 out of 108 (50%) healthy pregnancies and incorrectly categorized 0 out of 33 (0%) fetal AVB pregnancies as healthy.
NYU negative predictive value for fetal AVB
For those with NYU measurements, similar results were observed to the ARUP titers with the anti-Ro52 alone and joint decision rule with both anti-Ro52 and anti-Ro60 being optimal. Table 3 presents summaries for the anti-Ro/SSA NYU measurements and the overall performance of each approach. Figure 4A summarizes the overall performance of each strategy. For anti-Ro52 on its own, the threshold of < 440 EU identified 26 of 51 (51%) healthy pregnancies as healthy and 0 of 43 (0%) fetal AVB pregnancies as healthy.
Table 3:
Summary of outcomes using NYU and ARUP anti-Ro/SSA antibody titers presented as median (minimum, maximum).
| Group 1 | Group 2 | ||
|---|---|---|---|
| Overall | Healthy | AVB | |
| Outcome | (N=28) | (N=18) | (N=10) |
| ARUP Anti-Ro52 | 113.5 (2, 269) | 36.5 (2, 269) | 198.5 (111, 231) |
| ARUP Anti-Ro60 | 113.0 (0, 182) | 92.5 (0, 163) | 124.5 (100, 182) |
| NYU Anti-Ro52 | 1423 (72, 190516) | 210 (72, 98812) | 19350 (1116, 190516) |
| NYU Anti-Ro60 | 1915 (3, 68516) | 482 (3, 68516) | 23336 (3322, 56903) |
Key: ARUP = Associated Regional and University Pathologists; AVB = atrioventricular block; NYU = New York University.
Figure 4A.

NYU anti-Ro/SSA antibody titer threshold with 100% negative predictive value for fetal AVB and percent of pregnancies within fetal AVB and no fetal AVB groups (not including those with history of fetal AVB but no fetal AVB in current pregnancy) that would be excluded from surveillance.
Anti-Ro60 on its own did not perform as well, where the threshold of < 168 EU was the highest that maintained 100% NPV, but it only represented 13 of 51 (26%) healthy pregnancies predicted to be healthy.
The approach using both anti-Ro52 and 60 identified thresholds of anti-Ro52 < 650 EU and anti-Ro60 < 4060 EU predicting a healthy fetus. Using these cutoffs, 27 out of 51 (53%) healthy pregnancies would be correctly predicted to be healthy and 0 out of 43 to have fetal AVB. Figure 4B summarizes the model using both anti-Ro 52 and anti-Ro60 to predict fetal AVB from Group 1 (no AVB) and Group 2 (AVB).
Figure 4B:

NYU anti-Ro52 (x-axis) and anti-Ro60 (y-axis) titers of AVB and healthy subjects, with axes restricted to 0 to 5000 to highlight thresholds. Dashed lines show optimal antibody thresholds. A version without restricted axes is available in the supplementary materials.
Comparison of subjects with NYU and ARUP data
Only 28 subjects from Groups 1 and 2 had both ARUP and NYU titers evaluated. Given the small sample size, direct comparisons with the overall summaries were not made. The median (minimum, maximum) titer values are presented in Table 3.
Comments
Principal findings
With the goal of addressing the approach to surveillance of anti-Ro/SSA positive pregnancies, commercial and research assays were employed to define a threshold titer below which there is a very low likelihood of AVB. The strategy of using anti-Ro52 and anti-Ro60 antibody titers together correctly identified at least 50% of pregnancies with no fetal AVB while not misclassifying any fetal AVB cases. For ARUP, the specific thresholds used to exclude the possibility of fetal AVB were anti-Ro52 and anti-Ro60 < 110 AU/mL. For NYU, the thresholds were anti-Ro52 < 650 EU and anti-Ro 60 < 4060 EU. Levels of both antibodies to Ro52 and Ro60 were higher in subjects whose fetuses developed AVB during their current or past pregnancies than in subjects with healthy pregnancies and no history of fetal AVB.
Results in the context of what is known
There is substantial evidence that anti-Ro/SSA antibody titer predicts risk of fetal AVB. Leveraging data from the US Research Registry for Neonatal lupus, Reed and colleagues reported that umbilical cord blood levels of anti-Ro52 and anti-Ro60 were significantly higher in 59 neonates with fetal AVB compared to 29 without fetal AVB in the current or past pregnancies.10 Jaeggi and colleagues evaluated antibody titers in 146 healthy fetuses and 40 fetuses/neonates with a diagnosis of AVB, endocardial fibroelastosis or both.11 All cardiac complications were associated with moderate (≥50 U/mL; 15%) or high (≥100 U/mL; 85%) maternal anti-Ro/SSA antibody levels. There was a 5% event-rate of fetal 3°AVB in pregnancies with moderate maternal anti-Ro/SSA antibody titers (odds ratio: 7.8) compared to a 0% event rate in pregnancies with low maternal antibody titers (p < 0.0001). Isolated endocardial fibroelastosis (n = 1) and 1° or 2° (n = 4) or 3° (n = 4) fetal AVB were diagnosed in 9 (8%) pregnancies with anti-Ro/SSA titers >100 U/mL but none with lower titers (odds ratio 17.78; p = 0.004). In each of these reports, assays were limited to research laboratories, sources of antigens were distinct, and anti-Ro52 and anti-Ro60 responses were not considered in an algorithm incorporating both titers.
Clinical implications
From this study and others, anti-Ro/SSA antibody titers are not the sole predictors of fetal AVB.10 Recurrence rates are not 100%, and most fetuses exposed to even the highest levels of antibodies have normal conduction. Therefore, our finding that anti-Ro52 and anti-Ro60 titers are comparable in subjects with fetal AVB in current versus healthy pregnancies but fetal AVB in previous pregnancies is not unexpected. Given the rarity of fetal AVB even among high titer individuals, it is worth considering a streamlined approach to surveillance which excludes low titer subjects with no prior affected pregnancies. It is important to note that risk of fetal AVB is not influenced by parity per se in that in the same mother, fetal disease can occur in a first pregnancy or even a fifth pregnancy when all four previous pregnancies have been normal.17 Thus, having a normal child does not decrease the risk of fetal AVB in subsequent pregnancies.
The utility and cost of serial echocardiogram surveillance for all anti-Ro/SSA antibody positive pregnancies needs to be considered.18 For example, a patient undergoing weekly echocardiogram surveillance from 16–28 weeks’ gestation (as currently recommended by the American Heart Association)5 would spend approximately $18,880 on echocardiograms throughout a single pregnancy.19 These figures are accompanied by the logistical burden of frequent appointments for parents who may be trying to juggle multiple jobs or children at home. Given these considerations, the identification of a threshold with 100% NPV for fetal AVB assumes even greater clinical importance.
Research Implications
These data can provide an evidence-based framework for a prospective study risk stratifying surveillance of anti-Ro/SSA antibody positive pregnancies to only those pregnancies at risk of fetal AVB. Such a study is already underway (Clinical Trials NCT4474223). We have shown that hydroxychloroquine use does not affect anti-Ro/SSA antibody titers7 but rather decreases the pathologic effects of the antibodies via inhibition of downstream Toll like receptor signaling.20
Strengths and limitations
An important strength of this study is the use of data from both commercial (ARUP) and research (NYU) laboratories to develop 100% NPV thresholds for anti-Ro52 and anti-Ro60 antibody titers. Despite the differences in the sample prevalence for fetal AVB between the ARUP and NYU groups, the proportion of pregnancies without AVB were remarkably similar with approximately 50% below the threshold in each. Both datasets support the use of a combined anti-Ro52 and anti-Ro60 model, which performs similar to anti-Ro52 alone, but may better protect against false negatives. The distributions of titer levels in pregnancies affected by fetal AVB show that while one titer may be low, at least one is generally very high. Although this pattern is also present for some healthy pregnancies, we accomplished our goal to identify a strategy which could reduce healthcare burdens by decreasing intense surveillance of anti-Ro/SSA antibody exposed pregnancies with no likelihood of fetal AVB.
Limitations of this study include small sample sizes (<150) in each assay group, and an especially low number of subjects with antibody titers measured by both NYU and ARUP. Further prospective data collection is needed to validate the thresholds proposed by this study. Our study was limited to only one commercial assay; however, the authors are unaware of other CLIA approved laboratories that provide antibody titers rather than categorical (e.g., >8) or binary (i.e., positive/negative) results. Unfortunately, the NYU research laboratory is not CLIA approved and therefore not commercially available to clinicians caring for anti-Ro/SSA exposed pregnancies. Different scales used in ARUP analysis compared to NYU ELISAs limited direct comparisons between the two methods. The thresholds we report have 100% NPV for fetal AVB only; it is unclear whether these thresholds would demonstrate high NPV for extranodal disease without conduction system disease. However, isolated extranodal disease is a rare occurrence,17, 18 and there are no large studies documenting its natural history. Another limitation is that while hydroxychloroquine did not lead to significant differences in median titer levels, it may alter the risk of fetal AVB; treated subjects may therefore have higher titer levels that may indicate increased risk while being born without disease.
Conclusion
Antibody titer thresholds derived from clinical data can risk stratify anti-Ro/SSA antibody positive pregnancies in subjects with no prior fetal AVB and reduce the burden of expensive and time-consuming fetal echocardiogram surveillance. A threshold of < 110 AU/mL for both anti-Ro52 and anti-Ro60 antibody titers in a commercial laboratory successfully identified those subjects at no risk for fetal AVB. There is value in having both anti-Ro52 and anti-Ro60 in that one may have lower values while the other is higher, and that only collecting data on one may lead to false negatives, whereas low values on both are likely to provide additional evidence suggesting low risk for fetal AVB.
Supplementary Material
Figure 2:

Box plots comparing anti-Ro52 and anti-Ro60 levels by group (x-axis) and by titer(y-axis) for ARUP and NYU laboratories.
Figure 3B:

ARUP anti-Ro52 (x-axis) and anti-Ro60 (y-axis) titers of AVB and healthy subjects. Dashed lines show optimal antibody thresholds.
AJOG at a Glance:
A. Why was this study conducted?
To identify maternal anti-Ro52 and anti-Ro60 titer thresholds below which fetal atrioventricular block is unlikely to occur
B. What are the key findings?
The negative predictive value for of a clinically validated and FDA-approved solid-phase immunoassay for anti-Ro52 and anti-Ro60 <110 AU/mL is 100% for fetal atrioventricular block
C. What does this study add to what is already known?
Only 1–2% of anti-Ro/SSA positive mothers will experience fetal atrioventricular block yet echo surveillance protocols are not risk stratified and are costly, time-consuming, and ineffective. Identification of anti-Ro52 and anti-Ro60 titer thresholds below which fetal AVB is unlikely to develop can alleviate the surveillance burden for low-titer patients and reduce the patient load for medical professionals.
Acknowledgments
This work was funded by NICHD (R01 HD100925, J.P.B. and B.F.C), NIAMS (R37 AR042455, J.P.B; N01 AR42271, J.P.B.), and NHLBI (K01 HL151754, A.M.K.). ARUP provided a grant to perform the anti-Ro/SSA antibody assays from NYU subjects. The funding sources were not involved in study design, data collection, data analysis and interpretation, manuscript preparation, or manuscript submission.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflict of interest.
Preliminary findings were presented as an abstract at the Society for Maternal Fetal Medicine’s 41st Annual Pregnancy Meeting held virtually on January 25–30, 2021.
References
- 1.Brito-Zeron P, Izmirly PM, Ramos-Casals M, Buyon JP, Khamashta MA. The clinical spectrum of autoimmune congenital heart block. Nat Rev Rheumatol 2015;11:301–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brucato A, Frassi M, Franceschini F, et al. Risk of congenital complete heart block in newborns of mothers with anti-Ro/SSA antibodies detected by counterimmunoelectrophoresis: a prospective study of 100 women. Arthritis Rheum 2001;44:1832–5. [DOI] [PubMed] [Google Scholar]
- 3.Cimaz R, Spence DL, Hornberger L, Silverman ED. Incidence and spectrum of neonatal lupus erythematosus: a prospective study of infants born to mothers with anti-Ro autoantibodies. J Pediatr 2003;142:678–83. [DOI] [PubMed] [Google Scholar]
- 4.Llanos C, Izmirly PM, Katholi M, et al. Recurrence rates of cardiac manifestations associated with neonatal lupus and maternal/fetal risk factors. Arthritis Rheum 2009;60:3091–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donofrio MT, Moon-Grady AJ, Hornberger LK, et al. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation 2014;129:2183–242. [DOI] [PubMed] [Google Scholar]
- 6.Friedman DM, Kim MY, Copel JA, et al. Utility of cardiac monitoring in fetuses at risk for congenital heart block: the PR Interval and Dexamethasone Evaluation (PRIDE) prospective study. Circulation 2008;117:485–93. [DOI] [PubMed] [Google Scholar]
- 7.Izmirly P, Kim M, Friedman DM, et al. Hydroxychloroquine to Prevent Recurrent Congenital Heart Block in Fetuses of Anti-SSA/Ro-Positive Mothers. J Am Coll Cardiol 2020;76:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuneo BF, Sonesson SE, Levasseur S, et al. Home Monitoring for Fetal Heart Rhythm During Anti-Ro Pregnancies. J Am Coll Cardiol 2018;72:1940–51. [DOI] [PubMed] [Google Scholar]
- 9.Cuneo BF, Ambrose SE, Tworetzky W. Detection and successful treatment of emergent anti-SSA-mediated fetal atrioventricular block. Am J Obstet Gynecol 2016;215:527–8. [DOI] [PubMed] [Google Scholar]
- 10.Reed JH, Clancy RM, Lee KH, Saxena A, Izmirly PM, Buyon JP. Umbilical cord blood levels of maternal antibodies reactive with p200 and full-length Ro 52 in the assessment of risk for cardiac manifestations of neonatal lupus. Arthritis Care Res (Hoboken) 2012;64:1373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaeggi E, Laskin C, Hamilton R, Kingdom J, Silverman E. The importance of the level of maternal anti-Ro/SSA antibodies as a prognostic marker of the development of cardiac neonatal lupus erythematosus a prospective study of 186 antibody-exposed fetuses and infants. J Am Coll Cardiol 2010;55:2778–84. [DOI] [PubMed] [Google Scholar]
- 12.Buyon JP, Ben-Chetrit E, Karp S, et al. Acquired congenital heart block. Pattern of maternal antibody response to biochemically defined antigens of the SSA/Ro-SSB/La system in neonatal lupus. J Clin Invest 1989;84:627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kleinman CS, Hobbins JC, Jaffe CC, Lynch DC, Talner NS. Echocardiographic studies of the human fetus: prenatal diagnosis of congenital heart disease and cardiac dysrhythmias. Pediatrics 1980;65:1059–67. [PubMed] [Google Scholar]
- 14.Strasburger JF, Huhta JC, Carpenter RJ, Jr., Garson A, Jr., McNamara DG. Doppler echocardiography in the diagnosis and management of persistent fetal arrhythmias. J Am Coll Cardiol 1986;7:1386–91. [DOI] [PubMed] [Google Scholar]
- 15.Albon S, Bunn C, Swana G, Karim Y. Performance of a multiplex assay compared to enzyme and precipitation methods for anti-ENA testing in systemic lupus and systemic sclerosis. J Immunol Methods 2011;365:126–31. [DOI] [PubMed] [Google Scholar]
- 16.Koenker R. quantreg: Quantile Regression. R package version 5.86., 2021.
- 17.Solomon DG, Rupel A, Buyon JP. Birth order, gender and recurrence rate in autoantibody-associated congenital heart block: implications for pathogenesis and family counseling. Lupus 2003;12:646–7. [DOI] [PubMed] [Google Scholar]
- 18.Costedoat-Chalumeau N, Morel N, Fischer-Betz R, et al. Routine repeated echocardiographic monitoring of fetuses exposed to maternal anti-SSA antibodies: time to question the dogma. Lancet Rheumatology 2019;1:E187–E93. [DOI] [PubMed] [Google Scholar]
- 19.Evers PD, Alsaied T, Anderson JB, Cnota JF, Divanovic AA. Prenatal heart block screening in mothers with SSA/SSB autoantibodies: Targeted screening protocol is a cost-effective strategy. Congenit Heart Dis 2019;14:221–29. [DOI] [PubMed] [Google Scholar]
- 20.Clancy RM, Markham AJ, Reed JH, Blumenberg M, Halushka MK, Buyon JP. Targeting downstream transcription factors and epigenetic modifications following Toll-like receptor 7/8 ligation to forestall tissue injury in anti-Ro60 associated heart block. J Autoimmun 2016;67:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


