Abstract
Objective:
The objective was to compare the long-term overall survival (OS) of right versus left thoracic esophagectomy, and to evaluate whether surgical quality impacts comparison result.
Background:
Controversy regarding the optimal thoracic esophagectomy approach persists for esophageal squamous cell carcinoma (ESCC). No study has assessed the effect of surgical quality in comparison between right and left approaches.
Methods:
The authors consecutively recruited 5556 operable ESCC patients from two high-volume centers in China, of whom 2220 and 3336 received right and left thoracic esophagectomy, respectively. Cumulative sum was used to evaluate the learning curve for operation time of right approach, as the indicator of surgical proficiency.
Results:
With a median follow-up of 83.1 months, right approach, harvesting more lymph nodes, tended to have a better OS than left approach (Mean: 23.8 vs. 16.7 nodes; adjusted hazard ratio (HR)=0.93, 95% CI: 0.85–1.02). Subset analysis by the extent of lymphadenectomy demonstrated that right approach with adequate lymphadenectomy (≥15 nodes) resulted in statistically significant OS benefit compared with left approach (adjusted HR=0.86, 95% CI: 0.77–0.95), but not with limited lymphadenectomy. Subset analysis by surgical proficiency showed that proficient right approach conferred a better OS than left approach (adjusted HR=0.75, 95% CI: 0.64–0.88), but improficient right approach did not have such survival advantage.
Conclusions:
Surgical quality plays a crucial role in survival comparison between surgical procedures. Right thoracic esophagectomy performed with adequate lymphadenectomy and surgical proficiency, conferring more favorable survival than left approach, should be recommended as the preferred surgical procedure for localized ESCC.
Keywords: esophageal squamous cell carcinoma, learning curve, lymphadenectomy, right thoracic esophagectomy, surgical quality
Introduction
Highlights
Right thoracic esophagectomy can confer a better overall survival (OS) than left approach for esophageal squamous cell carcinoma.
Surgical quality determines the OS superiority of right over left approach.
Surgical quality should not be overlooked in OS comparison of surgical procedures.
Esophageal cancer (EC) is ranked as the 7th most common and 6th leading cause of cancer death in the world1. About 53% of new EC cases worldwide occur in China1. There are two main histologic subtypes of EC, namely squamous cell carcinoma (ESCC) and adenocarcinoma (EAC)2; more than 90% of EC cases in China are ESCC3,4. The overall 5-year survival for ESCC is about 20%5, and about 40–59% among surgically treated patients6–8. Transthoracic esophagectomy remains the cornerstone of curative treatment for localized ESCC in China and some other high-incidence regions.
Left and right thoracic esophagectomy represent two principal approaches to transthoracic esophagectomy for ESCC9–15. The right approach (Ivor-Lewis procedure and McKeown procedure) has been on the rise in the last decade for its substantially higher lymph node yield compared with left approach, which ensures both more accurate staging and radical tumor resection, potentially improving survival of ESCC patients16,17. However, the ʽtraditionalʼ left approach (Sweet procedure), which was the first approach adopted for ESCC surgery, continues to be widely applied for its technical simplicity (approximately 70% utilization in China8).
The current evidence concerning the optimal thoracic approach for improving long-term survival is inconsistent, which hinders the dissemination of the superior surgical approach for ESCC9–15. Previous observational studies (mostly limited by being single-center, low statistical power, and possible residual confounding) largely agreed that the right approach yielded more resected lymph nodes, but arrived at conflicting findings regarding long-term survival outcomes9–15. Only two RCTs have been conducted so far comparing the right and left thoracic approaches. The earlier one, being small and single-center, reported survival advantage of the right approach over the left approach, whereas the more recent 14-center one failed to find this survival benefit of right approach12,18.
It has been recognized that surgical quality (e.g. lymph node harvesting, learning curve effect) may affect survival and its variation may generate discordant comparative results19,20. Therefore, difference in surgical quality of emerging right thoracic esophagectomy, which is technically more demanding than the left approach, may account for the discrepancy in the findings of the previous studies. That being said, this issue has not yet been evaluated by high-quality studies.
Based on a large-scale, multicentered, real-world clinical cohort of ESCC patients in China with up to 12-year’s follow-up, this study aims to compare the long-term overall survival (OS) of right versus left thoracic esophagectomy, and to evaluate whether surgical quality factors (the extent of lymphadenectomy and surgical proficiency) impact comparison results. This study may provide high-level evidence for clinical decision-making and offer valuable insights for future comparative studies in the field of surgery.
Methods
Patients
This study included patients from two study centers in China, one being the Anyang Cancer Hospital located in Anyang City in Henan Province (hereinafter referred to as the Northern center) and the other Cancer Hospital of Shantou University Medical College in Shantou City in Guangdong Province (hereinafter referred to as the Southern center), as described in our previous papers21,22. Anyang City lies in the Taihang Mountain region of Northern Central China, a well-known high-incidence area of ESCC. In contrast, Shantou City is located in the southeastern coastal area of China, where ESCC incidence has been reported at the national average level23. Both centers are high-volume cancer hospitals with extensive experience in esophageal surgery.
In the study period from January, 2012 to December, 2017 at the Northern center and from August 2009 to December 2018 at the Southern center, we consecutively included patients who 1) were pathologically diagnosed with primary ESCC, characterized by the origin of neoplastic squamous cells from the esophageal squamous epithelium and invasion through the basement membrane of the esophagus24, and; 2) had undergone radical thoracic esophagectomy as initial treatment with or without adjuvant therapy. Patients were excluded if they had metastatic disease (M1, stage IV)25, or had received neoadjuvant therapy for primary ESCC, or had missing data on lymph node yield. The follow-up at the Northern center and the Southern center was up to August 2022 and June 2022, respectively. Patients with less than 6 months of follow-up were excluded from long-term survival analysis.
Data collection and processing
Baseline information, tumor characteristics, and treatment records of the included patients were extracted from the Hospital Information System of each center. Vital status of the patients was ascertained by standardized telephone interviews or outpatient clinic visits. Detailed description of data collection, processing, and quality control are provided in Supplementary Material S1 (Supplemental Digital Content 1, http://links.lww.com/JS9/B326). For missing data (all<5% missing proportion), the missing values in continuous variables were filled with the population median, and those in categorical variables were assigned to an additional ʽunknownʼ category. Pathologic staging was determined based on the seventh edition of American Joint Committee on Cancer TNM classification25. Adjuvant therapy was defined as at least one cycle of radiotherapy or chemotherapy after surgery, irrespective of dosage, regimen, or method of administration. Treatment regimens are categorized as follows: surgery alone (S), surgery followed by adjuvant chemotherapy (S + CT), surgery followed by adjuvant radiotherapy (S+ RT), and surgery followed by adjuvant chemoradiotherapy (S + CRT).
Surgical procedures
In this study, left thoracic esophagectomy included the Sweet procedure (one incision, left posterolateral thoracotomy) and modified Sweet procedure (two incisions, left posterolateral thoracotomy + left cervical incision)12. Right thoracic esophagectomy included the Ivor-Lewis procedure (two incisions, right posterolateral thoracotomy + midline laparotomy) and McKeown procedure (three incisions, right posterolateral thoracotomy + midline laparotomy + left cervical incision)26.
Outcomes
The primary outcome was long-term OS measured from the date of first admission into the study centers for ESCC to the date of death due to any cause or to the date of last follow-up contact, whichever occurred first. Secondary outcomes (short-term outcomes) included the number of lymph nodes harvested, duration of surgery, surgical margin status, intraoperative blood transfusion, postoperative all-cause 90-day mortality, and in-hospital surgery-related complications (e.g. leak, delayed gastric emptying and chylothorax) as detailed in Supplementary Material S1 (Supplemental Digital Content 1, http://links.lww.com/JS9/B326).
Surgical quality
Two dimensions of surgical quality were measured for right thoracic esophagectomy, that is, extent of lymphadenectomy (extended lymphadenectomy versus limited lymphadenectomy) and surgical proficiency (proficient esophagectomy versus improficient esophagectomy). Extended lymphadenectomy was defined as a procedure resecting at least 15 lymph nodes [recommended by the National Comprehensive Cancer Network (NCCN)26], and limited lymphadenectomy as resecting less than 15 nodes. Other cutoffs of resected lymph nodes (e.g. 20, 25, 30, 35, 40, and 45) were also used as sensitivity analysis.
Surgical proficiency was classified based on the learning curve of right thoracic esophagectomy. In specific, risk-adjusted-cumulative sum (RA-CUSUM) analysis for surgical failure (ra-cusum function, R) was conducted in the Southern center, which has a relatively complete learning process of this procedure. The surgical failure was defined as morbidity within 90 days after surgery, positive surgical margin, intraoperative blood transfusion, occurrence of in-hospital surgery-related complications, or less than 15 harvested lymph nodes. The probability of surgical failure was obtained from Logistic regression analysis, adjusting for age, sex, smoking, presence of comorbidities, tumor location, tumor morphology, pathological tumor-lymph node-metastasis (pTNM) stage, tumor grade, tumor size, and calendar year of operation. With all the included patients receiving right thoracic esophagectomy at the Southern center in chronological order, a graphical representation of the learning curve was depicted to identify the turning point (peak point). Right thoracic esophagectomies conducted before and after the turning point were categorized into the learning phase (improficient right approach) and the postlearning phase (proficient right approach), respectively.
Statistical analysis
To evaluate the comparability of groups at baseline, the χ2test was employed for categorical variables, while Student’s t-test was applied for continuous variables. In instances where the conditions necessary for the application of Student’s t-test—specifically, normality of data distribution and homogeneity of group variances as verified by the Kolmogorov–Smirnov test and Levene’s test, respectively—were found to be violated, the Wilcoxon-Mann–Whitney test was utilized instead.
OS was estimated using the nonparametric Kaplan–Meier (K–M) method and visualized by K–M curves. The hazard ratio (HR) estimates and the corresponding 95% CIs were obtained by multivariable Cox proportional-hazard regression models with a robust estimator, adjusting for margin status, postoperative treatment modality, study center, and other covariates listed above. Time-independent outcomes (e.g. lymph nodes harvested) were compared between groups using multivariable generalized linear models, adjusting for covariates listed above.
Subset analysis was used to assess the effect of surgical quality (i.e. the extent of lymphadenectomy and surgical proficiency) on OS comparison between left and right thoracic esophagectomies (extended right esophagectomy versus left approach, limited right esophagectomy versus left approach; proficient right esophagectomy versus left approach, improficient right esophagectomy versus left approach).
Sensitivity analyses
To assess the robustness of the results of above-described main analysis, we conducted the following sensitivity analyses (detailed in Supplementary Material S1, Supplemental Digital Content 1, http://links.lww.com/JS9/B326). First, we fitted propensity score-based Cox regression models, including covariate adjustment, stratification, weighting, and matching, to compare OS between left and right approaches (detailed in Supplementary Material S1, Supplemental Digital Content 1, http://links.lww.com/JS9/B326). Second, we used an alternative definition of surgical failure (morbidity within 90 days after surgery, positive surgical margin, or less than 15 harvested lymph nodes) for RA-CUSUM analysis and also employed the cumulative sum (CUSUM) analysis of operation time to ascertain the turning point of surgical proficiency gain curve. Third, we restricted analysis to patients with resectable middle and lower thoracic ESCC, and to patients who had undergone open esophagectomy. Lastly, comparisons were made with different sets of comparison groups by specifying the surgical procedures (e.g. McKeown vs. Sweet).
All statistical tests were two-sided, and a P-value of less than 0.05 was considered statistically significant. All analysis was done with Stata 16.0 (StataCorp.) and R 3.6.3 (R Foundation).
Ethics statement
This study was registered with Clinical-based prognosis prediction of upper gastrointestinal cancer (https://www.chictr.org.cn/showproj.html?proj=183318). This study was conducted according to the principles of the Declaration of Helsinki and approved by the Institutional Review Board of the Peking University School of Oncology, China (Approval number: 2018KT68). Data has been reported in line with strengthening the reporting of cohort, cross-sectional and case–control studies in surgery (STROCSS 2021 criteria, Supplemental Digital Content 2, http://links.lww.com/JS9/B32727.
Results
Study patients
A total of 5556 ESCC patients, 3692 from the Northern center and 1864 from the Southern center, met inclusion criteria for long-term survival analysis (Supplementary Figure S1, Supplemental Digital Content 3, http://links.lww.com/JS9/B328), among whom 2220 (40.0%) underwent right thoracic esophagectomy (McKeown: 2123, Ivor-Lewis: 97) and 3336 (60.0%) underwent left thoracic esophagectomy (Sweet: 1479, modified Sweet: 1857) (Supplementary Table S1, Supplemental Digital Content 1, http://links.lww.com/JS9/B326). Distributions of selected demographic and clinical characteristics of the included patients are shown in Table 1. Compared with the patients undergone a left approach, those who received a right approach were generally more likely to be younger, males, having tumors located in the upper section of the esophagus, at later stage at diagnosis, treated in the latter half of the study period (2014–2018), and treated with adjuvant therapy, but less likely to have comorbidities (Table 1).
Table 1.
Selected characteristics of ESCC patients receiving left and right esophagectomya.
| Characteristics | Left (N=3336) | Right (N=2220) | P b |
|---|---|---|---|
| Age (years) | |||
| <60 | 982 (29.4%) | 775 (34.9%) | <0.001 |
| 60–64 | 918 (27.5%) | 612 (27.6%) | |
| 65–69 | 792 (23.7%) | 471 (21.2%) | |
| 70–74 | 451 (13.5%) | 267 (12.0%) | |
| ≥75 | 193 (5.8%) | 95 (4.3%) | |
| Tumor size | |||
| <6 cm | 2671 (80.1%) | 1744 (78.6%) | 0.173 |
| ≥6 cm | 665 (19.9%) | 476 (21.4%) | |
| Sex | |||
| Male | 2129 (63.8%) | 1506 (67.8%) | 0.002 |
| Female | 1207 (36.2%) | 714 (32.2%) | |
| Comorbidity | |||
| No | 2056 (61.6%) | 1510 (68.0%) | <0.001 |
| Yes | 1280 (38.4%) | 710 (32.0%) | |
| Esophageal tumor location | |||
| Upper | 318 (9.5%) | 512 (23.1%) | <0.001 |
| Middle | 2255 (67.6%) | 1399 (63.0%) | |
| Lower | 763 (22.9%) | 309 (13.9%) | |
| pStage | |||
| 0–I | 696 (20.9%) | 331 (14.9%) | <0.001 |
| II | 1456 (43.6%) | 837 (37.7%) | |
| III | 1172 (35.1%) | 1049 (47.3%) | |
| Unknown | 12 (0.4%) | 3 (0.1%) | |
| Treatment | |||
| S | 2138 (64.1%) | 1320 (59.5%) | <0.001 |
| S+CT | 829 (24.9%) | 494 (22.3%) | |
| S+RT | 232 (7.0%) | 227 (10.2%) | |
| S+CRT | 137 (4.1%) | 179 (8.1%) | |
| Year of operation | |||
| 2009–2013 | 1451 (43.5%) | 714 (32.2%) | <0.001 |
| 2014–2018 | 1885 (56.5%) | 1506 (67.8%) | |
| Center | |||
| Anyang | 2593 (77.7%) | 1099 (49.5%) | <0.001 |
| Shantou | 743 (22.3%) | 1121 (50.5%) | <0.001 |
Data are shown in count (%).
Categorical variables were compared using the χ2test, and continuous variables were compared using the Student’s t-test.
ESCC, esophageal squamous cell carcinoma; S, surgery alone; S + CT, surgery and adjuvant chemotherapy; S+ RT, surgery and adjuvant. radiotherapy; S + CRT, surgery and adjuvant chemoradiotherapy.
OS comparison of right vs. left approach
The median follow-up for the entire included cohort was 83.1 months (95% CI: 82.3–86.7 months). A total of 933 (42.0%) deaths occurred in the right approach group and 1423 deaths (42.7%) in the left approach group. Right approach conferred a better OS than left approach, but with no statistical significance (adjusted HR=0.93, 95% CI: 0.85–1.02; Supplementary Figure S2, Supplemental Digital Content 4, http://links.lww.com/JS9/B329).
Subset OS comparison by the extent of lymphadenectomy
The number of lymph nodes retrieved was statistically significantly larger in right thoracic esophagectomy than in left thoracic approach [mean (SD): 23.8 (11.5) vs. 16.7 (7.4); P<0.001] (Fig. 1). The difference persisted when stratified by tumor site (upper, middle, and lower esophagus) (all P<0.0001; Fig. 1).
Figure 1.
Number of lymph nodes harvested by left and right thoracic esophagectomy for ESCC patients in total study population (A) and by tumor location (B–D). Comparison of lymph nodes between groups were estimated by multivariable generalized linear models. ESCC, esophageal squamous cell carcinoma.
In the subset analysis by the extent of lymphadenectomy of right thoracic esophagectomy, markedly better survival was observed for the right approach with extended lymphadenectomy compared with the left approach, but not for the right approach with limited lymphadenectomy (Fig. 2). Generally, extended lymphadenectomy defined with changing cutoff values of nodes resected in the range from 15 to 45 consistently conferred survival advantage for right approach over left approach (e.g. adjusted HR resected ≥15 nodes=0.86, 95% CI: 0.77–0.95, P=0.003; adjusted HR resected ≥30 nodes=0.79, 95% CI: 0.68–0.92, P=0.002; adjusted HR resected ≥45 nodes=0.70, 95% CI: 0.53–0.93, P=0.014) (Fig. 2).
Figure 2.
Subset analysis by the extent of lymphadenectomy for OS comparison of right vs. left thoracic esophagectomy among all ESCC patients (A) and limited to patients with middle and lower ESCC (B). Extended lymphadenectomy was defined as a procedure resecting at least 15 lymph nodes, and limited lymphadenectomy as resecting less than 15 nodes. Other cutoffs of resected lymph nodes (e.g. 20, 25, 30, 35, 40, and 45) were also used as sensitivity analysis. HR and 95% CIs representing the survival effect of extended (red lines) and limited (half-transparent red lines) right thoracic esophagectomy as compared with left thoracic esophagectomy, were obtained by multivariable Cox proportional-hazard models. ESCC, esophageal squamous cell carcinoma; HR, hazard ratio; OS, overall survival.
Subset OS comparison by surgical proficiency
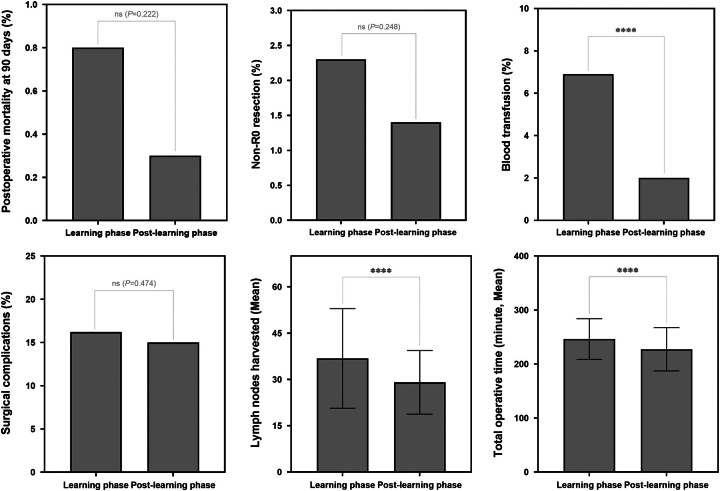
The proportion of right approach in the Southern center increased from 9.5% in 2009 to 84.9% in 2018 (Fig. 3A). Learning curve of right thoracic esophagectomy for that center showed the turning point for surgical failure occurring in April 2011, corresponding to the patient No. 125 (Fig. 3B). Right thoracic esophagectomy in the postlearning phase (i.e. proficient approach) showed shorter operation time, lower postoperative all-cause 90-day mortality, less positive surgical margin (non-R0 resection), lower proportion of intraoperative blood transfusion and in-hospital surgery-related complications than the right approach in the learning phase did (i.e. improficient approach), although some were without statistical significance (Fig. 4). In terms of lymph node yield, despite a decreasing trend, the average number of nodes resected in surgery during both learning and postlearning phases was considerably higher than the recommended threshold values of 15 nodes [mean (SD): 36.8 (16.1) vs. 29.1 (10.3), P<0.001].
Figure 3.
Subset analysis by the surgical proficiency for OS comparison of right vs. left thoracic esophagectomy for ESCC in Southern center. A. Trend in proportion of right thoracic esophagectomy for each admission year. B. Learning curve estimated by risk-adjusted-cumulative sum (RA-CUSUM) method [red point (patient No. 125) representing the highest point of the curve]. C–D. Kaplan–Meier survival curves for improficient right thoracic esophagectomy (conducted during the learning phase; red curve) versus left approach (blue curve) and proficient right left thoracic esophagectomy (conducted during the postlearning phase; red curve) versus left approach (blue curve). HR and 95% CIs were obtained by multivariable Cox proportional-hazard models. ESCC, esophageal squamous cell carcinoma; HR, hazard ratio; OS, overall survival.
Figure 4.
Changes in occurrence of secondary outcomes of ESCC patients receiving right thoracic esophagectomy during the learning period and the postlearning curve period in Southern center. Secondary outcomes were compared using multivariable generalized linear models, adjusting for age, sex, smoking, presence of comorbidities, tumor location, tumor morphology, pathological tumor-lymph node-metastasis (pTNM) stage grouping, tumor grade, and tumor size. ****P<0.001; ns, not statistically significant. ESCC, esophageal squamous cell carcinoma.
Overall, right approach was associated with increased OS compared with left approach in the Southern center (adjusted HR=0.78, 95% CI: 0.67–0.91, P=0.002) (Supplementary Figure S2, Supplemental Digital Content 4, http://links.lww.com/JS9/B329). In the subset analysis by surgical proficiency, even much better OS was observed for the proficient right approach versus left approach (adjusted HR=0.75, 95% CI: 0.64–0.88, P=0.001) (Fig. 3D), but not for the improficient right approach (adjusted HR=0.94, 95% CI: 0.71–1.24, P=0.666) (Fig. 3C).
Sensitivity analyses
Findings of sensitivity analyses for long-term OS and short-term outcomes were consistent across different analytic methods (propensity score-based Cox regressions, CUSUM analysis, RA-CUSUM analysis), tumor location (restricted to patients with middle and lower ESCC) (Fig. 2B), surgical techniques (restricted to patients receiving open esophagectomy), and surgical procedures (McKeown vs. left Sweet/Modified Sweet; McKeown vs. Sweet) (Supplementary Figure S3–S9, Supplemental Digital Content 5, http://links.lww.com/JS9/B330, Supplemental Digital Content 6, http://links.lww.com/JS9/B331, Supplemental Digital Content 7, http://links.lww.com/JS9/B332, Supplemental Digital Content 8, http://links.lww.com/JS9/B333, Supplemental Digital Content 9, http://links.lww.com/JS9/B334, Supplemental Digital Content 10, http://links.lww.com/JS9/B335, Supplemental Digital Content 11, http://links.lww.com/JS9/B336).
Discussion
For ESCC, the right thoracic esophagectomy is emerging in China and some other high-incidence regions, while the ʽtraditionalʼ left approach still prevails8. The right approach exhibits a significant advantage over the left approach in terms of harvesting more lymph nodes, which theoretically should improve survival assuming other conditions remain constant. However, previous studies comparing survival between right and left thoracic esophagectomy arrived at inconsistent results, hindering the adoption of the optimal surgical approach for ESCC. In this multicenter real-world cohort study, our findings demonstrated that right thoracic esophagectomy performed with adequate lymphadenectomy and surgical proficiency conferred much more favorable survival compared with left thoracic approach, hence should be recommended as the optimal surgical procedure for resectable ESCC patients. Moreover, the findings indicate that the assessment of surgical quality, which has been overlooked in prior studies, plays a determining role in comparison of long-term survival between surgical procedures.
Overall analysis in this study (i.e. ignoring surgical quality) showed more favorable survival in the right versus left approach, but without statistical significance, consistent with some previous studies14. However, by incorporating two dimensions of surgical quality, including extent of lymphadenectomy and surgical proficiency, we witnessed statistically significant survival superiority associated with the right thoracic approach over left approach. The black box was cracked open.
Subset analysis by the extent of lymphadenectomy showed that the survival benefit of right approach compared to left approach was largely dependent on the adequacy of lymph node dissection. A right approach can outperform a left approach for ESCC in terms of long-term OS if with adequate lymphadenectomy (adjusted HR resected ≥ 15 nodes=0.86, 95% CI: 0.77–0.95, P=0.003), but not if with limited lymphadenectomy. Since EC typically spreads via the lymphatic system, lymph node dissection is a major aspect of esophagectomy and an important index in evaluating the standardization of esophagectomy. In this study population, we have proved that the number of lymph nodes removed was an independent predictor of survival21, which adds weight to existing evidence that more extensive lymphadenectomy improves survival28. A potential mechanism could be that adequate lymph node dissection can ensure complete resection, accurate determination of the number of positive nodes (N staging), and facilitate informed choice of appropriate postoperative treatment26. In contrast with the left approach, the right approach can avoid the aortic arch and gain wide access to the thoracic and abdominal surgical fields, thus facilitating extended lymphadenectomy. However, the number of lymph node removed via right thoracic esophagectomy can be varied widely between hospitals and between surgeons within hospitals. This variation, particularly evident during the introduction phase of right thoracic esophagectomy, had been neglected by almost all of the previous studies and should be accounted for when evaluating the treatment effect of right versus left approach.
Subset analysis by surgical proficiency showed that proficient right approach conferred better OS than left approach (adjusted HR=0.75, 95% CI: 0.64–0.88, P=0.001), but improficient right approach did not have such survival advantage. Surgical proficiency may affect survival through increasing resection quality (e.g. more R0 resection margin) and guideline adherence29. Fewer complications and blood transfusion in esophagectomies performed during the postlearning phase might also reduce long-term morbidity, influencing nutritional and physical function. Additionally, with increasing proficiency, surgeons can refine the extent of lymphadenectomy to pursue the optimal balance between the benefit of lymph node dissection and the risk of surgical lesions30–33. Thus, surgical proficiency assessment is essential in studies comparing traditional and emerging techniques to fully adjust for the heterogeneity in surgical performance that may conceal the advantage, if it truly exists, of the emerging techniques.
Some limitations of this study are to be considered when interpreting its findings. First, patients treated with neoadjuvant therapy were not included in this study. Although neoadjuvant therapy is increasingly adopted to optimize outcomes for locally advanced ESCC following current clinical guidelines, its overall utilization remains generally limited in China (<10% in the two centers over the study period in this study). Second, the introduction of right thoracic esophagectomy in Northern center was earlier than the study period of the present study, rendering learning curve analysis impossible for that center.
Conclusion
In summary, based on large-scale real-world clinical data, we provide strong evidence that right thoracic esophagectomy performed with adequate lymphadenectomy and surgical proficiency can achieve much more favorable long-term survival for localized ESCC, as compared with the left approach. Our findings may expedite the broader adoption and dissemination of the superior right thoracic approach. Such a shift holds the promise of instigating positive transformations in the therapeutic landscape of ESCC in clinical practice. Our findings also illuminate that neglecting surgical quality in evaluations can obscure substantial differences in long-term prognosis. This revelation underscores the pressing need for future studies comparing surgical techniques to factor in the nuances of surgical quality.
Ethical approval
This study was conducted according to the principles of the Declaration of Helsinki. This study was approved by the Institutional Review Board of the Institutional Review Board of the Peking University School of Oncology, China (Approval number: 2018KT68).
Consent
Written informed consent was obtained from all participants.
Sources of funding
This work was supported by the National Key R&D Program of China (2021YFC2500405), the National Science & Technology Fundamental Resources Investigation Program of China (2019FY101102) and the Natural Science Foundation of Beijing Municipality (7182033).
Author contribution
Z.H. and Y.K.: conception and design; W.Y., L.C., R.X., J.K., B.H., L.Z., M.L., L.Z., F.Z., F.C., H.X., L.L., Y.H., M.L., Y.P., and Y.L.: acquisition of data; Z.H., F.L., W.Y., Y.H., and Z.L.: analysis and interpretation of data. All authors contributed in collection of data, manuscript writing, and final version approval.
Conflicts of interest disclosure
The authors declare no potential conflicts of interest.
Research registration unique identifying number (UIN)
1. Name of the registry: Clinical-based prognosis prediction of upper gastrointestinal cancer.
2. Unique identifying number or registration ID: “Chinese Clinical Trial Registry chictr.org.cn”. The registration ID is ChiCTR2200066670.
3. Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.chictr.org.cn/showproj.html?proj=183318.
Guarantor
Zhonghu He and Yang Ke.
Data availability statement
The primary data used to support the findings of this study are available from the corresponding author upon request.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge Dr. Michael A. McNutt for editing this manuscript.
Footnotes
Fangfang Liu, Wenlei Yang, Yu He, Wei Yang, Lei Chen, Ruiping Xu, and Zhen Liu contributed equally to this paper.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.lww.com/international-journal-of-surgery.
Published online 16 November 2023
Contributor Information
Fangfang Liu, Email: liufangfang919@foxmail.com.
Wenlei Yang, Email: yangwenlei@pku.edu.cn.
Yu He, Email: Yu.He1@lshtm.ac.uk.
Wei Yang, Email: yangwei_98@sina.com.
Lei Chen, Email: lei-chendoc@163.com.
Ruiping Xu, Email: Aywy05@126.com.
Zhen Liu, Email: liuzhen@bjmu.edu.cn.
Ji Ke, Email: 2211110729@stu.pku.edu.cn.
Bolin Hou, Email: houbl@linkdoc.com.
Liqun Zhang, Email: zh_liqun@163.com.
Miaoping Lin, Email: STLMP@163.com.
Linlin Liang, Email: lianglinlinlll@163.com.
Yi Huang, Email: 1109689755@qq.com.
Lixin Zhang, Email: zhanglx1000@126.com.
Fan Zhang, Email: lemon_fan@163.com.
Fen Cai, Email: caifen2012@126.com.
Huawen Xu, Email: 263420348@qq.com.
Mengfei Liu, Email: liumengfei1205@foxmail.com.
Yaqi Pan, Email: papi_727@126.com.
Ying Liu, Email: liuying88196762@126.com.
Zhonghu He, Email: zhonghuhe@foxmail.com.
Yang Ke, Email: keyang@bjmu.edu.cn.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 2. Arnold M, Soerjomataram I, Ferlay J, et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut 2015;64:381–387. [DOI] [PubMed] [Google Scholar]
- 3. Abnet CC, Arnold M, Wei W-Q. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology 2018;154:360–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liang H, Fan JH, Qiao YL. Epidemiology, etiology, and prevention of esophageal squamous cell carcinoma in China. Cancer Biol Med 2017;14:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391:1023–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rice TW, Rusch VW, Apperson-Hansen C, et al. Worldwide esophageal cancer collaboration. Dis Esophagus 2009;22:1–8. [DOI] [PubMed] [Google Scholar]
- 7. Watanabe M, Tachimori Y, Oyama T, et al. Comprehensive registry of esophageal cancer in Japan, 2013. Esophagus 2021;18:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mao YS, Gao SG, Wang Q, et al. Analysis of a registry database for esophageal cancer from high-volume centers in China. Dis Esophagus 2020;33:1–9. [DOI] [PubMed] [Google Scholar]
- 9. Xue Y, Chen D, Wang W, et al. Comparison of Ivor Lewis and Sweet esophagectomy for middle and lower esophageal squamous cell carcinoma: a systematic review and pooled analysis. EClinicalMedicine 2020;27:100497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li B, Hu H, Zhang Y, et al. Extended right thoracic approach compared with limited left thoracic approach for patients with middle and lower esophageal squamous cell carcinoma: three-year survival of a prospective, randomized, open-label trial. Ann Surg 2018;267:826–832. [DOI] [PubMed] [Google Scholar]
- 11. Zheng Y, Li Y, Liu X, et al. Right compared with left thoracic approach esophagectomy for patients with middle esophageal squamous cell carcinoma. Front Oncol 2020;10:536842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li B, Xiang J, Zhang Y, et al. Comparison of Ivor-Lewis vs Sweet esophagectomy for esophageal squamous cell carcinoma: a randomized clinical trial. JAMA Surg 2015;150:292–298. [DOI] [PubMed] [Google Scholar]
- 13. Wang J, Wei N, Jiang N, et al. Comparison of Ivor-Lewis versus Sweet procedure for middle and lower thoracic esophageal squamous cell carcinoma: A STROBE compliant study. Medicine 2019;98:e14416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mao Y, Yu Z, You B, et al. Society for Translational Medicine Expert consensus on the selection of surgical approaches in the management of thoracic esophageal carcinoma. J Thorac Dis 2019;11:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen D, Hu Y, Chen Y, et al. Comparison of outcomes between mckeown and sweet esophagectomy in the elderly patients for esophageal squamous cell carcinoma: a propensity score-matched analysis. Cancer Control : J Moffitt Cancer Center 2020;27:1073274820904700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peyre CG, Hagen JA, DeMeester SR, et al. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg 2008;248:549–556. [DOI] [PubMed] [Google Scholar]
- 17. Guo X, Wang Z, Yang H, et al. Impact of lymph node dissection on survival after neoadjuvant chemoradiotherapy for locally advanced esophageal squamous cell carcinoma: from the results of NEOCRTEC5010, a randomized multicenter study. Ann Surg 2023;277:259–266. [DOI] [PubMed] [Google Scholar]
- 18. Mao YS, Gao SG, Li Y, et al. Efficacy and safety of esophagectomy via left thoracic approach versus via right thoracic approach for middle and lower thoracic esophageal cancer: a multicenter randomized clinical trial (NST1501). Ann Transl Med 2022;10:904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Markar SR, Lagergren J. Surgical and surgeon-related factors related to long-term survival in esophageal cancer: a review. Ann Surg Oncol 2020;27:718–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heiden BT, Eaton DB, Jr, Chang SH, et al. Association between surgical quality metric adherence and overall survival among US veterans with early-stage non-small cell lung cancer. JAMA Surg 2023;158:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang W, Liu F, Xu R, et al. Is adjuvant therapy a better option for esophageal squamous cell carcinoma patients treated with esophagectomy? A prognosis prediction model based on multicenter real-world data. Ann Surg 2023;277:e61–e69. [DOI] [PubMed] [Google Scholar]
- 22. Liu F, Yang W, Yang W, et al. Minimally invasive or open esophagectomy for treatment of resectable esophageal squamous cell carcinoma? answer from a real-world multicenter study. Ann Surg 2023;277:e777–e784. [DOI] [PubMed] [Google Scholar]
- 23. Tian H, Yang W, Hu Y, et al. Estimating cancer incidence based on claims data from medical insurance systems in two areas lacking cancer registries in China. EClinicalMedicine 2020;20:100312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang GQ, Abnet CC, Shen Q, et al. Histological precursors of oesophageal squamous cell carcinoma: results from a 13 year prospective follow up study in a high risk population. Gut 2005;54:187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol 2010;17:1721–1724. [DOI] [PubMed] [Google Scholar]
- 26. National Comprehensive Cancer Network . Esophageal and Esophagogastric Junction Cancers. Version 2.2023 March 10, 2023. www.nccn.org/patients.
- 27. Mathew G, Agha R, Albrecht J, et al. STROCSS 2021: strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg 2021;96:106165. [DOI] [PubMed] [Google Scholar]
- 28. Visser E, Markar SR, Ruurda JP, et al. Prognostic value of lymph node yield on overall survival in esophageal cancer patients: a systematic review and meta-analysis. Ann Surg 2019;269:261–268. [DOI] [PubMed] [Google Scholar]
- 29. Markar SR, Mackenzie H, Lagergren P, et al. Surgical proficiency gain and survival after esophagectomy for cancer. J Clin Oncol 2016;34:1528–1536. [DOI] [PubMed] [Google Scholar]
- 30. Woo Y, Goldner B, Ituarte P, et al. Lymphadenectomy with optimum of 29 lymph nodes retrieved associated with improved survival in advanced gastric cancer: a 25,000-patient international database study. J Am Coll Surg 2017;224:546–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rizk NP, Ishwaran H, Rice TW, et al. Optimum lymphadenectomy for esophageal cancer. Ann Surg 2010;251:46–50. [DOI] [PubMed] [Google Scholar]
- 32. Moloney K, Janda M, Frumovitz M, et al. Development of a surgical competency assessment tool for sentinel lymph node dissection by minimally invasive surgery for endometrial cancer. Int J Gynecol Cancer 2021;31:647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McMasters KM, Wong SL, Chao C, et al. Defining the optimal surgeon experience for breast cancer sentinel lymph node biopsy: a model for implementation of new surgical techniques. Ann Surg 2001;234:292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The primary data used to support the findings of this study are available from the corresponding author upon request.